Introduction
Sepsis is an infection-induced systemic inflammatory
response syndrome that is aggressive, can rapidly progress to
septic shock or multiple organ dysfunction syndrome (MODS), is
difficult to treat and has a poor prognosis (1). Therefore, early identification, timely
diagnosis and effective treatment of sepsis may be the key to
preventing and treating MODS and improving the survival rate of
patients with sepsis (2). The
current treatment measures for sepsis are mainly symptomatic
treatment, anti-infection agents and organ support treatment
(3). Despite the continuous
development and advancement of clinical treatment measures, the
pathogenesis of sepsis is complicated, is closely associated with
the pathophysiological changes of multiple organs and contributes
to a high mortality rate (4).
Therefore, an in-depth exploration of the pathogenesis of sepsis,
active prevention of the occurrence and improvement of the cure
rate of patients with sepsis are important issues that need to be
resolved urgently in clinical practice and have important practical
significance.
As an important tissue and organ of the human body,
the intestine is not only the main place for the digestion,
absorption and transportation of nutrients, but also an important
immune protection site for the body (5). Recent studies have indicated that the
intestine serves an important role in the pathophysiological
development of critical illnesses with its unique physiological
environment (6). In addition to
causing an uncontrolled systemic inflammatory response, sepsis
often leads to the malfunction of a variety of organs of the body,
whereby the intestine is one of the most sensitive organs (7). During sepsis, bacteria and toxins in
the intestinal lumen can activate intestinal immune cells,
releasing a large number of inflammatory mediators, such as TNF-α,
IL-1β and IL-6, and causing intestinal mucosal damage (8). Simultaneously, inflammatory mediators
interact and form a network to cause mediator cascade effects,
leading to a vicious cycle, accelerating the course of the disease,
and even causing MODS (9). As a
result, the intestinal mucosa not only functions as a barrier but
can also produce a variety of inflammatory mediators after being
attacked (8). It is currently
proposed that intestinal function may not only be impaired due to
the development of sepsis, but also cause excessive release of
inflammatory mediators through the translocation of bacteria and
toxins, leading to dysfunction and even failure of other important
organs (10). Therefore,
suppressing the inflammatory response in the intestine is crucial
for preventing the initiation and progression of sepsis and
MODS.
Enhancer of zeste homolog 2 (EZH2) is an important
member of the polycomb-group protein gene family. Histone
methyltransferase homologous sequence 2 can catalyze the
trimethylation of amino acid K27 at position 27 of histone H3
(H3K27Me3) and regulate cell proliferation (11). In addition, the gene expression
product of EZH2 can silence tumor suppressor genes and serve an
important role in the evolution of various tumors. EZH2 is found to
be positively associated with poor clinical outcomes in a number of
aggressive tumors, and its inhibition has been indicated to
effectively inhibit cell proliferation and prevent tumor
progression (12,13). A previous study demonstrated that
inhibition of EZH2 suppressed the progression of lung injury and
alleviated inflammation induced by sepsis, suggesting that EZH2
could be a potential biomarker in predicting clinical outcome and a
novel target for therapeutic interference in sepsis (14). However, the role of EZH2 in
intestinal disorders caused by sepsis remains to be elucidated. The
present study aimed to investigate whether an inhibitor of EZH2,
GSK343, could protect the intestine against sepsis-induced injury
in mice that underwent cecal ligation and perforation (CLP)
operation.
Materials and methods
Animals
A total of 30 C57BL/6 mice (17-20 g) were purchased
from the Chinese Academy of Medical Sciences. All mice were kept in
a specific pathogen-free environment at room temperature under a
controlled 12/12 h light/dark cycle and received food and water
ad libitum. Male mice at 6-8 weeks of age were used for the
experiments. The animal study was approved by the Institutional
Animal Care and Use Committee of the Second People's Hospital of
Zhangye City.
Establishment of a sepsis model
A sepsis model was established by performing CLP on
mice. All mice were anesthetized with 2% isoflurane inhalation for
about 3 min until they were totally anesthetized before undergoing
surgical procedures, and their body temperatures were maintained at
36-38˚C with a heating pad. During the surgical procedure, 1.5%
isoflurane was used to maintain anesthesia. A 1.5 cm incision was
made on the abdominal wall, and the cecum was exposed and ligated
0.5 cm from the tip with a 4-0 silk suture. The cecum was
perforated by a single puncture with a 21-gauge needle and gently
squeezed to extrude a small amount of feces from the perforation
site. The cecum was replaced into the abdominal cavity, and the
exposed abdominal wall was closed in two layers with a running 4-0
silk suture. The sham-operated group (n=10) only underwent
laparotomy. Mice were resuscitated with subcutaneous injection of 1
ml normal saline.
Animal grouping and treatment
At 6 h after the CLP operation, sepsis could be
induced and the successfully modeled mice were selected and
randomly subdivided into CLP (n=10) and CLP + GSK343 (n=10) groups.
For GSK343 treatment, the septic mice were intravenously injected
with GSK343 (0.2 ml/20 g; MedChemExpress) suspended in anhydrous
ethanol diluted in PBS at 6 h post-CLP. For the CLP group, mice
were intravenously injected with equivalent amounts of anhydrous
ethanol. At 72 h after CLP or sham operation, all mice were
euthanized by cervical dislocation and small intestine samples and
serum were collected.
Histological staining
The small intestine samples were fixed with 4%
paraformaldehyde overnight at 4˚C, then dehydrated through an
alcohol-xylene series and finally embedded in paraffin and stored
at room temperature. Sections (3 µm thickness) were cut,
deparaffinized, rehydrated with gradient ethanol and stained with
hematoxylin and eosin (H&E; Abcam) or TUNEL. H&E staining
allows the identification of Paneth cells located at the base of
intestine crypt based on their distinctive granule staining
(15). TUNEL staining was used to
stain apoptotic cells according to the manufacturer's instructions
(Beyotime Institute of Biotechnology).
