Introduction
Diabetic encephalopathy is one of the major chronic
complications of diabetes mellitus (DM) (1). Symptoms of diabetic encephalopathy
include slow reaction times, cognitive and memory dysfunction,
severe cerebral thrombosis, stroke and Alzheimer's disease.
Although the pathogenesis of diabetic encephalopathy is not fully
understood, recent evidence suggestes that the formation and
accumulation of advanced glycation end products (AGEs) plays a
pivotal role. Acetylcholine (ACh) is important for the maintenance
of central nervous system function (2). A study found that a reduced level of
ACh may contribute to impaired learning and memory in diabetic rats
(3).
Oxidative stress has a close relationship with the
development of DM and diabetic encephalopathy (4). It has been demonstrated that ROS are a
class of highly reactive free radical molecules that can directly
damage cell viability and function (5), and that malondialdehyde (MDA) is one
of the most important products of membrane lipid peroxidation and
that its production can aggravate membrane damage (6). Superoxide dismutase (SOD) is an
important antioxidant enzyme in organisms that can scavenge free
radical molecules and glutathione peroxidase (GPX) is a
peroxide-degrading enzyme widely distributed in organisms that can
catalyze the reduction of toxic peroxides into non-toxic hydroxy
compounds thus protecting the structure and function of cell
membranes against peroxide damage (7,8). To
comprehensively evaluate effects of KWG against oxidative stress,
the above parameters were determined. It has also been demonstrated
that high concentrations of flavonoids are cytotoxic (9). On the other hand, cell death is
closely related with production of ROS (10). Cortex Mori [CM; Cortex Mori is the
dry root bark of Morus alba (Latin scientific name, Morus
alba L.)] is a traditional Chinese medicine believed to be
beneficial in the treatment of DM. A previous study found that CM
extract had an antidepressant-like effect on the rat hippocampus
(11). Flavonoids have been widely
reported to increase cell viability (12,13)
and they are the main components of CM (14). Several studies have demonstrated the
antiinflammatory, antioxidant and hypoglycemic effects of
flavonoids from CM (15-17),
but the specific molecule behind these effects is unknown. In a
previous study, Kuwanon G (KWG), a flavonoid derived from CM, was
suggested to have a beneficial effect against
lipopolysaccharide-induced inflammation and oxidative stress
(12). The antiinflammatory effect
of KWG has been demonstrated by a number of research groups
(9,18,19).
The HT22 cell line is a mouse hippocampal neuron
cell line. This cell line is a good model for studying the toxicity
of glutamate in vitro, and has good applications in many
neurodegenerative diseases, such as Alzheimer's and Parkinson's
disease (20,21). Therefore, HT22 cells were selected
for the present study.
In the present study, the neuroprotective effects of
KWG were explored in an in vitro model of a high glucose
environment and the potential mechanisms underlying its action were
investigated.
Materials and methods
Materials
Kuwanon G (Fig. 1A)
was supplied by Chengdu Pufeide Biotech Co., Ltd. AGEs were
prepared by incubating bovine serum albumin (BSA; Gibco; Thermo
Fisher Scientific, Inc.) with 50 mM D-glucose (GBCBIO Technologies,
Inc.) under sterile conditions in 5% CO2/95% air at 37˚C
for 3 months. Unincorporated glucose was then removed by dialysis
overnight against 0.01 M phosphate-buffered saline (PBS).
Unmodified BSA was incubated in the absence of glucose under the
same conditions, to be used as a control. AGEs were stored at 4˚C
until use (22-24).
Primary antibodies against Akt (cat. no. sc-514032), phosphorylated
(p)-Akt (cat. no. sc-8312), IκB-α (cat. no. sc-1643), p-IκB-α (cat.
no. sc-8404), glycogen synthase kinase 3 (GSK3) α/β (cat. no.
sc-7291), p-GSK3α/β (cat. no. sc-81496), GAPDH (cat. no. sc-47724)
and RIPA Lysis Buffer (cat. no. sc-24948) were supplied by Santa
Cruz Biotechnology, Inc. Antibodies for p38 MAPK (cat. no. 8690S),
p-p38 MAPK (cat. no. 9216S), NF-κB p65 (cat. no. 8242S) and p-NF-κB
p65 (cat. no. 3033S) were from Cell Signaling Technology, Inc.
Bcl-2 (cat.no. bs-0032R) and Bax (cat. no. bs-0127R) primary
antibodies, FITC- (cat. no. bs-0295D-FITC), Cy3- (cat. no.
bs-0296G-Cy3), goat anti-rabbit IgG/FITC (cat. no. bs-0295G-FITC)
and goat anti-mouse IgG/FITC (cat. no. bs-0296G-FITC) conjugated
secondary antibodies were bought from BIOSS. The ELISA kit for ACh
(cat. no. KTE70539) was purchased from Abbkine Scientific Co,. Ltd.
DAPI (cat. no. C1002) was supplied by GBCBIO Technologies, Inc. An
Annexin V-propidium iodide (AV-PI) kit (cat. no. KGA101) was
purchased from Nanjing KeyGen Biotech Co., Ltd. Glutathione
peroxidase (GPX; cat. no. S0056) and intracellular reactive oxygen
species (ROS; cat. no. S0033S) detection kits were supplied by
Beyotime Institute of Biotechnology. Superoxidase dismutase (SOD;
cat. no. A001-3-2) and choline acetyltransferase (ChAT) kits (cat.
no. A079-1-1) were supplied by Nanjing Jiancheng Bioengineering
Institute. Kits for malondialdehyde (MDA; cat. no. BC0025) and
acetylcholinesterase (AChE; cat. no. BC2025) were purchased from
Beijing Solarbio Science & Technology Co., Ltd. Dulbecco's
modified Eagle's medium (DMEM), fetal bovine serum (FBS), rabbit
serum, penicillin-streptomycin Solution and trypsin were obtained
from Gibco (Thermo Fisher Scientific, Inc.). Precision Plus
Protein™ Dual Color Standards (cat. no. 161-0374) was supplied by
Bio-Rad Laboratories, Inc.
