Introduction
Nursing personnel has an important role in the
pre-operative and post-operative management of patients in any
surgical practice. Enforcement of strict infection control measures
by them may significantly influence the rates of nosocomial
surgical site infections (SSIs) in a health-care setup.
SSIs are an important cause of increased morbidity
and mortality after cardiothoracic (CT) surgery (1). According to the literature, the
reported incidence of SSIs after CT surgery varies from 3 to 10.5%
(1-3).
A small proportion of these SSIs are able to progress to deep
sternal or mediastinal wound infection, which requires aggressive
medical and surgical therapy and the associated mortality rate is
up to 47% (4,5). Approximately 50% of SSIs after CT
surgery are caused by Staphylococcus aureus (S.
aureus), while coagulase-negative staphylococci, gram-negative
bacteria and yeast are less frequently implicated (3,6).
S. aureus is known to colonize the skin and
mucosae of 15-36% of the global population (7). Of the various sites reported, the nose
is considered to be an important reservoir in humans and nasal
colonization has been reported to be an independent risk factor for
SSIs (7-9).
To reduce the incidence of S. aureus infections, nasal
application of topical mupirocin has been widely reported in the
literature (10,11). In one of the earliest randomized
controlled trials (RCTs) on surgical patients, Bode et al
(12) reported a significantly
reduced incidence of SSIs in S. aureus carriers with the use
of pre-operative nasal mupirocin and chlorhexidine soap. A Cochrane
review by van Rijen et al (10), analyzing nine RCTs, indicated that
nasal mupirocin was associated with a statistically significant
reduction of S. aureus infection. The review, however,
included studies irrespective of the site of the surgical
procedure. The incidence of SSI may depend on several factors,
including patient characteristics, duration of surgical procedure
and factors specific to the operative site (3). While factors such as rheumatoid
arthritis, psoriasis, immunosuppression or history of surgery at
the site of the prosthesis may influence the risk of SSI in
orthopedic surgery, infections after CT surgery may be impacted by
the body mass index, hemodialysis, cardiogenic shock, perfusion
time, use of intra-aortic pump and the presence of >3
anastomoses (3). It is important to
further determine the risk of SSIs for specific surgical sites.
Evidence on the use of mupirocin-based
decolonization specifically in CT surgery has been rather
conflicting. Konvalinka et al (13) reported on an RCT that indicated no
beneficial effect of prophylactic nasal mupirocin in reducing the
incidence of SSI after CT surgery. On the other hand, in a recent
study, Lemaignen et al (14)
reported a significant reduction in the incidence of SSIs with the
use of nasal mupirocin-based decolonization protocol in their
13-year experience. Despite several reviews and meta-analyses
assessing the role of mupirocin-based decolonization in reducing
S. aureus infection (10,15-17),
only a small number of studies have analyzed evidence on the
efficacy of such an intervention in reducing SSIs specifically in
patients following CT surgery. A meta-analysis by Schweizer et
al (17) from 2013 assessed the
efficacy of bundled intervention consisting of nasal decolonization
(not restricted to mupirocin) and glycopeptide prophylaxis to
reduce SSIs in patients subjected to cardiac or orthopedic surgery.
They reported a statistically significant reduction of SSIs with
nasal decolonization in cardiac patients but did not separate
cardiac studies based on the targeted population treated with
mupirocin (i.e. S. aureus carriers or all patients).
Similarly, Ma et al (18)
performed a meta-analysis of pediatric and adult patients subjected
to CT surgery, pooling data of studies utilizing both targeted and
universal decolonization for reducing SSIs.
Considering the lacunae in the literature, the
purpose of the present review was to perform a systematic
literature search and pool evidence on the role of the
mupirocin-based decolonization protocol in reducing SSIs in
patients undergoing CT surgery based on their S. aureus
carrier status.
Materials and methods
Search strategy
A computerized literature search of the PubMed
(https://pubmed.ncbi.nlm.nih.gov), Embase
(https://www.embase.com), Ovid (https://ovidsp.ovid.com), BioMed Central (https://www.biomedcentral.com), Cochrane Central
Register of Controlled Trials (CENTRAL; https://www.cochranelibrary.com/central/about-central)
and Google scholar (https://scholar.google.com) databases was performed.
The search was conducted by two reviewers independently (QJ and
XH). The same reviewers independently screened the databases from
inception up to 1st March 2020. MeSH terms, as well as free-text
keywords, were used in the literature search. The entire search
protocol, as well as the results of the PubMed database, are
presented in Table SI. The search
results were screened by their titles and abstracts for each
database. Potentially relevant articles were then extracted and
subsequently screened by their full text. Both the reviewers
assessed individual studies based on inclusion criteria and
resolved any disagreement by discussion. After screening, the
bibliography of included studies, as well as review articles on the
subject, were hand searched for any additional references. The
Preferred Reporting Items for Systematic Reviews and Meta-analyses
guidelines were followed during the conduct of this systematic
review, except for protocol registration (19).
Inclusion criteria
All types of studies were included in this
systematic review. Included studies were to be performed on adult
patients (age, >18 years) undergoing any type of CT surgery
(‘Participants’). The study intervention was nasal decolonization
with mupirocin ointment in the study participants. Studies with
bundled interventions utilizing mupirocin-based nasal
decolonization were also included. Studies utilizing targeted
decolonization of S. aureus carriers as well as those
performing universal decolonization of all patients were included.
Studies were to compare the study group with a control group for
the incidence of SSIs. Studies not reporting relevant data or
separate data for CT surgery patients and those not specifying the
targeted population of mupirocin-based decolonization (carriers or
all) were excluded. Single-arm studies, case series, case reports,
abstracts, non-English language studies and review articles were
also excluded.
Data extraction and quality of
included studies
Using a pre-formatted abstraction form, the
reviewers extracted data from the included studies (QJ and XH).
Details including the name of the first author, publication year,
study type, study location, sample size, demographic details, use
of screening for carrier state and method of screening,
decolonization protocol, antibiotic prophylaxis use and incidence
of SSIs were extracted. The primary outcome variable was all SSIs
irrespective of the causative organism and site. Secondary outcomes
were superficial SSIs, deep SSIs, SSIs caused by S. aureus
(SA-SSIs) and SSIs caused by MRSA (MRSA-SSIs). Definitions of SSIs
were as per the included studies.
The risk of bias of RCTs was assessed using the
Cochrane Collaboration risk assessment tool for RCTs (20). Each study was assessed in the
following domains: Random sequence generation, allocation
concealment, blinding of participants and personnel, blinding of
outcome assessment, incomplete outcome data, selective reporting
and other biases. The risk of a bias assessment tool for
non-randomized studies was used to assess non-RCTs (21). Studies were rated as having low
risk, high risk or unclear risk of bias in the following
categories: Selection of participants, confounding variables,
intervention measurements, blinding of outcome assessment,
incomplete outcome data and selective outcome reporting.
Statistical analysis
Studies were divided into two groups based on the
decolonization protocol. The first group consisted of studies
utilizing decolonization of only carriers after appropriate
pre-operative screening (targeted decolonization), while the second
group consisted of studies carrying out decolonization of all
patients in the study group irrespective of the carrier state
(universal decolonization). Studies were pooled for a meta-analysis
only if they were conducted on coherent groups and reported data on
the same scale. The software ‘Open MetaAnalyst (version Yosemite
10.10)’ was used for the meta-analysis (22). To take methodological heterogeneity
of the included studies into account, a random-effects model was
used to calculate the pooled effect size for all analyses. The
incidence of SSIs was compared between study and control groups
using the Risk Ratio (RR) with 95% CI. A sub-group analysis was
also performed based on the use of other decolonization
interventions along with mupirocin for the primary outcome. A
leave-one-out analysis was performed to assess the influence of
each study on the pooled effect size for both the primary and
secondary outcome variables. Heterogeneity was assessed using the
I2 statistic. I2 values of 25-50% represented
low, values of 50-75% medium and >75% represented substantial
heterogeneity. Publication bias was not assessed due to the limited
number of studies included in the meta-analysis (<10) (20).