For immunofluorescent staining for ZO-1,
deparaffinized and rehydrated sections were blocked with 10% goat
serum (Gibco; Thermo Fisher Scientific, Inc.) at 37˚C for 1 h and
incubated with a primary antibody against ZO-1 (Abcam; cat. no.
ab190085; 1:2,000) at 4˚C overnight. Following incubation with
IgG-FITC secondary antibody (cat. no. ab6881; 1:5,000; Abcam) at
room temperature for 1 h in the dark, samples were stained with
DAPI at room temperature for 20 min in the dark and photographed
under a fluorescence microscope.
Western blot analysis
Intestinal tissues were pulverized in liquid
nitrogen and lysed in ice-cold RIPA buffer (Beyotime Institute of
Biotechnology) containing 0.01% protease and phosphatase inhibitor
(Sigma-Aldrich; Merck KGaA). Protein concentration in the
supernatant was measured using a BCA protein assay. Equal amounts
of protein (20 µg/lane) were subjected to 10% SDS-PAGE and
transferred to PVDF membranes. The transferred proteins were
blocked with 5% non-fat milk for 2 h at room temperature. Following
blocking, the membranes were incubated with anti-EZH2 (cat. no.
5246; 1:1,000), ZO-1 (cat. no. 13663; 1:1,000), occludin (cat. no.
91131; 1:1,000), claudin-1 (cat. no. 13255; 1:1,000), TNF-α (cat.
no. 11948; 1:1,000), IL-1β (cat. no. 31202; 1:1,000), IL-6 (cat.
no. 12912; 1:1,000), and anti-GAPDH (cat. no. 5174; 1:1,000)
primary antibodies (all, Cell Signaling Technology, Inc.) overnight
at 4˚C and then incubated with respective HRP-conjugated secondary
antibodies (anti-rabbit IgG; cat. no. 7074; 1:3,000; Cell Signaling
Technology, Inc.). Protein signals were visualized using the super
ECL detection reagent (Beyotime Institute of Biotechnology).
Image-Pro Plus software version 6.0 (Roper Technologies, Inc.) was
used for densitometry.
ELISA
Serum supernatant was separated after centrifugation
and the levels of TNF-α (cat. no. ab208348), IL-1β (cat. no.
ab197742) and IL-6 (cat. no. ab100713) (all from Abcam) were tested
using ELISA test kits in accordance with the manufacturer's
instructions. The optical density value was measured at an
excitation wavelength of 450 nm using a microplate reader (Bio-Rad