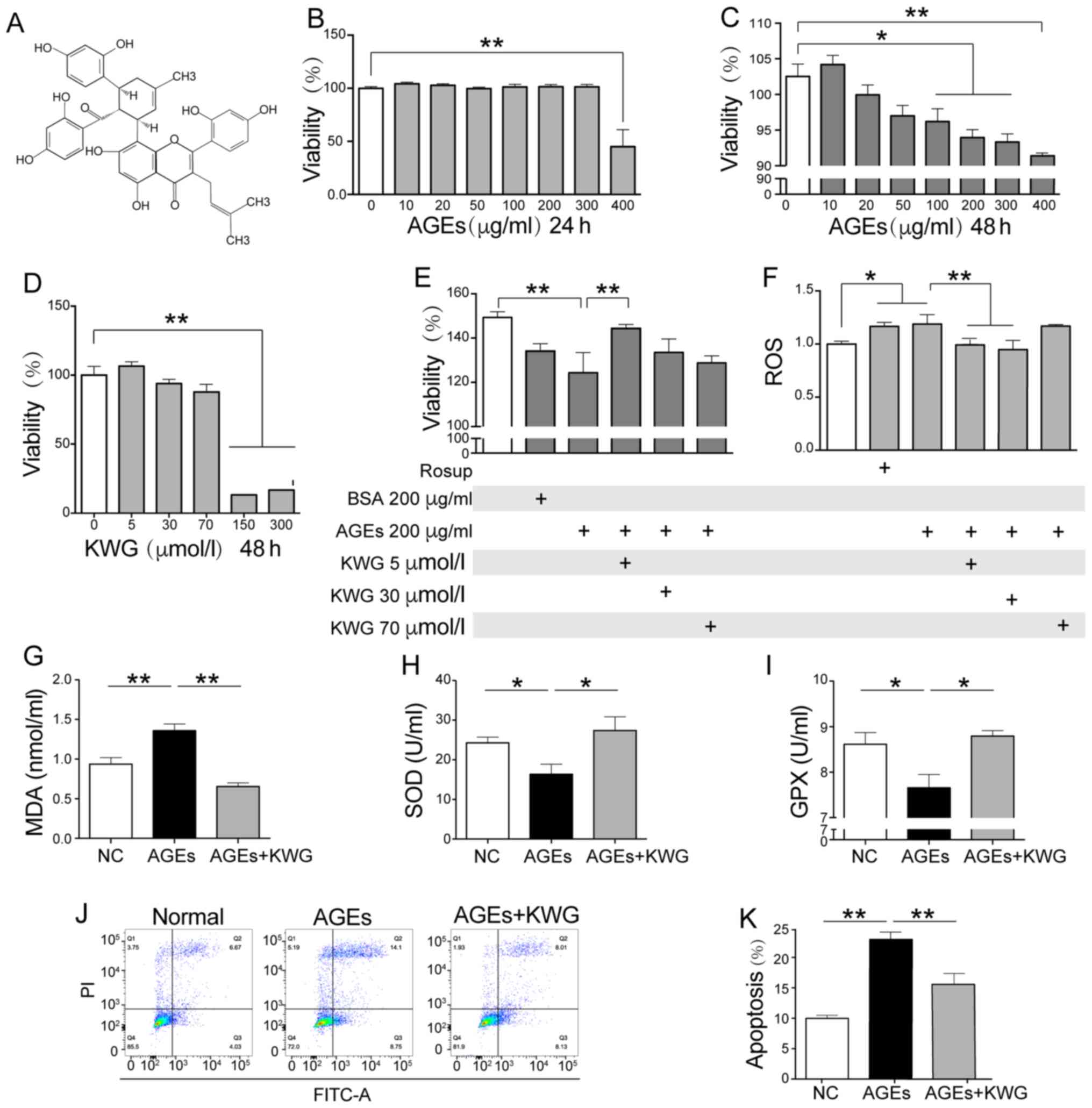 | Figure 1Effect of KWG on the viability and
AGE induced-oxidative stress of HT22 cells. (A) Chemical structure
of KWG. (B) Cell viability was determined by MTT after (B) 24-h and
(C) 48-h AGE treatment, (D) 48-h KWG treatment and (E) after a
combined treatment with AGE and KWG for Xh. (F) Levels of ROS, (G)
MDA, (H) SOD and (I) GPX were assayed. (J) HT22 cell apoptosis
levels were determined by flow cytometry and (K) quantified.
*P<0.05, **P<0.01. AGE, advanced
glycation end products; BSA, bovine serum albumin; GPX, glutathione
peroxidase; KWG, kuwanon G; MDA, malondialdehyde; NC, normal
control; ROS, reactive oxygen species ; SOD, superoxide
dismutase. |
Cell Culture
The HT22 cell line was a gift from Professor Lu Ming
(Nanjing Medical University, China). Cells were cultured in DMEM
supplemented with 10% heat-inactivated FBS at 37˚C in a humidified
atmosphere of 5% CO2 /95% air.
Cell viability evaluation by MTT
assay
HT22 cells at an exponential growth phase were
seeded on flat-bottomed 96-well plates, seeding density was
4x104 cells/ml. Cells were treated with PBS (vehicle),
AGEs, KWG or AGEs + KWG (the cells were incubated with AGEs for 30
min followed by KWG administration), as indicated for 24-48 h.
Cells were then incubated with
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (5
mg/ml) for 4 h in the cell culture incubator. Formazan was
dissolved with dimethyl sulfoxide. Absorbance values at a
wavelength of 490 nm were measured using a spectrophotometer (Tecan
Group Ltd.).
AV-FITC/PI cell apoptosis detection
assay
AV-PI assay was carried out to determine cell
apoptosis levels. After treatment, the cells were washed twice with
cold PBS, dissociated with EDTA-free trypsin (cat. no. PYG0107;
Boster Biologicals) and 1x106 cells/ml were harvested. A
5 µl volume of AV-FITC and 10 µl of PI were added sequentially.
Cells were incubated in the dark at room temperature for 15 min
with the stains before 400 µl of binding buffer was added to the
sample and the apoptosis rate measured by flow cytometry. The Q2
and Q3 quadrants were counted to determine the level of apoptosis.
For this aim, BD Aria III Flow Cytometer (BD Biosciences) and the
FlowJo software (7.6; FlowJo LLC) were applied for determination
and analysis.
Cell anti-oxidant activity
assessment
HT22 cells were seeded in 24-well plates, seeding
density was 1x105 cells/ml and vehicle, Rosup (used as
the positive control; Beyotime Institute of Biotechnology; cat. no.
S0033S; 1:1,000), AGEs (200 µg/ml) or AGEs (200 µg/ml) + KWG (5, 30
or 70 µM) were administered for 48 h. Levels of ROS (450 nm), SOD
(560 nm), GPX (412 nm) and MDA (532 nm) were determined by
colorimetric assay on a plate reader according to the protocol from
the product supplier.
Determination of ACh, ChAT and
AChE
HT22 cells were incubated with vehicle (as a
control), AGEs (200 µg/ml) or AGEs (200 µg/ml) + KWG (5 µM) for
12-48 h as indicated; the cell culture supernatant and
intracellular fluid (the intracellular fluid was obtained by lysing
the cells with RIPA lysis buffer, and then collected the
supernatant by centrifugation at 78,400 x g, 4˚C for 10 min) was
collected to evaluate levels of Ach (450 nm), ChAT (450 nm) and
AChE (450 nm) by colorimetric assay on a plate reader according to
the manufacturers' protocol.
Immunofluorescence
Cells were seeded on slides in 6-well cell culture
plates (seeding density, 1.2x105 cells/ml) and treated
with vehicle (PBS), AGEs (200 µg/ml) or AGEs (200 µg/ml) + KWG (5
µM) at 37˚C for 48 h. After washing with cold PBS, the cells were
fixed with 4% paraformaldehyde for 10 min at room temperature and
then permeabilized with 0.25% Triton X-100. Rabbit serum (1:20;
Gibco; Thermo Fisher Scientific, Inc.) was applied to block
unspecific antigens for 30 min at 37˚C. Cells were then incubated
with primary antibodies Bcl-2 (1:200), Bax (1:200), IκB-α (1:200)
and p-IκB-α (1:200) overnight at 4˚C. After washing with PBS, cells
were further incubated with FITC- or Cy3-conjugated secondary
antibodies at 4˚C for 12 h. DAPI was applied for nuclear staining.