Results
Study retrieval and quality
control
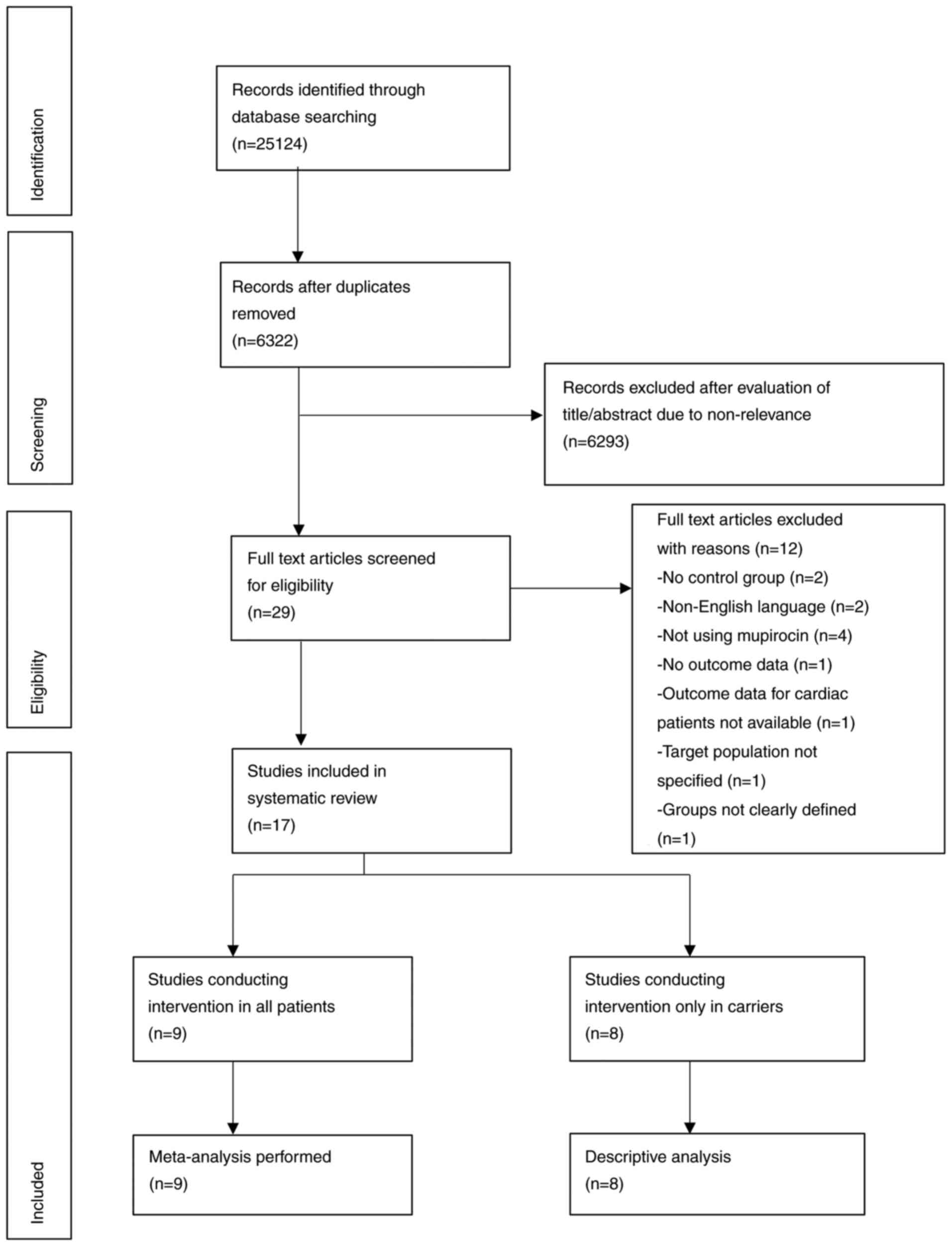
Initially, 6,322 unique records were identified in
the literature search. Of these, 29 articles were selected for
full-text review. A total of 12 studies were excluded as they did
not fulfill the inclusion criteria (Fig. 1). A total of 17 studies were
included (12-14,23-36).
Since studies (12,13,30-35)
on targeted mupirocin-based decolonization were not coherent with
respect to study groups and data presentation, only a descriptive
analysis was performed. Meta-analysis was performed for studies
(14,23-29,36)
conducting universal decolonization with the mupirocin-based
protocol. The results of the quality assessment of the included
studies are presented in Table
SII. The quality of RCTs was high but the overall quality of
all remaining studies was low. Owing to their study designs, only
two studies recruited patients in the same time period (31,35).
Multivariate analysis for confounding factors was performed only by
three studies (14,29,34).
Targeted mupirocin-based
decolonization
A total of 8 studies (12,13,30-35)
assessed the role of targeted mupirocin-based decolonization on
SSIs after a pre-operative screening of S. aureus carriers.
The characteristics of these studies are presented in Table I. Of these studies, 4 were
interrupted time-series (ITS) design studies (30,32-34),
1 was prospective (35), 1 was
retrospective (31) and 2 were RCTs
(12,13). All studies were conducted in
industrialized countries with 4 studies being carried out in the
USA (30,31,33,34).
There were differences in the carrier states between the study and
control groups in all 7 studies. Only carriers were included in
both the study and control groups of the 2 RCTs (12,13).
Nicholas et al (35) and
Shrestha et al (31)
compared mupirocin-based decolonization between a study group of
carriers and a control group of only non-carriers. In the remaining
four studies, both carriers and non-carriers constituted the study
and control groups (30,32-34),
but the intervention was restricted to only carriers in the study
group. S. aureus screening was carried out by either PCR or
culture in the included studies. Decolonization protocol was
restricted to the nasal application of mupirocin in three studies
(13,30,31)
while the remaining five studies also utilized topical
chlorhexidine (12,33-35)
or triclosan (32) in the study
groups. Duration of mupirocin use varied from 5-7 days. In four
studies mupirocin was also used in the control groups pending
results of carrier state screening (30-33).
Three ITS studies also utilized additional antibiotic prophylaxis
for carriers in the study group [vancomycin (33,34) or
teicoplanin (32)].
 | Table ICharacteristics of studies utilizing
mupirocin-based decolonization in carriers only. |
Table I
Characteristics of studies utilizing
mupirocin-based decolonization in carriers only.
