Introduction
Cataracts have a high morbidity rate worldwide
(1,2) and account for ~47.8% of the cases of
blindness in individuals (3).
Various factors result in the formation of cataracts, including
age, diabetes and ultraviolet light exposure, with aging remaining
the primary risk factor for cataract formation (4). For instance, age-related cataracts
affect 46% of individuals with visual impairment (5-7).
Therefore, it remains a priority to identify effective therapeutic
targets for the treatment of cataracts to decrease the incidence of
cataracts and blindness.
Currently, apoptosis has become a research hotspot
in the area of ophthalmology. As the lens develops during the
morphogenesis process, apoptosis serves as an important determinant
for sustaining the normal conditions in the lens (8). The induction or reduction of
apoptosis, due to genetic manipulation/mutations and/or
environmental factors, has been shown to generate abnormal lenses
or result in the absence of the ocular lens (9). In humans and animals, the presence of
apoptosis in LECs has been identified to be frequently involved in
the development of cataracts, which is a non-congenital condition
(10).
MicroRNAs (miRNAs/miRs) are a subgroup of small
non-coding RNAs of 20-25 nucleotides in length, which control
post-transcriptional gene expression (11). miRNAs regulate the translation or
degradation of target mRNAs by complementary binding to the
3'-untranslated region (UTR) of their target genes (12). miRNAs have been shown to serve roles
in cell proliferation, apoptosis and differentiation (13). Numerous miRNAs have been reported to
regulate the apoptosis of LECs in cataracts. For example, miR-221
induced LEC apoptosis by targeting sirtuin 1 (SIRT1) and
transcription factor E2F3(14), and
miR-23b-3p promoted LEC apoptosis and autophagy by targeting
SIRT1(15). In addition, the
expression levels of miR-23a were demonstrated to be upregulated in
cataractous lenses (16). However,
to the best of our knowledge, whether miR-23a-3p targets mRNAs in
cataracts remains unknown. Therefore, determining the role of
miR-23a-3p may provide a potential therapeutic target for the
treatment of patients with cataracts.
Materials and methods
Cell culture
HLE-B3 cells were obtained from the American Type
Culture Collection. HLE-B3 cells were cultured in minimum essential
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin, and maintained in a humidified incubator
with 5% CO2 at 37˚C.
Oxidants induce cell apoptosis and trigger the
development of cataracts (10). As
peroxidative damage is mediated by the toxic metabolites of oxygen,
such as hydroxide, H2O2 is frequently used to
induce the apoptosis of LECs in vitro. For the establishment
of an in vitro cataract model, HLE-B3 cells
(1x106 cells/well) were seeded into 6-well plates and
induced at 37˚C with 200 µmol/l H2O2
(Sigma-Aldrich; Merck KGaA) for 24 h, as previously described
(17,18), while cells in control group were
untreated.
Cell transfection
The miR-negative control (NC) mimic, miR-23a-3p
mimic, miR-NC inhibitor and miR-23a-3p inhibitor, in addition to
small interfering RNA (siRNA) targeting BCL2 (siRNA-BCL2) and
siRNA-NC, were all synthesized by Shanghai GenePharma Co., Ltd. The
50 nM miR-23a-3p mimic (5'-CCUUUAGGGACCGUUACACUA-3') or 100 nM
miR-23a-3p inhibitor (5'-UAGUGUAACGGUCCCUAAAGG-3') and their
respective NCs (miR-NC mimic, 5'-CGAGCUCACUGGACAACGCCG-3' and
miR-NC inhibitor, 5'-AGCUUAAGACAUUCCGAGGAAU-3') were transiently
transfected into HLE-B3 cells using Lipofectamine®
RNAiMAX reagent (Invitrogen; Thermo Fisher Scientific, Inc.), after
incubation at 37˚C for 48 h, cells were collected for the
subsequent experimentation. For the transient transfection of 50 nM
siRNA-NC (anti-sense, 5'-UGAGACAAUGCAUGCAGUACGG-3', sense,
5'-AUCGCAACAUAGACAGCUAACAG-3') and siRNA-BCL2 (anti-sense,
5'-UUCACAUUUAUAAACUAUUUGU-3', sense, 5'-AACAAAUAGUUUAUAAAUGUGAA-3')
into HLE-B3 cells, Lipofectamine 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used, after incubation at 37˚C for 48
h, cells were collected for the subsequent experimentation.
Cell treatment
Briefly, control or transiently transfected HLE-B3
cells were seeded (1x106 cells/well) in 6-well plates
and incubated overnight at 37˚C. Following which, HLE-B3 cells were
treated with or without 200 µmol/l H2O2 for
24 h at 37˚C before the conduction of the subsequent
experiments.
Dual luciferase reporter assay
Using the online software TargetScan 7.1 (www.targetscan.org/vert_71/), it was found that
miR-23a-3p was complementary to BCL2. The wild-type (WT) or mutant
(MUT) BCL2 3'-UTR containing the binding site for miR-23a-3p was
cloned into a pGL3 plasmid (Promega Corporation). The miR-23a-3p
mimic or miR-NC mimic were co-transfected with pGL3-WT-BCL2 or
pGL3-MUT-BCL2 into HLE-B3 cells using Lipofectamine®
2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.). After
incubation at 37˚C for 48 h, cells were collected. Luciferase
activity was measured using a dual-luciferase reporter assay system
(Promega Corporation) normalized to Renilla luciferase
activity in each group.
Cell proliferation assay
HLE-B3 cells were seeded into a 96-well plate and
incubated overnight as aforementioned. Subsequently, 10 µl Cell
Counting Kit-8 (CCK-8) reagent (Dojindo Molecular Technologies,
Inc.) was added to the HLE-B3 cells and incubated for 4 h. The cell
proliferation was measured at an absorbance of 450 nm using a
microplate reader (BioTek Instruments, Inc.).
Flow cytometric analysis of
apoptosis
HLE-B3 cell apoptosis was analyzed using an Annexin
V-FITC/propidium iodide (PI) apoptosis detection kit (BD
Biosciences). Briefly, 1x104 HLE-B3 cells/well were
cultured in six-well plates, digested using 0.25% trypsin without
EDTA and resuspended in 500 µl Annexin binding buffer.
Subsequently, the cells were incubated with 5 µl Annexin V-FITC and
5 µl PI in the dark for 15 min. Apoptotic cells were analyzed using
a fluorescence-activated cell sorting system (FACSVantage; BD
Biosciences) and CellQuest software (version 5.1; BD
Biosciences).