Laboratories, Inc.) with the blank well serving as the control.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA of the intestinal tissues was extracted
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). RT of the extracted RNA was completed with a
two-step method according to the kit instructions (SuperScript™ III
Two-Step RT-PCR system; Thermo Fisher Scientific, Inc.). A TaqMan
probe (Takara Bio, Inc.) was used for qPCR. The following primer
pairs were used for qPCR analysis: ZO-1 forward,
5'-GGAGCAGGCTTTGGAGGAG-3' and reverse, 5'-TGGGACAAAAGTCCGGGAAG-3';
occludin forward, 5'-GTGAATGGGTCACCGAGGG-3' and reverse,
5'-AGATAAGCGAACCTGCCGAG-3'; claudin-1 forward,
5'-GGCTTCTCTGGGATGGATCG-3' and reverse, 5'-GCAGCAGTT-CACAGGCAAAA-3'
and GAPDH forward, 5'-GGTCCCAGCTTAGGTTCATCA-3' and reverse,
5'-ATCCGTTCACACCGACCTTC-3'. The following thermocycling conditions
were used for the qPCR: Initial denaturation at 95˚C for 30 sec;
and 40 cycles of denaturation at 95˚C for 10 sec, annealing at 60˚C
for 20 sec and extension at 70˚C for 10 sec. GAPDH was used as the
internal reference gene and the 2-ΔΔCq method (16) was utilized to analyze the expression
of the target genes.
Statistical analysis
All experiments were performed at least three times.
Data were expressed as the mean ± SD and plotted and analyzed using
GraphPad Prism 5 (GraphPad Software, Inc.). Differences between
groups were analyzed using one-way ANOVA followed by Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
EZH2 is upregulated while tight
junction (TJ) proteins are downregulated in the intestinal tissue
of a CLP mice model
To determine whether EZH2 serves a role in
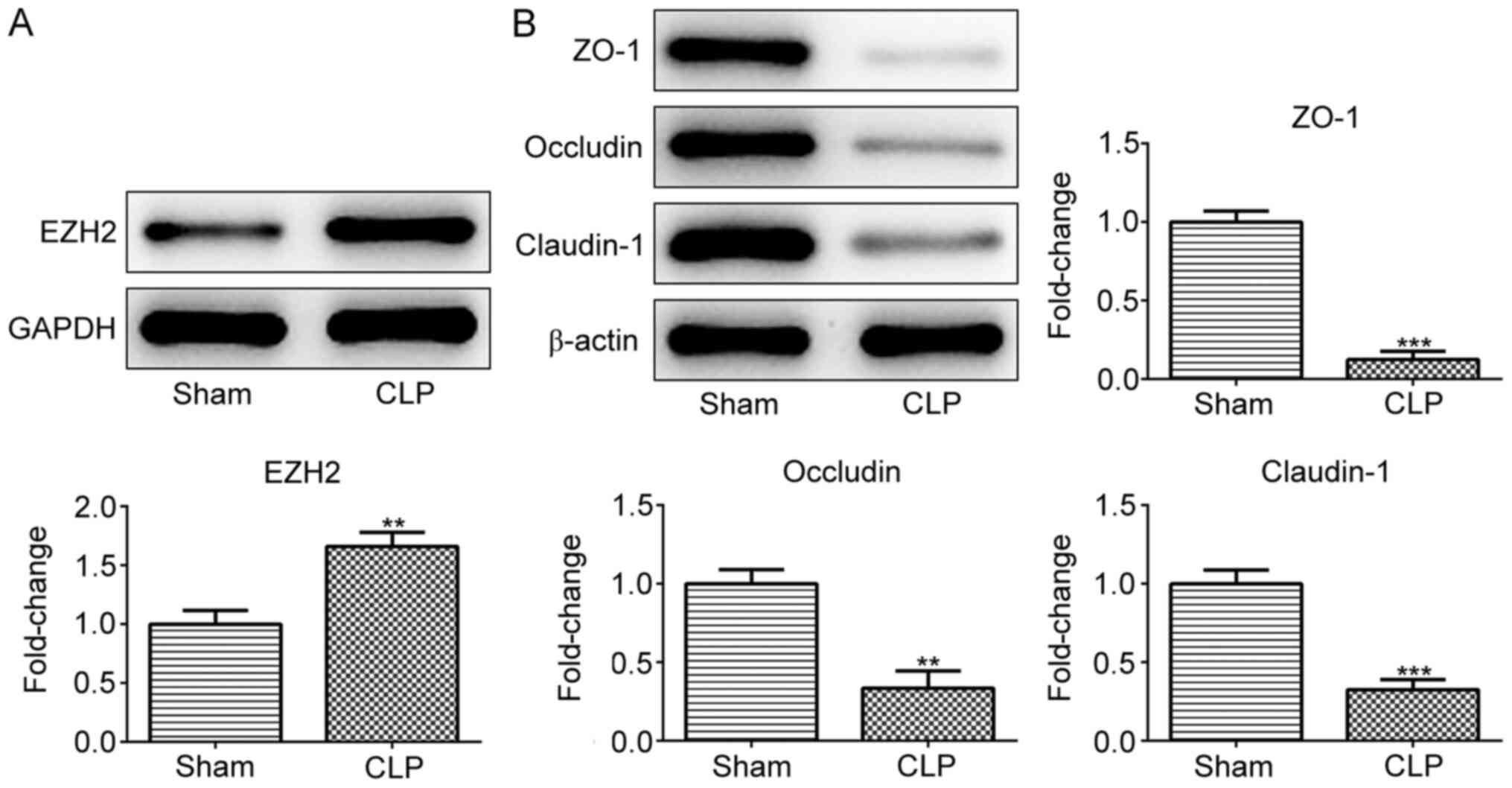
sepsis-induced intestinal disorders, EZH2 expression was detected
in CLP- or sham-operated mice. As presented in Fig. 1A, EZH2 expression was significantly
higher in the intestinal tissues of the CLP group compared with the
sham group, indicating the potential role of EZH2 in sepsis-induced
intestinal disorders. Subsequently, the expression of TJ proteins
including ZO-1, occludin and claudin-1 was measured in the
intestinal tissues of sham- or CLP-operated mice. The results