| | Sample size | Mean age
(years) | Diabetics (%) | | |
|---|
| Author (year) | Study type | Study location | CTS type | Carrier state in
study and control groups | Study | Control | Study | Control | Study | Control | Screening of
carrier state by | Decolonization
protocol | Antibiotic
prophylaxis | Outcomes | (Refs.) |
|---|
| Nicholas
(2020) | Prospective | France | CABG, valvular
surgery | Study: Carriers
Control: non-carriers | 43 | 315 | NA | NA | NA | NA | Culture | Nasal application
of mupirocin 2-3 times/day, CHX bath once/day, CHX oral rinse 2-3
times/day for 5 days. | Cefuroxime or
vancomycin | SA-SSI: 10.5% with
intervention failure and 0% with successful decolonization. Data of
control group NA. | (35) |
| Saraswat
(2017) | Interrupted
time-series study | USA | CABG, valvular
surgery | Studya: Carriers & non-carriers
Control: Carriers & non-carriers | 4038 | 2826 | 61.8 | 60.9 | 29 | 26 | PCR | Nasal application
of mupirocin 2 times/ day, CHX bath once/day for 5 days. | Cefazolin for all
patients. MRSA carriers also received a single dose of vancomycin
before surgery | All SSI: Reduced
incidence in study group (OR: 0.58, 95% CI: 0.39, 0.86) with
significantly reduced MRSA colonization. | (34) |
| Schweizer
(2015) | Interrupted
time-series study | USA | NA | Studya: Carriers & non-carriers
Control: Carriers & non-carriers | 3257 | 7576 | 67 | 67 | 42.3 | 39.9 | PCR/ culture | Nasal application
of mupirocin 2 times/ day, CHX bath once/day for 5 days. Mupirocin
in control group till test results were negative. | Cefazolin or
cefuroxime for all patients. MRSA carriers also received
vancomycin. | SA-SSI: No
difference between groups (IRR: 0.86, 95% CI: 0.47,1.57). | (33) |
| Bode (2010) | RCT | Netherlands | NA | Study: Carriers
Control: Carriers | 220 | 171 | NA | NA | NA | NA | PCR/ culture | Nasal application
of mupirocin, CHX soap bath bath for | NA | SA-SSI: Reduced
risk in study group (RR: 0.14, 95% CI: 0.04-0.51). | (12) |
| Jog (2008)
study | Interrupted
time-series | UK | NA | Studya: Carriers & non-carriers
Control: Carriers & non-carriers | 681 | 697 | NA | NA | NA | NA | PCR of
mupirocin | 5 days. Nasal
application for all patients. 3 times/day, triclosan 2% for 5 days.
Mupirocin & triclosan in control group till test results were
negative. | Gentamicin and
flucloxacillin in study group MRSA carriers also received a single
dose of teicoplanin prior to surgery. | MRSA-SSI: Reduced
risk (RR 0.77, 95% CI: 0.056, 0.95). | (32) |
| Shrestha
(2006) | Retrospective | USA | CABG, valvular
surgery | Study: Carriers
Control: Non-carriers | 1342 | 4992 | 60.7 | 62.1 | 26.6 | 23.6 | PCR | Nasal application
of mupirocin. Mupirocin in control group till test results were
negative for certain. patients | NA | MRSA infection: No
difference between groups (RR: 1.3, 95% CI: 0.71-2.39) All SSI: No
difference between groups (RR: 0.97, 95% CI: 0.69-1.36). | (31) |
| Nicholson
(2006) | Interrupted
time-series study | USA | NA | Studya: Carriers & non-carriers
Control: Carriers & non-carriers | 954 | 1077 | NA | NA | NA | NA | Culture | Nasal application
of mupirocin 2 times/day for 7 days. Mupirocin in control group
till test results were negative. | NA | SA-SSI: 0.37% in
study and 1.68% in control group. All SSI: 1.88% in study and 1.11%
in control group. | (30) |
| Konvalinka
(2006) | RCT | Canada | NA | Study: Carriers
Control: Carriers | 130 | 127 | 62.5 | 62.5 | 28.5 | 28.3 | Culture | Nasal application
of mupirocin 2 times/day for 7 days. | NA | SA-SSI: 13.8% in
study and 8.6% in control group. | (13) |
In the four ITS studies (30,32-34),
wherein screening and targeted mupirocin-based decolonization
protocol was introduced, and data of pre-and post-intervention were
compared; three reported a significant beneficial effect of the
intervention (30,32,34).
Outcomes were, however, different in all three studies. Saraswat
et al (34) reported
significantly reduced incidence of all-SSIs, Jog et al
(32) in MRSA-SSIs while Nicholson
et al (30) only in SA-SSIs.
Schweizer et al (33), in a
multi-centric ITS study conducted on orthopedic and cardiac surgery
patients, reported significantly reduced risk of SA-SSIs in the
entire cohort [Incidence rate ratio (IRR): 0.58, 95% CI:
0.37,0.92], but not specifically in patients undergoing cardiac
surgery (IRR: 0.86, 95% CI: 0.47,1.57). Amongst the 2 studies
comparing carriers and non-carriers, the study by Nicholson and
Huesman (30) did not report any
data on SA-SSIs in the control group. They instead analyzed the
efficacy of decolonization in the post-operative period and
reported that successful decolonization significantly reduced the
incidence of SA-SSIs. In another similar study, Shrestha et
al (31) reported no difference
in the incidence of SSIs between carriers and non-carriers and
concluded that targeted decolonization in carriers leads to a
significant reduction of mupirocin use without increasing SSIs in
non-carriers. Finally, the RCT of Konvalinka et al (13) reported no statistically significant
difference in SA-SSIs when mupirocin was used in a cohort of S.
aureus carriers. However, in the RCT of Bode et al
(12), a statistically significant
reduction of SA-SSIs was noted with the use of nasal mupirocin and
chlorhexidine soap.
Universal mupirocin-based
decolonization
A total of 9 studies were included in the sub-group
of studies using universal mupirocin-based decolonization (14,23-29,36).
Details of the studies are presented in Table II. All studies were ITS design
studies that introduced a universal mupirocin-based decolonization
protocol without carrier state screening in their respective
healthcare setups and analyzed its effect on the incidence of SSIs.
A total of 4 studies (23-25,36)
used only nasal application of mupirocin in the decolonization
protocol, while 4 studies (14,26,27,29)
additionally utilized anti-septic baths. In the study by Walsh
et al (28), pre-operative
screening of S. aureus carriers was performed and vancomycin
was added to the pre-operative prophylaxis of carriers only. On the
other hand, nasal mupirocin and coverage of chest tube and
mediastinal tube exit site with mupirocin was used in all patients
of the study group.
 | Table IICharacteristics of studies utilizing
mupirocin-based decolonization in all patients. |
Table II
Characteristics of studies utilizing
mupirocin-based decolonization in all patients.
| | Sample size | Mean age
(years) | Diabetics (%) | |
|---|
| Author (year) | Study type | Study location | CTS type | Carrier state in
study and control groups | Study | Control | Study | Control | Study | Control | Decolonization
protocol | Antibiotic
prophylaxis | (Refs.) |
|---|
| Lemaignen
(2018) | Interrupted
time-series study | France | CABG, valvular
surgery | Study: Carriers
& non-carriers Control: Carriers & non-carriers | 2629 | 5050 | NA | NA | NA | NA | Nasal application
of mupirocin 2 times/day, povidone iodine bath/wipes once/day, for
3 days. | Cefamandole | (14) |
| Kohler (2015) | Interrupted
time-series study | Switzerland | CABG, valvular
surgery | Study: Carriers
& non-carriers Control: Carriers & non-carriers | 646 | 945 | 64.4 | 65.2 | NA | NA | Nasal application
of mupirocin 2 times/day, CHX bath once/day or octenidine
impregnated washing gloves for bed-ridden patients. | Cefuroxime or
clindamycin | (29) |
| Thompson and
Houston (2013) | Interrupted
time-series study | USA | NA | Study: Carriers
& non-carriers Control: Carriers & non-carriers | 1995 | 643 | NA | NA | NA | NA | Nasal application
of mupirocin 2 times/day with daily CHX cloth bath starting on the
day before surgery and continued till post-operative day 5. | NA | (27) |
| Walsh (2011) | Interrupted
time-series study | USA | NA | Study: Carriers
& non-carriers Control: Carriers & non-carriers | 2496 | 2766 | NA | NA | NA | NA | Nasal application
of mupirocin 2 times/day for 5 days or until discharge. Coverage of
chest tube and mediastinal tube site with mupirocin. | Cefazolin for all.
