Introduction
Cervical cancer (CC) is the most common
gynecological cancer in women worldwide (1). In 2019, 13,170 new cases were
diagnosed and 4,250 disease-related deaths were predicted in the
USA (1). Furthermore, 530,000 new
cases are diagnosed yearly worldwide and 20-25% of cases are
reported in China (2). Although CC
also occurs during perimenopause, unlike endometrial cancer or
ovarian cancer, there are few reports on the association between
steroid hormone receptors and CC (3). Several in vitro studies have
investigated the interactions between estrogen/progesterone and
human papillomavirus (HPV), but the results are controversial
(4,5).
Estrogen serves a major role in several
hormone-dependent malignancies (6).
Estrogen and its analogs can activate estrogen-responsive genes by
binding to estrogen receptor α (ERα) (7). Previous genomic studies have
identified frequent mutations of the ERα gene (ESR1) in CC
(8,9). Additionally, it has been demonstrated
that viral-codified E6 and E7 proteins could directly interact with
androgen receptor (AR) (10).
However, to the best of our knowledge, the role of ERα and AR in
cervical carcinogenesis is not completely understood.
MircoRNAs (miRNAs/miRs) are a class of short, highly
conserved, non-coding RNAs that regulate gene expression by
inhibiting translation or inducing mRNA degradation at the
post-transcriptional level (11).
The aberrant expression of miRNAs has been reported in various
tumors (12), such as endometrial
cancer (13), ovarian cancer
(14), and CC (15). Our previous study demonstrated that
miR-107-5p could promote tumor proliferation and invasion by
targeting ERα in endometrial carcinoma (16); therefore, it was hypothesized that
specific miRNAs may control ERα and AR expression
post-transcriptionally during CC progression.
The aim of the present study was to investigate the
expression of miR-130a-3p, ERα and AR in healthy cervical and CC
tissues, and to explore whether miR-130a-3p could contribute to
tumor progression by suppressing ERα and AR.
Materials and methods
Clinical samples
The present study was approved (approval no. GKLW
2017-125) by the Human Investigation Ethical Committee of the
International Peace Maternity & Child Hospital Affiliated to
Shanghai Jiao Tong University School of Medicine (Shanghai, China).
Written informed consent was obtained from all patients. A total of
60 female patients (45-68 years old) who underwent radical
hysterectomy with lymph node dissection for CC at the International
Peace Maternity & Child Health Hospital Affiliated to Shanghai
Jiao Tong University School of Medicine between August 2014 and
April 2017 were included in the present study. The stages and
histological grades of the tumors were determined according to the
criteria of the Federation International of Gynecology and
Obstetrics Surgical staging system (2018) (17). A total of 20 healthy cervical tissue
samples (female subjects; 44-56 years old) were obtained from
patients who underwent a hysterectomy to treat other diseases
between April 2015 and April 2017 such as myoma or adenomyosis. In
addition, 20 cervical intraepithelial neoplasia (CIN) I, 20 CIN II
and 30 CIN III tissue samples (female subjects; 34-53 years old)
were collected from patients who underwent a cervical biopsy or
loop electrosurgical excision procedure between October 2015 and
April 2017. All the tissues were collected from each patient or
healthy control at the International Peace Maternity & Child
Health Hospital Affiliated to Shanghai Jiao Tong University School
of Medicine and immediately snap-frozen in liquid nitrogen until
further use. All the tissues were pathologically reviewed by two
pathologists before use.
Immunohistochemistry (IHC)
All tissue sections (4-µm thick) were processed for
IHC as previously described (18,19).
The following primary antibodies were used for IHC: Anti-ERα (cat.
no. ab75635; 1:200; Abcam) and AR (cat. no. ab74272; 1:250;
Abcam).
The expression of ERα and AR was evaluated in terms
of staining intensity: (Negative), 1 (weak), 2 (medium) or 3
(strong). The extent of staining was scored as 0 (0%), 1 (1-25%), 2
(26-50%), 3 (51-75%) or 4 (76-100%), according to the percentage of
positively stained areas in relation to the whole tumor area. The
final staining score (0-12) was calculated by multiplying the
intensity score and the extent score (19). The final staining score was used to
evaluate the expression of ERα and AR.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from frozen tissues using
TRI Reagent (Molecular Research Center). Total RNA was reverse
transcribed into mature miRNA using the TaqMan MicroRNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.), using the following RT temperature protocol: 42˚C for 15
min; 85˚C for 5 sec. Subsequently, qPCR was performed using TaqMan
MicroRNA assay primers (Applied Biosystems; Thermo Fisher
Scientific, Inc.) with TaqMan Universal PCR Master mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and an ABI Prism 7000
Sequence Detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The thermocycling conditions were as follows:
95˚C for 3 min; 95˚C for 30 sec and 60˚C for 30 sec for 35 cycles;
72˚C for 5 min and maintenance at 4˚C for further use. The
sequences of the primers used for qPCR are provided in Table I. miRNA expression levels were
normalized to the internal reference gene U6. Relative gene
expression was quantified using the 2-ΔΔCq method
(20).
 | Table IOligo sequences used in the present
study. |
Table I
Oligo sequences used in the present
study.
| Identifier | Sequence
(5'→3') |
|---|
| miR-130a | F:
TTGCGATTCTGTTTTGTGCT |
| | R:
GTGGGGTCCTCAGTGGG |
| U6 | F:
AGAGCCTGTGGTGTCCG |
| | R:
CATCTTCAAAGCACTTCCCT |
| miR-130a
inhibitor | F:
AUGCCCUUUUAACAUUGCACUG |
| | R:
UAGUGCAAUAGUAUCGUCGAGAC |
| miR-130a inhibitor
NC | F:
CAGUACUUUUGUGUAGUACAA |
| | R:
GUCCUGAGAAGGCUAGCAUAGAU |
| miR-130a mimic | F:
CAGUGCAAUGUUAAAAGGGCAU |
| | R:
AUAGCCCUGUACAAUGCUGCUUU |
| miR-130a mimic
NC | F:
UUCUCCGAACGUGUCACGUTT |
| | R:
ACUUUGACAAUACUAUAGUGGAA |
| ESR1-3'UTR-WT | F:
TAAACGCGTGTAACGTGAATACCAC |
| | R:
TCGATGCGTAACGATGTTCCTCAGTG |
| ESR1-3'UTR-MT | F:
TATTACCGATACGGAAAAGCAATGT |
| | R:
TGAAGCAACGGAAATGCATAGA |
| AR-3'UTR-WT | F:
TACGAAAAACTTGAATGACAATAC |
| | R:
GAAAATGAACTTGAGACAAATG |
| AR-3'UTR-MT | F:
GCGATGACACTGCTCCTATAGCGAAT |
| | R:
TGGGATCCCACTGCTCCCATGGCTTA |
Western blotting
Cells were harvested and proteins were extracted
using Mem-PER Eukaryotic Membrane Protein Extraction Reagent
(Pierce; Thermo Fisher Scientific, Inc.) containing complete mini
cocktail, NE-PER Nuclear and Cytoplasmic Extraction Reagents
(Pierce; Thermo Fisher Scientific, Inc.) and protease inhibitor
cocktail. A total of 20 µg proteins (determined using the BCA
method) were separated via 8% SDS-PAGE and transferred onto PVDF
membranes. The membranes were blocked for 1 h at room temperature
with 5% skimmed milk in TBS. Subsequently, the membranes were
incubated with rabbit polyclonal primary antibodies targeted
against: ERα (cat. no. ab75635; 1:1,000; Abcam), AR (cat. no.
ab74272; 1:1,000; Abcam) and β-actin (cat. no. 4970; 1:2,000; Cell
Signaling Technology, Inc.) in 10 ml of 5% skimmed milk and
incubated at 4˚C overnight. After washing, the membranes were
incubated with a horseradish peroxidase-conjugated goat anti-rabbit
IgG secondary antibody (cat. no. 7074; 1:5,000; Cell Signaling
Technology, Inc.) for 1 h at 37˚C. The results were visualized
using an enhanced chemiluminescence kit (ECL kit; Pierce; Thermo
Fisher Scientific, Inc.) using Kodak XAR-5 film (Sigma-Aldrich;
Merck KGaA). β-actin was used as the loading control.
