Introduction
Benign central airway stenosis, which refers to
airway stenosis caused by various benign lesions in the trachea,
left and right main bronchus and right middle bronchus, may result
in different degrees of dyspnea or even asphyxia in patients
(1). Benign airway stenosis is
primarily caused by the proliferation of granulation tissue in the
trachea and bronchi. Previously, endobronchial tuberculosis was the
primary cause of benign tracheal stenosis in China (2). In recent years, with the improvement
of various rescue techniques and respiratory support treatments,
the survival of patients with severe benign tracheal stenosis has
increased. Tracheal stenosis is currently caused by long-term
tracheal intubation or tracheotomy, and the proportion of patients
with tracheal stenosis is gradually increasing (2). At present, the primary methods for the
treatment of benign tracheal stenosis are surgery and endoscopic
intervention, however these treatments may induce secondary injury
(1,2). Excessive wound healing results in
restenosis of the trachea, and an increased restenosis rate often
requires repeated treatment (3-5),
which not only increases the discomfort of the patient but also
imposes a heavy financial burden on patients and their families.
Therefore, drugs designed to improve tracheal mucosal injury and
prevent benign tracheal stenosis and restenosis following
interventional therapy have received increased attention.
Previously, histone acetyltransferases (HATs) and
deacetylases (HDACs) have been indicated to function as ‘gene
switches’ in the nucleus, which may turn the transcription of
inflammatory genes on or off, thereby affecting the progression of
inflammatory diseases (6). HDAC2, a
subtype of class I HDACs, is located in the nucleus and is
primarily involved in the inhibition of inflammation (7,8). The
pathogenesis of benign tracheal stenosis is still unclear. The
activation and release of inflammatory cells and mediators and the
overexpression of inflammatory genes have been demonstrated to
promote the proliferative response of fibroblasts, resulting in the
proliferation and stenosis of tracheal granulation tissue (9,10).
Numerous studies have revealed that HDAC2 activity is reduced in
chronic airway inflammatory diseases, such as asthma and chronic
obstructive pulmonary disease (COPD), and is associated with
disease progression (11,12). However, whether an association
between HDAC2 and benign tracheal stenosis exists, requires further
study. A previous study demonstrated that erythromycin may enhance
the anti-inflammatory activity of budesonide in rats with COPD via
inhibiting the PI3K/AKT pathway and enhancing the activity of
HDAC2(13). Glucocorticoids
suppress the multiple inflammatory genes that are activated in
asthma via reversing the histone acetylation of activated
inflammatory genes, and this is initiated by the interaction of
ligand-bound glucocorticoid receptors with coactivator molecules
and the recruitment of HDAC2 to the activated inflammatory gene
transcription complex (14).
Vorinostat is a class I HDAC inhibitor, which has been demonstrated
to exhibit a strong inhibitory effect on HDAC2 and is clinically
used to treat malignant tumors (15-17).
In the present study, models of tracheal stenosis after injury were
established and drugs were administered to positively and
negatively regulate the expression of HDAC2. Subsequently, the
alterations in the degree of tracheal stenosis, the expression of
HDAC2, the inflammatory factor interleukin-8 (IL-8), and the
profibrotic factors transforming growth factor-β1 (TGF-β1),
vascular endothelial growth factor (VEGF) and collagen were
examined. Moreover, the effect of HDAC2 regulation on the
development of tracheal stenosis after injury was analyzed to
explore the possibility of using HDAC2 as a novel target for the
prevention and treatment of benign tracheal stenosis.
Materials and methods
Animals
A total of 24 New Zealand rabbits (4 weeks old; 12
males and 12 females; 2.5-3 kg) were purchased from Nanchang
Longping Rabbit Industry Co., Ltd. The rabbits were bred in a
specific-pathogen-free class barrier system, maintained in a room
(temperature, 18-22˚C; 60-70% humidity; normal atmospheric
pressure) with a 12 h light/dark cycle, with free access to food
and water. The present study followed international, national and
institutional guidelines for humane animal treatment and complied
to the relevant legislation (18).
The protocol was approved by the Animal Experimental Ethics
Committee of Guangxi Medical University with oversight of the
facility where the studies were conducted (approval no.
201806020).
Reagents and instruments
The following reagents and instruments were used:
Erythromycin enteric-coated tablets (cat. no. H42021990; Yichang
Renfu Pharmaceutical Co., Ltd.); vorinostat capsules (cat. no.
180509; Beijing Hengrui Kangda Pharmaceutical Sci. & Tech.
Development Co., Ltd.); budesonide suspension for inhalation (cat.
no. 8339000; AstraZeneca); hematoxylin dye solution (cat. no.
AR11800-1; Boster Biological Technology,); eosin staining solution
(cat. no. AR11800-2; Boster Biological Technology); Scott Blue
solution (cat. no. G1865; Beijing Solarbio Science & Technology
Co., Ltd.); rabbit anti-collagen III polyclonal antibody (cat. no.
bs-10423R; Bioss); rabbit anti-collagen I polyclonal antibody (cat.
no. bs-0549R; Bioss); horseradish-conjugated goat anti-rabbit IgG
(cat. no. ZB-2301; OriGene Technologies, Inc.); rabbit anti-HDAC2
polyclonal antibody (cat. no. bs-1813R; Bioss); goat anti-rabbit
IgG Cy3-conjugated (cat. no. CW0159S; CoWin Biosciences);
TRIzol® Reagent (cat. no. 15596026; Thermo Fisher
Scientific, Inc.); Ultrapure RNA extraction kit (cat. no. CW0581M;
CoWin Biosciences); HiFiScript complementary DNA (cDNA) synthesis
kit (cat. no. CW2569M; CoWin Biosciences); UltraSYBR mixture (cat.
no. CW0957M; CoWin Biosciences); optical microscope (cat. no. CX41,
Olympus Corporation); fluorescence microscope (cat. no. CKX53,
Olympus Corporation); and a CFX Connect™ Real-Time PCR Detection
System (Bole Medical Device Co., Ltd.).
Experimental groups and treatment
All rabbits were randomly divided into four groups
regardless of male or female sex. It was observed that sex had no
significant effect on the success rate of tracheal modeling and the
degree of tracheal stenosis during preliminary experiments
performed in the early stages of the study (19). All drug doses were based on body
surface area, calculated with the Meeh-Rubner formula: A=K x
(W2/3/104), where A is body surface area, W
is weight, and K is the constant (humans, 10.6; rabbits, 10.1)
(20). The rabbits in the control
group were not treated. In the erythromycin group, the rabbits were
treated with erythromycin (13.6 mg/kg) twice daily via oral gavage.
In the budesonide group, the rabbits were treated with a budesonide
suspension (0.05 mg/kg) twice daily via atomization inhalation. The
rabbits in the vorinostat group were treated with vorinostat (40
mg/kg) once daily via oral gavage. A total of six rabbits were
analyzed per group and samples were collected following ten
consecutive days of drug administration.
Tracheal stenosis model
The model was established according to the method of
Nakagishi et al (21). The
rabbits were fasted for 8 h prior to the establishment of the
model, and were administered with an intravenous injection of
pentobarbital sodium (30 mg/kg). To enhance analgesia, 2% lidocaine
hydrochloride (2 mg/kg) was injected into the anterior neck. After
anesthesia, the rabbits were placed in a supine position and
fastened on the operating table. The skin in the anterior cervical
region was disinfected twice with 0.5% iodophor. A longitudinal
incision in the skin of approximately 4-5 cm was performed, the
subcutaneous tissue and muscle were separated to expose the trachea
and annular tracheotomy was performed in cartilage spaces 3 and 4.
The length of the incision was 2/3 of the circumference of the
trachea to prevent injury of the tracheal cartilage. The proximal
end of the trachea was lifted to prevent suffocation caused by
blood flowback to the distal end of the trachea, and bleeding was
ceased via compressing the tracheal incision. A rigid nylon brush
was inserted into the distal trachea at ~1.5 cm depth through the
incision and rubbed back and forth 20x on the front and sidewalls
of the trachea. In case of intratracheal haemorrhage occurring due
to friction, a gauze was used for haemostasis. When no haemorrhage
was observed, a single no. 4.0 thread was used to carefully suture
the trachea layer by layer irregularly over three layers: The
muscular, subcutaneous tissue and skin layer. The wound was
disinfected and covered with sterile gauze. The rabbits were taken
back to their cages and observed for 1 h until natural
consciousness was restored.