Vancomycin only for MRSA carriers in study. group | (28) |
| Coskun (2005) | Interrupted
time-series study | Turkey | NA | Study: Carriers
& non-carriers Control: Carriers & non-carriers | 7555 | 4511 | NA | NA | NA | NA | Nasal application
of mupirocin for 3 days prior to surgery. | Cefazolin or
cefuroxime | (25) |
| Martorell
(2004) | Interrupted
time-series study | USA | NA | Study: Carriers
& non-carriers Control: Carriers & non-carriers | 469 | 466 | NA | NA | NA | NA | Nasal application
of mupirocin for 3 days and pre-operative CHX bath. | Cefazolin | (26) |
| Usry (2002) | Interrupted
time-series study | USA | NA | Study: Carriers
& non-carriers Control: Carriers & non-carriers | 1205 | 2595 | NA | NA | NA | NA | Nasal application
of mupirocin. | NA | (23) |
| Cimochowski
(2001) | Interrupted
time-series study | USA | NA | Study: Carriers
& non-carriers Control: Carriers & non-carriers | 854 | 992 | 61.1 | 64.7 | 31.2 | 27.9 | Nasal application
of mupirocin started on the night prior to surgery and continued
for 5 days. | Cefuroxime or
clindamycin | (24) |
| Kluytmans
(1996) | Interrupted
time-series study | Netherlands | CABG, valvular
surgery | Study: Carriers
& non-carriers Control: Carriers & non-carriers | 752 | 928 | 58.3 | 58.5 | NA | NA | Nasal application