Cell culture and transfections
Human HeLa and SiHa CC cell lines, and 293T cells
were obtained from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences. Cells were cultured in DMEM/F12 (cat.
no. 11030; Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS (cat. no. 16000-44; Gibco; Thermo Fisher Scientific, Inc.)
at 37˚C with 5% CO2.
miR-130a inhibitor (miR-130ai), miR-130a inhibitor
negative control (NC; miR-130ai NC), miR-130a mimic (miR-130am) and
miR-130a mimic negative control (miR-130am NC) were synthesized by
Shanghai GenePharma Co., Ltd. The oligo sequences are provided in
Table I. For transfection, cells
were seeded into 6-well plates at 70-80% confluence and grown
overnight. Subsequently, HeLa or SiHa cells were transfected with
100 nM miR-130ai, miR-130am, miR-130ai NC or miR-130am NC using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Transfection efficiency of miR-130ai and miR-130am was assessed via
RT-qPCR after 24 h (Fig. S1A and
B). For vector transduction,
ERα-overexpression vector (Ubi-MCS-3FLAG-ESR1), AR-overexpression
vector (Ubi-MCS-3FLAG-AR) and empty vector were purchased from
Shanghai GeneChem Co., Ltd. HeLa and SiHa cells were transfected
with 1 µg/ml Ubi-MCS-3FLAG-ESR1, Ubi-MCS-3FLAG-AR or empty vector
using Lipofectamine® 2000 according to the
manufacturer's instructions. Transfection efficiency of
Ubi-MCS-3FLAG-ESR1 and Ubi-MCS-3FLAG-AR were confirmed via western
blotting after 48 h (Fig. S1C and
D).
Cell Counting Kit-8 (CCK-8) and cell
clonogenic assays
To assess cell proliferation using the CCK-8 assay
at 70-80% confluence, transfected cells were serum-starved for 24 h
at 5% CO2, 37˚C and then cultured in 96-well plates
(1x103 cells/well). Subsequently, at selected time
points (24, 48, 72, 96 and 120 h), 20 µl CCK-8 reagent (Dojindo
Molecular Technologies, Inc.) were added in each well, and
incubated at 37˚C for 2 h to measure the rate of cell
proliferation. Absorbance was measured at a wavelength of 490 nm
using a SpectraMax 190 microplate reader (Bio-Rad Laboratories,
Inc.). For the cell clonogenic survival assays, transfected cells
were seeded (2x103 cells/plate) into 6-well plates.
Following culture for 2 weeks at 5% CO2, 37˚C, cell
colonies (-50 cells are visible from one cell) were fixed in 2%
formaldehyde in PBS for 2 min and the stained with 0.5% crystal
violet for 30 min (both at room temperature). Colonies were
visually counted.
Transwell invasion assay
Cells were plated (2x105 cells/well) in
serum-free medium in Transwell chambers (8-µm pore size; BD
Biosciences) pre-coated with Matrigel (4˚C for 2 h). Complete
DMEM/F12 medium containing 10% FBS was added to 24-well plates as a
chemoattractant (lower chamber). After 24 h of incubation at 5%
CO2, 37˚C, the cells were fixed with 4% paraformaldehyde
for 1 h at room temperature. The cells on the apical side of each
insert were removed by mechanical scraping. The cells that migrated
to the basal side of the membrane were stained with 0.1% crystal
violet at room temperature and visualized under a Leica DMI 3000B
light microscope (Leica Microsystems, Inc.; magnification,
x200).
Luciferase assays
By searching for potential miRNAs, the miR-130a-3p
targeting site was identified within the 3'untranslated region
(UTR) of ESR1 and AR, as detected by three software algorithms
(TargetScan v3.1, targetscan.org; Pictar, pictar.mdc-berlin.de; and MiRanda, miranda.org). To examine whether miR-130a-3p could
modulate ERα and AR expression, a DNA fragment comprising a partial
wild-type (WT) 3'UTR of ESR1or AR was constructed, as well as a
corresponding mutant (MT) 3'UTR of ESR1 or AR. The plasmids were
synthesized and cloned into the pGL3-REPORT luciferase vector
(Promega Corporation) containing the luciferase gene to generate
pGL3-ESR1/AR-3'UTR-WT and pGL3-ESR1/AR-3'UTR-MT. 293T cells
(5x104/well) were seeded into 24-well plates and
transfected with 0.2 µg of either pGL3-ESR1/AR-3'UTR-WT or
pGL3-ESR1/AR-3'UTR-MT. The luciferase reporter assay was performed
as previously described (16,21,22).
Xenograft tumor growth assays
The animal experiments were performed in strict
accordance with the Guideline for the Care and Use of Laboratory
Animals of China. The protocol was approved by the Committee on the
Ethics of Animal Experiments of Shanghai Jiaotong University
[approval no. SCXK (hu) 2018-0007]. All efforts were made to
minimize animal suffering. A total of 10 female BALB/c nude mice
(age, 5 weeks; weight, 15 g) were obtained from the Chinese Academy
of Sciences. Animals were maintained at standard controlled
conditions (room temperature, 22±1˚C; relative humidity, 50-60%) on
a 12 h light/dark cycle and free access to food and water. HeLa
cells were harvested and resuspended (5x106 cells/200
µl) in sterile saline. Mice (n=5 per group; miR-130a-targeting
antagomiR group and the control antagomiR group) were
subcutaneously injected with 200 µl HeLa cells in the subdermal
space on the medial side of the neck. After ~1 week, when tumors
reached an average volume of ~20 mm3, each tumor was
directly injected with antagomiR-130a (cat. no. B05001) or control
antagomiR (cat. no. B04007) (both from Cytiva; 40 ml PBS containing
1 µg antagomiR-130a or control antagomiR) on day 0 (when the tumors
reached an average volume of 20 mm3), 5 and 9(23). Tumor volume was measured every 7
days until the end of the experiment and was calculated using the
following formula: Volume=(largest diameter x smallest
diameter2) x0.5. At the end of the xenograft experiment
(day 21), mice were sacrificed by CO2 inhalation (30%
volume displacement per minute). Following sacrifice, tumor weight
was determined.
In silico analysis of ESR1, AR and
disease-free survival (DFS) of patients with CC
In silico analysis of the association between
ESR1 or AR and DFS of patients with cervical cancer was performed
using online published data obtained from Gene Expression Profiling
Interactive Analysis (GEPIA; gepia.cancer-pku.cn) and The Cancer Genome Atlas
(TCGA) network (cBioPortal for Cancer Genomics; cbioportal.org) (24-26).