Finally, images were obtained using a confocal laser scanning
microscope (Olympus Corporation; magnification, x800).
Western blotting
Total protein from HT22 cells was extracted with
RIPA lysis buffer (Santa Cruz Biotechnology, Inc.; cat. no.
sc-24948), and the concentration was determined using Bradford
assay. Proteins (30 µg/lane) were separated by sodium dodecyl
sulfate polyacrylamide gel electrophoresis minigel (10%) and then
electro-transferred onto PVDF membranes. After blocking with 5%
non-fat milk for 12 h at 4˚C, the membranes were incubated with
primary antibodies against Bcl-2 (1:1,000), Bax (1:1,000), Akt
(1:1,000), p-Akt (1:1,000), p38 MAPK (1:1,000), p-p38 MAPK
(1:1,000), NF-κB p65 (1:1,000), p-NF-κB p65 (1:1,000), IκB-α
(1:1,000), p-IκB-α (1:1,000), GSK3α/β (1:1,000) or p-GSK3α/β
(1:1,000) at 4˚C overnight and then incubated with goat anti-rabbit
IgG/FITC (1:6,000) or goat anti-mouse IgG/FITC (1:6,000)
horseradish peroxidase-conjugated secondary antibodies for 1 h at
room temperature. The protein bands were visualized using a Bio-Rad
Chemi Doc™ XRS system (Bio-Rad Laboratories, Inc.) and band density
was measured using ImageJ software (version 1.52; National
Institutes of Health). Relative expression of target proteins was
calculated based on the value of the internal control GAPDH.
Statistical analysis
Data are expressed as mean ± SD for at least 3
independent experiments. Differences between groups were analyzed
by SPSS 22.0 software (IBM Corp.) using one-way ANOVA with a
Tukey's post hoc test. P<0.05 was regarded as statistically
significant.
Results
KWG alleviates the oxidative stress
injury of HT22 cells caused by AGEs
To evaluate the influence of KWG on cell viability
was assayed by MTT. AGE administration concentration-dependently
induced HT22 cell death (Fig. 1B
and C), and co-incubation with 5-70
µM of KWG blocked this trend (Fig.
1D and E); the most significant
effect of KWG was observed at 5 µM. However, once the concentration
of KWG was higher than 70 µM, an inhibition effect of KWG on cell
viability was shown. The above findings were further verified by
flow cytometry according to Annexin V-FITC/PI double staining in
HT22 cells (Fig. 1J and K). The aim of the present study was to
explore effects of KWG on ACh production in HT22 cells. As 400
µg/ml AGEs was found to have significant effects on reducing cell
viability and a large number of cells were dead. Converging from
reports and previous studies, 200 µg/ml of AGEs were applied in the
present study (25).
In the present study, AGE-induced MDA and ROS
elevation was observed to be decreased by KWG treatment (Fig. 1F and G). At the same time, AGEs-induced SOD and
GPX reduction was reversed by KWG treatment (Fig. 1H and I). At the same time, a high dose of KWG
was also found to possess cytotoxic effects on viability of HT22
cells, while 5 µmol/l KWG inhibited effects of 200 µg/ml of AGEs on
cell viability. Thus, 5 µM of KWG was used in the present
study.
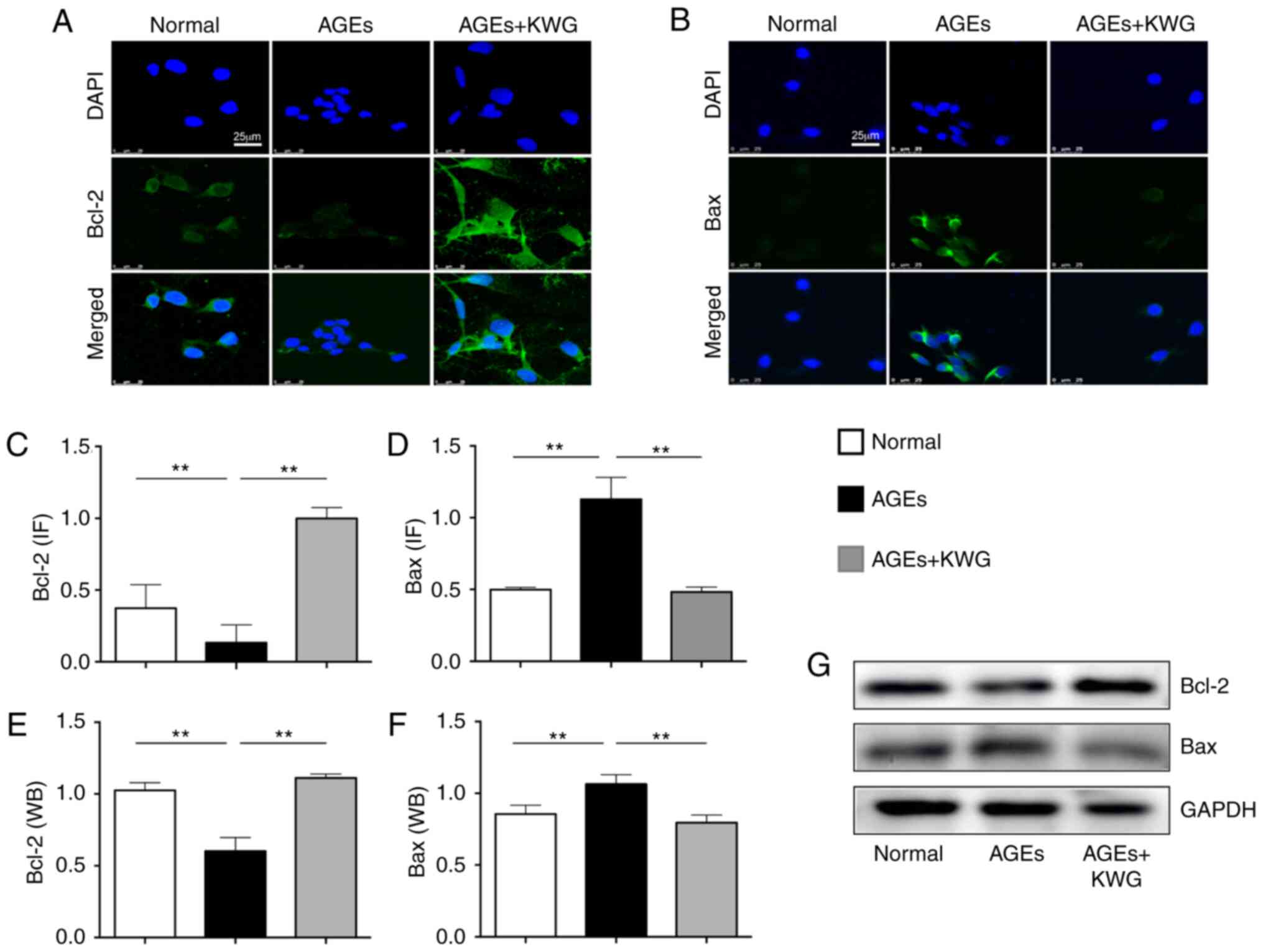
Kuwanon G (KWG) regulated expression
of Bcl-2/Bax proteins in AGE-treated cells
Bcl-2 family proteins play a pivotal role in
modulating cell viability (26).