Atomization inhalation
The animal atomization inhalation method, which was
reported by Lei et al (22)
was used. One end of a closed container, which was open at both
ends, was connected to the atomizer. When the container was filled
with atomizing gas and equilibrated, the rabbits were placed in the
container for atomizing inhalation, twice a day, 30 min each time,
for 10 consecutive days. On the first day of tracheal modeling,
rabbits were given the first nebulized inhalation 2 h after
surgery.
Sampling
All rabbits were fasted for 8 h before sampling and
were anaesthetized via an intravenous injection of sodium
pentobarbital (30 mg/kg) and an anterior neck injection of 2%
lidocaine hydrochloride. They were fastened on the operating table
and disinfected twice with 0.5% iodophor. The rabbits were
sacrificed via injecting air (30 ml/kg) into the ear vein. The skin
was cut at the anterior cervical region, the subcutaneous tissue
layer was separated layer by layer, the muscle layer was removed
and the trachea was exposed. The trachea was quickly cut within 2
cm from the original tracheotomy incision. The stenotic trachea was
collected and tracheal tissue specimens were stored separately at
-80˚C to meet the requirements for the subsequent tests.
Tracheal stenosis measurement
The trachea was removed to calculate tracheal
stenosis (S) using the following formula: S=[1-(r1 + r2)/(R1 + R2)]
x 100, where r1 and r2 are the long and short diameters of the
endotracheal ventilation chamber, respectively, and R1 and R2 are
the long and short diameters of the tracheal cartilage ring,
respectively (19).
Hematoxylin and eosin (H&E)
staining
The tissues were fixed with 100% methanol at 25˚C
for 30 sec, rinsed for 2 h and dehydrated with 70, 80 and 90%
ethanol solutions and a mixture of pure alcohol and xylene for 15
min, xylene I for 15 min, and xylene II for 15 min (until
transparent). The tissues were incubated in a mixture of xylene and
paraffin at 25˚C for 15 min, and paraffin I and paraffin II were
then added for 50-60 min each. The tissues were embedded in
paraffin and sliced. The paraffinized sections (5-µm thickness)
were dried in an incubator and then dewaxed and rehydrated. After
washing with distilled water, each slice was placed in an aqueous
solution of hematoxylin at 25˚C for 3 min, incubated in an ethanol
differentiation solution at 25˚C for 15 sec, washed slightly with
water, incubated in Scott Blue solution at 25˚C for 15 sec, rinsed
with water, stained with eosin for 3 min at 25˚C, and again rinsed
with water. The slices were then dehydrated until transparent,
sealed with neutral resin and examined by optical microscopy at
x400 magnification (Olympus CX41; Olympus Corporation).
Reverse transcription-quantitative
(RT-q) PCR analysis
Each group of tracheal tissues was ground in liquid
nitrogen for RNA extraction using the ultrapure RNA extraction kit,
and cDNA was synthesized with a RT kit (temperature and duration:
70˚C for 10 min, 50˚C for 15 min and 85˚C for 5 min) and used as a
template for a reaction system containing UltraSYBR mixture for
amplification and detection on a CFX Connect™ Real-Time PCR
Detection System (Bole Medical Device Co., Ltd.). The following
thermocycling conditions were used for the qPCR: Initial
denaturation at 95˚C for 10 min, followed by 40 cycles of 95˚C for
10 sec and 57˚C for 30 sec and a final extension of 72˚C for 30
sec. The following primer sequences were used: TGF-β1, forward
(5'-AAGGACCTGGGCTGGAA-3') and reverse (5'-CGGGTTGTGCTGGTTGTA-3');
VEGF, forward (5'-CGAGTACATATTCAAGCCTTCC-3') and reverse
(5'-CTTGCTCTGTCTTTCTTTGGTC-3'); IL-8, forward
(5'-CTGTTGGTCAGGCCATGAGT-3') and reverse
(5'-AAAGTGCTTCCATGTGCCCT-3'); HDAC2, forward
(5'-AGGAGACTTGAGGGATATTGG-3') and reverse
(5'-CATTTAGCATGACCTTTGACTG-3'); GAPDH, forward
(5'-GCCGCCCAGAACATCAT-3') and reverse (5'-TGCCTGCTTCACCACCTT-3').
All primers were purchased from Sangon Biotech Co., Ltd. and the
accession numbers of the genes used were as follows: TGF-β1
(XM_008249704.2), VEGF (XM_017345155.1 ), IL-8 (NM_001082293.1) and
HDAC2 (AB232682.1). The relative mRNA expression levels of TGF-β1,
VEGF, IL-8 and HDAC2 in each group of samples were calculated using
GAPDH as an internal control. Gene expression was presented as the
relative expression, which was calculated using the
2-ΔΔCq method (23).
Immunofluorescence assay
The tissue sections (8-µm thick) were left to dry at
25˚C and fixed with 100% acetone at 4˚C for 10 min. Tissues were
then submerged in xylene for 10 min and subsequently submerged in
100% ethanol twice, 95% ethanol, 80% ethanol and purified water for
5 min each. Antigen retrieval was performed using a citrate buffer
(pH 6.0) at 121˚C for 2 min. After blocking with 5% BSA (Thermo
Fisher Scientific, Inc.) at 37˚C for 30 min, the tissue slices were
incubated with rabbit anti-HDAC2 polyclonal antibody (1:400)
overnight at 4˚C. The slices were washed 3x with PBS and incubated
with goat anti-rabbit IgG Cy3-conjugated (1:200) at 37˚C for 30
min. DAPI was added to the tissue slices and incubated at 25˚C for
5 min in the dark. Excess DAPI was washed with PBS, and the slices
were finally washed with water for 1 min. The tissue slices were
sealed with ultra-clean high-grade sealant (Hai Besuo Biotechnology
Co., Ltd.) and observed with a fluorescence microscope (400x at
magnification).
Immunohistochemical detection
Briefly, the tracheal tissues were embedded in
paraffin using the conventional method. The tissues were cut into
5-µm slices and incubated with a 0.3% endogenous peroxidase
blocking solution at 25˚C for 20 min following dewaxing and
rehydration. Subsequently, the sections were incubated with a 3%
hydrogen peroxide methanol solution at at 25˚C for 10 min and
washed 3 times with PBS (3 min/wash). Antigen retrieval was
performed using a citrate buffer (pH 6.0) at 121˚C for 2 min.
Following blocking at 37˚C for 30 min with 5% BSA (Thermo Fisher
Scientific, Inc.), the tissues were incubated with a primary
polyclonal antibody [anti-collagen I (1:400) and anti-collagen III
(1:400)] overnight at 4˚C. They were then incubated at 37˚C for 30
min with horseradish peroxidase-conjugated goat anti-rabbit IgG
(1:100) according to the manufacturer's instructions, and mounted
with epoxy resin. Tissue sections were observed with an optical
microscope at x400 magnification (Olympus CX41; Olympus
Corporation).
Statistical analysis
Experiments were repeated three times for each group
of samples. All data are presented as the mean ± standard
deviation. One-way ANOVA followed by Tukey's post-hoc test was used
to analyze differences among multiple groups. P<0.05 was
considered to indicate a statistically significant difference. All
analyses were conducted using SPSS v19.0 software (IBM Corp.).