revealed that ZO-1, occludin and claudin-1 protein expression was
reduced upon CLP stimulation (Fig.
1B).
GSK343 inhibits CLP-induced intestinal
pathological injury and inflammation
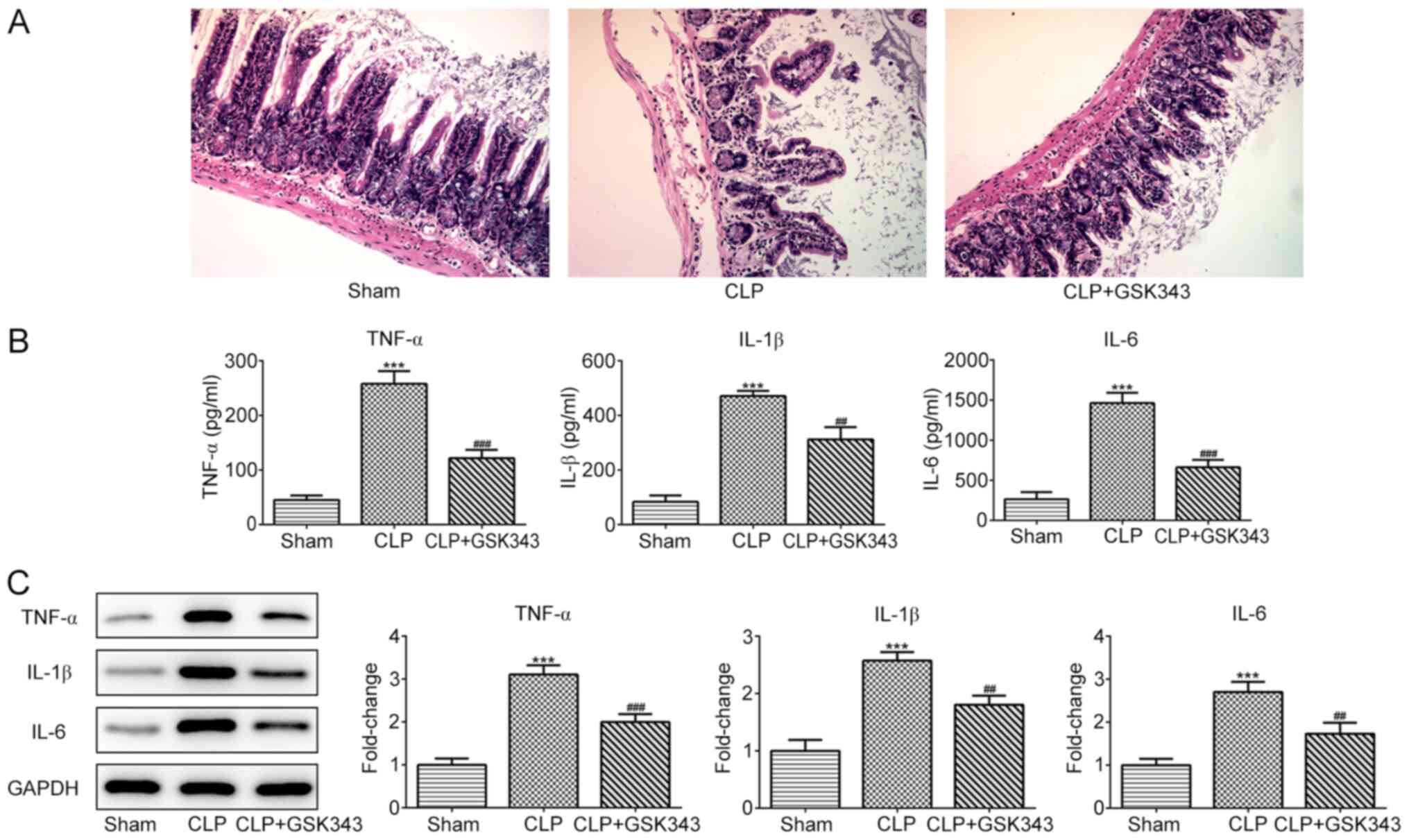
GSK343, which is an EZH2 inhibitor, was used to
treat CLP mice to observe whether inhibition of EZH2 could protect
the intestine against sepsis-induced injury. At the end of the
experiment, animals were sacrificed and H&E staining was
performed on small intestinal tissue sections. Fig. 2A demonstrates the results of H&E
staining in the intestinal tissues of mice in different groups. The
ileum tissue structure of the mice in the sham operation group was
normal, the intestinal villi were arranged neatly and the villi
structure was clear. In the CLP group, however, evident edema,
hyperemia, necrosis and inflammatory cell infiltration were
observed, accompanied by missing apical epithelial cells of the
villi and thinner and shorter microvilli. Compared with the CLP
group, the intestinal structure of the mice in the GSK343 treatment
group was relatively normal, with relieved intestinal villi edema
and inflammation. The levels of inflammatory cytokines, including
TNF-α, IL-1β and IL-6, in the serum and intestinal tissues of mice
in different groups are presented in Fig. 2B and C. It was indicated that CLP stimulation
significantly promoted the production of all inflammatory
cytokines, and this increase was reversed by GSK343 treatment.
GSK343 represses CLP-induced
intestinal tissue apoptosis
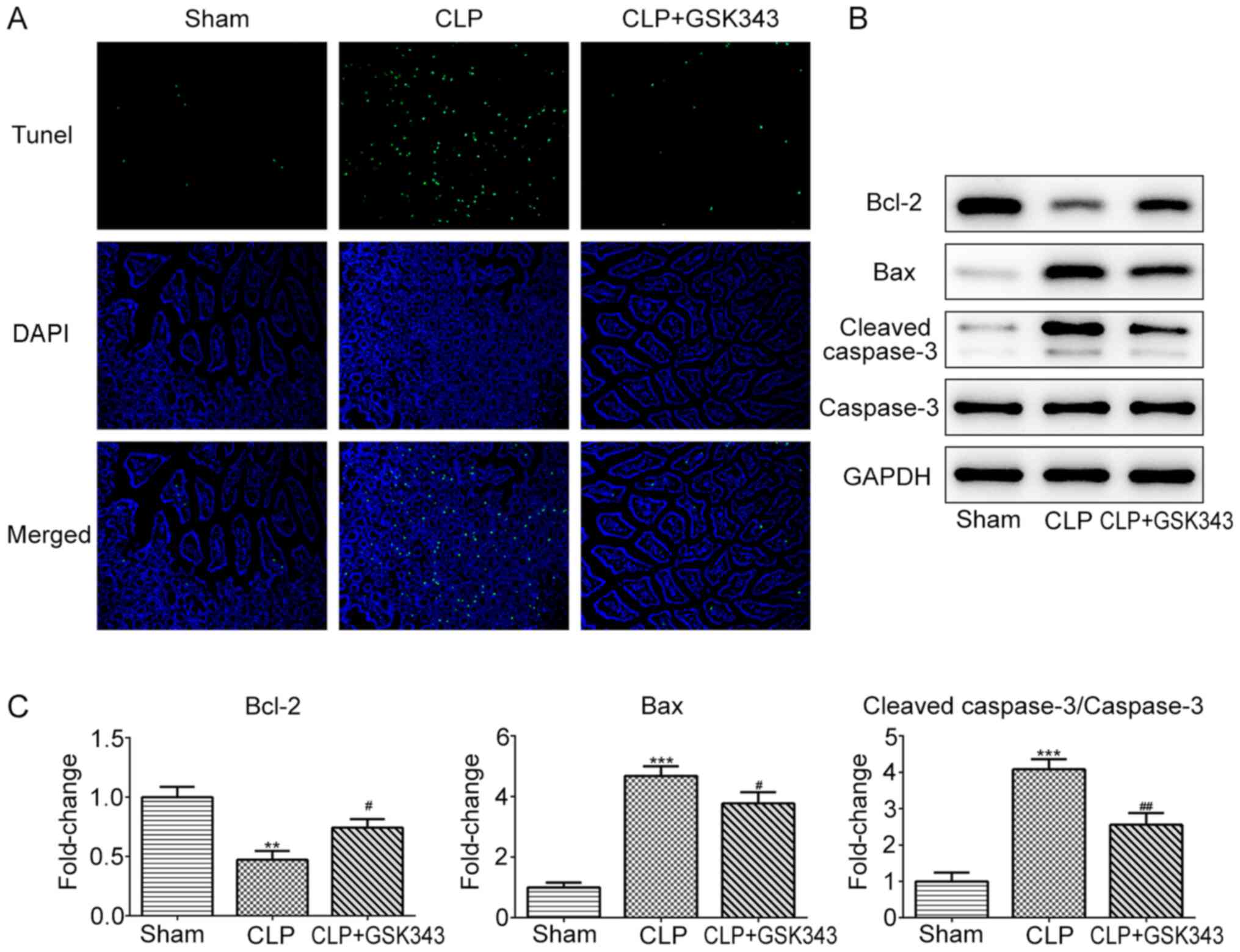
Subsequently, the cell apoptosis of animal
intestinal tissue in different groups was assessed. As presented in
Fig. 3A, CLP operation markedly
increased the number of apoptotic cells compared with the sham
operation. However, in contrast to the CLP group, GSK343 treatment
markedly reduced the number of apoptotic cells in the intestinal
tissue of CLP mice. Similar results were observed in Fig. 3B and C, where CLP reduced anti-apoptotic protein
Bcl-2 expression and increased the expression of pro-apoptotic
proteins Bax and cleaved-caspase-3. Meanwhile, GSK343 treatment
attenuated the effects of CLP on apoptotic protein expression.