Western blotting and immunofluorescence were used to evaluate
influence of KWG on Bcl-2 family protein expression (Fig. 2A-G). The results indicated that AGE
treatment significantly increased expression of pro-apoptotic Bax
and decreased anti-apoptotic Bcl-2 expression in comparison with
control treatment and that KWG administration was able to alleviate
the AGE-induced effects.
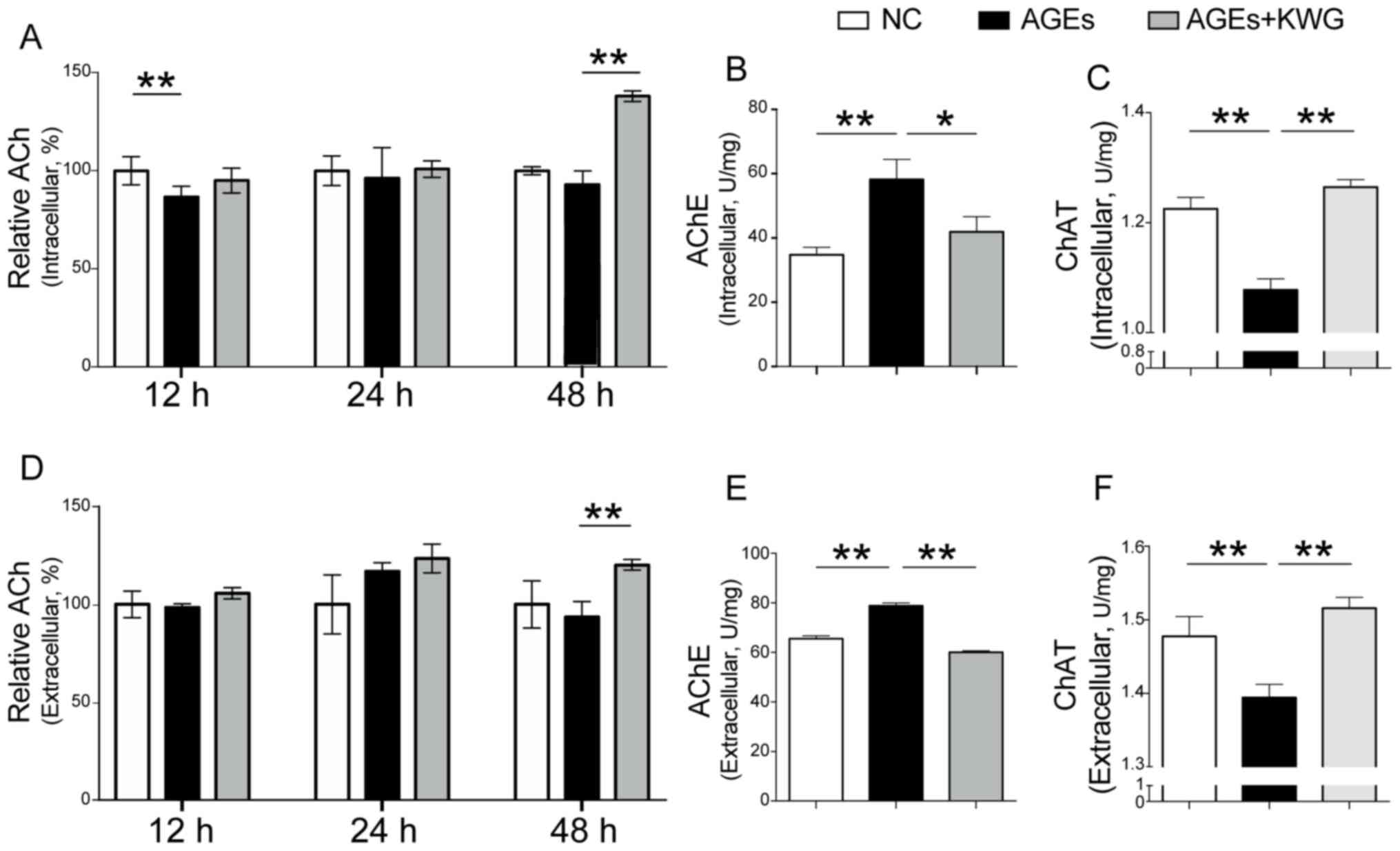
Acetylcholine (ACh) production was
increased by KWG
Reduction of Ach is believed to be responsible for
brain regression disease, including diabetic encephalopathy
(27-30).
Intracellular and extracellular levels of ACh after AGE
administration and KWG treatment were determined by ELISA kits. As
indicated in Fig. 3A and B, AGEs significantly reduced ACh content
within the cells compared to control and KWG administration could
restore the level of ACh both in- and outside of HT22 cells. The
expression of AchE and ChAT were assessed and the results showed
that AGEs significantly reduced AChE content within the cells, and
KWG restored the level of AChE both in- and -outside of HT22 cells
(Fig. 3C and D). At the same time, KWG reduced the
secretion of ChAT induced by AGEs both in- and -outside of HT22
cells (Fig. 3E and F). This finding suggests that KWG may
protect against diabetic encephalopathy via increasing ACh
production.
