Introduction
Heart failure (HF) refers to a syndrome in which
venous reflux is normal and cardiac output is reduced due to
primary cardiac damage, which cannot meet the requirements of
tissue metabolism. Pulmonary circulation and/or systemic
circulation congestion and inadequate tissue perfusion are the
major characteristics of clinical HF; thus, it is also called
congestive HF and is the final stage of heart disease due to
various causes (1). With an aging
population, HF-associated morbidity and mortality have exhibited an
upward trend in the United States and globally (2). It has been indicated that certain
patients with HF still maintained a relatively normal left
ventricular ejection fraction (LVEF), which is known as HF with
preserved ejection fraction (HFPEF) (3). Patients with HFPEF accounted for ~50%
of all cases of HF and their clinical prognosis is poor (4). Echocardiography is a non-invasive
examination method with simple operation, so it is widely adopted
in the clinic to assess heart function. However, HFPEF cannot
always be detected at the early stages, whilst early diagnosis is
important for saving the life of affected patients and easing the
disease burden. With the fast development in biomedical technology,
biomarker-based determination has received increasing attention.
Plasma pro-brain natriuretic peptide (BNP), a hormonal biologically
active substance secreted by left ventricular wall cells, is the
major serum marker to evaluate the severity of HF (5).
As a class of non-coding transcripts, long
non-coding RNAs (lncRNAs) are >200 nt in length and regulate
gene expression at epigenetic, transcriptional and
post-transcriptional levels (6,7).
Aberrant expression levels of various lncRNAs in the serum have
recently been utilized as diagnostic biomarkers, e.g. for cardiac
diseases (8). For instance,
long-intergenic noncoding RNA predicting cardiac remodeling was
able to predict survival in patients with HF (9). HOX transcript antisense RNA is an
essential mediator of acute myocardial infarction (10). Antisense non-coding RNA in the INK4
locus is of important diagnostic value for in-stent restenosis
(11). The lncRNA taurine
upregulated gene 1 (TUG1) is a newly discovered lncRNA, which was
originally detected in mouse retinal cells treated with taurine-β
(12). However, the molecular
function of TUG1 in patients with HFPEF and hypertension has
remained elusive. In the present study, the role of circulating
TUG1 in the diagnosis of HFPEF in subjects with hypertension was
investigated.
Materials and methods
Participants and samples
Between January 2017 and January 2019, 80
aged/elderly hypertensive patients (Table I) with HFPEF were recruited as the
‘observation group’ at The First Affiliated Hospital of Jinzhou
Medical University (Jinzhou, China). Written informed consent was
provided by all study participants. The present study was approved
by the ethics committee of The First Affiliated Hospital of Jinzhou
Medical University. The diagnostic criteria for HFPEF were
according to the European Society of Cardiology guidelines for the
diagnosis and treatment of acute and chronic HF from 2016(13): i) Signs or symptoms of HF; ii)
elevated levels of BNPs (BNP>35 pg/ml and/or NT-proBNP>125
pg/ml); iii) evidence of cardiac structural alterations (left
atrial volume index >34 ml/m2 or a left ventricular
mass index ≥115 g/m² for males and ≥95 g/m² for females) or
functional features of diastolic dysfunction [ratio of early
diastolic mitral flow velocity to early diastolic mitral ring
velocity (E/e') ≥13 and a mean E' septal and lateral wall <9
cm/sec]; iv) normal or slightly abnormal LVEF (>50%) and the
left ventricle was not enlarged; and v) left ventricular diastolic
dysfunction. The patients in the observation group were included if
they fulfilled the following criteria: i) Age, 60-75 years; ii)
systolic blood pressure >150 mmHg and normal diastolic blood
pressure; iii) conformed to the diagnostic criteria of HFPEF.