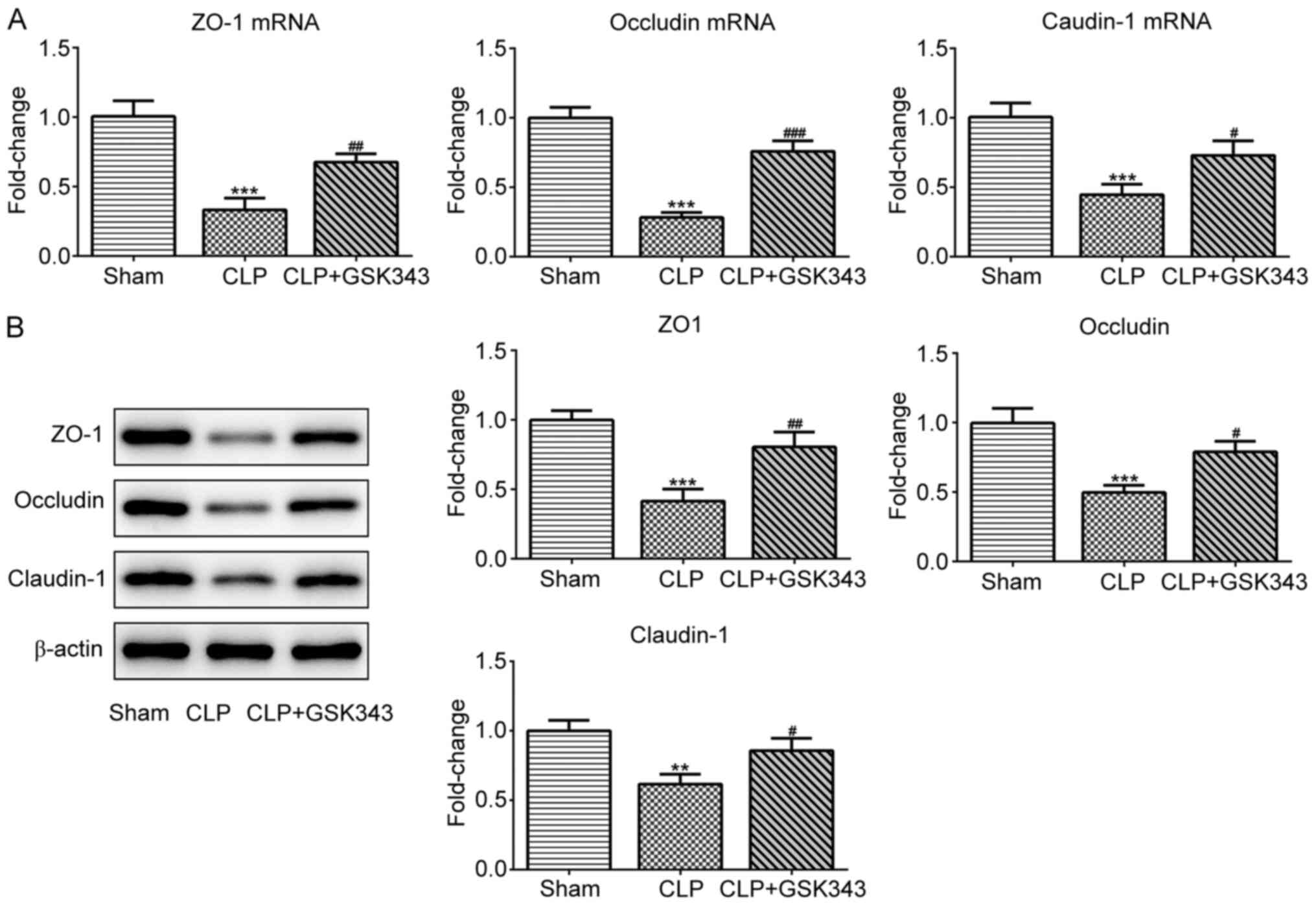
GSK343 promotes TJ protein expression
and the number of Paneth cells in the intestinal tissue of a CLP
mice model
The expression of TJ proteins including ZO-1,
occludin and claudin-1 in the intestinal tissue of mice was also
detected. The results indicated in Fig.