Overall survival (OS), DFS, disease-specific survival (DSS) and
progression-free survival (PFS) were analyzed using the cBioPortal
for Cancer Genomics database (25,26).
Statistical analysis
Comparisons between two groups were analyzed using
an unpaired Student's t-test. Comparisons among multiple groups
were analyzed using one-way ANOVA followed by Tukey's post hoc
test. DFS was assessed by using the Kaplan-Meier method with the
log-rank test Data are presented as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using SPSS
software (version 16.0; SPSS, Inc.). All experiments were carried
out in triplicate and repeated at least three times.
Results
In silico analysis of ESR1 and AR
expression, and association with survival of patients with CC
To establish whether steroid hormone receptors
affected CC progression, the GEPIA database was used to examine
cross-cancer alteration summaries of ESR1 and AR. Patients with CC
displayed notably lower ESR1 and AR expression levels compared with
healthy individuals (Fig. 1A and
B). The associations between ESR1
and AR expression and DFS of patients with CC (n=292) were assessed
in an independent dataset obtained from the GEPIA database
(24). The DFS of the high-ESR1
group [hazard ratio (HR), 0.73; 95% confidence interval (CI),
0.61-0.90] and the low-ESR1 group (95% CI, 0.62-0.82) are presented
in Fig. 1C. The DFS of the high-AR
group (HR, 0.78; 95% CI, 0.72-0.88) and the low-AR group (95% CI,
0.63-0.83) are presented in Fig.
1D. In another TCGA network (cBioPortal for Cancer Genomics)
(25,26), the OS, DFS, DSS and PFS of patients
with CC from the cBioPortal database were also analyzed (data not
shown). Although not significant, patients with CC and lower ESR1
and AR expression displayed poorer survival rates compared with
patients with CC and higher ESR1 and AR expression.
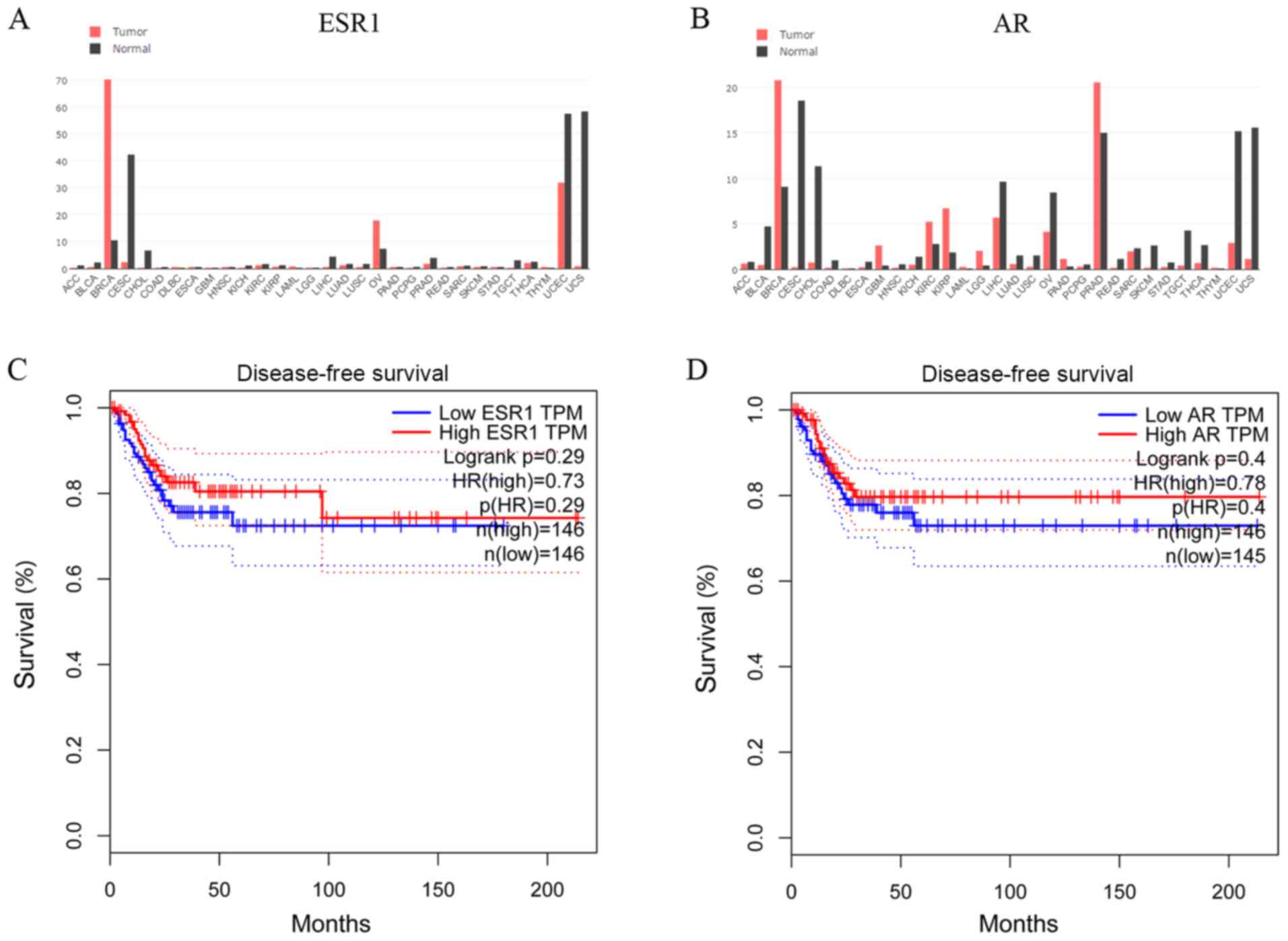 | Figure 1In silico analysis of ESR1 and
AR expression, and the association with patient survival in CC.
Genomics of cross-cancer alteration summary of (A) ESR1 and (B) AR
genes in tumor and healthy tissues. (C) DFS of the high-ESR1 (HR,
0.73; 95% CI, 0.61-0.90) and low-ESR1 (95% CI, 0.62-0.82) groups.
(D) DFS of the high-AR (HR, 0.78; 95% CI, 0.72 to 0.88) and low-AR
(95% CI, 0.63-0.83) groups. ESR1, estrogen receptor α gene; AR,
androgen receptor; DFS, disease-free survival; CI, confidence
interval; HR, hazard ratio. |
ERα and AR expression is significantly
decreased in CC
The protein expression levels of ERα and AR in CC
were analyzed via IHC. ERα- and AR-positive immunostaining was
observed in the cell nucleus (Fig.
2A and B). The protein
expression levels of ERα and AR were decreased in a sequential
manner from healthy cervical to CIN and further to CC tissues
(Fig. 2C), suggesting that low ERα
and AR expression was associated with high-grade lesions.