Patients were excluded under the following circumstances: i) LVEF
<50%; ii) chronic obstructive pulmonary disease, valvular heart
disease, hypertrophic cardiomyopathy, restrictive cardiomyopathy,
pericardial disease or diabetes mellitus; iii) hematological and
neoplastic disorders or autoimmune diseases; iv) severe liver or
kidney dysfunction. Furthermore, 80 aged/elderly hypertensive
patients (age, 60-75 years; systolic blood pressure >150 mmHg;
not combined with any other diseases) were enrolled as a control
group. Furthermore, according to the New York Heart Association
(NYHA) cardiac function classification (14), the patients in the observation group
were divided into four groups with NYHA I-IV, respectively
(Table II). After overnight
fasting, 5 ml peripheral blood samples were collected from the
brachial vein of each patient. Whole blood was centrifuged at 1,500
x g for 5 min at room temperature and the supernatant was
immediately frozen and stored at -80˚C for further analysis.
 | Table IClinical characteristics of patients
in the control and observation groups. |
Table I
Clinical characteristics of patients
in the control and observation groups.
| Variable | Control group
(n=80) | Observation group
(n=80) | P-value |
|---|
| Age (years) | 68.24±6.13 | 66.87±7.44 | 0.125 |
| Male sex (%) | 48 (60%) | 46 (57.5%) | 0.748 |
| BMI
(kg/m2) | 26.21±2.58 | 25.78±2.97 | 0.528 |
| Smoking (%) | 50 (62.5%) | 49 (61.25%) | 0.871 |
| History of
hypertension (years) | 10.11±2.81 | 11.36±1.44 | 0.215 |
| Blood glucose
(mmol/l) | 6.25±1.13 | 6.12±1.44 | 0.853 |
| Serum creatinine
(µmol/l) | 82.56±10.54 | 75.58±9.38 | 0.521 |
| Hyperlipemia (%) | 16 (25%) | 24 (30%) | 0.479 |
| NYHA | | | |
|
II | / | 34 | |
|
III | / | 26 | |
|
IV | / | 20 | |
 | Table IIEchocardiographic indexes in the
control and observation groups. |
Table II
Echocardiographic indexes in the
control and observation groups.
| Variable | Control group
(n=80) | Observation group
(n=80) | P-value |
|---|
| LVEDD (mm) | 50.56±10.52 | 51.32±9.78 | 0.425 |
| LAD (mm) | 38.56±7.63 | 48.21±5.96 | 0.001 |
| LVEF (%) | 0.64±0.08 | 0.63±0.05 | 0.358 |
| LVPWT (mm) | 9.26±1.38 | 9.19±1.71 | 0.481 |
| IVS (mm) | 9.87±1.85 | 9.17±1.31 | 0.412 |
| E/A | 1.67±0.81 | 0.89±0.47 | 0.001 |
Measurement of NT-proBNP
The concentration of NT-proBNP in the serum of each
participant was determined using an ELISA kit (cat. no. DY3604-05;
R&D Systems, Inc.) according to the manufacturer's protocol. A
microplate reader was used to determine the absorbance at 450
nm.
Assessment of cardiac function
The patients were kept in a left lateral position
for echocardiography examinations. M-mode echocardiography was used
for the determination of the left ventricular end diastolic
diameter (LVEDD), left atrial diameter (LAD), left ventricular
posterior wall thickness and interventricular septal thickness
(LVPWT), interventricular septal thickness (IVS). Pulse Doppler
echocardiography was adopted to measure the ratio of the peak flow
velocity in the early diastolic phase to the peak flow velocity in
the late diastolic phase (E/A).
Reverse transcription-quantitative
(RT-q)PCR analysis
Total RNA in the serum of all paticipants was
isolated using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) following the manufacturer's protocol.
Complementary DNA synthesis was performed using the TaqMan MicroRNA
RT kit (Applied Biosystems; Thermo Fisher Scientific, Inc.). Total
RNA was reverse transcribed into cDNA at 37˚C for 2 h, at 95˚C for
15 min and then was cooled on ice. Real-time PCR was performed
using SYBR® Premix Ex Taq™ II (Takara Bio, Inc.). The
relative expression was analyzed using the 2-ΔΔCq method
(15) with normalization to GAPDH.