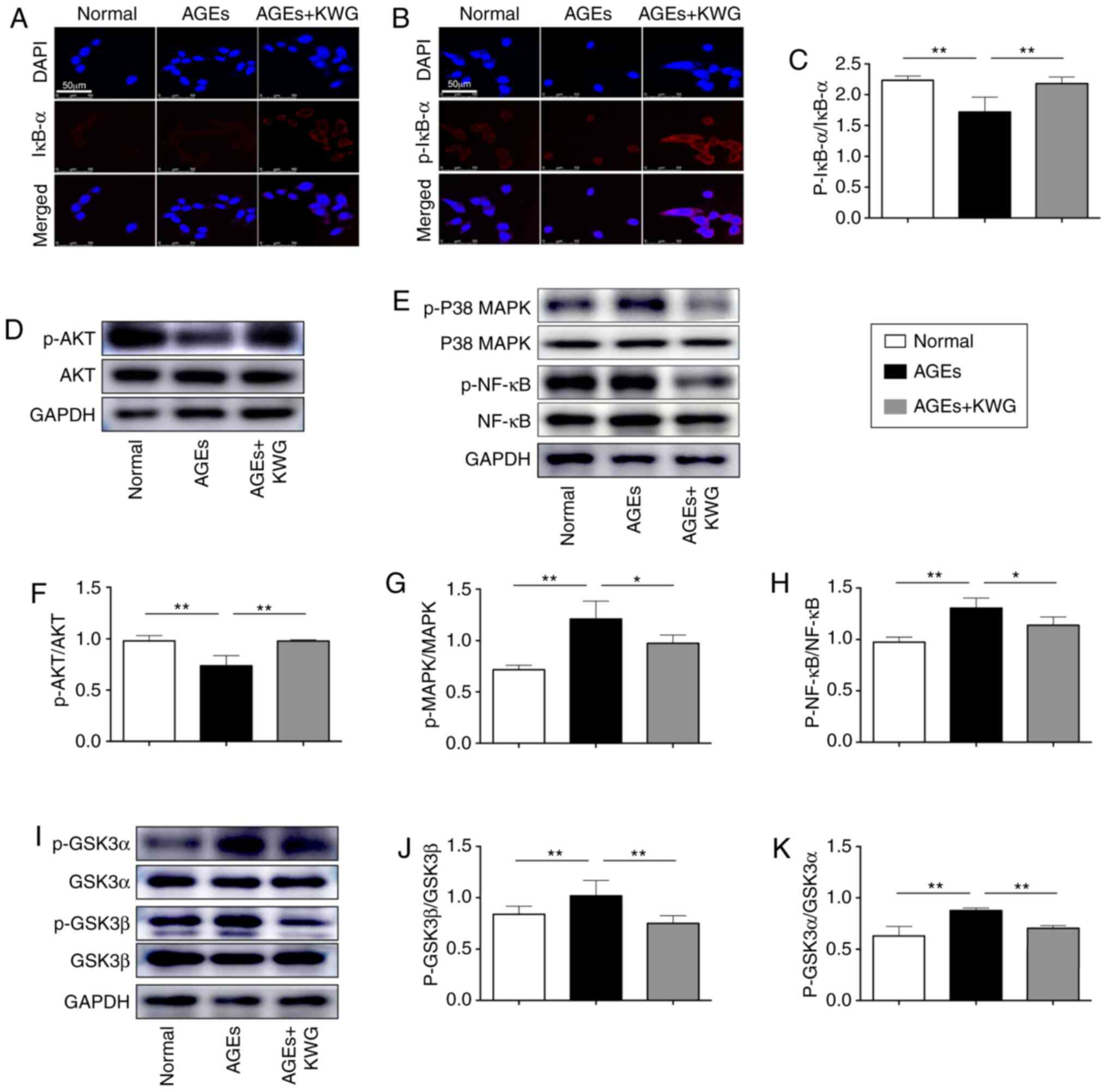
Effects of Kuwanon G on AGEs-induced
of PI3K/AKT/GSK3αβ/P38 MAPK/NF-κB p65/Iκb-α signaling pathway in
HT22 cells
In order to further explore the underlying
mechanism, some intracellular signaling pathway proteins were
evaluated by western blotting. As shown in Fig. 4A-K, expression and activation of AKT
and IκB-α was restored by KWG administration, while AGE induced
phosphorylation of signaling proteins including GSK3α/β, p38 MAPK
and NF-κB p65 was reduced by KWG.
Discussion
The diabetic population are at significant risk of
developing neurodegenerative disease. Although both peripheral and
central nerves can be influenced, greater focus should be on the
prevention of diseases causing brain dysfunction, as cognitive and
memory impairment will seriously influence patient quality of life
(25,31). The accumulation of AGEs has been
demonstrated to contribute to diabetic encephalopathy (32). The results of the present study
suggested that Kuwanon G (KWG) may be able to inhibit AGE-induced
neuron loss and dysfunction and the possible mechanism was also
explored.
Diabetic encephalopathy is a condition closely
related to both aging and hyperglycemia. Although the underlying
pathophysiological mechanism of this condition remains to be
elucidated, existing evidence suggests that accumulation of AGEs
may be a related factor (33). AGEs
refer to a group of stable metabolic products of macromolecules
including proteins, amino acids, lipids or nucleic acids in the
body that are glycated following exposure to sugars under
non-enzymatic conditions (34-36).
The two pivotal factors suggested to have a role in AGE production
are hyperglycemia and oxidative stress (37,38).
In the present study AGEs were applied in order to construct an
in vitro diabetic encephalopathy model in HT22 cells
(39,40), a mouse hippocampal neuron cell line
commonly used to study neurodegenerative disease (41,42).
Reports concerning correlation between AGEs and diabetes are
abundant and AGEs have been demonstrated to play a role in
promoting diabetic neuropathy (32,40,43).
However, interventions specifically addressing AGEs remain very
limited.
Cortex Mori (CM) is a TCM commonly thought to
be beneficial in the treatment of diabetes (44-46).
However, its active components are not well understood. KWG is a
flavonoid that can be found in CM, but there has been limited study
of its pharmacological activity. A recent finding suggested that
KWG inhibited α-glucosidase activity (14). This finding was verified by Paudel
et al (9) in HepG2 cells
where it was additionally shown that as well as inhibiting
α-glucosidase, KWG could also inhibit PTP1B, promote binding
between insulin and the insulin receptor and alleviate insulin
resistance. The above findings suggest the possibility that KWG
might be a beneficial therapy for diabetes. Our previous study also
demonstrated that KWG had a protective effect against diabetic
endotoxemia via reducing inflammatory cytokine expression and
alleviating oxidative stress (12).
This supplies further evidence that KWG may have anti-inflammatory
and anti-aging effects.
In the present study, the aim to was explore the
potential therapeutic effect of KWG on diabetic encephalopathy. To
first exclude a possibility that KWG may increase viability of HT22
cells, MTT assay was carried out. KWG at 5 µM did not have
significant effect on cell viability; but KWG at 5 µM could inhibit
effect of AGEs on reducing cell viability as well as ROS
production, suggesting that KWG has an effect against AGE-induced
cell damage. Further study found that KWG protected HT22 cells
against AGE-induced damage via anti-oxidative stress and
anti-inflammation related signaling pathways. Although further work
remains to be carried out, the present findings support the
conclusion that KWG may promote the production and secretion of ACh
and protect hippocampal neurons from oxidative toxicity induced by
AGEs.
As oxidative stress can facilitate accumulation and
function of AGEs and therefore contribute to progression of neuron
loss and diabetic encephalopathy (47-49),
whether KWG could reduce AGE-induced ROS production was
investigated. Additionally, levels of SOD, GPX and MDA were
assessed and the results indicated that KWG increased the
expression of SOD, GPX and reduced the secretion of MDA following
AGE administration. This is in line with findings from both our
group (12) and other research
groups (50). Another factor that
accounts for cell viability loss is apoptosis. By both flow
cytometry and immunocytochemistry, it was demonstrated that KWG
could inhibit HT22 cell apoptosis induced by AGEs via increasing
anti-apoptotic Bcl-2 and decreasing pro-apoptotic Bax expressions.
Preservation of cell number may inhibit progression of
neurodegenerative disease.
ACh is a major neurotransmitter in the brain and its
deficition leads to cognitive dysfunction. Dysfunction in the
production and/or secretion of ACh is the basic variation that
contributes to memory loss (31,51,52).