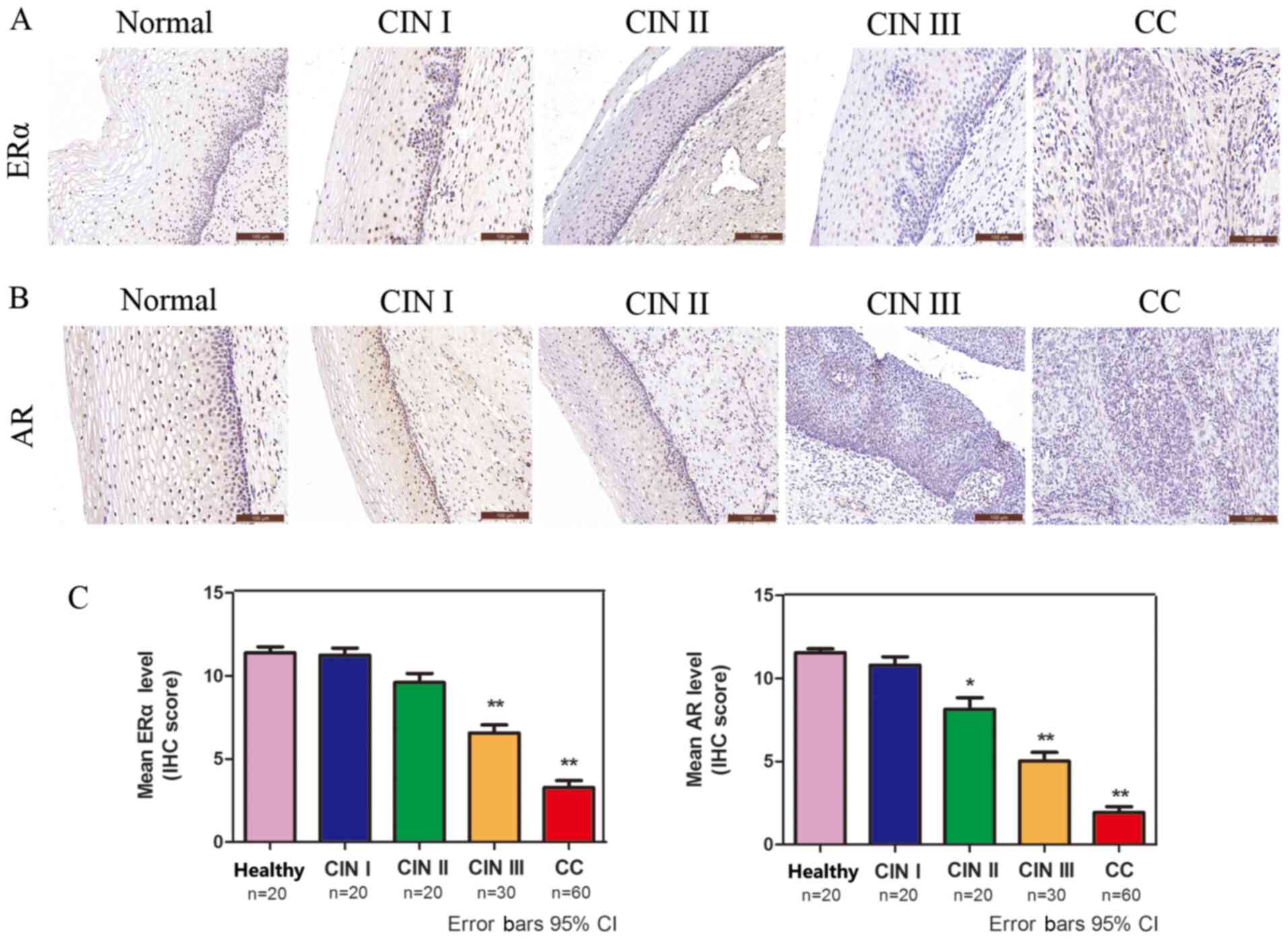 | Figure 2ERα and AR are significantly
decreased in CC. Immunohistochemical analysis of (A) ERα and (B) AR
in healthy cervical, CIN and squamous cell cervical carcinoma
tissues (magnification, x200). (C) Quantification of
immunohistochemical staining in healthy cervical epithelium (n=20),
CIN I (n=20), CIN II (n=20), CIN III (n=30) and CC (n=60) tissues.
*P<0.05 and **P<0.01. ERα, estrogen
receptor α; AR, androgen receptor; CC, cervical cancer; CIN,
cervical intraepithelial neoplasia; IHC, immunohistochemistry; CI,
confidence interval. |
ERα and AR are targets of
miR-130a-3p
miRNAs can regulate gene expression by inhibiting
translation or inducing mRNA degradation at the
post-transcriptional level (27).
The miR-130a-3p targeting site was identified within the 3'UTRs of
ESR1 and AR (Fig. 3A). 293T cells
were co-transfected with WT or MT vector and miR-130am or miR-130am
NC. miR-130am significantly decreased the relative luciferase
activity of ESR1-3'UTR-WT and AR-3'UTR-WT compared with miR-130am
NC. By contrast, miR-130am did not significantly alter the
luciferase activity of ESR1-3'UTR-MT or AR-3'UTR-MT compared with
miR-130am NC (Fig. 3B and C).
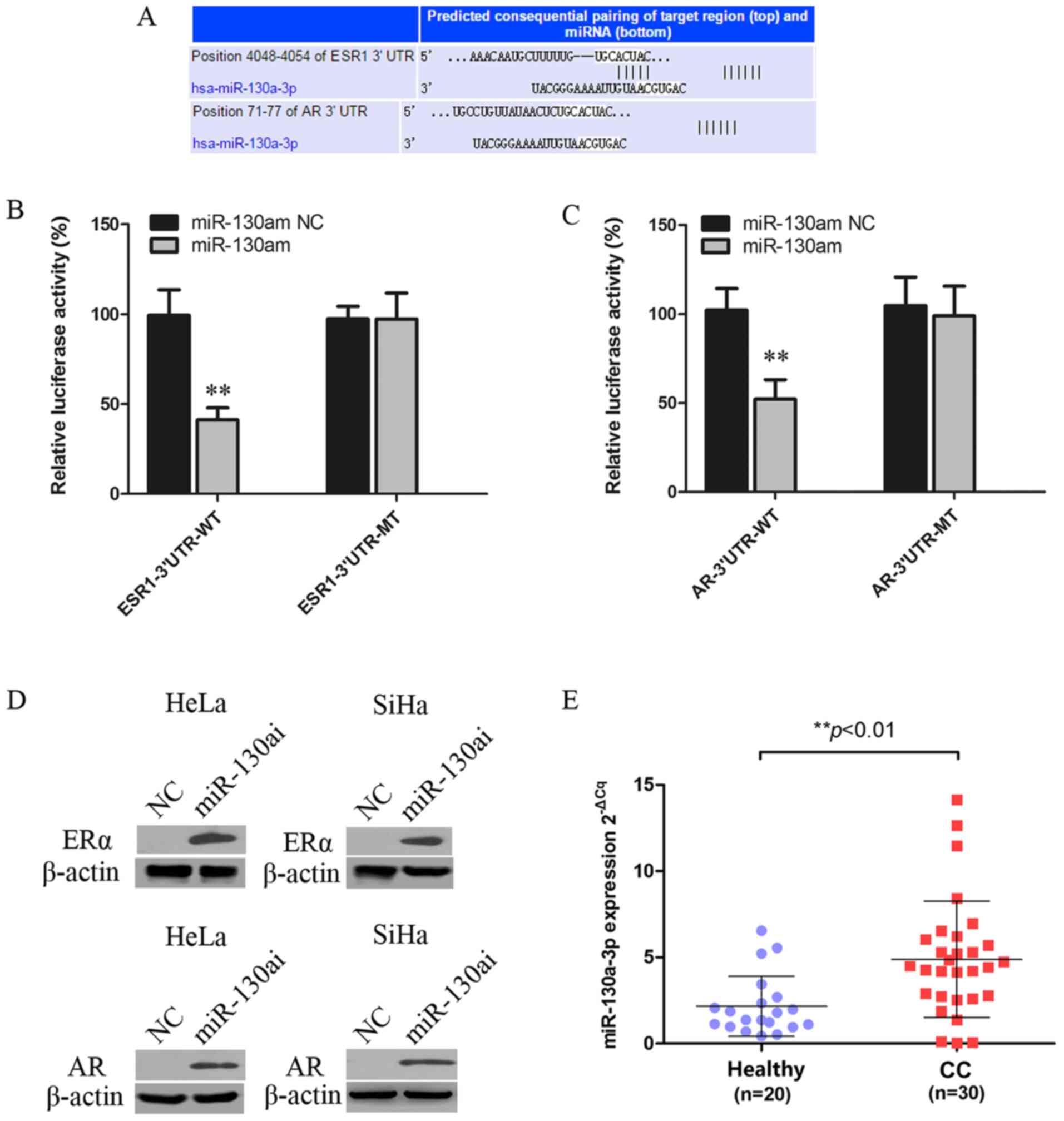 | Figure 3ERα and AR are targets of
miR-130a-3p. (A) Putative miR-130a-3p binding site in the 3'UTRSs
of ESR1 and AR, as predicted by TargetScan, Pictar and MiRanda.