Results
Establishment of the tracheal stenosis
model
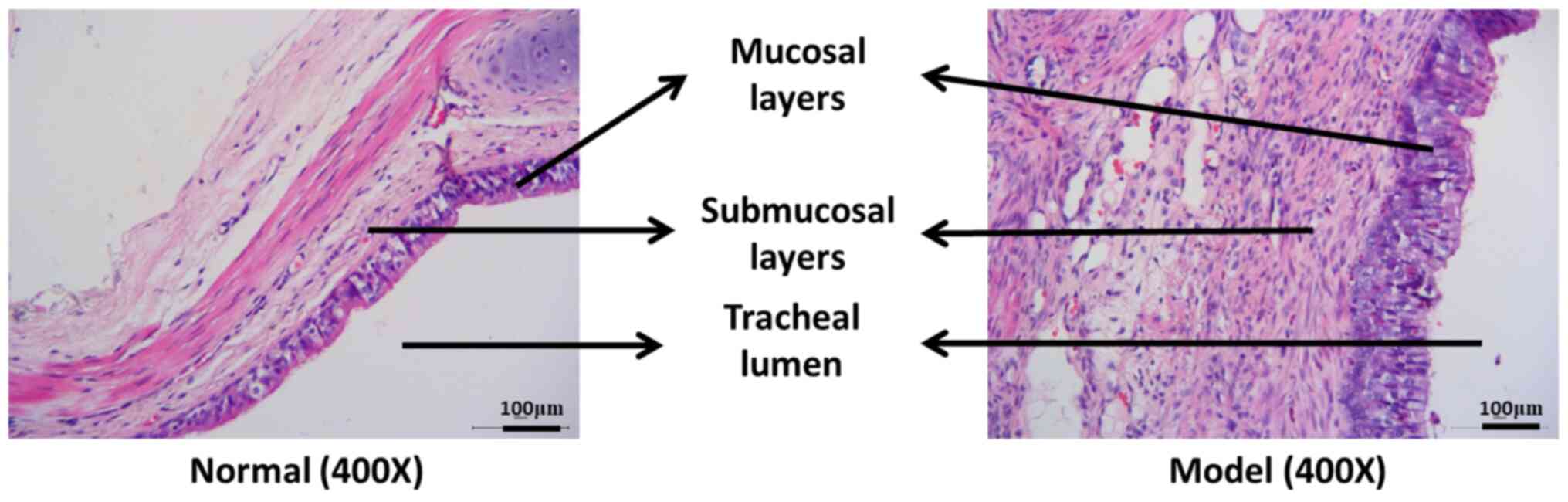
As presented in Fig.
1, in the normal group, the mucosa and submucosa, the goblet
and basal cells were visible, and the connective tissue of the
submucosal fibers were loose. Mucosal epithelial hyperplasia,
submucosal fibroblast proliferation, collagen fiber thickening,
disorganization and inflammatory cell infiltration were observed in
the model group, indicating that the tracheal stenosis model had
been successfully established.
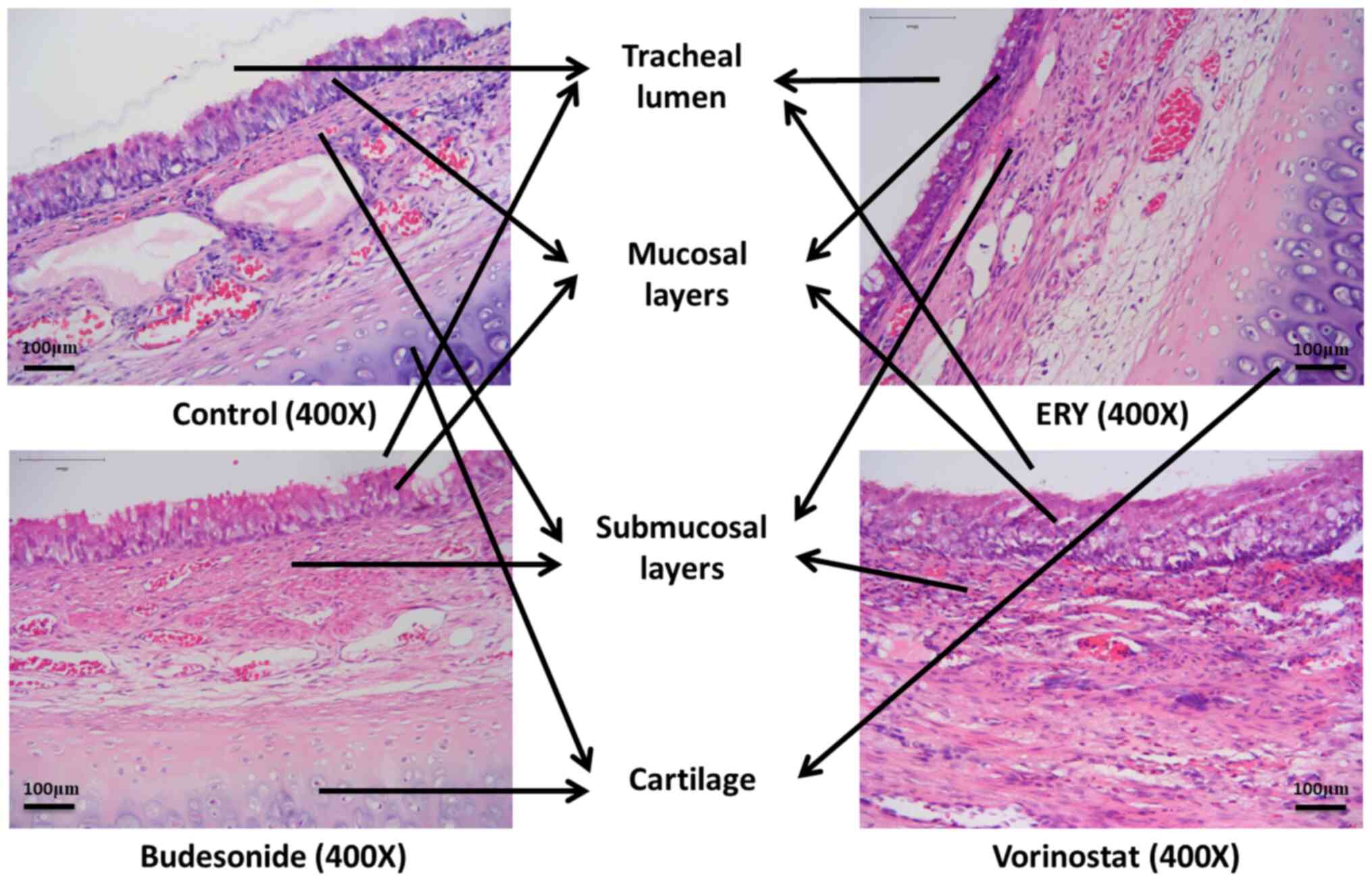
H&E staining results
The tracheal lumen of the control, the erythromycin,
the budesonide and the vorinostat group exhibited different degrees
of stenosis, tissue hyperplasia and mucosal epithelial hyperplasia
(Fig. 2). Compared with the control
group, mucosal epithelial hyperplasia in the erythromycin group was
substantially improved, exhibiting only mild hyperplasia, while
that in the budesonide group was only slightly improved. On the
contrary, mucosal epithelial hyperplasia in the vorinostat group
was substantially aggravated (Fig.
2).