Reverse transcription-quantitative
PCR
Total RNA was extracted from HLE-B3 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA was reverse transcribed into cDNA using a
RevertAid RT reverse transcription kit (Invitrogen; Thermo Fisher
Scientific, Inc.), incubated at 25˚C for 5 min, 60 min at 42˚C,
then terminated at 70˚C for 5 min. qPCR was subsequently performed
using a SYBR-Green PCR kit (Takara Bio, Inc.). The following
thermocycling conditions were used: Initial denaturation at 95˚C
for 10 min, and 35 cycles of 95˚C for 10 sec and annealing at 60˚C
for 30 sec, after which a melting curve analysis was set from 60˚C
to 90˚C. The following primers were used: BCL2 forward,
5'-AACAAATAGTTTATAAATGTGAA-3' and reverse,
5'-TTCACATTTATAAACTATTTGTT-3'; miR-23a-3p forward,
5'-CCTTTAGGGACCGTTACACTA-3' and reverse
5'-TAGTGTAACGGTCCCTAAAGG-3'; GAPDH forward,
5'-AAGAAGGTGGTGAAGCAGGC-3' and reverse 5'-GTCAAAGGTGGAGGAGTGGG-3';
and U6 forward, 5'-CTCGCTTCGGCAGCACATA-3' and reverse,
5'-CAGTGCAGGGTCCGAGGTA-3'. The expression levels were quantified
using the 2-∆∆Cq method (19) and the relative expression levels of
BCL2 and miR-23a-3p were normalized to GAPDH and U6,
respectively.
Western blotting
Total protein was extracted from HLE-B3 cells using
RIPA lysis buffer supplemented with a protein inhibitor cocktail
(Roche Applied Science). Protein concentration determination was
carried out using a BCA kit (Thermo Fisher Scientific, Inc.).
Protein samples (15 µg per lane) were separated via 8% SDS-PAGE and
the separated proteins were transferred onto PVDF membranes. The
PVDF membranes were blocked with 5% non-fat milk at room
temperature for 1 h and then incubated with anti-BCL2 (cat. no.
4223; 1:1,000; Cell Signaling Technology, Inc.), anti-caspase-3
(cat. no. 14220, 1:1,000; Cell Signaling Technology, Inc.),
anti-caspase-8 (cat. no. 4790; 1:1,000; Cell Signaling Technology,
Inc.) and anti-GAPDH (cat. no. 2118; 1:1,000; Cell Signaling
Technology, Inc.) primary antibodies overnight at 4˚C. Following
the primary antibody incubation, the membranes were incubated with
a horseradish peroxidase-conjugated IgG secondary antibody (cat.
no. 5127; 1:2,000; Cell Signaling Technology, Inc.) at room
temperature for 2 h. Protein bands were visualized using an ECL
chemiluminescence Substrate Reagent kit (Pierce; Thermo Fisher
Scientific, Inc.). The densitometry of protein was normalized to
GAPDH and analyzed using ImageJ (version 1.5.2; National Institutes
of Health).
Statistical analysis
Each experiment was repeated ≥3 times and data are
presented as the mean ± SD. Statistical differences between two
groups were analyzed using a two-tailed unpaired Student's t-test,
whereas comparisons among three groups were analyzed using one-way
ANOVA followed by Newman-Keuls test. P<0.05 was considered to
indicate a statistically significant difference.
Results
MiR-23a-3p expression levels are
upregulated in H2O2-induced HLE-B3 cells
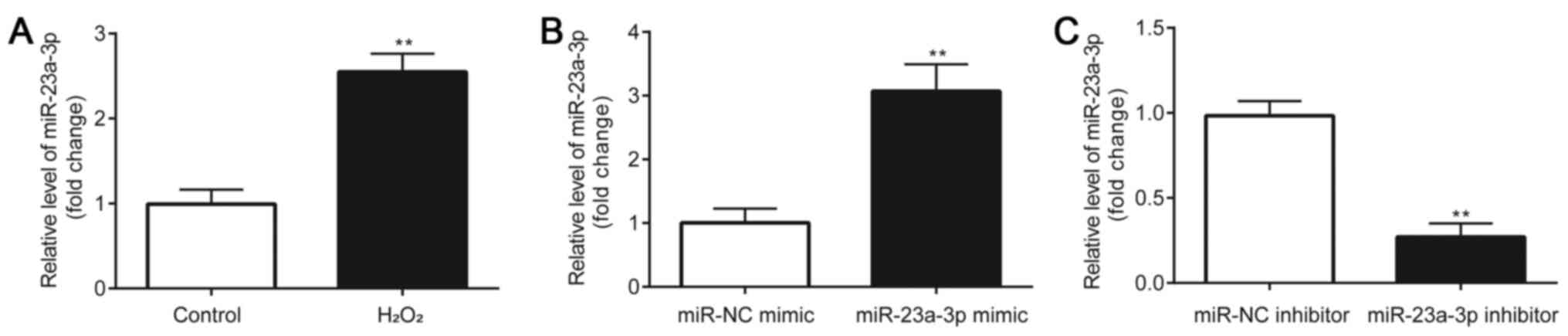
A significant upregulation of miR-23a-3p expression
levels was observed in H2O2-induced HLE-B3
cells compared with the control group (Fig. 1A). Subsequently, the effects of the
inhibition of miR-23a-3p expression in
H2O2-induced HLE-B3 cells were investigated.
HLE-B3 cells were first transfected with a miR-23a-3p mimic or
inhibitor and the transfection efficiency was verified. Compared
with the miR-NC mimic group, HLE-B3 cells transfected with the
miR-23a-3p mimic had significantly increased miR-23a-3p expression
levels (Fig. 1B). Conversely,
compared with the miR-NC inhibitor group, HLE-B3 cells transfected
with the miR-23a-3p inhibitor had significantly downregulated
expression levels of miR-23a-3p (Fig.
1C). These results indicated the successful transfection of the
miR-23a-3p mimic or inhibitor into HLE-B3 cells.