Luciferase assay of 293T cells co-transfected with (B)
pGL3-ESR1-3'UTR-WT, pGL3-ESR1-3'UTR-MT, (C) pGL3-AR-3'UTR-WT or
pGL3-AR-3'UTR-MT and miR-130am or NC. (D) ERα and AR protein
expression levels in HeLa and SiHa cells following transfected with
miR-130ai NC or miR-130ai. (E) miR-130a-3p expression levels in CC
(n=30) and healthy cervical (n=20) tissues. **P<0.01
vs. NC. EERα, estrogen receptor α; AR, androgen receptor; miR,
microRNA; 3'UTR, 3'untranslated region; WT, wild-type; MT, mutant;
miR-130am, miR-130a mimics; miR-130ai, miR-130a inhibitor; NC,
negative control; CC, cervical cancer. |
To further investigate the functional role of
deregulated miR-130a-3p in CC cells, the effects of miR-130ai on
the expression of ERα and AR were examined. miR-130ai notably
increased the protein expression levels of ERα and AR compared with
miR-130ai NC in HeLa and SiHa cells (Fig. 3D), supporting its role as a
functional suppressor of ERα and AR. Additionally, the levels of
miR-130a-3p in 20 healthy cervical tissues and 30 CC tissues were
detected via RT-qPCR. The results indicated that miR-130a-3p
expression levels were significantly higher in CC tissues compared
with healthy cervical tissues (Fig.
3E).
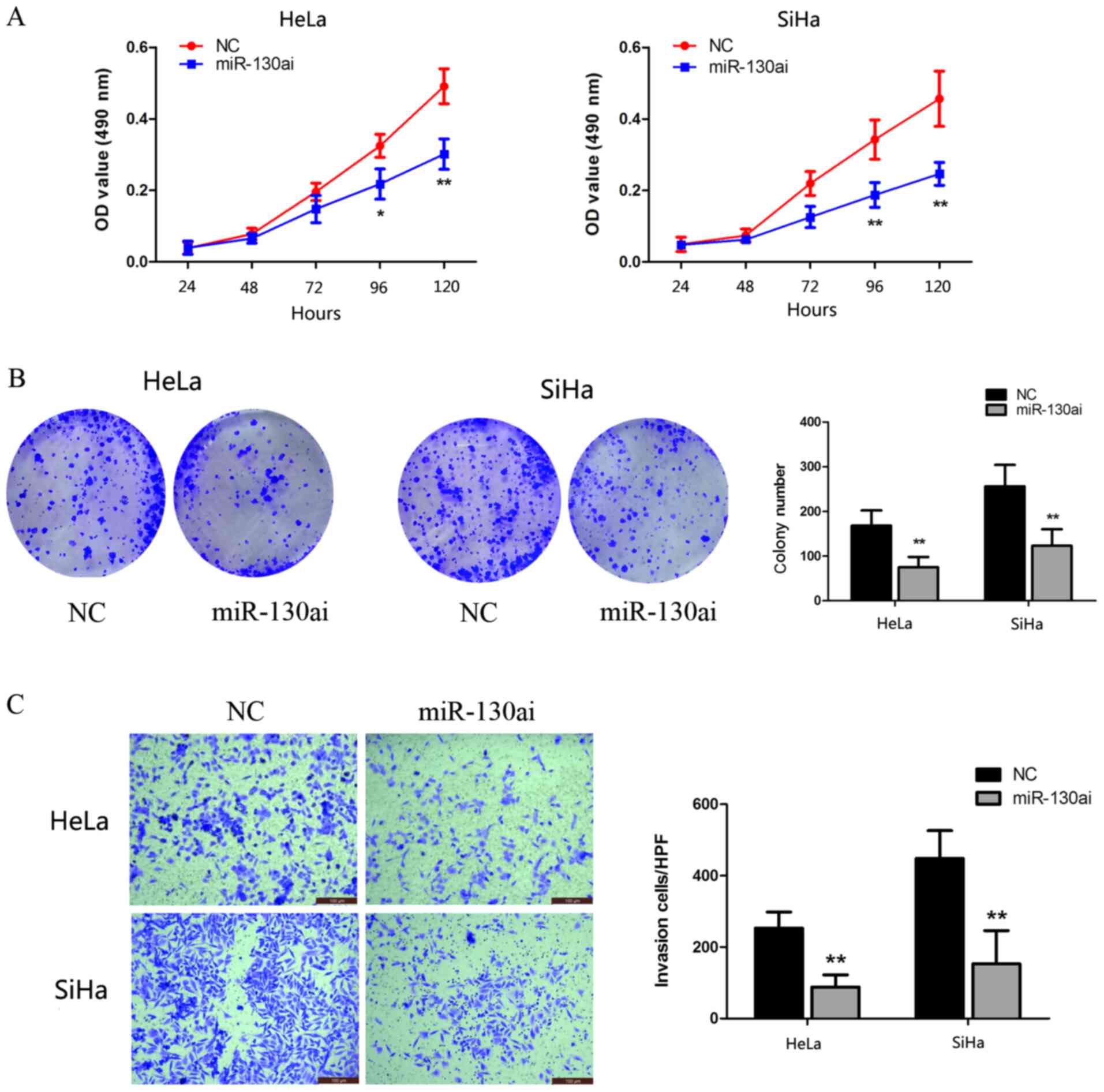
miR-130a-3p knockdown inhibits CC cell
proliferation and invasion
To identify the biological function of miR-130a-3p,
HeLa and SiHa cells were transfected with miR-130ai. Compared with
miR-130ai NC, miR-130ai significantly inhibited HeLa and SiHa cell
proliferation, as indicated by the CCK-8 and clonogenic assay
results (Fig. 4A and B). Subsequently, the role of miR-130a-3p
in the HeLa and SiHa cell invasion was investigated following
transfection with miR-130ai. miR-130ai significantly decreased cell
invasion compared with miR-130ai NC (Fig. 4C), suggesting that miR-130a-3p
enhanced cell invasion.
