Introduction
Coronavirus disease 2019 (COVID-19) is a novel type
of respiratory pneumonia and persistent systemic illness caused by
the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
This disease has spread globally, and according to the COVID-19
Situation Report-73 from the World Health Organization, until April
2, 2020, there have been 896,450 confirmed cases and 45,542 deaths
globally (1). The rapid measures
taken by the Chinese government to control this outbreak have been
highly successful.
Studies investigating the epidemiological and
clinical characteristics indicate that the Chinese population are
susceptible to COVID-19, as reported in The Diagnosis and Treatment
of Coronavirus disease 2019 (Version 7) of National Health
Commission of the P.R. China (2,3). Its
transmission route is through the respiratory system and close
person-to-person contact, and it may involve aerosol dispersion;
the incubation period is 1-14 days (3). Fever and cough are the most common
symptoms, whereas hypertension and diabetes are the most prevalent
comorbidities in patients with COVID-19 infection (4,5). A
meta-analysis reported a fatality rate of 7% among COVID-19
patients, which is lower compared with that of the other two
coronavirus species, namely the severe acute respiratory syndrome
coronavirus (SARS-COV) and Middle East respiratory syndrome
coronavirus (MERS-COV), which have fatality rates of >10 and
35%, respectively (6). The high
death rate is due to the high proportion of severe cases resulting
in acute respiratory distress syndrome and multiorgan failure
(3,4).
Patients with severe COVID-19 have different
clinical characteristics and case fatality rates compared with
those with moderate infection. Laboratory medicine plays a key role
in clinical decision making in several other infectious diseases.
By contrast, there are no characteristic differences in laboratory
abnormalities between patients with moderate and those with severe
COVID-19. In the present study, laboratory results were assembled
through a systematic search. Information on the incidence of
increased or reduced white blood cell count, lymphocyte count,
C-reactive protein (CRP), procalcitonin (PCT), lactic dehydrogenase
(LDH) and aspartate aminotransferase (AST) levels was included. The
differences were analyzed, and the data were examined to
investigate whether the variations may play a role in
distinguishing patients with severe or moderate COVID-19 disease
for clinical intervention.
Data and methods
Literature search and selection
A systematic literature search was conducted on
studies published in online databases, including PubMed, Embase and
Cochrane Library, between December 2019 and May 2020. The keywords
included ‘Coronavirus Disease 2019’ OR ‘2019-nCoV’ OR ‘COVID-19’
AND ‘clinical characteristics’, without date or language
restrictions. EndNote X6.0 software (Thomson Reuters Corporation)
was used to manage the records and exclude duplicates. The
reference lists were also checked for other potentially eligible
studies to ensure the comprehensiveness of the research.
Severe disease was defined in this analysis
following the national treatment guideline of COVID-19 (in Chinese)
or according to the patient's need for admission to the intensive
care unit (ICU) (7). Thus, patients
in different studies were divided into severe and non-severe
groups, or ICU and non-ICU groups. The inclusion criteria were as
follows: i) Study population: Patients with diagnosed COVID-19. ii)
Study design: Case studies involving infected individuals with
severe and moderately severe symptoms. iii) Outcome measures: At
least one reported change in laboratory indicators among
leukocytosis, leukocytopenia, CRP, PCT, AST and LDH. The exclusion
criteria were as follows: i) Abstracts from conferences and
commentary articles; and ii) case reports, family-based studies and
solely pediatric cases.
Data extraction and quality
assessment
Evaluation of data extraction and quality of the
literature was conducted independently by two researchers (HY and
YYZ). Disagreements were resolved by consulting with a third
investigator (LLM) or by reaching consensus.
Although the Newcastle-Ottawa Scale (NOS) is the
most frequently reported tool for non-randomized studies (8), the methodological index for
non-randomized studies (MINORS) was selected to evaluate the risk
of bias in the present analysis (9). As it was difficult to evaluate the
exposure factors in the studies included in this analysis, except
for the items related to exposure factors, the remaining NOS items
were similar to the MINORS items. The ideal global score of MINORS
would be 16 for non-comparative studies. Article quality was
assessed as follows: Low quality=0-8; moderate quality=9-12; and
high quality=13-16.
Statistical analysis
Studies were divided into two separate groups for
analysis, namely severe and moderate groups. The meta-analysis was
conducted by calculating the individual and pooled odds ratios
(ORs) with their relative 95% confidence intervals (95% CIs) by
Review Manager 5.3 (Copenhagen: The Nordic Cochrane Centre, The
Cochrane Collaboration, 2014). Heterogeneity among studies was
tested using Cochran's χ2 test and I2; when
I2<50%, a fixed-effects model was used; by contrast,
when I2>50%, a random-effects model was selected.
Since approaches to detecting publication bias would have exhibited
limited efficacy, publication bias was not assessed in the present
report, as only a limited number of studies were included.