of mupirocin started on the day prior to surgery and continued 2
times/day for 5 days. | Cefuroxime or
clindamycin | (36) |
Except for the study by Thompson and Houston
(27), all studies reported data on
all SSIs in their respective cohorts. Analysis of the data of
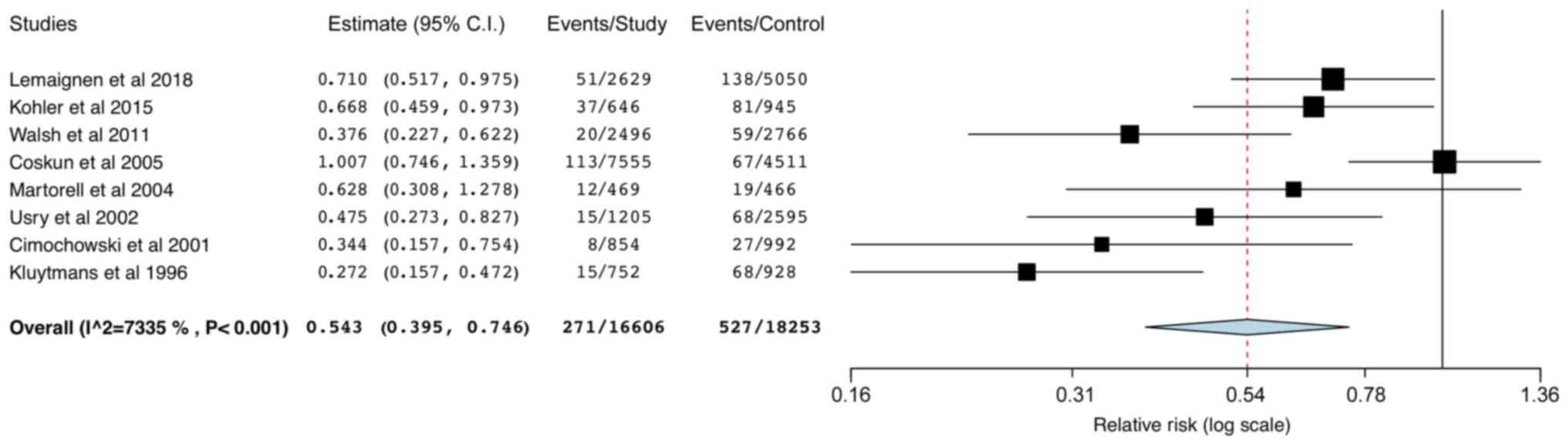
34,859 patients revealed that the mupirocin-based universal
decolonization protocol was associated with a significantly reduced
risk of all SSIs (RR: 0.54; 95% CI: 0.40,0.75;
I2=73.35%; Fig. 2). The
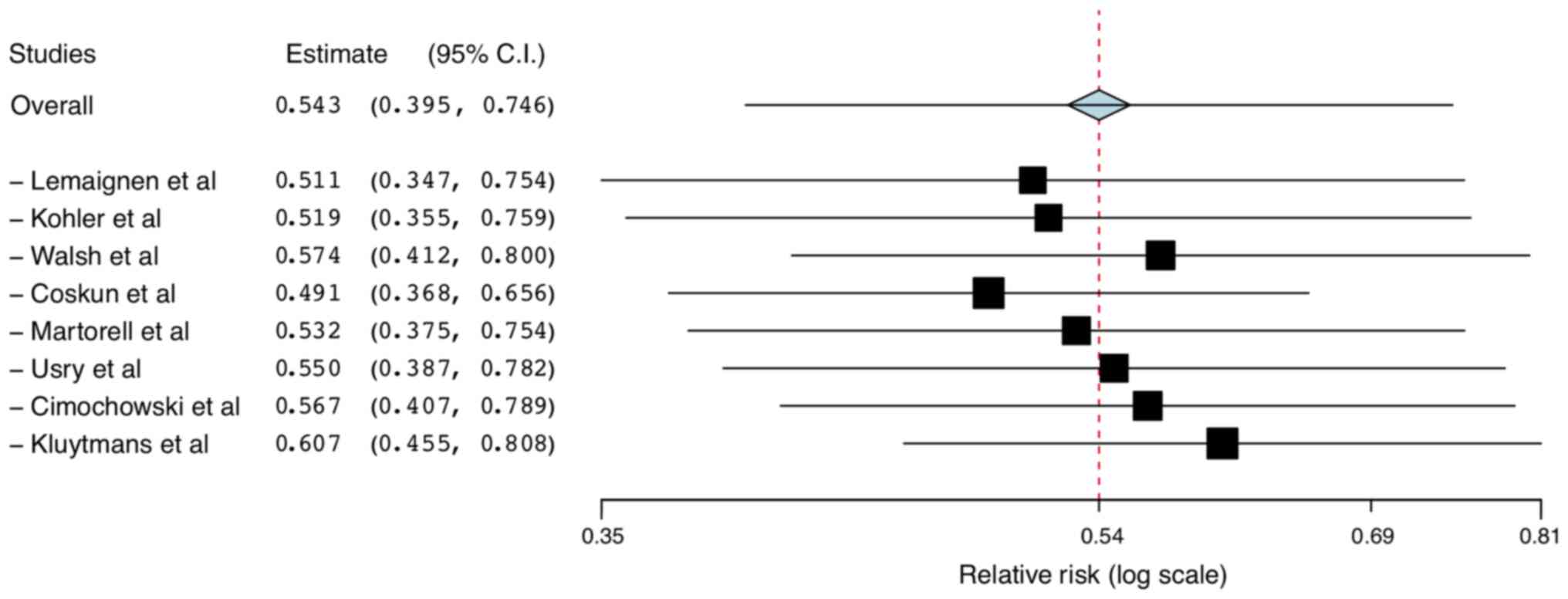
‘leave-one-out analysis’ did not demonstrate any significant change
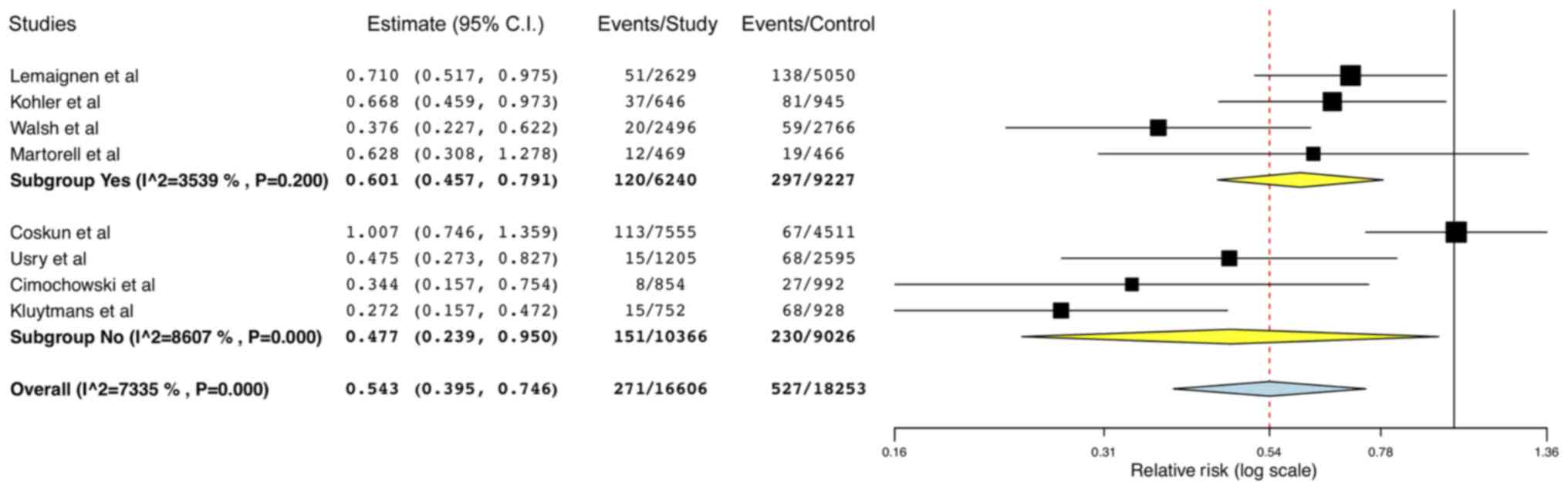
in the outcome after the exclusion of one study at a time (Fig. 3). Sub-group analysis based on use of
other decolonization interventions with mupirocin (Yes vs. No)
demonstrated reduction of all SSIs in both sub-groups (Yes-RR:
0.60; 95% CI: 0.46,0.79; I2=35.39% and No-RR: 0.48; 95%
CI: 0.24,0.95; I2=86.07%; Fig. 4).
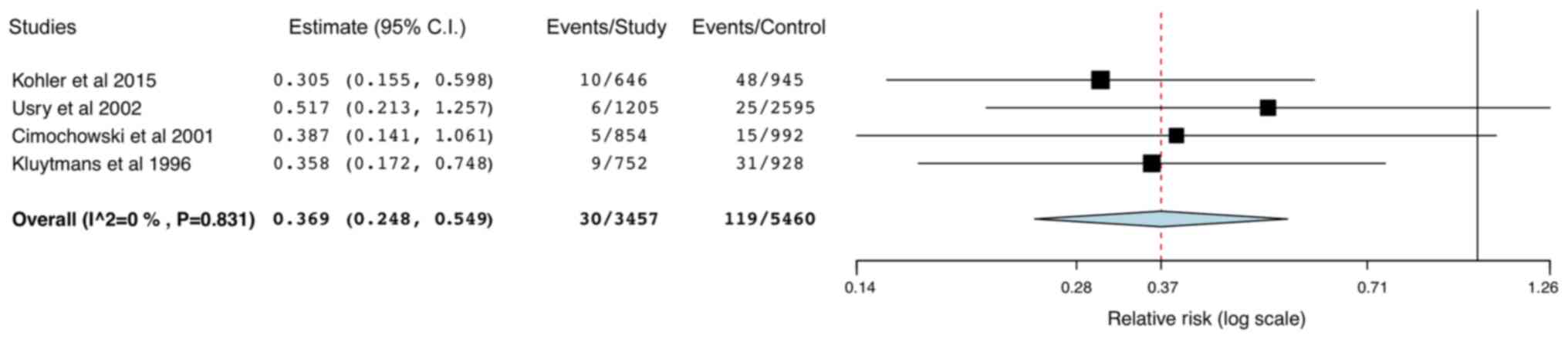
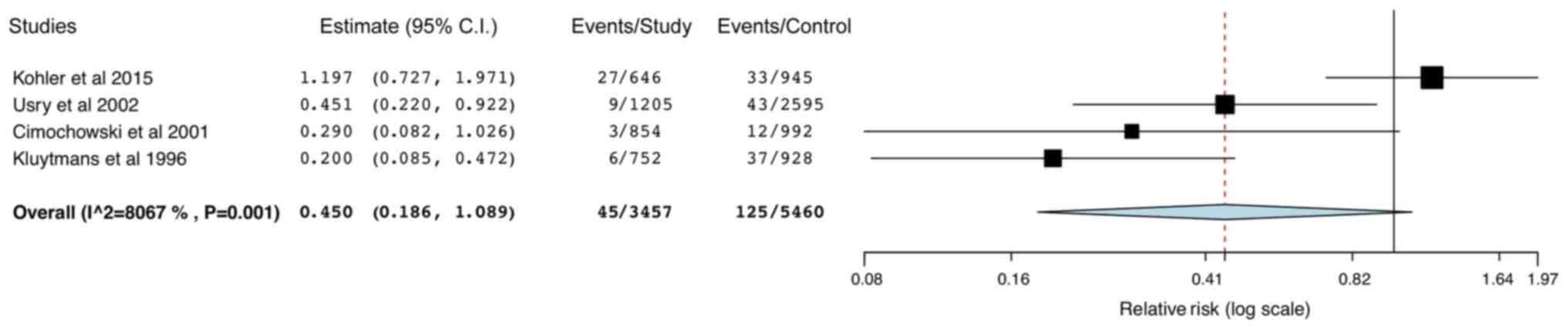
A total of 4 studies reported data on superficial
and deep SSIs. Pooled analysis indicated a statistically
significant reduction in the risk of superficial SSIs (RR: 0.37 95%
CI: 0.25,0.55; I2=0%; Fig.