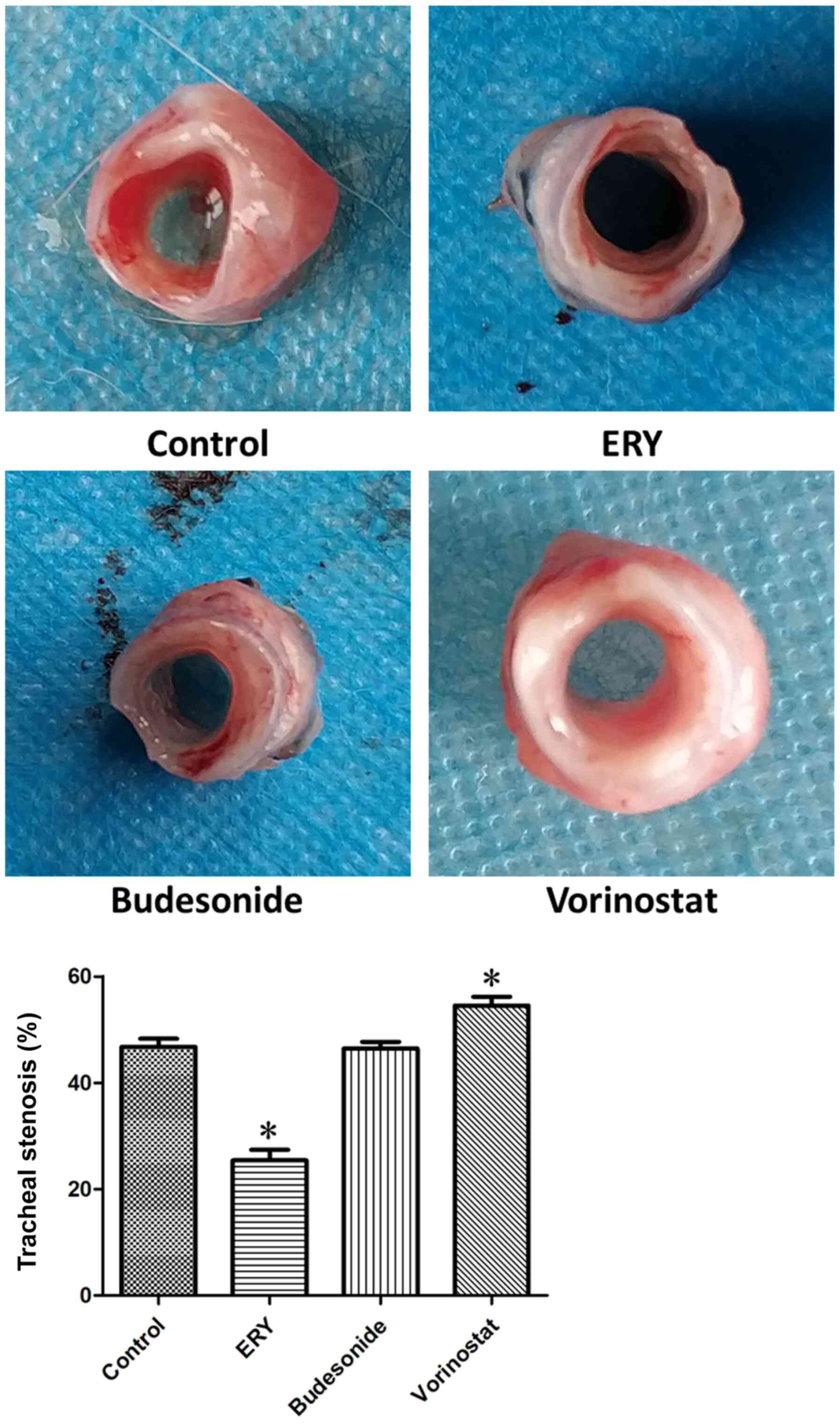
Detection of tracheal stenosis
As illustrated in Fig.
3, the degrees of tracheal stenosis in the control,
erythromycin, budesonide and vorinostat group were 46.76±3.71,
25.43±4.78, 46.45±2.97 and 54.48±4.09%, respectively. Compared with
the control group, tracheal stenosis in the erythromycin group was
reduced (P<0.05), while no difference was observed in in the
budesonide group. By contrast, tracheal stenosis was increased in
the vorinostat group (P<0.05).
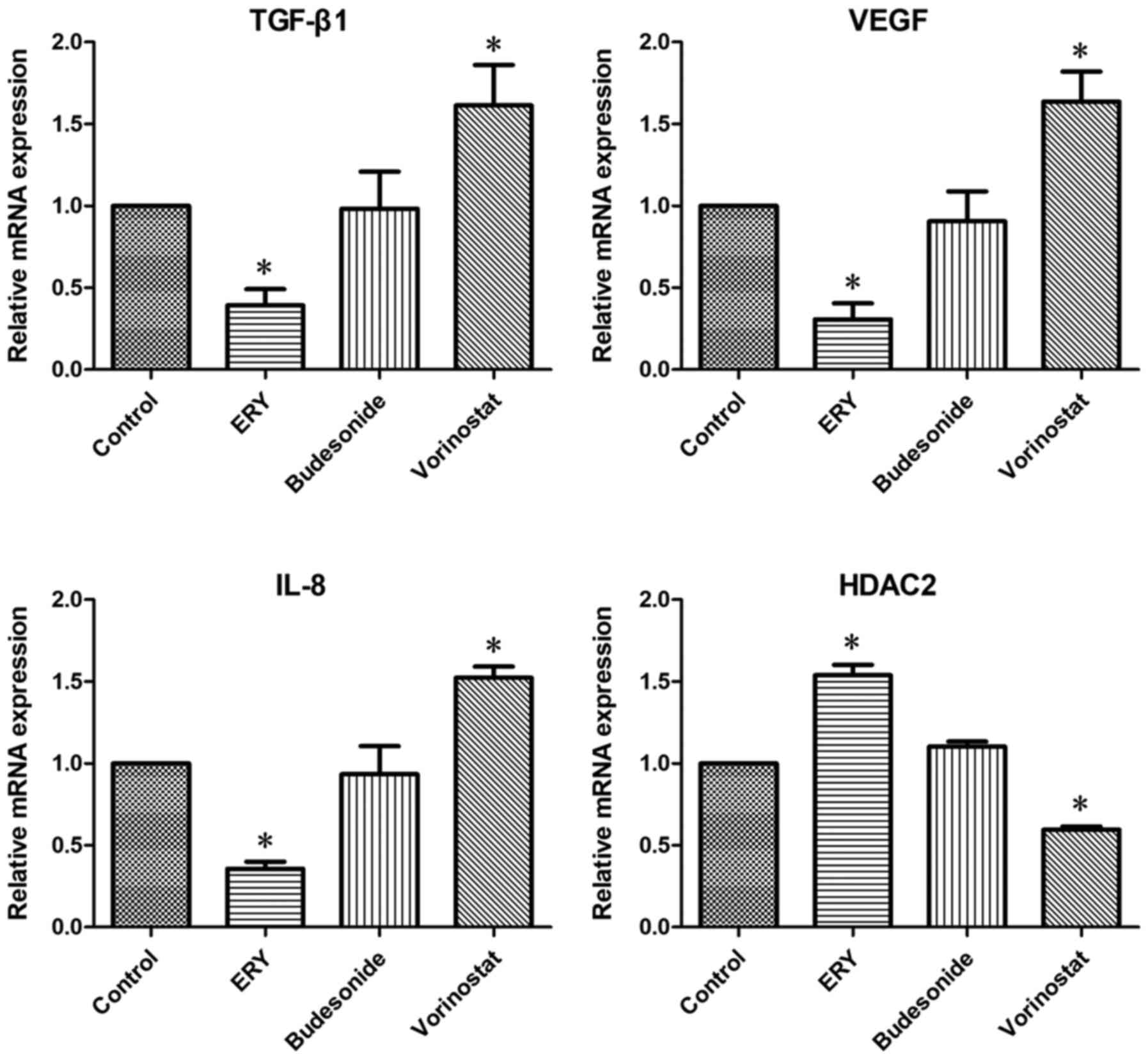
mRNA expression levels of TGF-β1,
VEGF, IL-8 and HDAC2
As demonstrated in Fig.
4, the mRNA expression of TGF-β1, VEGF and IL-8 in the
erythromycin group was decreased compared with that in the control
group, while the mRNA expression of HDAC2 was increased
(P<0.05). Compared with the control group, the mRNA expression
of TGF-β1, VEGF and IL-8 in the vorinostat group was increased,
while the mRNA expression of HDAC2 was decreased (P<0.05). No
difference was observed in the mRNA expression of TGF-β1, VEGF,
IL-8 and HDAC2 between the budesonide and the control group.