Inhibition of miR-23a-3p attenuates
the H2O2-induced decrease in proliferation of
HLE-B3 cells
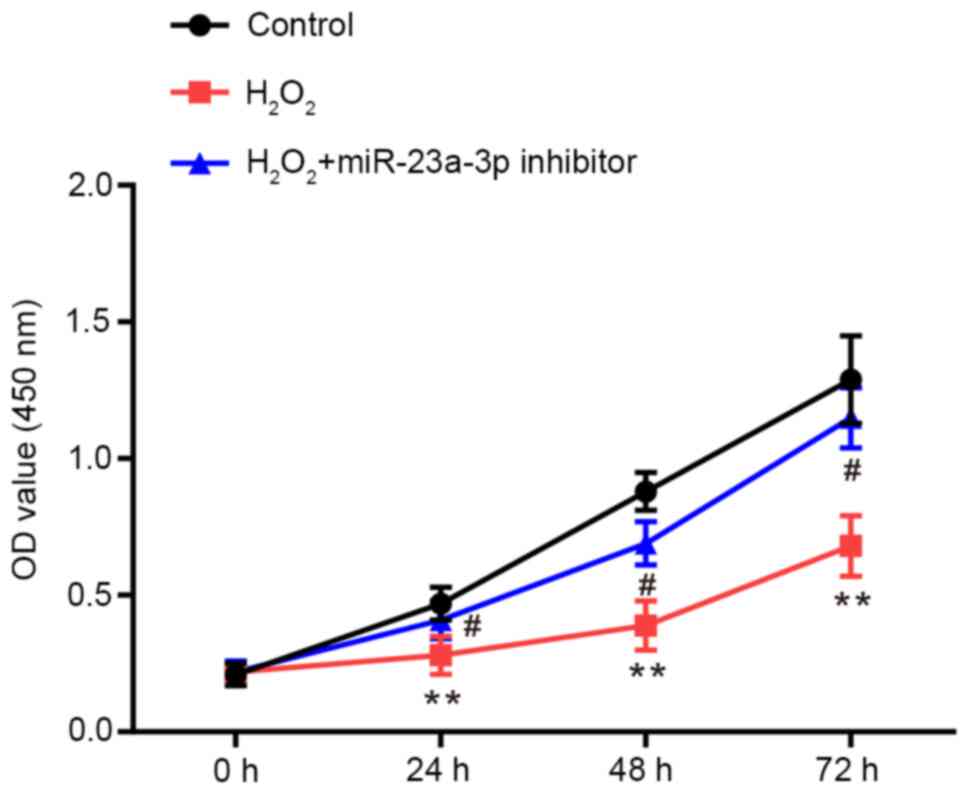
CCK-8 assays were performed to determine the
proliferative ability of HLE-B3 cells. Compared with the control
group, the proliferative rate of HLE-B3 cells was significantly
repressed by H2O2, which was rescued by the
transfection with the miR-23a-3p inhibitor (Fig. 2).
Inhibition of miR-23a-3p attenuates
H2O2-induced apoptosis in HLE-B3 cells
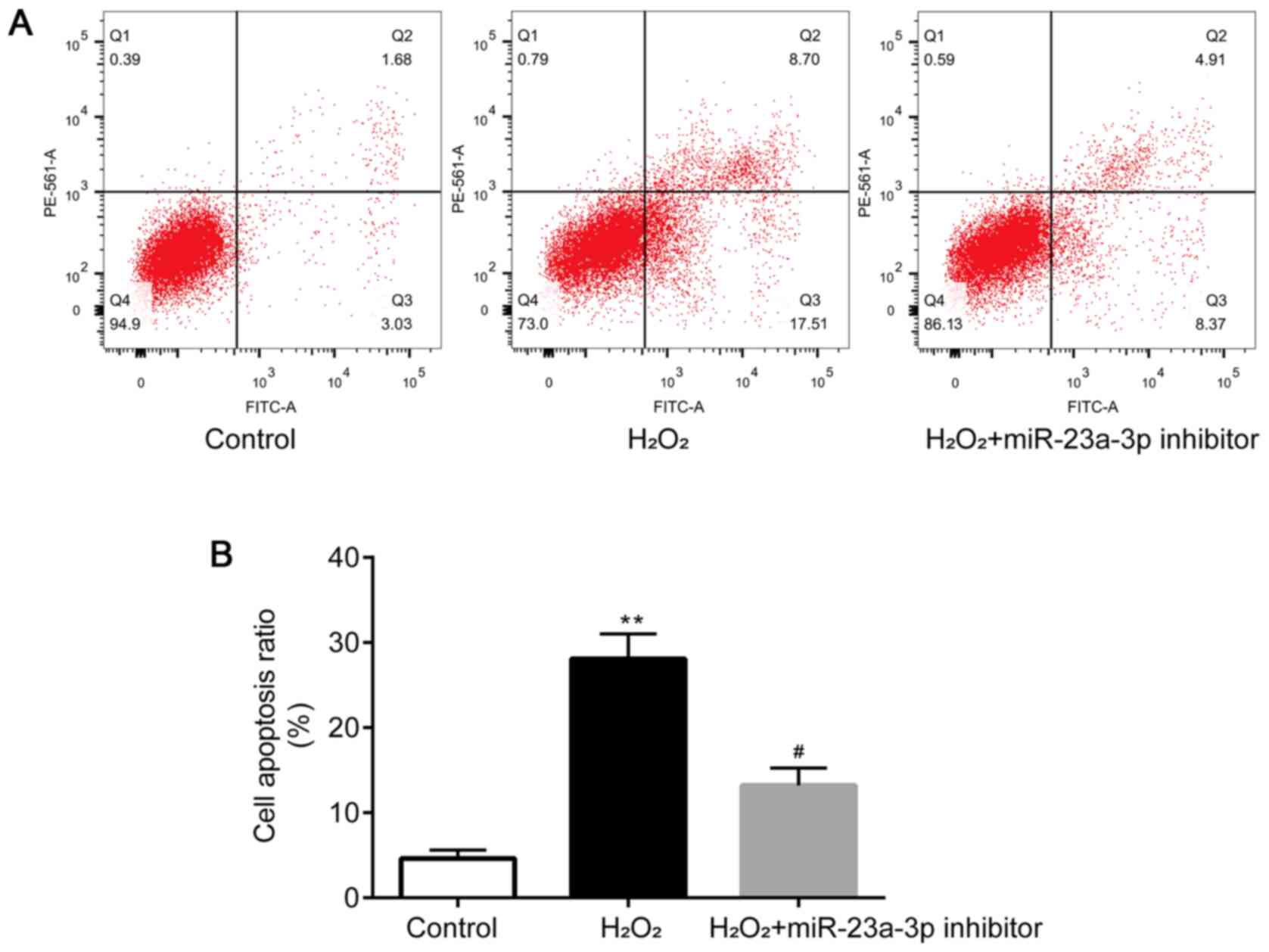
Flow cytometry was performed to determine the levels
of apoptosis in HLE-B3 cells. Compared with the control group,
HLE-B3 cell apoptosis was significantly induced by
H2O2, which was then attenuated by the
transfection with the miR-23a-3p inhibitor (Fig. 3A and B). Taken together, these findings
suggested that the miR-23a-3p inhibitor may protect HLE-B3 cells
from H2O2-induced injury.