The primer sequences used for qPCR were shown as follows: TUG1
forward, 5'-TAGCAGTTCCCCAATCCTTG-3' and reverse,
5'-CACAAATTCCCATCATTCCC-3' and GAPDH forward,
5'-GGGAGCCAAAAGGGTCAT-3' and reverse,
5'-GTGGTTTGAGGGCTCTTACTCCTT-3'. The qPCR thermocycling conditions
used were as follows: Initial denaturation at 95˚C for 10 min,
followed by denaturation at 94˚C for 15 sec, annealing at 59˚C for
30 sec and extension at 70˚C for 30 sec, for 40 cycles.
Statistical analysis
Statistical analyses were performed using SPSS
software (version no. 21.0; IBM Corp.). All measurement data were
expressed as the mean ± standard deviation. Differences were
calculated using Student's t-test or one-way ANOVA followed by
Tukey's post hoc test. The correlation analysis was performed using
Spearman's correlation method. The diagnostic efficacy of the
plasma indexes was determined using receiver operating
characteristics (ROC) curve analyses. P<0.05 was considered to
indicate statistical significance.
Results
NT-proBNP concentration is increased
in serum of hypertensive patients with HFPEF
The general demographic and clinicopathological data
of all patients are listed in Table
I. There were no significant differences in clinical materials
of these two groups including age, gender and some risk factors
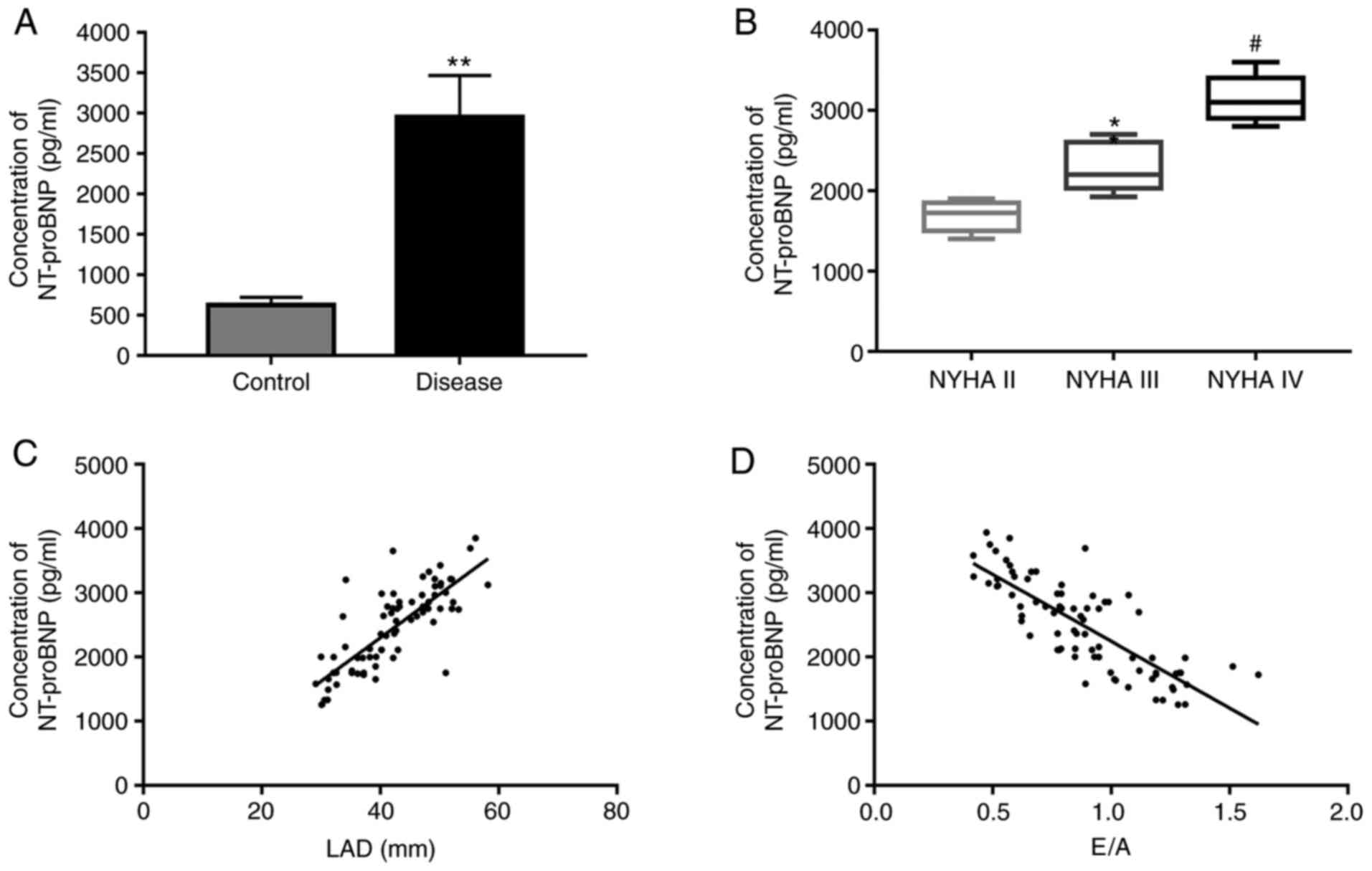
(P>0.05). First, the NT-proBNP concentration in the serum of
patients was measured using ELISA. The results demonstrated that
the NT-proBNP concentration was significantly upregulated in the
observation group as compared with that in the control group
(Fig. 1A). Furthermore, the
NT-proBNP concentration was significantly increased with the
severity degree of HF (Fig. 1B).
Spearman's rank correlation analysis demonstrated that the
NT-proBNP concentration was positively correlated with the LAD and
negatively correlated with the E/A (Fig. 1C and D, respectively).
Serum TUG1 is elevated in hypertensive
patients with HFPEF
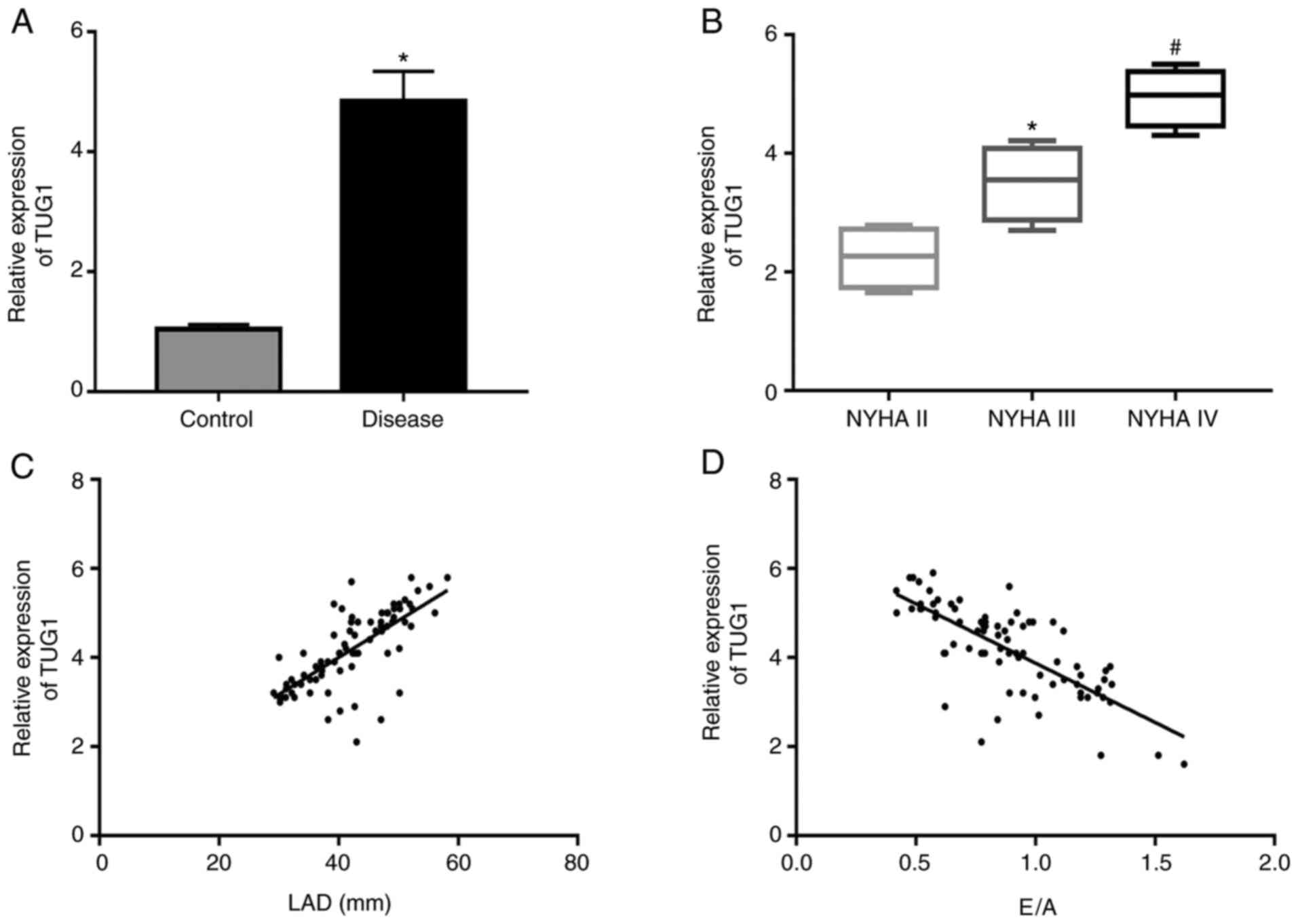
The levels of TUG1 in the serum of patients were
then assessed using RT-qPCR. The levels of TUG1 were significantly
enhanced in the observation group in comparison with those in the
control group (Fig. 2A).
Furthermore, TUG1 expression levels were significantly augmented
with the increase in the severity degree of HF (Fig. 2B). Spearman's correlation analysis
demonstrated that TUG1 was positively correlated with the LAD and
negatively correlated with the E/A (Fig. 2C and D, respectively).
TUG1 and NT-proBNP are useful
diagnostic biomarkers for HFPEF in a hypertensive population
A positive correlation between the serum levels of
TUG1 and NT-proBNP was confirmed (Fig.
3A). ROC curve analysis suggested that TUG1 and NT-proBNP are
useful biomarkers for the diagnosis of HFPEF among subjects with
hypertension, with area under the curve (AUC) values of 0.73 (95%
CI, 0.67-0.79; cut-off value, 0.43) and 0.76 (95% CI: 0.71-0.85;
cut-off value, 0.45). In addition, the combination of the two
parameters slightly improved the diagnostic power, with an AUC of
0.83 (95% CI, 0.74-0.89; cut-off value, 0.48).