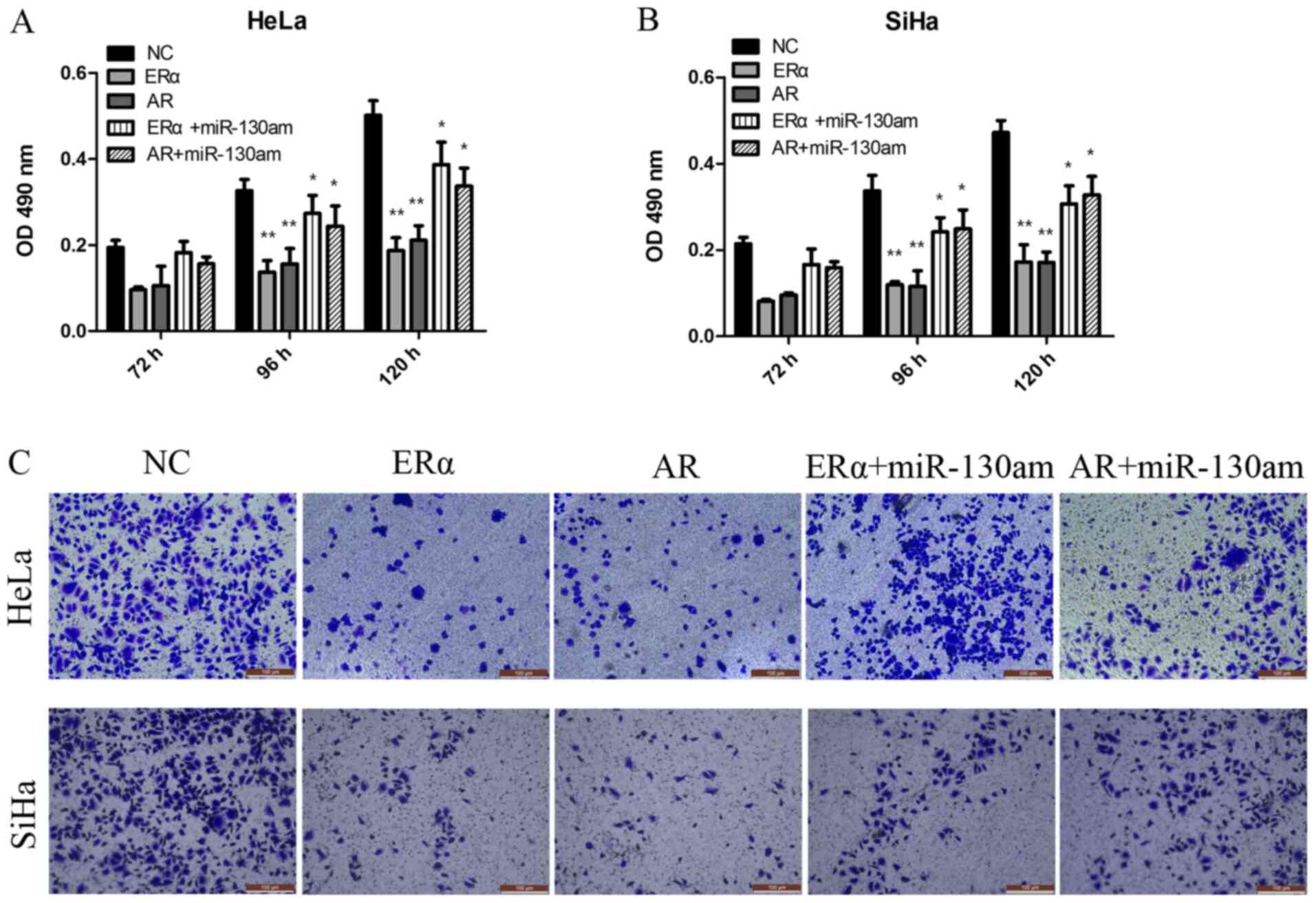
ERα and AR are functionally targets of
miR-130a-3p, and are involved in CC cell proliferation and
invasion
To investigate the biological function of ERα and
AR, vector transduction was performed to overexpress ERα and AR in
HeLa and SiHa cells. Compared with the NC group, ERα overexpression
and AR overexpression significantly inhibited HeLa and SiHa cell
proliferation and invasion, as assessed by performing CCK-8 and
Transwell assays (Fig. 5).
To address whether the biological function effects
of ERα and AR expression were predominately due to the regulation
of miR-130a-3p, the present study investigated whether miR-130a-3p,
ERα and AR functioned via the same signaling pathway to modulate CC
cell proliferation and invasion. The results suggested that
miR-130am significantly rescued CC cell proliferation and invasion
in ERα- and AR-overexpression HeLa and SiHa cells (Fig. 5). The results suggested that ERα and
AR were functional targets of miR-130a-3p in CC cells.
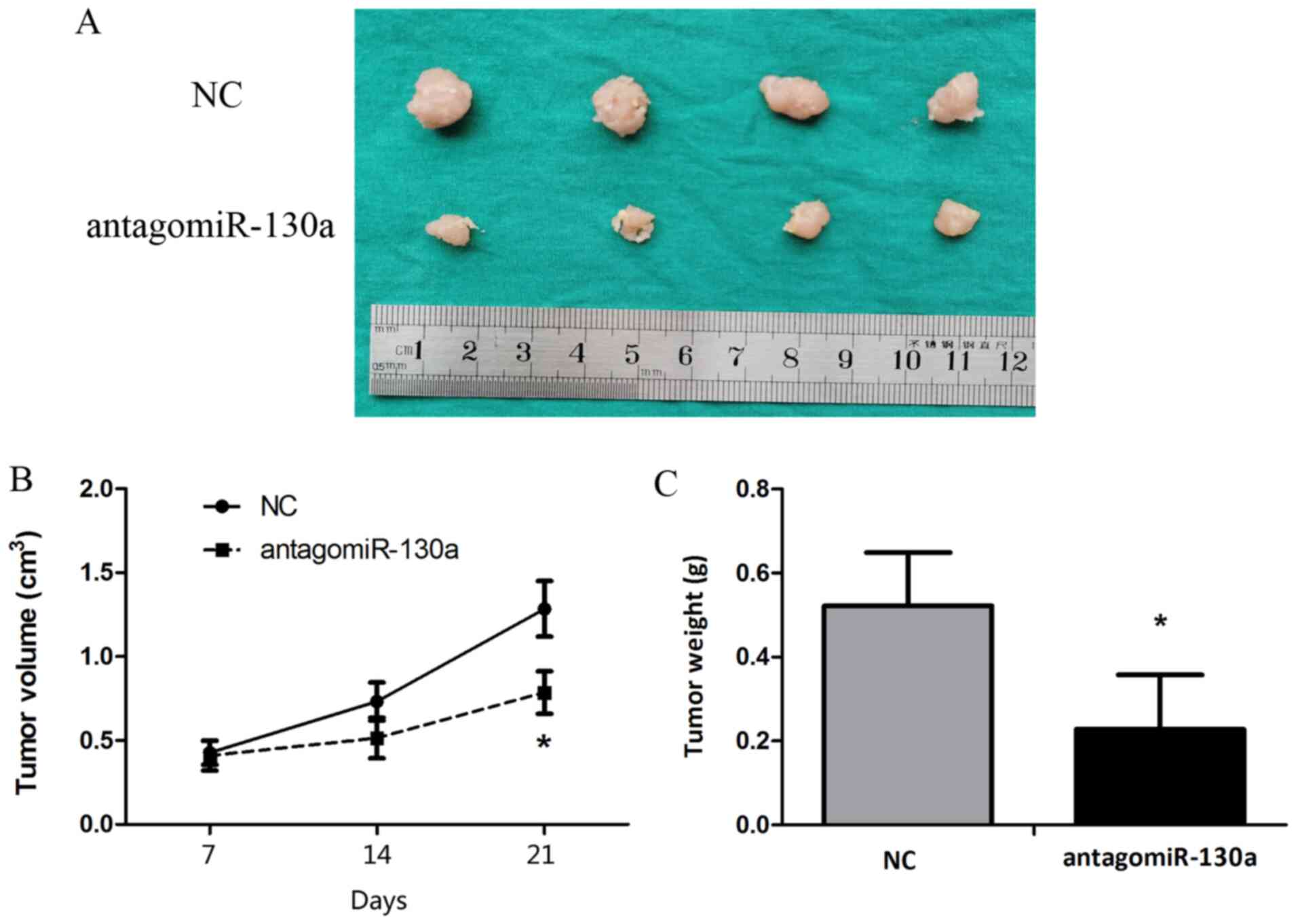
Blocking miR-130a-3p inhibits
tumorigenicity in vivo
Animal studies were conducted to assess the effect
of miR-130a-3p on tumor growth in nude mice. HeLa cells were
subcutaneously injected into subdermal space on the medial side of
the neck of nude mice to establish tumors, which were treated by
direct intratumoral injection when they became clearly palpable.