5) in the study group, but no difference in deep SSIs between
the two groups (RR: 0.45; 95% CI: 0.19,1.09; I2=80.67%;
Fig. 6). The leave-one-out analysis
did not change the results of superficial SSIs, but on the
exclusion of the study by Kohler et al (29), the results for deep SSIs were
significantly in favor of mupirocin-based decolonization (RR: 0.31;
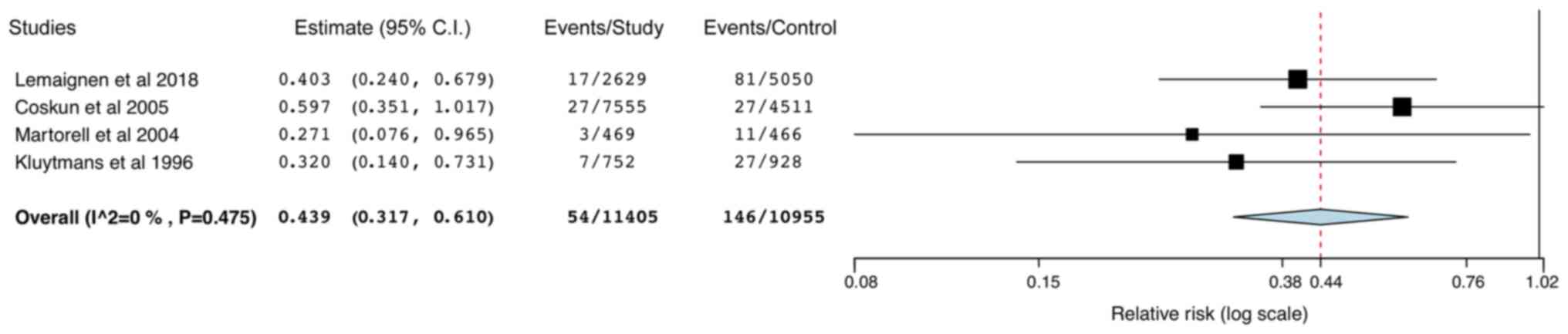
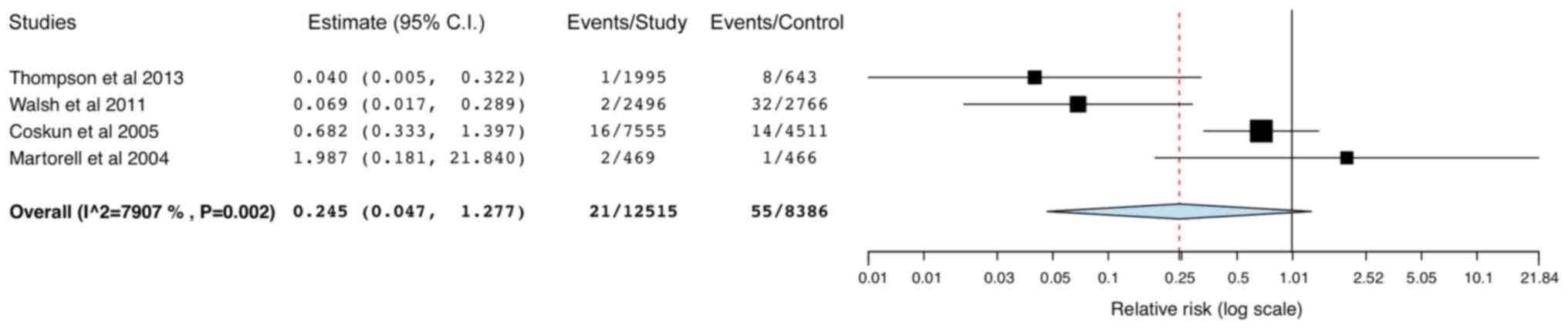
95% CI: 0.18,0.52; data not shown). Data from 4 studies were pooled
for SA-SSIs and MRSA-SSIs and the results indicated a significantly
reduced risk of SA-SSIs in the study group (RR: 0.44; 95% CI:
0.32,0.61; I2=0%; Fig.
7) but not for MRSA-SSIs (RR: 0.25; 95% CI: 0.05,1.28;
I2=79.07%; Fig. 8). The
results were stable in the leave-one-out analysis (data not
shown).
Discussion
The present systematic review and meta-analysis of
17 studies separately analyzed evidence on the efficacy of targeted
and universal mupirocin-based decolonization in preventing SSI in
patients subjected to CT surgery. The role of targeted
mupirocin-based decolonization remains inconclusive with
contrasting evidence from RCTs and retrospective studies. Limited
data from low-quality ITS studies suggested that universal
mupirocin-based decolonization may be effective in reducing all
SSIs, superficial SSIs and SA-SSIs with no effect on deep SSIs and
MRSA-SSIs.
Since colonization with S. aureus has been
known to increase the risk of nosocomial infections (7-9),
decolonization protocols to reduce carriage and infection rates
have been widely reported in the medical and surgical literature.
According to a systematic review and meta-analysis by Gebreselassie
et al (37), mupirocin with
whole-body decolonization is highly effective in eradicating MRSA
infestation in hemodialysis patients. Another study focusing on
non-surgical patients reported a 59% reduced risk of S.
aureus infection in patients decolonized using nasal mupirocin
(38). Orthopedic procedures
including implant placement and joint replacement surgeries are
associated with a high risk of developing SSI due to foreign body
placement. Meta-analysis studies have demonstrated the
effectiveness of nasal decolonization in reducing gram-positive
SSIs and MRSA-SSIs in such patients (17,18).
Mupirocin-based decolonization has not only been
used to reduce transmission and infections in MRSA carriers but
also to reduce all gram-positive SSIs (25,26).
Thus, controversy exists regarding whether such a decolonization
strategy should be restricted to only high-risk carriers or whether
a more universal decolonization protocol should be followed
(39). To answer this question,
Huang et al (40) conducted
a large RCT involving 43 hospitals with 74,256 intensive care unit
patients. They concluded that universal decolonization was more
effective than screening and targeted decolonization to reduce
bloodstream infections caused by any pathogen.
To provide comprehensive and clear evidence on the
efficacy of both protocols in patients subjected to CT, studies
assessing targeted and universal mupirocin-based decolonization
were separated for the present review. Descriptive analysis of
targeted decolonization in CT surgical patients indicated
contrasting results among the included studies. Amongst the RCTs,
Bode et al (12) reported a
significantly reduced risk of SA-SSIs but Konvalinka et al
(13) indicated no significant
difference in their study sample. The slightly larger sample size
and use of chlorhexidine soap may have influenced outcomes in the
trial performed by Bode et al (12). Similarly, for ITS-design studies on
mupirocin-based targeted decolonization, owing to differences in
study outcomes and varying results, it was not possible to draw any
strong conclusions. Saraswat et al (34) reported a reduced risk of all-SSIs in
their cohort but no such effect was noted by Nicholson and Huesman
(30). Similarly, results were not
coherent for the incidence of SA-SSIs and only Jog et al
(32) reported data on MRSA-SSIs.
Differences in the study population, decolonization protocol and
other infection control measures in the included studies may have
contributed to these differences.
Targeted decolonization has been recommended to
reduce the emergence of mupirocin resistance (41). The literature on the level of
mupirocin resistance following its universal use is, however,
scarce. The REDUCE-MRSA trial (42)
compared targeted and universal decolonization with mupirocin and
chlorhexidine and determined that the odds of mupirocin resistance
were not different across treatment arms. The results were not
conclusive, as the 95% CIs of the outcomes were wide. In a recent
meta-analysis, Dadashi et al (41) reported an increased prevalence of
mupirocin resistance in both S. aureus and MRSA, indicating
that reduced efficacy of mupirocin poses a risk for invasive
infection. In one of the included studies, Shrestha et al
(31) demonstrated that limiting
mupirocin to only carriers does not increase the risk of MRSA and
all-SSIs in non-carriers. In recent years, due to the increased
awareness of mupirocin resistance, research is underway to identify
alternative nasal decolonization agents. Alcohol and iodine-based
agents are in use but their efficacy is still under investigation
(15).
Despite the drawback of anti-microbial resistance,
universal decolonization with mupirocin offers certain distinct
advantages. MRSA surveillance and the requirement for contact
precautions may be eliminated with universal decolonization, which
may reduce hospital costs and contribute to better patient care
(40,43). It may also reduce the total
microbial burden of the patient and contribute to a greater
reduction in hospital infection rates (40). The present analysis of 9 studies
revealed that mupirocin-based decolonization was effective in
reducing the risk of all SSIs, superficial SSIs and SA-SSIs in CT
surgical patients but not in reducing deep SSIs and MRSA-SSIs. The
lack of significant results for MRSA-SSIs may be due to the limited
number of studies reporting data on MRSA-SSIs. In addition, the
exact number of MRSA carriers in the study and control groups was
unknown. An insufficient number of carriers in these studies may
have led to reduced statistical power.