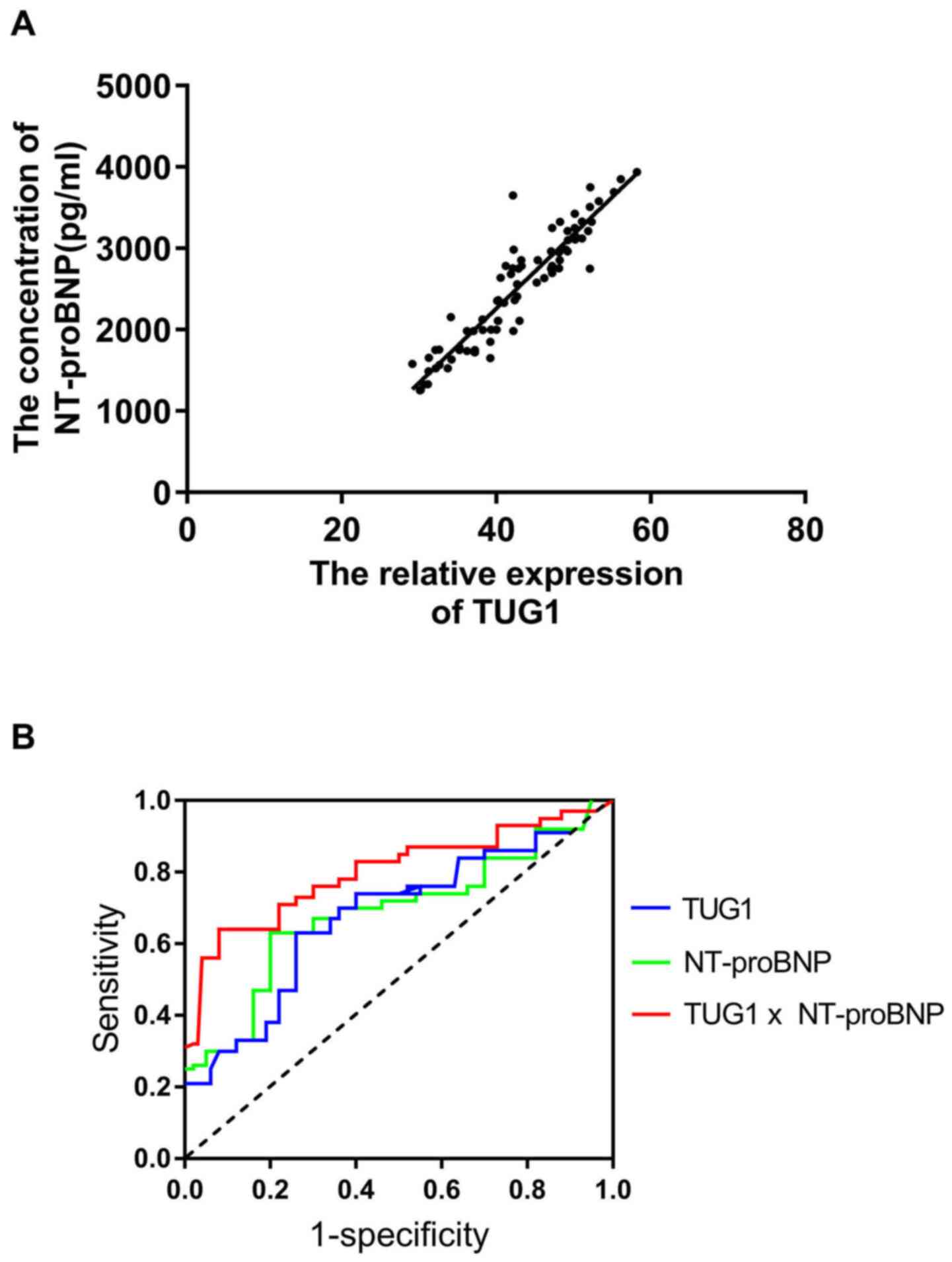 | Figure 3TUG1 and NT-proBNP may be useful
biomarkers for the diagnosis of HFPEF in a hypertensive population.