After monitoring tumor growth for 21 days, the antagomiR-130a group
displayed significantly decreased tumor size and weight compared
with the control antagomiR group (Fig.
6).
Discussion
To the best of our knowledge, the present study
suggested for the first time that miR-130a-3p promoted CC cell
proliferation and invasion by directly targeting ERα and AR.
Despite advances in diagnostic and screening techniques, and the
availability of vaccines, CC remains the fourth largest cause of
cancer-related deaths in women worldwide (1). Numerous established risk factors of CC
have been reported, including exposure to HPV, a high number of
sexual partners and young age at onset of sexual activity (2). Risk factors that are associated with
progression from HPV infection to pre-cancer include oral
contraceptive use and smoking (28). The importance of estrogen and ERα at
all carcinogenic steps was strongly supported in certain mouse
models (29,30); however, few human studies have been
conducted. Therefore, the exact role of hormonal factors in
progression to pre-cancer and cancer is not completely
understood.
ERα is usually expressed in healthy cervical
tissues, but its expression is decreased or absent in invasive CC,
which indicated that ERα expression is lost during the development
of CC (31). Zhai et al
(32) reported that restoration of
ESR1 expression in ERα-negative CC reduced cell invasiveness in
cell culture, and concluded that loss of ERα expression serves a
major role in mediating CC invasion and progression. However, other
studies have indicated that estrogenic stimulation can influence
cervical tumorigenesis (32,33) in
agreement with previous studies, the present study suggested that
the expression of ERα and AR was decreased in a sequential manner
from healthy cervical tissues to CIN tissues and further to CC
tissues, suggesting that the loss of ERα and AR served a major role
in mediating CC progression. The role of AR in CC is not completely
understood. A previous study reported that long-term androgen
treatment of female-to-male transsexuals is associated with
morphological alterations of the cervix (34). In another study, Noël et al
(10) demonstrated that the loss of
AR expression is a frequent and common event in high-grade squamous
intraepithelial lesion and invasive squamous CC, resulting from
complex interactions between high risk HPVs and AR. However, the
underlying regulatory mechanisms and the downstream biological
effects of the loss of ERα and AR in CC malignancy are not
completely understood.
Aberrant expression of miR-130a has been reported in
several types of cancer, including gastric (35), esophageal (36) and breast cancer (21), as well as in CC (15,37).
However, the role of miR-130a in tumor development and progression
is contradictory, as it can serve as an oncogene or tumor
suppressor gene by regulating various canonical signaling pathways
or target genes (38,39). miR-130a is a potential oncomiR
candidate in adriamycin-resistant breast cancer and
platinum-resistant ovarian cancer, whereas it serves as a
suppressive miRNA in cisplatin-resistant hepatoma cells (39). To date, the role of miR-130a in the
progression of CC carcinogenesis is not completely understood. Yin
et al (40) reported that
high expression of miR-130a was significantly associated with lymph
node metastasis and an advanced clinical stage of CC. The results
of the present study indicated that the expression of miR-130a in
CC tissues was obviously higher compared with healthy cervical
tissues, and miR-130a knockdown inhibited CC cell proliferation and
invasion in vitro and in vivo compared with miR-130ai
NC and control antagomiR, respectively. Moreover, the present study
indicated that miR-130a could directly bind to the 3'UTR of ESR1
and AR mRNA, suggesting that miR-130a mediated CC progression by
functionally regulating ERα and AR.
In conclusion, the present study indicated that
miR-130a-3p may contribute to tumor progression by suppressing ERα
and AR, and it may serve as a promising candidate target for the
treatment of patients with CC.
Supplementary Material
Verification of transfection
efficiency. Transfection efficiency of (A) miR-130am, (B)
miR-130ai, (C) Erα-overexpression vector and (D) AR-overexpression
vector in HeLa and SiHa cells. **P<0.01 vs. NC.
miR-130am, miR-130a mimics; miR-130ai, miR-130a inhibitor; ERα,
estrogen receptor α; AR, androgen receptor; NC, negative
control.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 81172477, 81572547 and 81402135),
the Project of Shanghai Key Clinical Department (grant no.
shslczdzk06302), the Project of the Science and Technology
Commission of Shanghai Municipality (grant nos. 11ZR1440800 and
13JC1401303), the Project of Outstanding Subject Leaders of the
Shanghai Health System (grant no. XBR2013097) and the Shanghai Jiao
Tong University Medicine-Engineering Fund (grant no.
YG2017MS41).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QF and YW conceived and designed the study. QF, TH,
XS, XY, JW, YaL, TN and SG performed the experiments. QF, TH, XS
and YuL analyzed the data. QF, TH and YW wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no. GKLW
2017-125) by the Human Investigation Ethical Committee of the
International Peace Maternity & Child Hospital Affiliated to
Shanghai Jiao Tong University School of Medicine (Shanghai, China).
The animal experiment protocol was approved by the Committee on the
Ethics of Animal Experiments of Shanghai Jiaotong University
[approval no. SCXK (hu) 2018-0007].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhao F and Qiao Y: Cervical cancer
prevention in China: A key to cancer control. Lancet. 393:969–970.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Brasseur K, Gevry N and Asselin E:
Chemoresistance and targeted therapies in ovarian and endometrial
cancers. Oncotarget. 8:4008–4042. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wu MH, Huang CJ, Liu ST, Liu PY, Ho CL and
Huang SM: Physical and functional interactions of human
papillomavirus E2 protein with nuclear receptor coactivators.
Biochem Biophys Res Commun. 356:523–528. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Remoue F, Jacobs N, Miot V, Boniver J and
Delvenne P: High intraepithelial expression of estrogen and
progesterone receptors in the transformation zone of the uterine
cervix. Am J Obstet Gynecol. 189:1660–1665. 2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liang J and Shang Y: Estrogen and cancer.