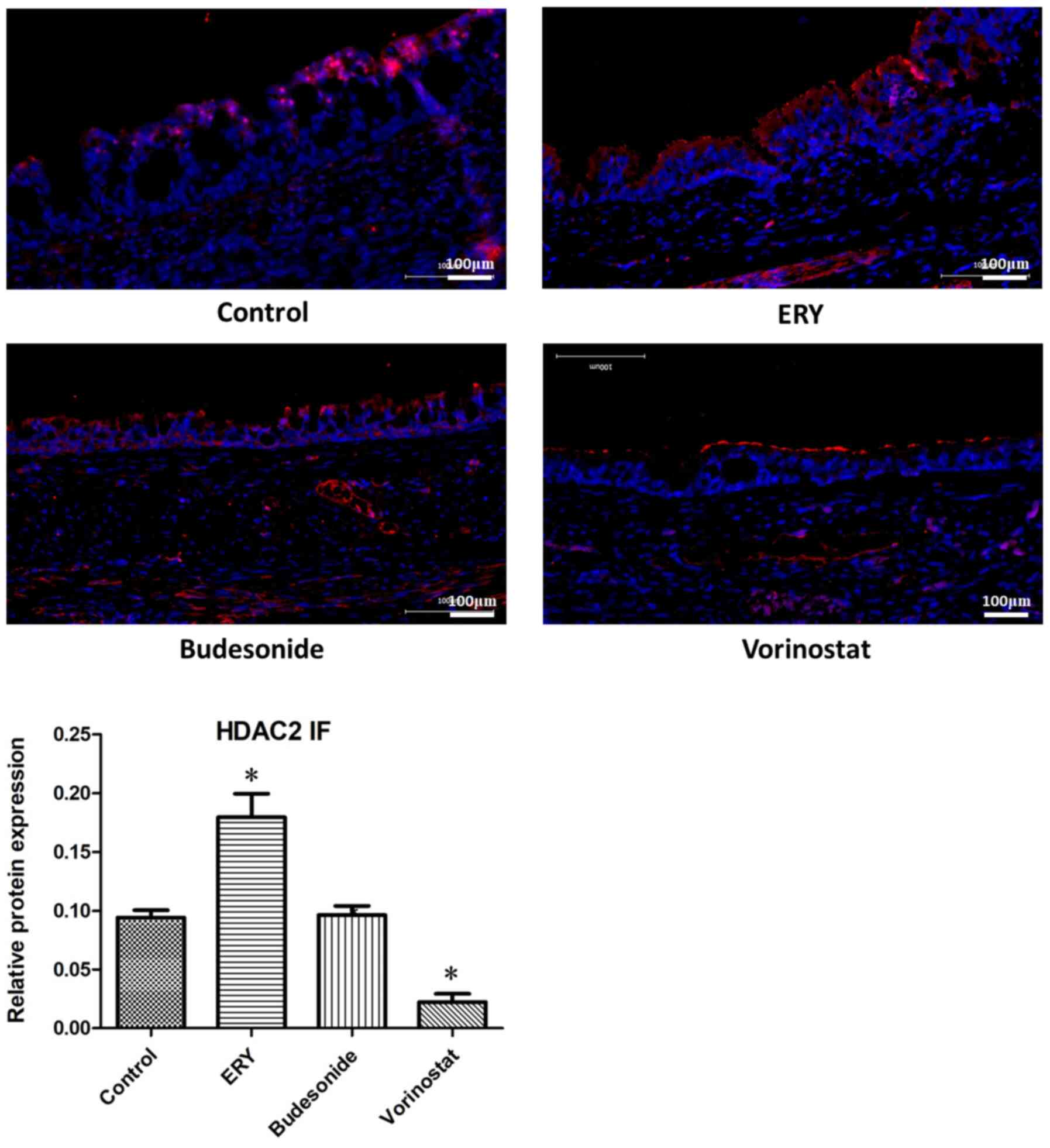
The protein expression levels of HDAC2
were examined via immunofluorescence
As indicated in Fig.
5, the protein expression of HDAC2 was increased in the
erythromycin and decreased in the vorinostat group, compared with
the control group (P<0.05). No difference was observed in HDAC2
protein expression between the budesonide and the control
group.
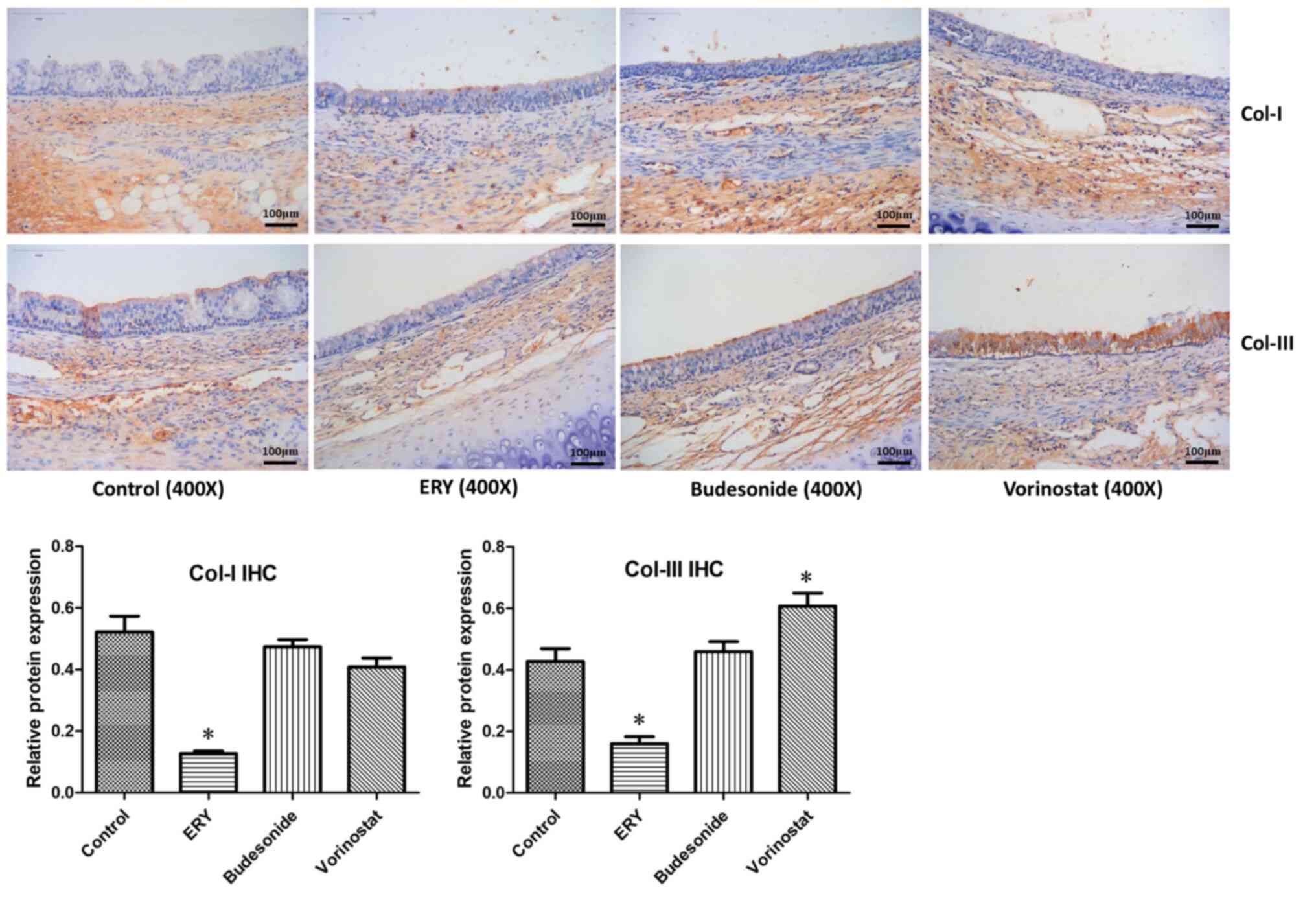
The protein expression levels of
collagen I and III were examined via immunohistochemistry
As illustrated in Fig.
6, the protein expression levels of collagen I and III in the
erythromycin group were decreased, while the expression level of
collagen III in the vorinostat group was increased compared with
that in the control group (P<0.05). Compared with the control
group, there was no significant change in the level of collagen I
expression in the vorinostat group. No difference was observed in
the protein expression of collagen I and III between the budesonide
and the control group.
Discussion
Benign tracheal stenosis mainly occurs in the
central airway or other bronchi and may affect the patient's
pulmonary ventilation function, resulting in obstructive pneumonia
at the distal end of the narrow airway and death due to severe
respiratory failure (1,24). The incidence of benign tracheal
stenosis increases annually in China (2). Tracheal intubation and tracheotomy are
the primary causes of benign tracheal stenosis (25,26).
Surgical treatments, such as segmental resection and end-to-end
anastomosis that are used to reconstruct the airway, have
previously been used to treat benign tracheal stenosis (24). With the evolution of interventional
pulmonology, bronchoscopy interventional therapy is gradually
becoming the principal method for the treatment of benign tracheal
stenosis, as it results in low degree of trauma, and rapid relief
of the patient's symptoms (1).