(A) A positive correlation was confirmed between the serum levels
of TUG1 and NT-proBNP in hypertensive patients with hypertension
and with heart failure with preserved ejection fraction (r=0.823,
P<0.05). (B) Receiver operating characteristic curves for
evaluating the ability of TUG1, NT-proBNP and their combination to
identify patients with heart failure with preserved ejection
fraction in a hypertensive population. TUG1 had an AUC value of
0.73 (95% CI, 0.67-0.79; cut-off value, 0.43), NT-proBNP had an AUC
value of 0.76 (95% CI, 0.71-0.85; cut-off value, 0.45) and the
combination of the two parameters had an AUC value of 0.83 (95% CI,
0.74-0.89; cut-off value, 0.48). HFPEF, heart failure with
preserved ejection fraction; NT-proBNP, N-terminal pro-brain
natriuretic peptide; TUG1, taurine upregulated 1; AUC, area under
the curve. |
Discussion
HFPEF is caused by decreased cardiac smooth muscle
relaxation during the process of left ventricular diastole. When
HFPEF occurs, the myocardial compliance is impaired and the
diastolic capacity of the ventricular muscle is weakened, resulting
in decreased filling function of the left ventricle during the
diastolic period and decreased output per stroke, which then leads
to an increase of the filling pressure at the end of the diastolic
period of the left ventricle, finally inducing HF (16). HFPEF has numerous causes and the
most common etiology is hypertension in the elderly (17). By determining the two-dimensional
ultrasonic diagnostic index, the size of each atrioventricular
cavity of the heart may be rapidly measured, which is helpful for
the diagnosis of HFPEF. Blood flow Doppler and tissue Doppler are
sensitive for the measurement of mitral valve diastole, systolic
blood flow velocity and mitral annulus root movement velocity
(18). In the present study, it was
observed that the LAD was significantly thicker in hypertensive
patients with HFPEF compared with that in the control group of
patients with hypertension. Furthermore, the E/A was markedly
decreased in these patients. NT-proBNP is a precursor of BNP, which
is mainly synthesized and secreted by ventricular myocytes. It may
reflect the functions of left ventricular systole and remodeling
and is of important diagnostic value for HF (19). The present results suggested that
the concentration of NT-proBNP was significantly elevated in
hypertensive patients with HFPEF compared with that in the control
group of patients with hypertension. The NYHA classification is the
basis for the classification of the severity of clinical HF and the
major criterion for judging the severity of the disease in the
clinic (20). The present results
indicated that NT-proBNP levels were increased with the severity
degree of HF. Furthermore, Spearman's correlation analysis
demonstrated the NT-proBNP concentration was positively correlated
with the LAD and negatively correlated with the E/A. These data
suggested that NT-proBNP may be used as one of the hematological
diagnostic indexes for hypertensive patients with HFPEF.
As a novel lncRNA, TUG1 has been reported to
participate in the development of cardiac diseases, including
cardiac hypertrophy (21), ischemic
myocardial injury (22) and
atherosclerosis (23). Therefore,
in the present study, the role of TUG1 in HF was investigated and
it was determined that the serum levels of TUG1 in hypertensive
patients with HFPEF were significantly higher than those in
hypertensive controls. In addition, TUG1 levels were positively
correlated with the LAD and negatively correlated with the E/A. A
positive correlation was confirmed between the serum levels of TUG1
and NT-proBNP. Furthermore, ROC curve analysis revealed that the
plasma levels of TUG1 and NT-proBNP are useful biomarkers for the
diagnosis of HFPEF among hypertensive subjects and the combination
of these two indexes slightly improved the diagnostic power.
In conclusion, the present study indicated that the
NT-proBNP concentration and TUG1 expression levels were increased
in the serum of hypertensive patients with HFPEF. Furthermore, TUG1
and NT-proBNP were determined to be useful plasma biomarkers for
the diagnosis of HFPEF among hypertensive subjects.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
RJ and BL performed the experiments. SZ designed
experiments analyzed the data and wrote the manuscript. RJ and SZ
can authenticate the raw data. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
Written informed consent was provided by all study
participants. The present study was approved by the ethics
committee of The First Affiliated Hospital of Jinzhou Medical
University (Jinzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Savarese G and Lund LH: Global public
health burden of heart failure. Card Fail Rev. 3:7–11.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Benjamin EJ, Muntner P, Alonso A,
Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR,
Cheng S, Das SR, et al: Heart disease and stroke statistics-2019
update: A report from the american heart association. Circulation.
139:e556–e528. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yancy CW, Jessup M, Bozkurt B, Butler J,
Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi
JL, et al: 2013 ACCF/AHA guideline for the management of heart
failure: A report of the American college of cardiology
foundation/American heart association task force on practice
guidelines. J Am Coll Cardiol. 62:e147–e239. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Owan TE, Hodge DO, Herges RM, Jacobsen SJ,
Roger VL and Redfield MM: Trends in prevalence and outcome of heart
failure with preserved ejection fraction. N Engl J Med.