BCL2 is a target of miR-23a-3p in
HLE-B3 cells
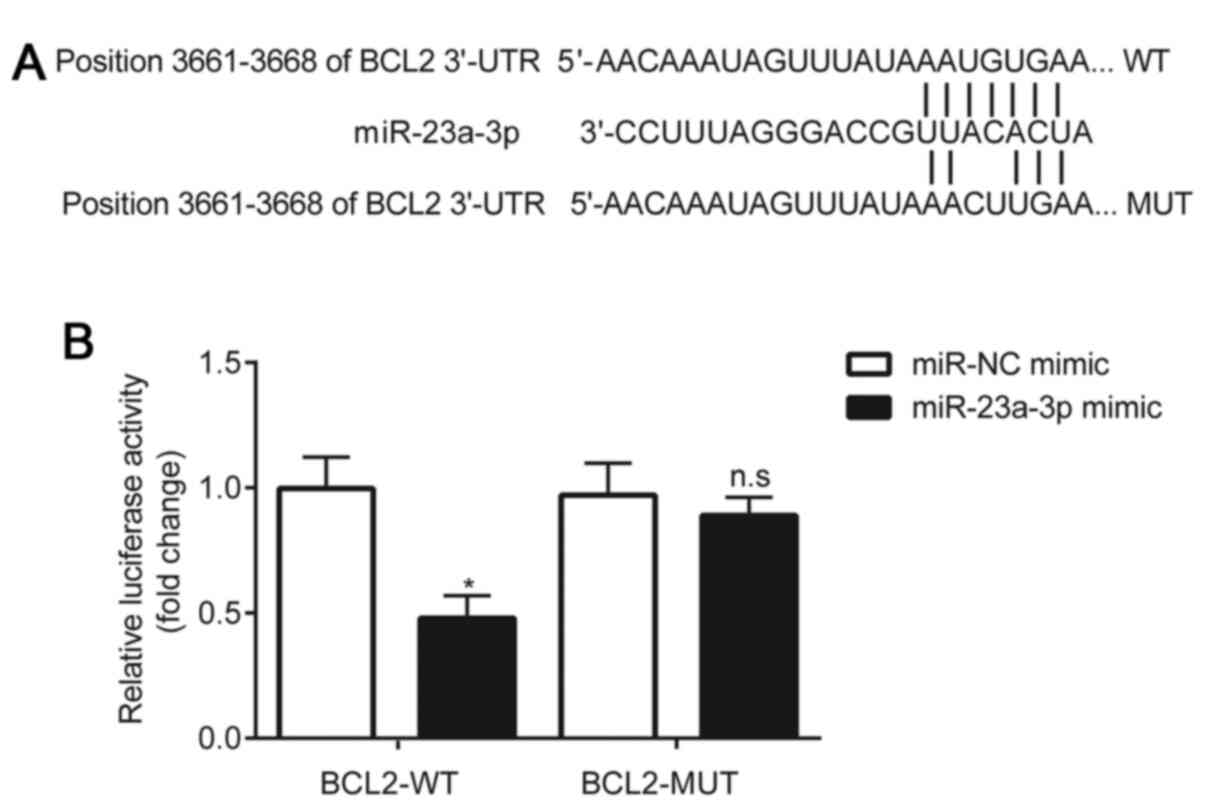
Using the online software, TargetScan 7.1, the
3'-UTR of BCL2 was predicted to be complementary to miR-23a-3p
(Fig. 4A). A dual luciferase
reporter assay was subsequently performed to validate the
interaction between miR-23a-3p and BCL2. The results demonstrated
that compared with the miR-NC mimic, the miR-23a-3p mimic
significantly reduced the relative luciferase activity of the
HLE-B3 cells transfected with pGL3-WT-BCL2. However, in HLE-B3
cells transfected with pGL3-MUT-BCL2, no significant differences
were observed in the relative luciferase activity between the
miR-NC mimic and miR-23a-3p mimic groups (Fig. 4B).
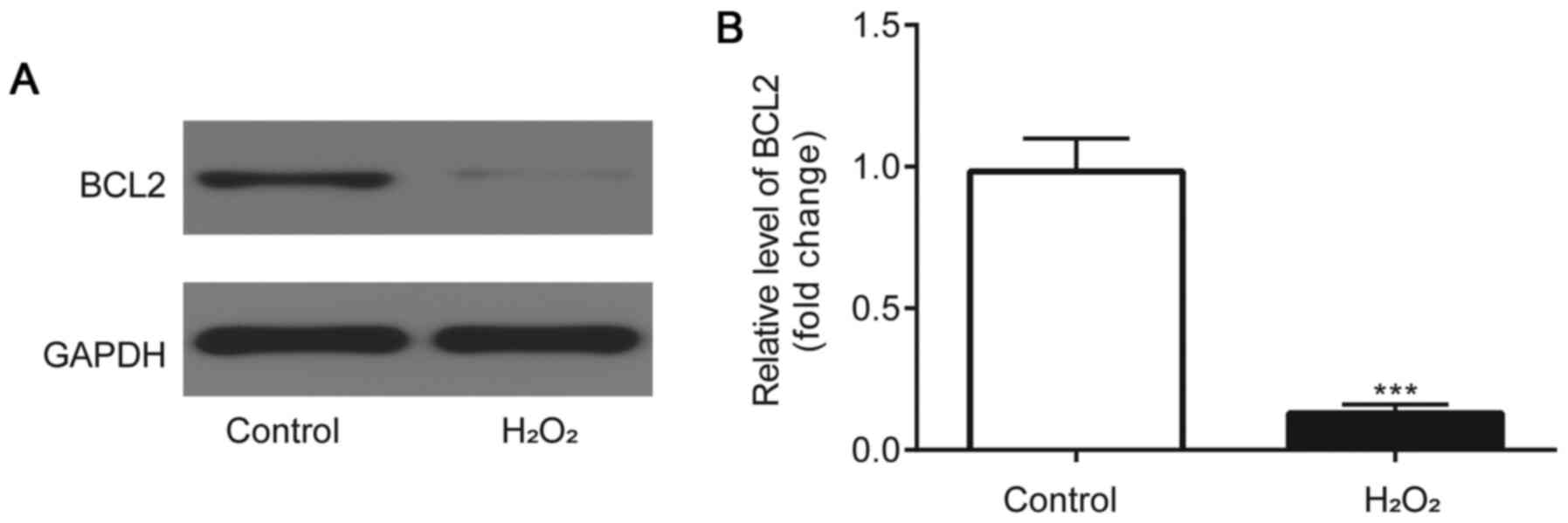
BCL2 expression levels are
downregulated in H2O2-induced HLE-B3
cells
Western blotting was used to analyze BCL2 protein
expression levels. Compared with the control group, BCL2 protein
expression levels were identified to be significantly downregulated
in the HLE-B3 cells incubated with H2O2
(Fig. 5A and B).