4 revealed that mRNA and protein expression of these TJ
proteins were significantly downregulated upon CLP stimulation, but
effectively partially recovered by GSK343 treatment. Consistently,
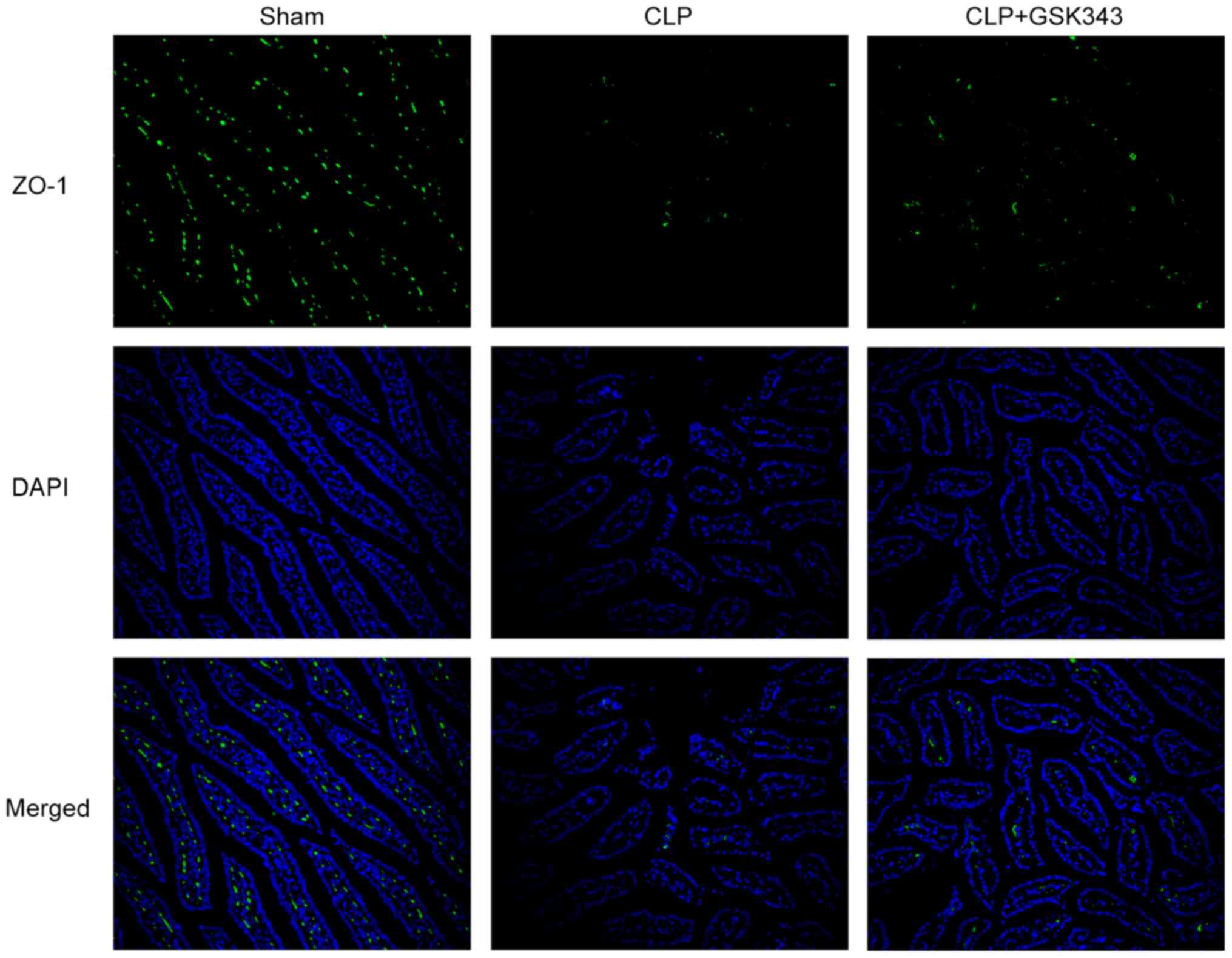
as presented in Fig. 5, ZO-1
immunofluorescent staining results showed the decreased expression
of ZO-1 upon CLP stimulation, but effectively recovered expression
of ZO-1 after GSK343 treatment.
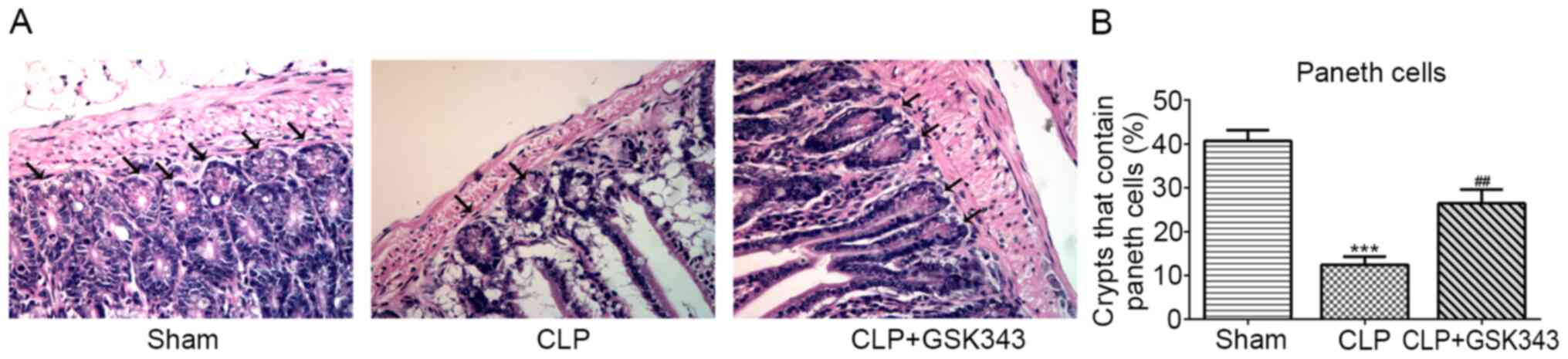
Finally, the distribution of Paneth cells at the
base of the intestine crypt was observed based on their distinctive
granule staining. As presented in Fig.
6, CLP mice showed a significant decrease in the number of
crypts with positive Paneth cells in the small intestine compared
with Sham mice. By contrast, animals treated with GSK343 exhibited
an increased number of Paneth cells compared with CLP mice.
Discussion
Intestinal mucosal destruction, intestinal wall
congestion and necrosis caused by sepsis are the main causes of
intestinal dysfunction (17). In
the present study, a mice sepsis model was constructed by CLP
operation. Following which, the mucosal morphology was markedly
damaged, the expression of TJ proteins was decreased, in
conjunction with the production of a large amount of inflammatory
cytokines and occurrence of apoptosis. TJ proteins serve an
important role in the physiology and disease biology of intestinal
disease (18). TJ proteins exert
their functional role as integral proteins in forming barriers in
the gut (19). However, TJ proteins
can also serve important functional roles in the signaling,
trafficking and regulation of gene expression (20). In addition, the number of Paneth
cells were significantly decreased upon CLP stimulation. Paneth
cells are physiologically found at the bottom of small intestinal
crypts and are characterized by their apically located granules
(21). The intestinal crypts have
been identified as a significant source of antimicrobial peptides
and proteins that are important in host defense and in shaping the
composition of the commensal microbiota (22). The dysfunction of Paneth cells and
molecular mechanisms underlying the secretory disorders of Paneth
cells have been demonstrated to be highly associated with
inflammatory bowel disease (23).