The results of the present analysis concur with
previous reviews. Schweizer et al (17), in a review of studies published up
to January 2012, reported a significantly reduced risk of
gram-positive SSIs and SA-SSIs with nasal decolonization in cardiac
surgery patients but no difference in MRSA infection rates. In
2017, Ma et al (18), in a
meta-analysis of 9 studies on pediatric and adult cardiac surgical
patients, determined a significantly reduced risk of all SSIs and
SA-SSIs with nasal decolonization. In contrast to the present
analysis, these reviews were not focussed on mupirocin-based
decolonization and included a mix of studies employing both
targeted and universal decolonization.
The decolonization protocol was not identical among
the included studies. Anti-septic baths consisting of chlorhexidine
and povidone-iodine were used with mupirocin in half of the
included studies. It is known that S. aureus may colonize
other regions of the body, including the pharynx, groin, perianal
region or axilla and contribute to SSIs (12). Therefore, it is plausible that the
use of chlorhexidine for whole-body decolonization with mupirocin
for nostrils may lead to a greater reduction of SSIs (44). However, the current evidence on the
role of such anti-septic baths for reducing SSIs is weak.
Meta-analysis studies have reported no significant reduction in the
risk of SSIs with anti-septic baths (45,46). A
total of 4 studies included in the present review also used
additional glycopeptide antibiotics for MRSA carriers along with
mupirocin-based decolonization. Saleh et al (47) demonstrated that the addition of
glycopeptide antibiotics significantly reduces the risk of S.
aureus infections in patients undergoing cardiac, vascular and
orthopedic surgery. To analyze the role of such additional
interventions on the pooled outcomes of the present meta-analysis,
sub-group and leave-one-out analyses were performed. The exclusion
of the single study by Walsh et al (28), in which vancomycin was added to MRSA
carriers in the universal decolonization study, did not change the
significance of the overall results. In addition, on subgroup
analysis, no difference in the risk of all SSIs was identified for
studies using these additional interventions.
The limitations of the present review require to be
mentioned. First, due to the limited number of coherent studies, it
was not possible to perform any meta-analysis for targeted
mupirocin-based decolonization. Furthermore, the results of the
pooled analysis of data from retrospective ITS studies may be
limited due to the high risk of bias. Time-based variation in the
hospital infection control protocol, as well as differences in
surgical technique, compliance of intervention or level of
post-operative care may have skewed the results. Geographical
variation in the patient population with baseline differences in
patient comorbidities and the type of surgical procedure may also
have influenced infection rates. In addition, the decolonization
protocol was not similar across studies with the use of antiseptic
baths and glycopeptide antibiotic prophylaxis in certain studies.
Finally, only four studies were included in the meta-analysis of
secondary outcomes, which limited the power of the analysis.
However, the present study provides up-to-date and
comprehensive evidence on the role of mupirocin-based
decolonization in CT surgical patients. Studies were segregated
based on the decolonized population, analyzing them separately for
available evidence. Appropriate sub-group and sensitivity analyses
were performed to take into account differences among the included
studies.
To conclude, the present review indicated that
currently available evidence on the role of targeted
mupirocin-based decolonization to reduce SSIs after CT surgery is
non-coherent and inconclusive. Analysis of low-quality
retrospective studies suggested that universal mupirocin-based
decolonization may reduce all SSIs, superficial SSIs and SA-SSIs
but not deep SSIs and MRSA-SSIs in CT surgical patients. Further,
high-quality RCTs with a homogenous decolonization protocol on
patients subjected to CT surgery are required to strengthen the
current evidence.
Supplementary Material
Search strategy and PubMed database
search results.
Risk of bias in included studies.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW conceived and designed the study. QJ and XH
collected the data and performed the literature search. LW was
involved in the writing of the manuscript. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Loop FD, Lytle BW, Cosgrove DM, Mahfood S,
McHenry MC, Goormastic M, Stewart RW, Golding LA and Taylor PC: J
Maxwell Chamberlain memorial paper. Sternal wound complications
after isolated coronary artery bypass grafting: Early and late
mortality, morbidity, and cost of care. Ann Thorac Surg.
49:179–186; discussion 186-7. 1990.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lepelletier D, Perron S, Bizouarn P,
Caillon J, Drugeon H, Michaud JL and Duveau D: Surgical-site
infection after cardiac surgery: Incidence, microbiology, and risk
factors. Infect Control Hosp Epidemiol. 26:466–472. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Saadatian-Elahi M, Teyssou R and Vanhems
P: Staphylococcus aureus, the major pathogen in orthopaedic
and cardiac surgical site infections: A literature review. Int J
Surg. 6:238–245. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Abboud CS, Wey SB and Baltar VT: Risk
factors for mediastinitis after cardiac surgery. Ann Thorac Surg.
77:676–683. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Grossi EA, Culliford AT, Krieger KH, Kloth
D, Press R, Baumann FG and Spencer FC: A survey of 77 major
infectious complications of median sternotomy: A review of 7,949
consecutive operative procedures. Ann Thorac Surg. 40:214–223.
1985.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Filsoufi F, Castillo JG, Rahmanian PB,
Broumand SR, Silvay G, Carpentier A and Adams DH: Epidemiology of
deep sternal wound infection in cardiac surgery. J Cardiothorac
Vasc Anesth. 23:488–494. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Verhoeven PO, Gagnaire J, Botelho-Nevers
E, Grattard F, Carricajo A, Lucht F, Pozzetto B and Berthelot P:
Detection and clinical relevance of Staphylococcus aureus
nasal carriage: An update. Expert Rev Anti Infect Ther. 12:75–89.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gagnaire J, Verhoeven PO, Grattard F,
Rigaill J, Lucht F, Pozzetto B, Berthelot P and Botelho-Nevers E:
Epidemiology and clinical relevance of Staphylococcus aureus
intestinal carriage: A systematic review and meta-analysis. Expert
Rev Anti Infect Ther. 15:767–785. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Katayanagi T: Nasal methicillin-resistant
S aureus is a major risk for mediastinitis in pediatric
cardiac surgery. Ann Thorac Cardiovasc Surg. 21:37–44.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
van Rijen M, Bonten M, Wenzel R and
Kluytmans J: Mupirocin ointment for preventing Staphylococcus
aureus infections in nasal carriers. Cochrane Database Syst
Rev. 4(CD006216)2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Langenberg JCM, Kluytmans JAJW, Mulder
PGH, Romme J, Ho GH and Van Der Laan L: Peri-Operative nasal
eradication therapy prevents staphylococcus aureus surgical
site infections in aortoiliac surgery. Surg Infect (Larchmt).
19:510–515. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bode LG, Kluytmans JA, Wertheim HF,
Bogaers D, Vandenbroucke-Grauls CM, Roosendaal R, Troelstra A, Box
AT, Voss A, van der Tweel I, et al: Preventing surgical-site
infections in nasal carriers of Staphylococcus aureus. N
Engl J Med. 362:9–17. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Konvalinka A, Errett L and Fong IW: Impact
of treating Staphylococcus aureus nasal carriers on wound
infections in cardiac surgery. J Hosp Infect. 64:162–168.
2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lemaignen A, Armand-Lefevre L, Birgand G,
Mabileau G, Lolom I, Ghodbane W, Dilly MP, Nataf P and Lucet JC:
Thirteen-year experience with universal Staphylococcus
aureus nasal decolonization prior to cardiac surgery: A
quasi-experimental study. J Hosp Infect. 100:322–328.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sakr A, Brégeon F, Rolain JM and Blin O:
Staphylococcus aureus nasal decolonization strategies: A
review. Expert Rev Anti Infect Ther. 17:327–340. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu Z, Norman G, Iheozor-Ejiofor Z, Wong
JK, Crosbie EJ and Wilson P: Nasal decontamination for the
prevention of surgical site infection in Staphylococcus
aureus carriers. Cochrane Database Syst Rev.