miR-23a-3p inhibitor attenuates the
H2O2-induced reduction of proliferation of
HLE-B3 cells by targeting BCL2
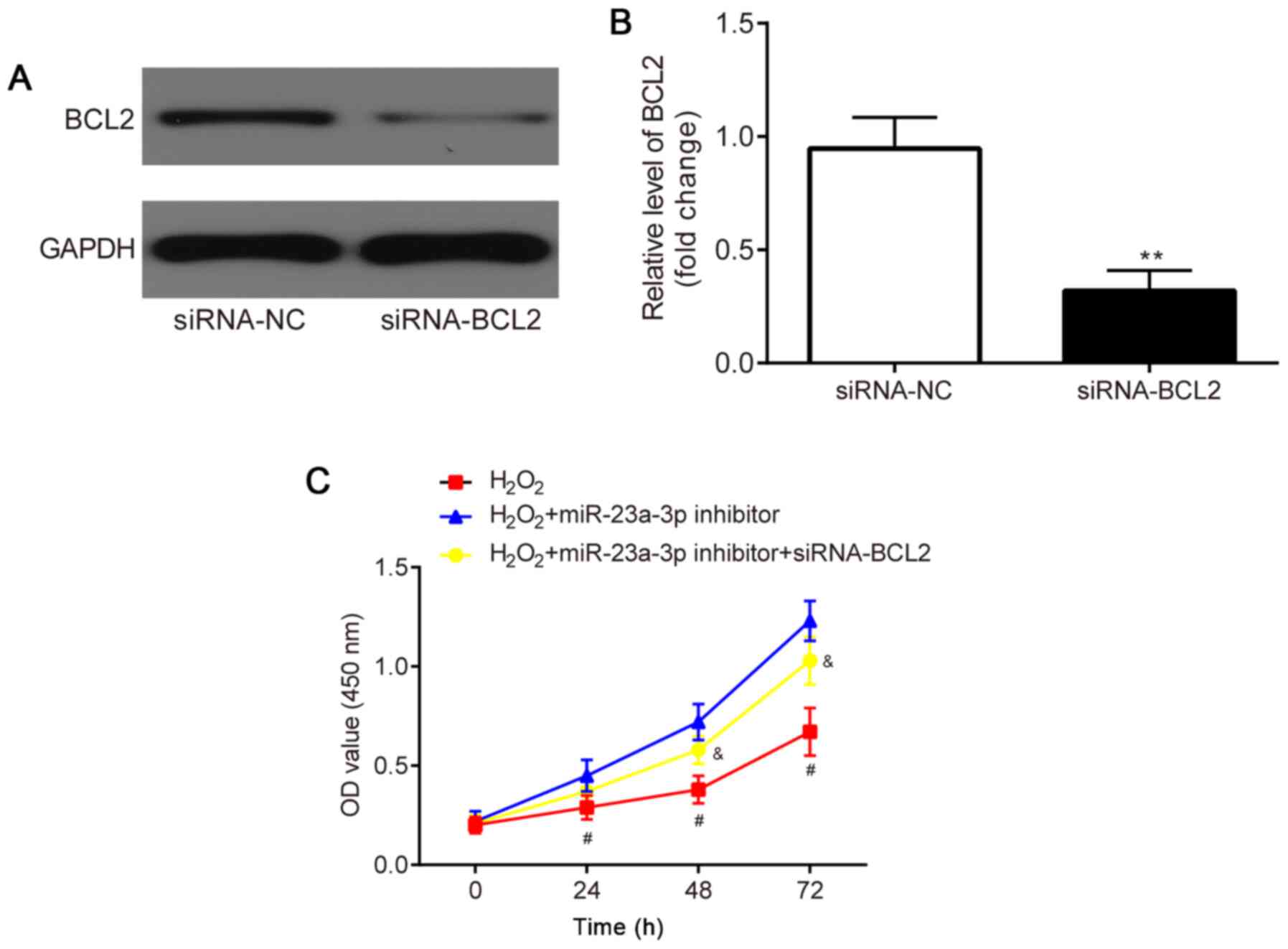
The effects of the co-transfection of siRNA-BCL2 and
miR-23a-3p inhibitor in H2O2-induced HLE-B3
cells were subsequently investigated. HLE-B3 cells were first
transfected with siRNA-NC or siRNA-BCL2 to verify the transfection
efficacy. The results revealed that compared with the siRNA-NC
group, the protein expression levels of BCL2 were significantly
downregulated in the siRNA-BCL2 group (Fig. 6A and B).
A CCK-8 assay was performed to determine the
proliferative ability of the HLE-B3 cells. Compared with the
H2O2 group, the miR-23a-3p inhibitor
increased the proliferation of the HLE-B3 cells, which was
subsequently partially reversed through the co-transfection with
siRNA-BCL2 (Fig. 6C).