To evaluate whether KWG could preserve ACh levels in AGE treated
HT22 cells, its content was determined by an ELISA kit. AGE
administration significantly reduced the level of ACh within the
cells, suggesting that accumulation of AGEs within brain tissue may
promote neuro-dysfunction. Dementia and diabetes share some
pathological process, including neuroinflammation, abnormal AChE
levels, insulin resistance and decreased glucose metabolism
(53). Studies found that during
the course of diabetic encephalopathy, it was usually accompanied
by decreased secretion of ACh, and increased production of AChE
(28,54). The expression levels of ChAT and
AChE were also assessed, and the results suggested that KWG
administration could restore the level of ChAT both in- and
-outside of HT22 cells following AGE treatment. At the same time,
KWG reduced the secretion of AChE induced by AGEs both in- and
-outside of cells. It was also observed that KWG increased the
production of ChAT while inhibiting the secretion of AChE. This
result shows that KWG may be beneficial in preventing the
occurrence of diabetic encephalopathy.
Direct correlation between inflammation and
cognitive impairment has been demonstrated in numerous studies
(55-59).
In diabetes, the interaction between AGE and its receptors results
in the production of ROS and activation of signaling pathways,
including that of p38 MAPK and NF-κB (60). A previous study indicated that
increased formation of AGEs also leads to inactivation of PI3K/AKT
signaling cascade, which further activates the GSK3 pathway and
promotes production of pro-inflammatory cytokines (61). Activation of GSK3 signaling pathways
and oxidative stress in the brain may inhibit the expression of
Bcl-2(62). In this sense, AGEs,
ROS, PI3K/AKT and MAPK activation are linked and are key
contributors in the pathogenesis of diabetic cognitive dysfunction.
The expression and activation of the above signaling pathway
proteins was assessed in the present study. The results suggested
that KWG increased the phosphorylation of AKT and IκB-α and
decreased the phosphorylation of GSK3α, GSK3β, P38 and MAPK/NF-κB
p65. Phosphorylation of IκB-α is generally believed to be related
to NF-κB p65 nuclear translocation and inflammatory events
(63). In the present study, KWG
administration appeared to increase activation of AKT. It has been
previously demonstrated that phosphorylation of AKT will contribute
to p-IκB-α activation (64). In the
present sudy KWG increased both AKT and phosphorylation of IκB-α,
this is in line with conventional findings. However, NF-κB p65
activation was inhibited by KWG. This is obviously conflict with
that of p-IκB-α. To explain this phenomenon, activation of GSK3,
which plays pivotal role in inflammation, was assessed within
cells. It has been demonstrated that GSK3 plays key role in
modulating NF-κB p65 activation in that p65 is phosphorylated in
vitro by this kinase (65), in
this sense, GSK3 modulated activation of p65 after IκB. However,
the underlying mechanism deserves further investigation.
One consideration is the bioavailability of KWG to
neurons, i.e. its permeability across biological barriers including
the blood-brain barrier (BBB). Permeability of flavonoids across
the BBB is affected by their lipophilicity and interaction with
efflux transporters. Studies from Youdim et al (66,67)
suggested two flavonoids, naringenin and quercetin, can cross BBB
in vivo. Both clinical and experimental studies have also
suggested that the integrity of both the gut-barrier and the BBB is
damaged and that permeability of these two barriers is increased
(12,66-68),
suggesting that greater quantities of flavonoids may cross
biological barriers under diabetic settings. However, more work
should be carried out to further demonstrate this hypothesis.
In conclusion, the present study suggests that KWG
can promote both the production and secretion of ACh and can
protect hippocampal neurons from oxidative toxicity induced by
AGEs. The present findings indicate that KWG may be a potential
candidate for the prevention of diabetic neurodegenerative
disease.
Acknowledgements
The authors would like to thank Professor Lu Ming
from the Department of Pharmacology (Nanjing Medical University,
China) for providing the HT22 cell line.
Funding
The present study was supported by the Science and Technology
Development Fund of Macau (grant. no. FDCT: 0093/2018/A3).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WJG, CLG, DKG and YHX designed the experiments. WJG,
CLG, WQZ and JLG carried out the western blotting and
immunofluorescence experiments. WJG, TTZ, HLG, LLY and LFL carried
out ELISA. HZ, YX and YHX analyzed the data. WJG, CLG and YHX
drafted the manuscript. HZ, YX and DKG revised the manuscript. All
authors read and approved the final manuscript.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Markowicz-Piasecka M, Sikora J, Szydłowska
A, Skupień A, Mikiciuk-Olasik E and Huttunen KM: Metformin - a
future therapy for neurodegenerative diseases: Theme: Drug
Discovery, Development and Delivery in Alzheimer's Disease Guest
Editor: Davide Brambilla. Pharm Res. 34:2614–2627. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gireesh G, Kumar TP, Mathew J and Paulose
C: Enhanced muscarinic M1 receptor gene expression in the corpus
striatum of streptozotocin-induced diabetic rats. J Biomed Sci.
16(38)2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Saliu JA, Oboh G, Omojokun OS, Rocha JBT,
Schetinger MR, Guterries J, Stefanello N, Carvalho F, Schmatz R,
Morsch VM, et al: Effect of dietary supplementation of Padauk
(Pterocarpus soyauxii) leaf on high fat diet/streptozotocin
induced diabetes in rats’ brain and platelets. Biomed Pharmacother.
84:1194–1201. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhao J, Liu L, Li X, Zhang L, Lv J, Guo X,
Chen H and Zhao T: Neuroprotective effects of an Nrf2 agonist on
high glucose-induced damage in HT22 cells. Biol Res.
52(53)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Waslo C, Bourdette D, Gray N, Wright K and
Spain R: Lipoic acid and other antioxidants as therapies for
multiple sclerosis. Curr Treat Options Neurol.
21(26)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Eckert GP, Eckert SH, Eckmann J, Hagl S,
Muller WE and Friedland K: Olesoxime improves cerebral
mitochondrial dysfunction and enhances Aβ levels in preclinical
models of Alzheimer's disease. Exp Neurol.
329(113286)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Balamurugan M, Santharaman P, Madasamy T,
Rajesh S, Sethy NK, Bhargava K, Kotamraju S and Karunakaran C:
Recent trends in electrochemical biosensors of superoxide
dismutases. Biosens Bioelectron. 116:89–99. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jiao Y, Wang Y, Guo S and Wang G:
Glutathione peroxidases as oncotargets. Oncotarget. 8:80093–80102.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Paudel P, Yu T, Seong SH, Kuk EB, Jung HA
and Choi JS: Protein tyrosine phosphatase 1B inhibition and glucose
uptake potentials of mulberrofuran G, Albanol B, and Kuwanon G from
root bark of Morus alba L. in insulin-resistant HepG2 cells:
An in vitro and in silico study. Int J Mol Sci.
19(E1542)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nourazarian AR, Kangari P and Salmaninejad
A: Roles of oxidative stress in the development and progression of
breast cancer. Asian Pac J Cancer Prev. 15:4745–4751.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lee MS, Park WS, Kim YH, Kwon SH, Jang YJ,
Han D, Morita K and Her S: Antidepressant-like effects of Cortex
Mori Radicis extract via bidirectional phosphorylation of
glucocorticoid receptors in the hippocampus. Behav Brain Res.
236:56–61. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Guo H, Xu Y, Huang W, Zhou H, Zheng Z,
Zhao Y, He B, Zhu T, Tang S and Zhu Q: Kuwanon G preserves
LPS-induced disruption of gut epithelial barrier in vitro.