355:251–259. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Paul B, Soon KH, Dunne J and De Pasquale
CG: Diagnostic and prognostic significance of plasma
N-terminal-pro-brain natriuretic peptide in decompensated heart
failure with preserved ejection fraction. Heart Lung Circ.
17:497–501. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Geisler S and Coller J: RNA in unexpected
places: Long non-coding RNA functions in diverse cellular contexts.
Nat Rev Mol Cell Biol. 14:699–712. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Mattick JS and Rinn JL: Discovery and
annotation of long noncoding RNAs. Nat Struct Mol Biol. 22:5–7.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shi Q and Yang X: Circulating MicroRNA and
long noncoding RNA as biomarkers of cardiovascular diseases. J Cell
Physiol. 231:751–755. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kumarswamy R, Bauters C, Volkmann I, Maury
F, Fetisch J, Holzmann A, Lemesle G, de Groote P, Pinet F and Thum
T: Circulating long noncoding RNA, LIPCAR, predicts survival in
patients with heart failure. Circ Res. 114:1569–1575.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gao L, Liu Y, Guo S, Yao R, Wu L, Xiao L,
Wang Z, Liu Y and Zhang Y: Circulating long noncoding RNA HOTAIR is
an essential mediator of acute myocardial infarction. Cell Physiol
Biochem. 44:1497–1508. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang F, Su X, Liu C, Wu M and Li B:
Prognostic value of plasma long noncoding RNA ANRIL for in-stent
restenosis. Med Sci Monit. 23:4733–4739. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Young TL, Matsuda T and Cepko CL: The
noncoding RNA taurine upregulated gene 1 is required for
differentiation of the murine retina. Curr Biol. 15:501–512.
2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ponikowski P, Voors AA, Anker SD, Bueno H,
Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP,
Jankowska EA, et al: 2016 ESC Guidelines for the diagnosis and
treatment of acute and chronic heart failure: The Task Force for
the diagnosis and treatment of acute and chronic heart failure of
the European Society of Cardiology (ESC)developed with the special
contribution of the heart failure association (HFA) of the ESC. Eur
Heart J. 37:2129–2200. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Elasfar A: Correlation between plasma
N-terminal pro-brain natriuretic peptide levels and changes in New
York Heart Association functional class, left atrial size, left
ventricular size and function after mitral and/or aortic valve
replacement. Ann Saudi Med. 32:469–472. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bhargava S, Ali A, Manocha A, Kankra M,
Das S and Srivastava LM: Homocysteine in occlusive vascular
disease: A risk marker or risk factor. Indian J Biochem Biophys.
49:414–420. 2012.PubMed/NCBI
|
|
17
|
Di Palo KE and Barone NJ: Hypertension and
heart failure: Prevention, targets, and treatment. Heart Fail Clin.
16:99–106. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fitzgibbons TP, Meyer TE and Aurigemma GP:
Mortality in diastolic heart failure: An update. Cardiol Rev.
17:51–55. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Negi SI, Jeong EM, Shukrullah I, Raicu M
and Dudley SC Jr: Association of low plasma adiponectin with early
diastolic dysfunction. Congest Heart Fail. 18:187–191.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Poss J, Link A and Bohm M: Acute and
chronic heart failure in light of the new ESC guidelines. Herz.
38:812–820. 2013.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
21
|
Zou X, Wang J, Tang L and Wen Q: LncRNA
TUG1 contributes to cardiac hypertrophy via regulating miR-29b-3p.
In Vitro Cell Dev Biol Anim. 55:482–490. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Su Q, Liu Y, Lv XW, Dai RX, Yang XH and
Kong BH: LncRNA TUG1 mediates ischemic myocardial injury by
targeting miR-132-3p/HDAC3 axis. Am J Physiol Heart Circ Physiol.
318:H332–H344. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li FP, Lin DQ and Gao LY: LncRNA TUG1
promotes proliferation of vascular smooth muscle cell and
atherosclerosis through regulating miRNA-21/PTEN axis. Eur Rev Med
Pharmacol Sci. 22:7439–7447. 2018.PubMed/NCBI View Article : Google Scholar
|