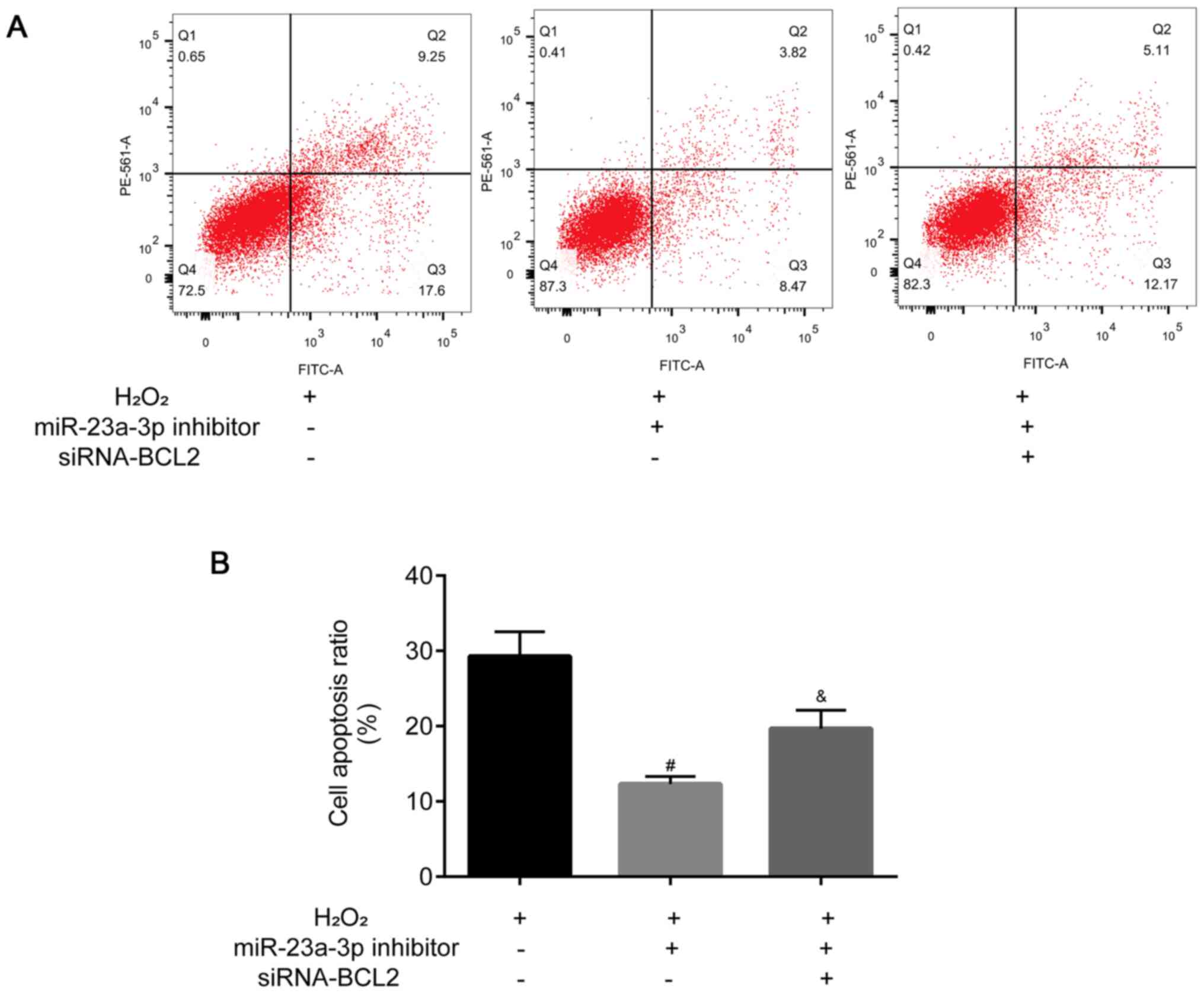
miR-23a-3p inhibitor attenuates
H2O2-induced apoptosis in HLE-B3 cells by
targeting BCL2
Flow cytometric analysis was used to analyze the
levels of apoptosis in HLE-B3 cells. The levels of HLE-B3 cell
apoptosis were decreased following the transfection with the
miR-23a-3p inhibitor compared with the H2O2
group, which was then partially reversed by the co-transfection
with siRNA-BCL2 (Fig. 7A and
B).
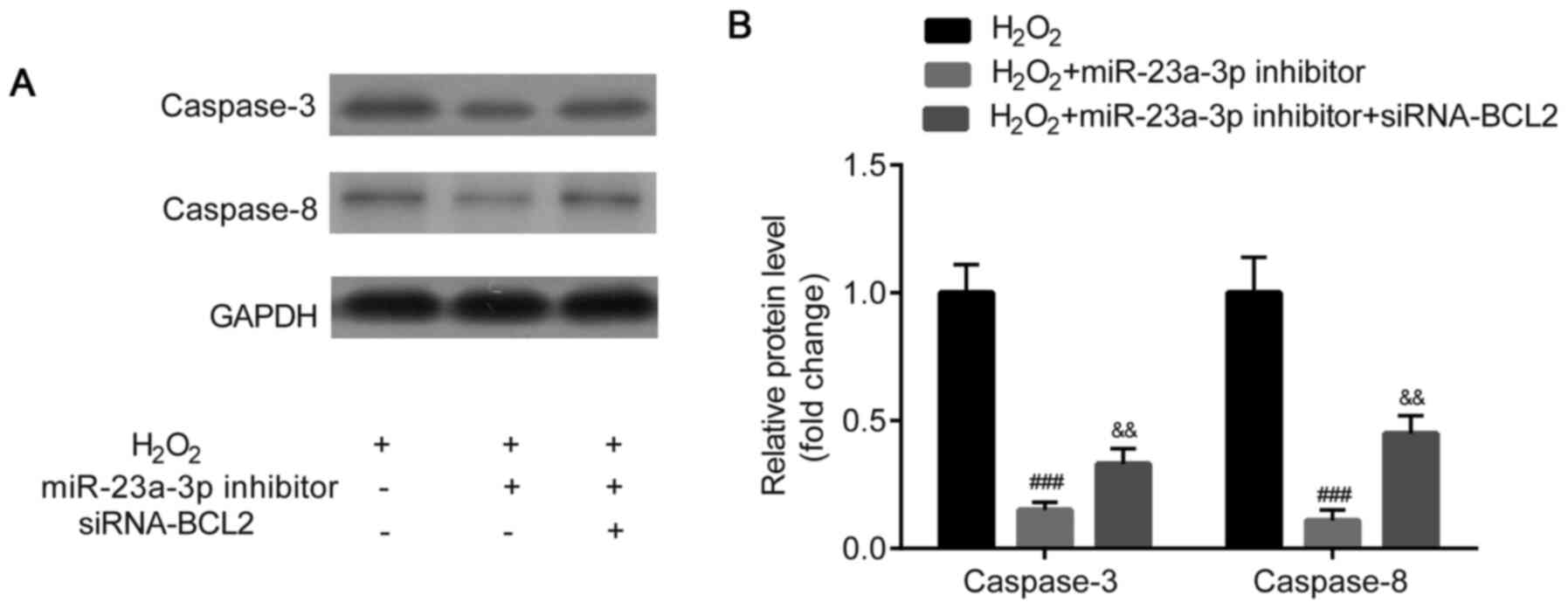
Western blotting was used to analyze caspase-3 and
caspase-8 protein expression levels. Caspase-3 and caspase-8
protein expression levels were identified to be significantly
downregulated in the HLE-B3 cells following the transfection with
the miR-23a-3p inhibitor compared with the
H2O2 group, which was then partially reversed
by the co-transfection with siRNA-BCL2 (Fig. 8A and B).