Molecules. 21(E1597)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
You S and Kim GH: Protective effect of
Mori Cortex radicis extract against high glucose-induced oxidative
stress in PC12 cells. Biosci Biotechnol Biochem. 83:1893–1900.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen Z, Du X, Yang Y, Cui X, Zhang Z and
Li Y: Comparative study of chemical composition and active
components against α-glucosidase of various medicinal parts of
Morus alba L. Biomed Chromatogr. 32(e4328)2018.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Wei J, Chen JR, Pais EMA, Wang TY, Miao L,
Li L, Li LY, Qiu F, Hu LM, Gao XM, et al: Oxyresveratrol is a
phytoestrogen exerting anti-inflammatory effects through NF-κB and
estrogen receptor signaling. Inflammation. 40:1285–1296.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lee HJ, Lyu H, Koo U, Nam KW, Hong SS, Kim
KO, Kim KH, Lee D and Mar W: Protection of prenylated flavonoids
from Mori Cortex Radicis (Moraceae) against nitric oxide-induced
cell death in neuroblastoma SH-SY5Y cells. Arch Pharm Res.
35:163–170. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ma LL, Yuan YY, Zhao M, Zhou XR, Jehangir
T, Wang FY, Xi Y and Bu SZ: Mori Cortex extract ameliorates
nonalcoholic fatty liver disease (NAFLD) and insulin resistance in
high-fat-diet/streptozotocin-induced type 2 diabetes in rats. Chin
J Nat Med. 16:411–417. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu XX, Zhang XW, Wang K, Wang XY, Ma WL,
Cao W, Mo D, Sun Y and Li XQ: Kuwanon G attenuates atherosclerosis
by upregulation of LXRα-ABCA1/ABCG1 and inhibition of NFκB activity
in macrophages. Toxicol Appl Pharmacol. 341:56–63. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kuk EB, Jo AR, Oh SI, Sohn HS, Seong SH,
Roy A, Choi JS and Jung HA: Anti-Alzheimer's disease activity of
compounds from the root bark of Morus alba L. Arch Pharm Res.
40:338–349. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gong M, Ye S, Li WX, Zhang J, Liu Y, Zhu
J, Lv W, Zhang H, Wang J, Lu A, et al: Regulatory function of Pja2
mediated by the P2rx3/P2rx7 axis in mouse hippocampal neuronal
cells. Am J Physiol Cell Physiol. 318:C1123–C1135. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cancela S, Canclini L, Mourglia-Ettlin G,
Hernández P and Merlino A: Neuroprotective effects of novel
nitrones: In vitro and in silico studies. Eur J Pharmacol.
871(172926)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xu Y, Wang S, Feng L, Zhu Q, Xiang P and
He B: Blockade of PKC-beta protects HUVEC from advanced glycation
end products induced inflammation. Int Immunopharmacol.
10:1552–1559. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xu Y, Feng L, Wang S, Zhu Q, Lin J, Lou C,
Xiang P, He B, Zheng Z, Tang D, et al: Phytoestrogen
calycosin-7-O-β-D-glucopyranoside ameliorates advanced
glycation end products-induced HUVEC damage. J Cell Biochem.
112:2953–2965. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xu Y, Feng L, Wang S, Zhu Q, Zheng Z,
Xiang P, He B and Tang D: Calycosin protects HUVECs from advanced
glycation end products-induced macrophage infiltration. J
Ethnopharmacol. 137:359–370. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Leng J, Li X, Tian H, Liu C, Guo Y, Zhang
S, Chu Y, Li J, Wang Y and Zhang L: Neuroprotective effect of
diosgenin in a mouse model of diabetic peripheral neuropathy
involves the Nrf2/HO-1 pathway. BMC Complement Med Ther.
20(126)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Voehringer DW: BCL-2 and glutathione:
Alterations in cellular redox state that regulate apoptosis
sensitivity. Free Radic Biol Med. 27:945–950. 1999.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Scremin OU, Roch M, Norman KM, Djazayeri S
and Liu YY: Brain acetylcholine and choline concentrations and
dynamics in a murine model of the Fragile X syndrome: Age, sex and
region-specific changes. Neuroscience. 301:520–528. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhou X, Zhu Q, Han X, Chen R, Liu Y, Fan H
and Yin X: Quantitative-profiling of neurotransmitter abnormalities
in the disease progression of experimental diabetic encephalopathy
rat. Can J Physiol Pharmacol. 93:1007–1013. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liapi C, Kyriakaki A, Zarros A,
Galanopoulou P, Al-Humadi H, Dontas I, Voumvourakis K and Tsakiris
S: Choline-deprivation alters crucial brain enzyme activities in a
rat model of diabetic encephalopathy. Metab Brain Dis. 25:269–276.
2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Aykac A, Ozbeyli D, Uncu M, Ertaş B,
Kılınc O, Şen A, Orun O and Sener G: Evaluation of the protective
effect of Myrtus communis in scopolamine-induced Alzheimer
model through cholinergic receptors. Gene. 689:194–201.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Argente-Pla M, Pérez-Lázaro A,
Martinez-Millana A, Del Olmo-García MI, Espí-Reig J,
Beneyto-Castello I, López-Andújar R and Merino-Torres JF:
Simultaneous pancreas kidney transplantation improves
cardiovascular autonomic neuropathy with improved valsalva ratio as
the most precocious test. J Diabetes Res.
2020(7574628)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhu X, Cheng YQ, Lu Q, Du L, Yin XX and
Liu YW: Enhancement of glyoxalase 1, a polyfunctional defense
enzyme, by quercetin in the brain in streptozotocin-induced
diabetic rats. Naunyn Schmiedebergs Arch Pharmacol. 391:1237–1245.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lu M, Xu L, Li B, Zhang W, Zhang C, Feng
H, Cui X and Gao H: Protective effects of grape seed
proanthocyanidin extracts on cerebral cortex of
streptozotocin-induced diabetic rats through modulating
AGEs/RAGE/NF-kappaB pathway. J Nutr Sci Vitaminol (Tokyo).
56:87–97. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Luévano-Contreras C, Gómez-Ojeda A,
Macías-Cervantes MH and Garay-Sevilla ME: Dietary advanced
glycation end products and cardiometabolic risk. Curr Diab Rep.
17(63)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Singh R, Barden A, Mori T and Beilin L:
Advanced glycation end-products: A review. Diabetologia.
44:129–146. 2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gurav AN: Advanced glycation end products:
A link between periodontitis and diabetes mellitus? Curr Diabetes
Rev. 9:355–361. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Shumilina J, Kusnetsova A, Tsarev A, Janse
van Rensburg HC, Medvedev S, Demidchik V, Van den Ende W and Frolov
A: Glycation of plant proteins: Regulatory roles and interplay with
sugar signalling? Int J Mol Sci. 20(2366)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bellier J, Nokin MJ, Lardé E, Karoyan P,
Peulen O, Castronovo V and Bellahcène A: Methylglyoxal, a potent
inducer of AGEs, connects between diabetes and cancer. Diabetes Res
Clin Pract. 148:200–211. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dafre AL, Schmitz AE and Maher P:
Methylglyoxal-induced AMPK activation leads to autophagic
degradation of thioredoxin 1 and glyoxalase 2 in HT22 nerve cells.