However, the occurrence of secondary trauma is still possible and
tracheal restenosis may occur (4).
Benign tracheal stenosis is intractable because of its complex
pathogenesis. In a multigenetic background, benign tracheal
stenosis may have a variety of causes, such as low immunity,
inflammation, cell proliferation, differentiation, apoptosis and
microvessel formation (27,28). The pathogenesis of benign tracheal
stenosis includes angiogenesis and remodeling following ischemia
and hypoxia of the tracheal mucosa, infection and inflammation,
production of proinflammatory and profibrotic cytokines involved in
repair processes, fibroblast cell proliferation and inhibition of
apoptosis, and extracellular matrix deposition caused by an
imbalance in collagen synthesis and degradation (27,29).
Therefore, the efficient control of the tracheal inflammatory
response via reducing or preventing the proliferation of
fibroblasts and the synthesis of collagen fibers is an important
component of the prevention and treatment of tracheal stenosis
after injury.
The activation and release of inflammatory cells and
mediators occur via the transcription and regulation of
inflammatory genes (6). Histone
acetylation and deacetylation function as ‘switches’ that regulate
the transcription of inflammatory genes (6). HATs acetylate histones, which result
in chromatin unwinding at specific sites, thereby increase the
binding of transcription factors and RNA polymerase to DNA and
promote inflammatory gene transcription, while HDACs remove the
acetyl groups from the histone tails and inhibit the transcription
of inflammatory genes (30,31). Endotracheal intubation or
tracheotomy may cause an airway inflammatory response that is
associated with changes in HDAC (mainly HDAC2) activity (19,32).
Distinct subtypes of HDACs catalyze different patterns of
deacetylation and regulate different genes, thereby generating
different effects (33). HDAC2
activity was revealed to be slightly reduced in bronchial biopsy
specimens and alveolar macrophages of patients with mild asthma,
but it was demonstrated to be increased in severe and smoking
asthma patients (34,35). Oxidative stress is gradually
aggravated during the onset of COPD, and inflammation is amplified
in acute COPD episodes that are induced by infection (36). It has been previously demonstrated
that the mRNA and protein expression of HDAC2 was decreased the
most among HDAC subtypes, and the activity of HDAC3 and HDAC5 were
reduced, while no substantial alteration was observed in the
activity of other HDAC subtypes (6). The expression of HDAC2 and
interleukin-17A (IL-17A) in the sputum and lung tissue samples from
patients with COPD is associated with a thickening of the bronchial
wall and collagen deposition (37).
Activation of HDAC2 and/or the inhibition of IL-17A expression was
had been revealed to prevent the development of airway remodelling
by inhibiting airway inflammation and modulating fibroblast
activation (37). Therefore, HDAC2,
among all HDACs, is most closely associated with chronic airway
inflammatory diseases.
Erythromycin is a macrolide antibiotic with strong
anti-inflammatory activities, immunomodulatory effects and
antibacterial effects (38). A
previous study demonstrated that erythromycin enhanced the activity
of HDAC2 in monocytes under oxidative stress, thereby reducing the
expression of NF-κB and IL-8(39).
Budesonide, which is widely used as an inhaled glucocorticoid in
bronchial asthma and COPD, regulates airway inflammation and
reduces airway hyperresponsiveness (40). The activated glucocorticoid receptor
recruits HDAC2 to the promoter region of the inflammatory gene,
which promotes the deacetylation of histones in the promoter and
inactivates the inflammatory gene (6). Subsequently, activated HDAC2
deacetylates and activates the glucocorticoid receptor, further
inhibiting the expression of inflammatory genes (8). Vorinostat is the first HDAC inhibitor
drug to be approved by the US Food and Drug Administration for the
treatment of cutaneous T-cell lymphoma with a significant
inhibitory effect on class I HDACs (17). In the present study, the mRNA and
protein expression levels of HDAC2 in the erythromycin group were
increased, the levels in the vorinostat group were decreased and
those in the budesonide group exhibited no difference, compared
with the control group of the tracheal stenosis model. The mRNA
expression of IL-8 in the erythromycin group was decreased and
expression in the vorinostat group was elevated, while there was no
substantial alteration in IL-8 mRNA expression in the budesonide
group. This finding suggests that erythromycin inhibited the
expression of the inflammatory factor IL-8 via upregulating HDAC2,
and vorinostat promoted the expression of the inflammatory factor
IL-8 by downregulating HDAC2.
Airway epithelial integrity and function are
impaired following airway trauma and/or infection, and decreased
HDAC2 activity promotes NF-κB transcription, resulting in an
enhanced activity of inflammatory factors, including IL-8 and tumor
necrosis factor-α (TNF-α) (11,41).
These inflammatory factors induce epithelial cells to release
TGF-β1, which is a potent extracellular matrix inducer that
functions as a chemoattractant for fibroblasts and
polymorphonuclear cells (42,43)
and a mitogen for fibroblasts (44). TGF-β1 induces the production of
downstream effectors, including VEGF, fibroblast growth factor and
connective tissue growth factor, stimulates local capillary
angiogenesis, formation and remodeling, and promotes a
proliferative response in epithelial cells and fibroblasts,
collagen synthesis and extracellular matrix deposition, resulting
in subepithelial fibrosis, tracheal granulation tissue
proliferation, thickening of the tracheal wall and enhanced airway
remodeling (19,45-47).
In the current study, the expression of TGF-β1, VEGF and type I and
III collagen was decreased in the erythromycin group, while the
expression of TGF-β1, VEGF and type III collagen was increased in
the vorinostat group and was not altered in the budesonide group,
compared with the control group. When the pathological
manifestations of tracheal stenosis were examined, tracheal
epithelial hyperplasia was improved, the degree of hyperplasia
being the lowest, and tracheal stenosis was reduced in the
erythromycin group compared with the control group, and in the
vorinostat group, tracheal epithelial hyperplasia was aggravated
and stenosis was increased, while no substantial alteration was
observed in the budesonide group.
It has been reported that IL-8 is an important
pro-inflammatory signal that promotes the migration of neutrophils
to the wound area at the early stage of wound healing, which alters
the local immune environment and initiates the granulation tissue
formation to repair the wound (48). A previous study on biomarkers
predicting tracheal stenosis, which was caused by metal stent
implantation, demonstrated that increased IL-8 expression levels
may predict the development of tracheal stenosis in rabbits
(10). IL-8 is a multifunctional
inflammatory factor, which is widely involved in acute and chronic
inflammation. It may promote fibroblast proliferation and collagen
synthesis, inhibit collagen fiber decomposition and facilitate the
deposition of extracellular matrix, the formation of granulation
tissue and fibrosis (49). In human
tracheobronchial epithelial cells, carbocisteine, which is a
mucoactive drug, reduced IL-8 expression by increasing HDAC2
activity (50). Erythromycin may
increase the activity of HDAC2 via inhibiting the PI3K/Akt pathway
and reducing the release of inflammatory factors, such as IL-8,
thereby increasing the anti-inflammatory activity of budesonide
(13). In human THP-1 cells
stimulated with lipopolysaccharide and primary human peripheral
blood mononuclear cells, pre-treatment with the HDAC inhibitor
trichostatin A or gene silencing of HDAC1 and HDAC2 inhibited the
anti-inflammatory effects of isoflurane and increased the
expression of TNF-α, IL-8 and interleukin-1β (51). These studies indicated that
increasing the expression/activity of HDAC2 reduces the release of
the inflammatory factor IL-8 and enhances the anti-inflammatory
effect, while inhibiting the expression/activity of HDAC2 increases
the release of the inflammatory factor IL-8 and aggravates the
inflammatory response (13,50,51). A
clinical study revealed that mechanical damage, which was triggered
via tracheal intubation induced an increased expression of TGF-β,
VEGF, and basic fibroblast growth factor, which resulted in
enhanced expression of collagen type I and III, tracheal
granulation hyperplasia and tracheal stenosis (52). Lee et al (45) demonstrated that the expression of
TGF-β1 and VEGF and the accumulation of fibroblasts was increased
in the tracheal granulation tissue, which was isolated via
interventional bronchoscopy after tracheostomy, while the
submucosal layer of the granulation tissue was thicker than the
epithelial layer. Low concentrations of erythromycin (1 mg/ml),
rather than dexamethasone, inhibited the TGF-β1-induced VEGF
production. In a study of a rat COPD model, carbocisteine
ameliorated the airway remodeling via increasing the HDAC2
expression/activity, including the airway epithelial and smooth
muscle thickness, airway fibrosis and the α-smooth muscle antibody
and TGF-β1 levels (53). Multiple
lines of evidence have suggested that erythromycin and
carbocisteine upregulate HDAC2 expression, reduce the release of
inflammatory factors, such as IL-8, to achieve anti-inflammatory
effects, decrease the expression of the fibrosis-related factors
TGF-β1, VEGF, type I and III collagen and inhibit airway fibrosis
(13,45,50,52,53).