Discussion
Previous microarray analysis reported the
dysregulation of multiple miRNAs in cataractous lenses, including
miR-23a (16); however, to the best
of our knowledge, the exact function of miR-23a-3p in cataracts
remains undetermined.
Oxidants have been shown to induce apoptosis and
result in the development of cataracts (10). Therefore, to establish an in
vitro cataract model in the present study, HLE-B3 cells were
induced with H2O2, as described in a previous
study (17,18). miR-23a-3p expression levels were
revealed to be upregulated in H2O2-induced
HLE-B3 cells, which suggested the potential involvement of
miR-23a-3p in cataract development and provided further evidence
for the role of miR-23a-3p in cataracts, as previously reported
(16).
The apoptosis of LECs, which is induced by oxidative
stress, is a cellular mechanism frequently occurring in cataracts
(20). Accumulating evidence
suggests the involvement of miRNAs in the apoptosis of LECs; for
example, in cataracts, miR-let-7b promoted LEC apoptosis by
targeting leucine-rich repeat containing G protein-coupled receptor
4(21); miR-378a was shown to
increase LEC apoptosis by targeting the superoxide dismutase 1 gene
(22); and miR-26a and miR-26b
reduced lens fibrosis by regulating the Jagged-1/Notch signaling
pathway (23). The present study
demonstrated that the inhibition of miR-23a-3p expression levels
reduced the H2O2-induced apoptosis of HLE-B3
cells. However, to the best of our knowledge, the potential target
mRNAs of miR-23a-3p remained to be investigated.
In the present study, miR-23a-3p was predicted and
verified to target BCL2, an anti-apoptotic gene family member, in
HLE-B3 cells, which may improve the current understanding of the
role of miR-23a-3p in numerous types of human disease (24,25).
In a previous study, BCL2 reduced cell apoptosis by acting via
cellular signal transduction pathways or inhibiting lipid oxidation
via inhibition of oxygen free radicals (26). BCL2 protein expression level was
lower in the lens epithelium of elderly individuals compared with
that of human fetuses and children (27). BCL2 was reported to be associated
with cell apoptosis in oxidative stress-induced cataracts; for
example, BCL2 protein expression levels were reduced in LECs if
cell apoptosis was induced (28),
and anthocyanin was shown to protect HLECs against oxidative damage
and prevent the H2O2-induced downregulation
of BCL2(29). In addition, the
downregulation of Smac expression levels attenuated the
H2O2-induced apoptosis and downregulation of
BCL2 expression levels in HLECs (30). Furthermore, ELL-associated factor 2
prevented HLECs from oxidative stress-induced apoptosis and the
downregulation of BCL2 expression levels by targeting the Wnt
signaling pathway (31).
Previously, the 3'-UTR of BCL2 was discovered to be targeted by
several miRNAs in cataracts. For example, miR-34a induced HLEC
apoptosis by targeting BCL2(32)
and miR-15a-3p repressed the proliferation and promoted the
apoptosis of HLECs by targeting BCL2 (17,33).
However, to the best of our knowledge, whether miR-23a-3p can
regulate the formation of cataracts by targeting BCL2 remained
undetermined. In the present study, BCL2 protein expression levels
were significantly downregulated in
H2O2-induced HLE-B3 cells. In addition, the
miR-23a-3p inhibitor was found to attenuate
H2O2-induced apoptosis and the inhibition of
proliferation in HLE-B3 cells by targeting BCL2. However, the
present study was an in vitro investigation, which suggested
that targeting BCL2 may be useful for treating cataracts;
therefore, further in vivo studies are required to confirm
these findings.
Caspase-3 and caspase-8 were previously demonstrated
to be positively associated with the apoptosis of HLECs (34,35).
Therefore, the protein expression levels of caspase-3 and caspase-8
were also evaluated in the present study. The results revealed that
caspase-3 and caspase-8 protein expression levels were
downregulated following the transfection with the miR-23a-3p
inhibitor compared with the H2O2 group;
however, the downregulated expression levels were reversed
following the transfection with siRNA-BCL2.
In conclusion, the findings of the present study
indicated that the inhibition of miR-23a-3p expression levels may
attenuate H2O2-induced injury of human lens
epithelial cells by targeting Bcl-2 in an in vitro model of
cataract by targeting BCL2, thus providing a novel therapeutic
target for the treatment of patients with cataracts.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PY conceived the study, performed the experiments
and analyzed the data. XM analyzed the data. JJ, ZC, YH and YW
performed the experiments and analyzed the data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee CM and Afshari NA: The global state of
cataract blindness. Curr Opin Ophthalmol. 28:98–103.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liu YC, Wilkins M, Kim T, Malyugin B and
Mehta JS: Cataracts. Lancet. 390:600–612. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Khairallah M, Kahloun R, Bourne R, Limburg
H, Flaxman SR, Jonas JB, Keeffe J, Leasher J, Naidoo K, Pesudovs K,
et al: Number of people blind or visually impaired by cataract
worldwide and in world regions, 1990 to 2010. Invest Ophthalmol Vis
Sci. 56:6762–6769. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hodge WG, Whitcher JP and Satariano W:
Risk factors for age-related cataracts. Epidemiol Rev. 17:336–346.