Free Radic Biol Med. 108:270–279. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sung SK, Woo JS, Kim YH, Son DW, Lee SW
and Song GS: Sildenafil ameliorates advanced glycation end
products-induced mitochondrial dysfunction in HT-22 hippocampal
neuronal cells. J Korean Neurosurg Soc. 59:259–268. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li Z, Chen X, Lu W, Zhang S, Guan X, Li Z
and Wang D: Anti-oxidative stress activity is essential for
Amanita caesarea mediated neuroprotection on
glutamate-induced apoptotic HT22 cells and an Alzheimer's disease
mouse model. Int J Mol Sci. 18(E1623)2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liu J, Li L and Suo WZ: HT22 hippocampal
neuronal cell line possesses functional cholinergic properties.
Life Sci. 84:267–271. 2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Singh VP, Bali A, Singh N and Jaggi AS:
Advanced glycation end products and diabetic complications. Korean
J Physiol Pharmacol. 18:1–14. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chen F, Nakashima N, Kimura I and Kimura
M: Hypoglycemic activity and mechanisms of extracts from mulberry
leaves (folium mori) and cortex mori radicis in
streptozotocin-induced diabetic mice. Yakugaku Zasshi. 115:476–482.
1995.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
45
|
Lemus I, García R, Delvillar E and Knop G:
Hypoglycaemic activity of four plants used in Chilean popular
medicine. Phytother Res. 13:91–94. 1999.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Cai S, Sun W, Fan Y, Guo X, Xu G, Xu T,
Hou Y, Zhao B, Feng X and Liu T: Effect of mulberry leaf (Folium
Mori) on insulin resistance via IRS-1/PI3K/Glut-4 signalling
pathway in type 2 diabetes mellitus rats. Pharm Biol. 54:2685–2691.
2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Samarghandian S, Azimi-Nezhad M and Samini
F: Ameliorative effect of saffron aqueous extract on hyperglycemia,
hyperlipidemia, and oxidative stress on diabetic encephalopathy in
streptozotocin induced experimental diabetes mellitus. BioMed Res
Int. 2014(920857)2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Jiang L, Wang J, Wang Z, Huang W, Yang Y,
Cai Z and Li K: Role of the glyoxalase system in Alzheimer's
disease. J Alzheimers Dis. 66:887–899. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Joubert M, Manrique A, Cariou B and Prieur
X: Diabetes-related cardiomyopathy: The sweet story of glucose
overload from epidemiology to cellular pathways. Diabetes Metab.
45:238–247. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Abbas GM, Abdel Bar FM, Baraka HN, Gohar
AA and Lahloub MF: A new antioxidant stilbene and other
constituents from the stem bark of Morus nigra L. Nat Prod
Res. 28:952–959. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Tzavara ET, Bymaster FP, Overshiner CD,
Davis RJ, Perry KW, Wolff M, McKinzie DL, Witkin JM and Nomikos GG:
Procholinergic and memory enhancing properties of the selective
norepinephrine uptake inhibitor atomoxetine. Mol Psychiatry.
11:187–195. 2006.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kumamoto E and Kuba K: Sustained rise in
ACh sensitivity of a sympathetic ganglion cell induced by
postsynaptic electrical activities. Nature. 305:145–146.
1983.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Agatonovic-Kustrin S, Kustrin E and Morton
DW: Essential oils and functional herbs for healthy aging. Neural
Regen Res. 14:441–445. 2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Pandey S and Garabadu D: Piracetam
facilitates the anti-amnesic but not anti-diabetic activity of
metformin in experimentally induced type-2 diabetic encephalopathic
rats. Cell Mol Neurobiol. 37:791–802. 2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Song IU, Chung SW, Kim YD and Maeng LS:
Relationship between the hs-CRP as non-specific biomarker and
Alzheimer's disease according to aging process. Int J Med Sci.
12:613–617. 2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Gregor MF and Hotamisligil GS:
Inflammatory mechanisms in obesity. Annu Rev Immunol. 29:415–445.
2011.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Gorska-Ciebiada M, Saryusz-Wolska M,
Borkowska A, Ciebiada M and Loba J: Serum levels of inflammatory
markers in depressed elderly patients with diabetes and mild
cognitive impairment. PLoS One. 10(e0120433)2015.PubMed/NCBI View Article : Google Scholar
|
|
58
|
John CM, Mohamed Yusof NIS, Abdul Aziz SH
and Mohd Fauzi F: Maternal cognitive impairment associated with
gestational diabetes mellitus: A review of potential contributing
mechanisms. Int J Mol Sci. 19(E3894)2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Meng Y, Wang W, Kang J, Wang X and Sun L:
Role of the PI3K/AKT signalling pathway in apoptotic cell death in
the cerebral cortex of streptozotocin-induced diabetic rats. Exp
Ther Med. 13:2417–2422. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Parveen A, Kim JH, Oh BG, Subedi L, Khan Z
and Kim SY: Phytochemicals: Target-based therapeutic strategies for
diabetic retinopathy. Molecules. 23(E1519)2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Liang W, Zhang D, Kang J, Meng X, Yang J,
Yang L, Xue N, Gao Q, Han S and Gou X: Protective effects of rutin
on liver injury in type 2 diabetic db/db mice. Biomed Pharmacother.
107:721–728. 2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Butterfield DA, Di Domenico F and Barone
E: Elevated risk of type 2 diabetes for development of Alzheimer
disease: A key role for oxidative stress in brain. Biochim Biophys
Acta. 1842:1693–1706. 2014.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Han S, Gao H, Chen S, Wang Q, Li X, Du LJ,
Li J, Luo YY, Li JX, Zhao LC, et al: Procyanidin A1 Alleviates
Inflammatory Response induced by LPS through NF-κB, MAPK, and
Nrf2/HO-1 Pathways in RAW264.7 cells. Sci Rep.
9(15087)2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Xu L, Botchway BOA, Zhang S, Zhou J and
Liu X: Inhibition of NF-κB signaling pathway by resveratrol
improves spinal cord injury. Front Neurosci. 12(690)2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Ghosh S and Hayden MS: New regulators of
NF-kappaB in inflammation. Nat Rev Immunol. 8:837–848.
2008.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Youdim KA, Qaiser MZ, Begley DJ,
Rice-Evans CA and Abbott NJ: Flavonoid permeability across an in
situ model of the blood-brain barrier. Free Radic Biol Med.
36:592–604. 2004.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Youdim KA, Dobbie MS, Kuhnle G,
Proteggente AR, Abbott NJ and Rice-Evans C: Interaction between
flavonoids and the blood-brain barrier: In vitro studies. J
Neurochem. 85:180–192. 2003.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Bogush M, Heldt NA and Persidsky Y: Blood
brain barrier injury in diabetes: Unrecognized effects on brain and
cognition. J Neuroimmune Pharmacol. 12:593–601. 2017.PubMed/NCBI View Article : Google Scholar
|