5(CD012462)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Schweizer M, Perencevich E, McDanel J,
Carson J, Formanek M, Hafner J, Braun B and Herwaldt L:
Effectiveness of a bundled intervention of decolonization and
prophylaxis to decrease Gram positive surgical site infections
after cardiac or orthopedic surgery: Systematic review and
meta-analysis. BMJ. 346(f2743)2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ma N, Cameron A, Tivey D, Grae N, Roberts
S and Morris A: Systematic review of a patient care bundle in
reducing staphylococcal infections in cardiac and orthopaedic
surgery. ANZ J Surg. 87:239–246. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Moher D, Liberati A, Tetzlaff J, Altman DG
and PRISMA Group: Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. BMJ.
339(b2535)2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Higgins JPT, Thomas J, Chandler J,
Cumpston M, Li T, Page MJ and Welch VA: Cochrane Handbook for
Systematic Reviews of Interventions. Version 6.1 (updated September
2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
|
|
21
|
Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS,
Hahn S, Jang BH and Son HJ: Testing a tool for assessing the risk
of bias for nonrandomized studies showed moderate reliability and
promising validity. J Clin Epidemiol. 66:408–414. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wallace BC, Schmid CH, Lau J and
Trikalinos TA: Meta-analyst: Software for meta-analysis of binary,
continuous and diagnostic data. BMC Med Res Methodol.
9(80)2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Usry GH, Johnson L, Weems JJ Jr and
Blackhurst D: Process improvement plan for the reduction of sternal
surgical site infections among patients undergoing coronary artery
bypass graft surgery. Am J Infect Control. 30:434–436.
2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cimochowski GE, Harostock MD, Brown R,
Bernardi M, Alonzo N and Coyle K: Intranasal mupirocin reduces
sternal wound infection after open heart surgery in diabetics and
nondiabetics. Ann Thorac Surg. 71:1572–1578; discussion 1578-9.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Coskun D and Aytac J: Decrease in
Staphylococcus aureus surgical site infections following
cardiovascular surgery. J Hosp Infect. 60:287–289. 2005.
|
|
26
|
Martorell C, Engelman R, Corl A and Brown
RB: Surgical site infections in cardiac surgery: An 11-year
perspective. Am J Infect Control. 32:63–68. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Thompson P and Houston S: Decreasing
methicillin-resistant Staphylococcus aureus surgical site
infections with chlorhexidine and mupirocin. Am J Infect Control.
41:629–633. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Walsh EE, Greene L and Kirshner R:
Sustained reduction in methicillin-resistant Staphylococcus
aureus wound infections after cardiothoracic surgery. Arch
Intern Med. 171:68–73. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kohler P, Sommerstein R, Schönrath F,
Ajdler-Schäffler E, Anagnostopoulos A, Tschirky S, Falk V, Kuster
SP and Sax H: Effect of perioperative mupirocin and antiseptic body
wash on infection rate and causative pathogens in patients
undergoing cardiac surgery. Am J Infect Control. 43:e33–e38.
2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nicholson MR and Huesman LA: Controlling
the usage of intranasal mupirocin does impact the rate of
Staphylococcus aureus deep sternal wound infections in
cardiac surgery patients. Am J Infect Control. 34:44–48.
2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shrestha NK, Banbury MK, Weber M, Cwynar
RE, Lober C, Procop GW, Karafa MT and Gordon SM: Safety of targeted
perioperative mupirocin treatment for preventing infections after
cardiac surgery. Ann Thorac Surg. 81:2183–2188. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jog S, Cunningham R, Cooper S, Wallis M,
Marchbank A, Vasco-Knight P and Jenks PJ: Impact of preoperative
screening for meticillin-resistant Staphylococcus aureus by
real-time polymerase chain reaction in patients undergoing cardiac
surgery. J Hosp Infect. 69:124–130. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Schweizer ML, Chiang HY, Septimus E, Moody
J, Braun B, Hafner J, Ward MA, Hickok J, Perencevich EN, Diekema
DJ, et al: Association of a bundled intervention with surgical site
infections among patients undergoing cardiac, hip, or knee surgery.
JAMA. 313:2162–2171. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Saraswat MK, Magruder JT, Crawford TC,
Gardner JM, Duquaine D, Sussman MS, Maragakis LL and Whitman GJ:
Preoperative staphylococcus aureus screening and targeted
decolonization in cardiac surgery. Ann Thorac Surg. 104:1349–1356.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nicolas R, Carricajo A, Morel J, Rigaill
J, Grattard F, Guezzou S, Audoux E, Campisi S, Favre JP, Berthelot
P, et al: Evaluation of effectiveness and compliance with the
mupirocin nasal ointment part of Staphylococcus aureus
decolonization in real life using UPLC-MS/MS mupirocin
quantification. J Antimicrob Chemother. 75:1623–1630.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kluytmans JA, Mouton JW, VandenBergh MF,
Manders MJ, Maat AP, Wagenvoort JH, Michel MF and Verbrugh HA:
Reduction of surgical-site infections in cardiothoracic surgery by
elimination of nasal carriage of staphylococcus aureus.
Infect Control Hosp Epidemiol. 17:780–785. 1996.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gebreselassie HM, Lo Priore E and
Marschall J: Effectiveness of meticillin-resistant
Staphylococcus aureus decolonization in long-term
haemodialysis patients: A systematic review and meta-analysis. J
Hosp Infect. 91:250–256. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Nair R, Perencevich EN, Blevins AE, Goto
M, Nelson RE and Schweizer ML: Clinical effectiveness of mupirocin
for preventing staphylococcus aureus infections in
nonsurgical settings: A meta-analysis. Clin Infect Dis. 62:618–630.
2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wenzel RP and Edmond MB: Infection
control: The case for horizontal rather than vertical
interventional programs. Int J Infect Dis. 14 (Suppl 4):S3–S5.
2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Huang SS, Septimus E, Kleinman K, Moody J,
Hickok J, Avery TR, Lankiewicz J, Gombosev A, Terpstra L, Hartford
F, et al: Targeted versus universal decolonization to prevent ICU
infection. N Engl J Med. 368:2255–2265. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Dadashi M, Hajikhani B, Darban-Sarokhalil
D, van Belkum A and Goudarzi M: Mupirocin resistance in
Staphylococcus aureus: A systematic review and
meta-analysis. J Glob Antimicrob Resist. 20:238–247.
2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hayden MK, Lolans K, Haffenreffer K, Avery
TR, Kleinman K, Li H, Kaganov RE, Lankiewicz J, Moody J, Septimus
E, et al: Chlorhexidine and mupirocin susceptibility of
methicillin-resistant staphylococcus aureus isolates in the
REDUCE-MRSA trial. J Clin Microbiol. 54:2735–2742. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Morgan DJ, Diekema DJ, Sepkowitz K and
Perencevich EN: Adverse outcomes associated with contact
precautions: A review of the literature. Am J Infect Control.
37:85–93. 2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
McConeghy KW, Mikolich DJ and LaPlante KL:
Agents for the decolonization of methicillin-resistant
Staphylococcus aureus. Pharmacotherapy. 29:263–280.
2009.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Webster J and Osborne S: Preoperative
bathing or showering with skin antiseptics to prevent surgical site
infection. Cochrane Database Syst Rev CD004985, 2015.
|
|
46
|
Franco LM, Cota GF, Pinto TS and Ercole
FF: Preoperative bathing of the surgical site with chlorhexidine
for infection prevention: Systematic review with meta-analysis. Am
J Infect Control. 45:343–349. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Saleh A, Khanna A, Chagin KM, Klika AK,
Johnston D and Barsoum WK: Glycopeptides versus β-lactams for the
prevention of surgical site infections in cardiovascular and
orthopedic surgery: A mata-analysis. Ann Surg. 261:72–80.
2015.PubMed/NCBI View Article : Google Scholar
|