1995.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kempen JH, Sugar EA, Varma R, Dunn JP,
Heinemann MH, Jabs DA, Lyon AT and Lewis RA: Studies of Ocular
Complications of AIDS Research Group. Risk of cataract among
subjects with acquired immune deficiency syndrome free of ocular
opportunistic infections. Ophthalmology. 121:2317–2324.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Keel S and He M: Risk factors for
age-related cataract. Clin Exp Ophthalmol. 46:327–328.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jiang H, Yin Y, Wu CR, Liu Y, Guo F, Li M
and Ma L: Dietary vitamin and carotenoid intake and risk of
age-related cataract. Am J Clin Nutr. 109:43–54. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim B, Kim SY and Chung SK: Changes in
apoptosis factors in lens epithelial cells of cataract patients
with diabetes mellitus. J Cataract Rcfract Surg. 38:1376–1381.
2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yan Q, Liu JP and Li DW: Apoptosis in lens
development and pathology. Differentiation. 74:195–211.
2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li WC, Kuszak JR, Dunn K, Wang RR, Ma W,
Wang GM, Spector A, Leib M, Cotliar AM, Weiss M, et al: Lens
epithelial cell apoptosis appears to be a common cellular basis for
non-congenital cataract development in humans and animals. J Cell
Biol. 130:169–181. 1995.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kim VN: MicroRNA biogenesis: Coordinated
cropping and dicing. Nat Rev Mol Cell Biol. 6:376–385.
2005.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297.
2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gong W, Li J, Wang Y, Meng J and Zheng G:
miR-221 promotes lens epithelial cells apoptosis through
interacting with SIRT1 and E2F3. Chem Biol Interact. 306:39–46.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhou W, Xu J, Wang C, Shi D and Yan Q:
miR-23b-3p regulates apoptosis and autophagy via suppressing SIRT1
in lens epithelial cells. J Cell Biochem. 120:19635–19646.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wu C, Lin H, Wang Q, Chen W, Luo H, Chen W
and Zhang H: Discrepant expression of microRNAs in transparent and
cataractous human lenses. Invest Ophthalmol Vis Sci. 53:3906–3912.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li Q, Pan H and Liu Q: MicroRNA-15a
modulates lens epithelial cells apoptosis and proliferation through
targeting B-cell lymphoma-2 and E2F transcription factor 3 in
age-related cataracts. Biosci Rep. 39(BSR20191773)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ren H, Tao H, Gao Q, Shen W, Niu Z, Zhang
J, Mao H, Du A and Li W: miR-326 antagomir delays the progression
of age-related cataract by upregulating FGF1-mediated expression of
betaB2-crystallin. Biochem Biophys Res Commun. 505:505–510.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang ZF, Zhang J, Hui YN, Zheng MH, Liu
XP, Kador PF, Wang YS, Yao LB and Zhou J: Up-regulation of NDRG2 in
senescent lens epithelial cells contributes to age-related cataract
in human. PLoS One. 6(e26102)2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dong Y, Zheng Y, Xiao J, Zhu C and Zhao M:
MicroRNA let-7b induces lens epithelial cell apoptosis by targeting
leucine-rich repeat containing G protein-coupled receptor 4 (Lgr4)
in age-related cataract. Exp Eye Res. 147:98–104. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu Y, Li HH and Liu Y: microRNA-378a
regulates the reactive oxygen species (ROS)/Phosphatidylinositol
3-Kinases (PI3K)/AKT signaling pathway in human lens epithelial
cells and cataract. Med Sci Monit. 25:4314–4321. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen X, Xiao W, Chen W, Liu X, Wu M, Bo Q,
Luo Y, Ye S, Cao Y and Liu Y: MicroRNA-26a and -26b inhibit lens
fibrosis and cataract by negatively regulating Jagged-1/Notch
signaling pathway. Cell Death Differ. 24:1431–1442. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Thomadaki H and Scorilas A: BCL2 family of
apoptosis-related genes: Functions and clinical implications in
cancer. Crit Rev Clin Lab Sci. 43:1–67. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vogler M, Walter HS and Dyer MJS:
Targeting anti-apoptotic BCL2 family proteins in haematological
malignancies-from pathogenesis to treatment. Br J Haematol.
178:364–379. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Frenzel A, Grespi F, Chmelewskij W and
Villunger A: Bcl2 family proteins in carcinogenesis and the
treatment of cancer. Apoptosis. 14:584–596. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Weng J and Zhang H: The characteristics of
bcl-2 and PCNA expression in the lens epithelium of human being.
Zhonghua Yan Ke Za Zhi. 37:197–199. 2001.PubMed/NCBI(In Chinese).
|
|
28
|
Yu Y, Xing K, Badamas R, Kuszynski CA, Wu
H and Lou MF: Overexpression of thioredoxin-binding protein 2
increases oxidation sensitivity and apoptosis in human lens
epithelial cells. Free Radic Biol Med. 57:92–104. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mok JW, Chang DJ and Joo CK: Antiapoptotic
effects of anthocyanin from the seed coat of black soybean against
oxidative damage of human lens epithelial cell induced by
H2O2. Curr Eye Res. 39:1090–1098.
2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kong DQ, Liu Y, Li L and Zheng GY:
Downregulation of Smac attenuates
H2O2-induced apoptosis via endoplasmic
reticulum stress in human lens epithelial cells. Medicine
(Baltimore). 96(e7419)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Feng K and Guo HK: Eaf2 protects human
lens epithelial cells against oxidative stress-induced apoptosis by
Wnt signaling. Mol Med Rep. 17:2795–2802. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li QL, Zhang HY, Qin YJ, Meng QL, Yao XL
and Guo HK: MicroRNA-34a promoting apoptosis of human lens
epithelial cells through down-regulation of B-cell lymphoma-2 and
silent information regulator. Int J Ophthalmol. 9:1555–1560.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu SJ, Wang WT, Zhang FL, Yu YH, Yu HJ,
Liang Y, Li N and Li YB: miR-15a-3p affects the proliferation,
migration and apoptosis of lens epithelial cells. Mol Med Rep.
19:1110–1116. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ma T, Chen T, Li P, Ye Z, Zhai W, Jia L,
Chen W, Sun A, Huang Y, Wei S and Li Z: Heme oxygenase-1 (HO-1)
protects human lens epithelial cells (SRA01/04) against hydrogen
peroxide (H2O2)-induced oxidative stress and
apoptosis. Exp Eye Res. 146:318–329. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sundararajan M, Thomas PA, Teresa PA,
Anbukkarasi M and Geraldine P: Regulatory effect of chrysin on
expression of lenticular calcium transporters, calpains, and
apoptotic-cascade components in selenite-induced cataract. Mol Vis.
22:401–423. 2016.PubMed/NCBI
|