In the present study, it was demonstrated that
erythromycin upregulated the expression of HDAC2, inhibited the
expression of inflammatory factors, such as IL-8, and reduced the
inflammatory response. Moreover, erythromycin inhibited the release
of TGF-β1 and VEGF in tracheal epithelial tissue, alleviated the
proliferation of epithelial cells and fibroblasts, reduced collagen
synthesis and local capillary formation, thereby inhibiting the
proliferation of tracheal granulation tissue and improving tracheal
stenosis development. In contrast, vorinostat downregulated the
expression of HDAC2, which aggravated the inflammatory response and
worsened tracheal stenosis. Budesonide did not have a prominent
regulatory effect on HDAC2 expression, and as a result no
improvement in benign tracheal stenosis was observed. The degree of
granulation tissue proliferation and tracheal stenosis was
associated with the level of HDAC2 expression.
In conclusion, the current study demonstrated that
the development of stenosis after tracheal injury differed
depending on the positive and negative regulation of HDAC2
expression in animal models. These results indicated that
regulating the expression of HDAC2 may become a novel potential
strategy for the treatment of benign tracheal stenosis, via
reducing the inflammatory response and inhibiting the proliferation
of tracheal granulation tissue. In previous studies, the
pathogenesis and treatment of benign tracheal stenosis was
investigated (19,39,54,55).
Due to the limited sample size of the current study, a preliminary
discussion of the aforementioned arguments were performed. In the
future, more diverse samples should be collected, including
tracheal tissue, serum, bronchoalveolar lavage fluid, and specific
HDAC2 agonists and targeted HDAC2 gene blocking methods should be
used. Future studies should also involve patient subjects and may
validate the aforementioned results from multiple perspectives,
such as screening for more efficient HDAC2 agonists and optimal
doses, and whether HDAC2 affects oxidative stress and immunity in
the treatment of benign tracheal stenosis.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Nature Science
Foundation of China (grant no. 81760001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZH and GL designed the experiments. ZH and PW
performed the experiments. ZH, PW, LG and WL recorded, classified,
and compiled the original experimental data. TZ, CQ and ZC analyzed
the data and ZH validated the analysis. ZH prepared the manuscript
and GL revised the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The current study was approved by the Animal
Experimental Ethics Committee of Guangxi Medical University
(approval no. 201806020).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Oberg CL, Holden VK and Channick CL:
Benign central airway obstruction. Semin Respir Crit Care Med.
39:731–746. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Su ZQ, Wei XQ, Zhong CH, Chen XB, Luo WZ,
Guo WL, Wang YZ and Li SY: The cause and efficacy of benign
tracheal stenosis. Zhonghua Jie He He Hu Xi Za Zhi. 36:651–654.
2013.PubMed/NCBI(In Chinese).
|
|
3
|
Zhang J, Wang J, Wang T, Xu M, Dang BW,
Pei YH and Zhang CY: A pilot study on interventional bronchoscopy
in the management of airway stenosis with benign hyperplasia.
Zhonghua Jie He He Hu Xi Za Zhi. 34:334–338. 2011.PubMed/NCBI(In Chinese).
|
|
4
|
Zhang J, Wang T, Wang J, Pei YH, Xu M,
Wang YL, Zhang X and Wang C: Effect of three interventional
bronchoscopic methods on tracheal stenosis and the formation of
granulation tissues in dogs. Chin Med J (Engl). 123:621–627.
2010.PubMed/NCBI
|
|
5
|
Tsakiridis K, Darwiche K, Visouli AN,
Zarogoulidis P, Machairiotis N, Christofis C, Stylianaki A,
Katsikogiannis N, Mpakas A, Courcoutsakis N and Zarogoulidis K:
Management of complex benign post-tracheostomy tracheal stenosis
with bronchoscopic insertion of silicon tracheal stents, in
patients with failed or contraindicated surgical reconstruction of
trachea. J Thorac Dis. 4 (Suppl 1):S32–S40. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Barnes PJ: Histone deacetylase-2 and
airway disease. Ther Adv Respir Dis. 3:235–243. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang Q, Zhao K, Shen Q, Han Y, Gu Y, Li
X, Zhao D, Liu Y, Wang C, Zhang X, et al: Tet2 is required to
resolve inflammation by recruiting Hdac2 to specifically repress
IL-6. Nature. 525:389–393. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ito K, Yamamura S, Essilfie-Quaye S, Cosio
B, Ito M, Barnes PJ and Adcock IM: Histone deacetylase 2-mediated
deacetylation of the glucocorticoid receptor enables NF-kappaB
suppression. J Exp Med. 203:7–13. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kim EY, Park YS, Shin JH, Cho YJ, Shin DH,
Yoon HK and Song HY: The effectiveness of erythromycin in reducing
stent-related tissue hyperplasia: An experimental study with a rat
esophageal model. Acta Radiol. 53:868–873. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Arellano-Orden E, Serrano C,
Montes-Worboys A, Sánchez-López V, Laborda A, Lostalé F, Lahuerta
C, Rodríguez-Panadero F and de Gregorio MÁ: Stent-induced tracheal
stenosis can be predicted by IL-8 expression in rabbits. Eur J Clin
Invest. 47:84–92. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Barnes PJ: Role of HDAC2 in the
pathophysiology of COPD. Annu Rev Physiol. 71:451–464.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ito K, Lim S, Caramori G, Chung KF, Barnes
PJ and Adcock IM: Cigarette smoking reduces histone deacetylase 2
expression, enhances cytokine expression, and inhibits
glucocorticoid actions in alveolar macrophages. FASEB J.
15:1110–1112. 2001.PubMed/NCBI
|
|
13
|
Miao L, Gao Z, Huang F, Huang S, Zhang R,
Ma D, Wu Q, Li F, Chen H and Wang J: Erythromycin enhances the
anti-inflammatory activity of budesonide in COPD rat model. Int J
Clin Exp Med. 8:22217–22226. 2015.PubMed/NCBI
|
|
14
|
Barnes PJ: Glucocorticosteroids. Handb Exp
Pharmacol. 237:93–115. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yu Q, Feng N, Hu Y, Luo F, Zhao W, Zhao W,
Liu Z, Li M, Xu L, Wu L and Liu Y: Suberoylanilide hydroxamic acid
(SAHA) alleviates the learning and memory impairment in rat
offspring caused by maternal sevoflurane exposure during late
gestation. J Toxicol Sci. 44:177–189. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen WY, Zhang H, Gatta E, Glover EJ,
Pandey SC and Lasek AW: The histone deacetylase inhibitor
suberoylanilide hydroxamic acid (SAHA) alleviates depression-like
behavior and normalizes epigenetic changes in the hippocampus
during ethanol withdrawal. Alcohol. 78:79–87. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mann BS, Johnson JR, Cohen MH, Justice R
and Pazdur R: FDA approval summary: Vorinostat for treatment of
advanced primary cutaneous T-cell lymphoma. Oncologist.
12:1247–1252. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kilkenny C, Browne WJ, Cuthill IC, Emerson
M and Altman DG: Improving bioscience research reporting: The
ARRIVE guidelines for reporting animal research. Plos Biol.
8(e1000412)2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li LH, Xu MP, Gan LM, Li Y, Liang YL, Li
WT, Qin EY, Gan JH and Liu GN: Effect of low dose erythromycin on
the proliferation of granulation tissue after tracheal injury.