Consistent with previous reports (22,24,25),
the present results demonstrated sepsis-induced intestinal injury
and the involvement of Paneth cell dysfunction.
EZH2 serves crucial roles in regulating a variety of
cellular functions, including development and differentiation
(26). Consequently, the majority
of previous studies regarding EZH2 have focused on its modulatory
effects on a variety of cancer types, such as prostate cancer
(27), ovarian cancer (28), gastric cancer (29) and head and neck cancer (30).
Recently, several studies revealed the involvement
of EZH2 in the initiation and progression of sepsis (14,31-33).
EZH2 was indicated to accelerate inflammation and apoptosis, and
EZH2 inhibition was demonstrated to exert protective effects
against sepsis (14,31,34).
For example, a study confirmed that GSK343, which is an inhibitor
of EZH2, could mitigate fibrosis and inflammation (35). Moreover, EZH2 was indicated to
accelerate skeletal muscle cell apoptosis and the inflammatory
response in sepsis (31). However,
the effects of EZH2 on intestinal dysfunction during sepsis and the
underlying epigenetic mechanisms are still unclear. It has been
illustrated that epithelial EZH2 was responsible for maintaining
the integrity of the epithelial cell barrier and homeostasis,
indicating that it has a protective effect on colitis (36). By contrast, the inhibition of EZH2
with GSK126 in vitro has been reported to restore intestinal
homeostasis by restoring Paneth cell population, implicating that
EZH2 has a destructive role in intestinal homeostasis (37). These seemingly contradictory
conclusions reveal the complex and diverse role of EZH2 in
intestinal diseases. The present study observed a significant
upregulation of EZH2 in CLP-induced septic animal models,
suggesting the modulatory effects of EZH2 on intestinal dysfunction
during sepsis. Subsequently, septic mice were treated with the EZH2
inhibitor, GSK343. The results indicated that the application of
GSK343 significantly improved the morphological injury of
intestinal tissues, reduced the level of inflammatory cytokines in
serum and intestinal tissues, inhibited the occurrence of apoptosis
in intestinal tissues and rescued the expression of TJ proteins in
intestinal tissues. Furthermore, the decreased population of Paneth
cells caused by CLP operation was also significantly increased by
GSK343 treatment. These results revealed that intestine injury
during sepsis was predominantly caused by inflammation and
apoptosis, the relieving of which by EZH2 inhibition was sufficient
to prevent the progression of intestinal injury. In addition, the
protective effects of EZH2 inhibition against intestine injury
during sepsis may mainly rely on suppressing inflammation and
apoptosis as well as recovering TJ protein expression and the
number of Paneth cells in the intestine. However, there are still
some shortcomings in the current study. Firstly, only a CLP-induced
mice model was employed in the present study since the laboratory
was equipped with mature CLP modeling conditions and technology.
Establishment of an LPS-induced mice model should be performed in
the future research. The use of clinical samples will strengthen
conclusions, and add evidence from clinical samples should be
included in future research.
In conclusion, the current study demonstrated that
inhibition of EZH2 could relieve intestinal injury during sepsis
via suppressing inflammation, apoptosis and at the same time
recovering TJ protein expression and the number of Paneth cells in
the intestinal tissues. Therefore, targeting EZH2 with
pharmacological inhibitors may represent an effective and
economical therapeutic approach that may be used in the treatment
of intestinal injury caused by sepsis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FW and DY contributed to study conception or design;
DY, ZW and YY contributed to acquisition of data; ZH and GL
contributed to analysis or interpretation of data; DY and FW
drafted the work and revised it critically for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of the Second People's Hospital of Zhangye City.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hotchkiss RS, Moldawer LL, Opal SM,
Reinhart K, Turnbull IR and Vincent JL: Sepsis and septic shock.
Nat Rev Dis Primers. 2(16045)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fleischmann C, Scherag A, Adhikari NK,
Hartog CS, Tsaganos T, Schlattmann P, Angus DC and Reinhart K:
International Forum of Acute Care Trialists. Assessment of global
incidence and mortality of hospital-treated sepsis. Current
estimates and limitations. Am J Respir Crit Care Med. 193:259–272.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rello J, Valenzuela-Sánchez F,
Ruiz-Rodriguez M and Moyano S: Sepsis: A review of advances in
management. Adv Ther. 34:2393–2411. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lelubre C and Vincent JL: Mechanisms and
treatment of organ failure in sepsis. Nat Rev Nephrol. 14:417–427.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mowat AM and Agace WW: Regional
specialization within the intestinal immune system. Nat Rev
Immunol. 14:667–685. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Brown EM, Sadarangani M and Finlay BB: The
role of the immune system in governing host-microbe interactions in
the intestine. Nat Immunol. 14:660–667. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Fay KT, Ford ML and Coopersmith CM: The
intestinal microenvironment in sepsis. Biochim Biophys Acta Mol
Basis Dis. 1863:2574–2583. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hwang JS, Kim KH, Park J, Kim SM, Cho H,
Lee Y and Han IO: Glucosamine improves survival in a mouse model of
sepsis and attenuates sepsis-induced lung injury and inflammation.
J Biol Chem. 294:608–622. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hu Q, Ren H, Li G, Wang D, Zhou Q, Wu J,
Zheng J, Huang J, Slade DA, Wu X and Ren J: STING-mediated
intestinal barrier dysfunction contributes to lethal sepsis.
EBioMedicine. 41:497–508. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhou Q and Verne GN: Intestinal
hyperpermeability: A gateway to multi-organ failure? J Clin Invest.
128:4764–4766. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tremblay-LeMay R, Rastgoo N, Pourabdollah
M and Chang H: EZH2 as a therapeutic target for multiple myeloma
and other haematological malignancies. Biomark Res.