Annual Rev Physiol. 75:225–240. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rosenfeld MG and Glass CK: Coregulator
codes of transcriptional regulation by nuclear receptors. J Biol
Chem. 276:36865–36868. 2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yang XM, Wu ZM, Huang H, Chu XY, Lou J, Xu
LX, Chen YT, Wang LQ and Huang OP: Estrogen receptor 1 mutations in
260 cervical cancer samples from Chinese patients. Oncol Lett.
18:2771–2776. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sood S, Patel FD, Ghosh S, Arora A,
Dhaliwal LK and Srinivasan R: Epigenetic alteration by DNA
methylation of ESR1, MYOD1 and hTERT Gene promoters is useful for
prediction of response in patients of locally advanced invasive
cervical carcinoma treated by chemoradiation. Clin Oncol (R Coll
Radiol). 27:720–727. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Noël JC, Bucella D, Fayt I, Simonart T,
Buxant F, Anaf V and Simon P: Androgen receptor expression in
cervical intraepithelial neoplasia and invasive squamous cell
carcinoma of the cervix. Int J Gynecol Pathol. 27:437–441.
2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297.
2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bao W, Wang HH, Tian FJ, He XY, Qiu MT,
Wang JY, Zhang HJ, Wang LH and Wan XP: A TrkB-STAT3-miR-204-5p
regulatory circuitry controls proliferation and invasion of
endometrial carcinoma cells. Mol Cancer. 12(155)2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lv T, Song K, Zhang L, Li W, Chen Y, Diao
Y, Yao Q and Liu P: miRNA-34a decreases ovarian cancer cell
proliferation and chemoresistance by targeting HDAC1. Biochem Cell
Biol. 96:663–671. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
He L, Wang HY, Zhang L, Huang L, Li JD,
Xiong Y, Zhang MY, Jia WH, Yun JP, Luo RZ and Zheng M: Prognostic
significance of low DICER expression regulated by miR-130a in
cervical cancer. Cell Death Dis. 5(e1205)2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bao W, Zhang Y, Li S, Fan Q, Qiu M, Wang
Y, Li Y, Ji X, Yang Y, Sang Z, et al: miR1075p promotes tumor
proliferation and invasion by targeting estrogen receptoralpha in
endometrial carcinoma. Oncol Rep. 41:1575–1585. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wright JD, Matsuo K, Huang Y, Tergas AI,
Hou JY, Khoury-Collado F, St Clair CM, Ananth CV, Neugut AI and
Hershman DL: Prognostic performance of the 2018 international
federation of gynecology and obstetrics cervical cancer staging
guidelines. Obstet Gynecol. 134:49–57. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fan Q, Qiu MT, Zhu Z, Zhou JH, Chen L,
Zhou Y, Gu W, Wang LH, Li ZN, Xu Y, et al: Twist induces
epithelial-mesenchymal transition in cervical carcinogenesis by
regulating the TGF-β/Smad3 signaling pathway. Oncol Rep.
34:1787–1794. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bao W, Qiu H, Yang T, Luo X, Zhang H and
Wan X: Upregulation of TrkB promotes epithelial-mesenchymal
transition and anoikis resistance in endometrial carcinoma. PLoS
One. 8(e70616)2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pan Y, Wang R, Zhang F, Chen Y, Lv Q, Long
G and Yang K: MicroRNA-130a inhibits cell proliferation, invasion
and migration in human breast cancer by targeting the RAB5A. Int J
Clin Exp Pathol. 8:384–393. 2015.PubMed/NCBI
|
|
22
|
Zheng L, Kang Y, Zhang L and Zou W:
MiR-133a-5p inhibits androgen receptor (AR)-induced proliferation
in prostate cancer cells via targeting FUsed in Sarcoma (FUS) and
AR. Cancer Biol Ther. 21:34–42. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mercatelli N, Coppola V, Bonci D, Miele F,
Costantini A, Guadagnoli M, Bonanno E, Muto G, Frajese GV, De Maria
R, et al: The inhibition of the highly expressed miR-221 and
miR-222 impairs the growth of prostate carcinoma xenografts in
mice. PLoS One. 3(e4029)2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6(pl1)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Laengsri V, Kerdpin U, Plabplueng C,
Treeratanapiboon L and Nuchnoi P: Cervical cancer markers:
Epigenetics and microRNAs. Lab Med. 49:97–111. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Luhn P, Walker J, Schiffman M, Zuna RE,
Dunn ST, Gold MA, Smith K, Mathews C, Allen RA, Zhang R, et al: The
role of co-factors in the progression from human papillomavirus
infection to cervical cancer. Gynecol Oncol. 128:265–270.
2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chung SH, Wiedmeyer K, Shai A, Korach KS
and Lambert PF: Requirement for estrogen receptor alpha in a mouse
model for human papillomavirus-associated cervical cancer. Cancer
Res. 68:9928–9934. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chung SH, Franceschi S and Lambert PF:
Estrogen and ERalpha: Culprits in cervical cancer? Trends
Endocrinol Metab. 21:504–511. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lopez-Romero R, Garrido-Guerrero E,
Rangel-Lopez A, Manuel-Apolinar L, Piña-Sánchez P, Lazos-Ochoa M,
Mantilla-Morales A, Bandala C and Salcedo M: The cervical malignant
cells display a down regulation of ER-α but retain the ER-β
expression. Int J Clin Exp Pathol. 6:1594–1602. 2013.PubMed/NCBI
|
|
32
|
Zhai Y, Bommer GT, Feng Y, Wiese AB,
Fearon ER and Cho KR: Loss of estrogen receptor 1 enhances cervical
cancer invasion. The American journal of pathology. 177:884–895.
2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Deligeoroglou E, Michailidis E and
Creatsas G: Oral contraceptives and reproductive system cancer.
Annals of the New York Academy of Sciences. 997:199–208.
2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Miller N, Bedard YC, Cooter NB and Shaul
DL: Histological changes in the genital tract in transsexual women
following androgen therapy. Histopathology. 10:661–669.
1986.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jiang H, Yu WW, Wang LL and Peng Y:
miR-130a acts as a potential diagnostic biomarker and promotes
gastric cancer migration, invasion and proliferation by targeting
RUNX3. Oncology reports. 34:1153–1161. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu SG, Qin XG, Zhao BS, et al:
Differential expression of miRNAs in esophageal cancer tissue.
Oncology letters. 5:1639–1642. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chi C, Mao M, Shen Z, Chen Y, Chen J and
Hou W: HOXD-AS1 Exerts Oncogenic Functions and Promotes
Chemoresistance in Cisplatin-Resistant Cervical Cancer Cells. Human
gene therapy. 29:1438–1448. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hofsjo A, Bohm-Starke N, Bergmark K,
Masironi B and Sahlin L: Sex steroid hormone receptor expression in
the vaginal wall in cervical cancer survivors after radiotherapy.
Acta oncologica. 58:1107–1115. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhang HD, Jiang LH, Sun DW, Li J and Ji
ZL: The role of miR-130a in cancer. Breast cancer. 24:521–527.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yin S, Zhang Q, Wang Y, Li S and Hu R:
MicroRNA-130a regulated by HPV18 E6 promotes proliferation and
invasion of cervical cancer cells by targeting TIMP2. Experimental
and therapeutic medicine. 17:2837–2846. 2019.PubMed/NCBI View Article : Google Scholar
|