Zhonghua Yi Xue Za Zhi. 97:777–781. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
20
|
Spiers DE and Candas V: Relationship of
skin surface area to body mass in the immature rat: A
reexamination. J Appl Physiol Respir Environ Exerc Physiol.
56:240–243. 1984.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nakagishi Y, Morimoto Y, Fujita M, Ozeki
Y, Maehara T and Kikuchi M: Rabbit model of airway stenosis induced
by scraping of the tracheal mucosa. Laryngoscope. 115:1087–1092.
2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lei H, Qian GS, Wu GM and Huang GJ:
Transfection of human beta defensin 2 gene into the lung by aerosol
inhalation: Experiment with rats. Zhonghua Yi Xue Za Zhi.
88:1425–1428. 2008.PubMed/NCBI(In Chinese).
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Stoelben E, Koryllos A, Beckers F and
Ludwig C: Benign stenosis of the trachea. Thorac Surg Clin.
24:59–65. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lorenz RR: Adult laryngotracheal stenosis:
Etiology and surgical management. Curr Opin Otolaryngol Head Neck
Surg. 11:467–472. 2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Stauffer JL, Olson DE and Petty TL:
Complications and consequences of endotracheal intubation and
tracheotomy. A prospective study of 150 critically ill adult
patients. Am J Med. 70:65–76. 1981.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Puyo CA and Dahms TE: Innate immunity
mediating inflammation secondary to endotracheal intubation. Arch
Otolaryngol Head Neck Surg. 138:854–858. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lawrence DA, Branson B, Oliva I and
Rubinowitz A: The wonderful world of the windpipe: A review of
central airway anatomy and pathology. Can Assoc Radiol J. 66:30–43.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang J, Li Q, Bai C, Han Y and Huang Y:
Inhalation of TGF-beta1 antibody: A new method to inhibit the
airway stenosis induced by the endobronchial tuberculosis. Med
Hypotheses. 73:1065–1066. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yao H and Rahman I: Current concepts on
oxidative/carbonyl stress, inflammation and epigenetics in
pathogenesis of chronic obstructive pulmonary disease. Toxicol Appl
Pharmacol. 254:72–85. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Adenuga D, Yao H, March TH, Seagrave J and
Rahman I: Histone deacetylase 2 is phosphorylated, ubiquitinated,
and degraded by cigarette smoke. Am J Respir Cell Mol Biol.
40:464–473. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Huang Z, Wei P, Gan L, Zeng T, Qin C and
Liu G: Role of erythromycin-regulated histone deacetylase-2 in
benign tracheal stenosis. Can Respir J.
2020(4213807)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Peterson CL: HDAC's at work: Everyone
doing their part. Mol Cell. 9:921–922. 2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bhavsar P, Hew M, Khorasani N, Torrego A,
Barnes PJ, Adcock I and Chung KF: Relative corticosteroid
insensitivity of alveolar macrophages in severe asthma compared
with non-severe asthma. Thorax. 63:784–790. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ito K, Caramori G, Lim S, Oates T, Chung
KF, Barnes PJ and Adcock IM: Expression and activity of histone
deacetylases in human asthmatic airways. Am J Respir Crit Care Med.
166:392–396. 2002.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bowler RP, Barnes PJ and Crapo JD: The
role of oxidative stress in chronic obstructive pulmonary disease.
COPD. 1:255–277. 2004.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lai T, Tian B, Cao C, Hu Y, Zhou J, Wang
Y, Wu Y, Li Z, Xu X, Zhang M, et al: HDAC2 suppresses
IL17A-mediated airway remodeling in human and experimental modeling
of COPD. Chest. 153:863–875. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Giamarellos-Bourboulis EJ: Macrolides
beyond the conventional antimicrobials: A class of potent
immunomodulators. Int J Antimicrob Agents. 31:12–20.
2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Li M, Zhong X, He Z, Wen M, Li J, Peng X,
Liu G, Deng J, Zhang J and Bai J: Effect of erythromycin on
cigarette-induced histone deacetylase protein expression and
nuclear factor-κB activity in human macrophages in vitro. Int
ImmunopharmacoL. 12:643–650. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Tashkin DP, Lipworth B and Brattsand R:
Correction to: Benefit: Risk profile of budesonide in obstructive
airways disease. Drugs. 79(1911)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Altenburg J, de Graaff CS, van der Werf TS
and Boersma WG: Immunomodulatory effects of macrolide antibiotics -
part 1: Biological mechanisms. Respiration. 81:67–74.
2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hannigan M, Zhan L, Ai Y and Huang CK: The
role of p38 MAP kinase in TGF-beta1-induced signal transduction in
human neutrophils. Biochem Biophys Res Commun. 246:55–58.
1998.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Postlethwaite AE, Keski-Oja J, Moses HL
and Kang AH: Stimulation of the chemotactic migration of human
fibroblasts by transforming growth factor beta. J Exp Med.
165:251–256. 1987.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Perng DW, Wu YC, Chang KT, Wu MT, Chiou
YC, Su KC, Perng RP and Lee YC: Leukotriene C4 induces TGF-beta1
production in airway epithelium via p38 kinase pathway. Am J Respir
Cell Mol Biol. 34:101–107. 2006.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lee YC, Hung MH, Liu LY, Chang KT, Chou
TY, Wang YC, Wu YC, Lai CL, Tsai CC, Su KC and Perng DW: The roles
of transforming growth factor-β1 and vascular endothelial growth
factor in the tracheal granulation formation. Pulm Pharmacol Ther.
24:23–31. 2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Bissonnette ÉY, Madore AM, Chakir J,
Laviolette M, Boulet LP, Hamid Q, Bergeron C, Maghni K and Laprise
C: Fibroblast growth factor-2 is a sputum remodeling biomarker of
severe asthma. J Asthma. 51:119–126. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wójcik-Pszczoła K, Jakieła B, Plutecka H,
Koczurkiewicz P, Madeja Z, Michalik M and Sanak M: Connective
tissue growth factor regulates transition of primary bronchial
fibroblasts to myofibroblasts in asthmatic subjects. Cytokine.
102:187–190. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ellis S, Lin EJ and Tartar D: Immunology
of wound healing. Curr Dermatol Rep. 7:350–358. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
David JM, Dominguez C, Hamilton DH and
Palena C: The IL-8/IL-8R axis: A double agent in tumor immune
resistance. Vaccines (Basel). 4(22)2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Song Y, Chi DY, Yu P, Lu JJ, Xu JR, Tan
PP, Wang B, Cui YY and Chen HZ: Carbocisteine improves histone
deacetylase 2 deacetylation activity via regulating sumoylation of
histone deacetylase 2 in human tracheobronchial epithelial cells.
Front Pharmacol. 10(166)2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Guo X, Deng J, Zheng B, Liu HM, Zhang Y,
Ying Y, Jia J and Ruan X: HDAC1 and HDAC2 regulate
anti-inflammatory effects of anesthetic isoflurane in human
monocytes. Immunol Cell Biol. 98:318–331. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Cai Z, Li H, Zhang H, Han S, An R and Yan
X: Novel insights into the role of hypoxiainducible factor1 in the
pathogenesis of human postintubation tracheal stenosis. Mol Med
Rep. 8:903–908. 2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Song Y, Yu P, Lu JJ, Lu HZ, Zhu L, Yu ZH,
Chen HZ and Cui YY: A mucoactive drug carbocisteine ameliorates
steroid resistance in rat COPD model. Pulm Pharmacol Ther.
39:38–47. 2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Enyuan Q, Mingpeng X, Luoman G, Jinghua G,
Yu L, Wentao L, Changchun H, Lihua L, Xiaoyan M, Lei Z and Guangnan
L: Erythromycin combined with corticosteroid reduced inflammation
and modified trauma-induced tracheal stenosis in a rabbit model.
Ther Adv Respir Dis. 12(1753466618773707)2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Liang YL, Liu GN, Zheng HW, Li Y, Chen LC,
Fu YY, Li WT, Huang SM and Yang ML: Management of benign tracheal
stenosis by small-diameter tube-assisted bronchoscopic balloon
dilatation. Chin Med J Engl. 128:1326–1330. 2015.PubMed/NCBI View Article : Google Scholar
|