6(34)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Margueron R and Reinberg D: The Polycomb
complex PRC2 and its mark in life. Nature. 469:343–349.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gan L, Yang Y, Li Q, Feng Y, Liu T and Guo
W: Epigenetic regulation of cancer progression by EZH2: From
biological insights to therapeutic potential. Biomark Res.
6(10)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang Q, Sun H, Zhuang S, Liu N, Bao X,
Liu X, Ren H, Lv D, Li Z, Bai J, et al: Novel pharmacological
inhibition of EZH2 attenuates septic shock by altering innate
inflammatory responses to sepsis. Int Immunopharmacol.
76(105899)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cazorla SI, Maldonado-Galdeano C, Weill R,
De Paula J and Perdigón GD: Oral administration of probiotics
increases paneth cells and intestinal antimicrobial activity. Front
Microbiol. 9(736)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen S, He Y, Hu Z, Lu S, Yin X, Ma X, Lv
C and Jin G: Heparanase mediates intestinal inflammation and injury
in a mouse model of sepsis. J Histochem Cytochem. 65:241–249.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dokladny K, Zuhl MN and Moseley PL:
Intestinal epithelial barrier function and tight junction proteins
with heat and exercise. J Appl Physiol (1985). 120:692–701.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Günzel D and Fromm M: Claudins and other
tight junction proteins. Compr Physiol. 2:1819–1852.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zeisel MB, Dhawan P and Baumert TF: Tight
junction proteins in gastrointestinal and liver disease. Gut.
68:547–561. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Clevers HC and Bevins CL: Paneth cells:
Maestros of the small intestinal crypts. Annu Rev Physiol.
75:289–311. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Schmitt M, Schewe M, Sacchetti A, Feijtel
D, van de Geer WS, Teeuwssen M, Sleddens HF, Joosten R, van Royen
ME, van de Werken HJ, et al: Paneth cells respond to inflammation
and contribute to tissue regeneration by acquiring stem-like
features through SCF/c-Kit signaling. Cell Rep. 24:2312–2328.e2317.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Holly MK and Smith JG: Paneth cells during
viral infection and pathogenesis. Viruses. 10(225)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Han SJ, Kim M, D'Agati VD and Lee HT:
Norepinephrine released by intestinal Paneth cells exacerbates
ischemic AKI. Am J Physiol Renal Physiol. 318:F260–F272.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lee HT, Kim M, Kim JY, Brown KM, Ham A,
D'Agati VD and Mori-Akiyama Y: Critical role of interleukin-17A in
murine intestinal ischemia-reperfusion injury. Am J Physiol
Gastrointest Liver Physiol. 304:G12–G25. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gulati N, Béguelin W and Giulino-Roth L:
Enhancer of zeste homolog 2 (EZH2) inhibitors. Leuk Lymphoma.
59:1574–1585. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bai Y, Zhang Z, Cheng L, Wang R, Chen X,
Kong Y, Feng F, Ahmad N, Li L and Liu X: Inhibition of enhancer of
zeste homolog 2 (EZH2) overcomes enzalutamide resistance in
castration-resistant prostate cancer. J Biol Chem. 294:9911–9923.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jones BA, Varambally S and Arend RC:
Histone methyltransferase EZH2: A therapeutic target for ovarian
cancer. Mol Cancer Ther. 17:591–602. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pan YM, Wang CG, Zhu M, Xing R, Cui JT, Li
WM, Yu DD, Wang SB, Zhu W, Ye YJ, et al: STAT3 signaling drives
EZH2 transcriptional activation and mediates poor prognosis in
gastric cancer. Mol Cancer. 15(79)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yamagishi M and Uchimaru K: Targeting EZH2
in cancer therapy. Curr Opin Oncol. 29:375–381. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yong H, Wu G, Chen J, Liu X, Bai Y, Tang
N, Liu L and Wei J: lncRNA MALAT1 accelerates skeletal muscle cell
apoptosis and inflammatory response in sepsis by decreasing BRCA1
expression by recruiting EZH2. Mol Ther Nucleic Acids. 19:97–108.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhao D, Li Z, Liu X, Liu N, Bao X, Sun H,
Meng Q, Ren H, Bai J, Zhou X and Tang L: Lymphocyte expression of
EZH2 is associated with mortality and secondary infectious
complications in sepsis. Int Immunopharmacol.
89(107042)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yong H, Wu G, Chen J, Liu X, Bai Y, Tang
N, Liu L and Wei J: lncRNA MALAT1 accelerates skeletal muscle cell
apoptosis and inflammatory response in sepsis by decreasing BRCA1
expression by recruiting EZH2. Mol Ther Nucleic Acids.
21:1120–1121. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yu Z, Rayile A, Zhang X, Li Y and Zhao Q:
Ulinastatin protects against lipopolysaccharide-induced cardiac
microvascular endothelial cell dysfunction via downregulation of
lncRNA MALAT1 and EZH2 in sepsis. Int J Mol Med. 39:1269–1276.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang Q, Xu L, Zhang X, Liu D and Wang R:
GSK343, an inhibitor of EZH2, mitigates fibrosis and inflammation
mediated by HIF-1α in human peritoneal mesothelial cells treated
with high glucose. Eur J Pharmacol. 880(173076)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu Y, Peng J, Sun T, Li N, Zhang L, Ren
J, Yuan H, Kan S, Pan Q, Li X, et al: Epithelial EZH2 serves as an
epigenetic determinant in experimental colitis by inhibiting
TNFα-mediated inflammation and apoptosis. Proc Natl Acad Sci USA.
114:E3796–E3805. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Nakanishi Y, Reina-Campos M, Nakanishi N,
Llado V, Elmen L, Peterson S, Campos A, De SK, Leitges M, Ikeuchi
H, et al: Control of paneth cell fate, intestinal inflammation, and
tumorigenesis by PKCλ/ι. Cell Rep. 16:3297–3310. 2016.PubMed/NCBI View Article : Google Scholar
|