Results
Study selection
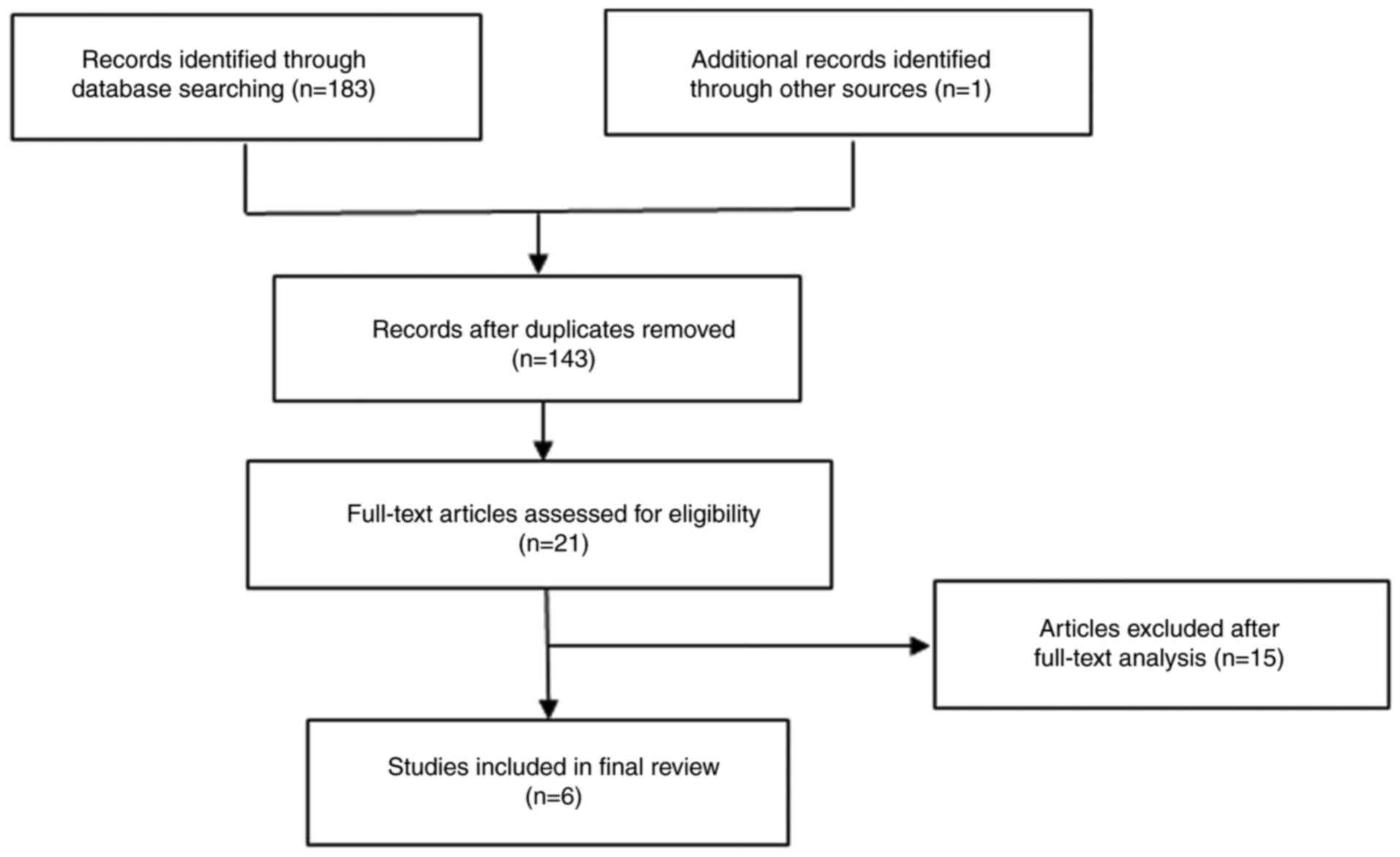
A total of 184 articles were compiled from the
online databases. Following the deletion of duplicate records, 143
records were retained. The titles and abstracts were checked and
the full texts were reviewed. A total of 6 studies (10-15)
eventually met the eligibility criteria and were processed for
inclusion in the final meta-analysis. Although there were no ethnic
restrictions, the relevant studies retrieved during the literature
search between December 2019 and May 2020 were all published in
China; thus, all the studies covered in our analysis are in
Chinese. An outline of the literature search process is shown in
Fig. 1.
All the selected articles were assessed for
methodological quality. The quality score of each study is
presented in Table I. Of the 6
studies, 3 were of high quality and 3 were of moderate quality. The
characteristics of the included studies are summarized in Table II.
 | Table IBias risk assessment. |
Table I
Bias risk assessment.
| Study (Refs.) | Clearly stated
aim | Inclusion of
consecutive patients | Prospective
collection of data | Endpoints appropriate
for study aim | Unbiased assessment
of study endpoint | Follow-up appropriate
for study aim | Loss to follow-up
<5% | Prospective
calculation of study size | Score |
|---|
| Chen et al
(10) | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 12 |
| Guan et al
(11) | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 10 |
| Huang et al
(12) | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 13 |
| Wang et al
(13) | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 13 |
| Zhang et al
(14) | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 0 | 12 |
| Zhang et al
(15) | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 13 |
 | Table IICharacteristics of the included
studies. |
Table II
Characteristics of the included
studies.
| Study (Refs.) | Country | No. of patients
(severe/moderate) | Sex (male/
female) | Mean age (years) | Study design | Laboratory
results |
|---|
| Chen et al
(10) | China | 21 (11/10) | 17/4 | 56 | Retrospective | Leukocytosis,
lymphocytopenia, increased CRP, PCT and LDH |
| Guan et al
(11) | China | 1099 (173/926) | 637/459 | 47 | Retrospective | Leukocytosis,
lymphocytopenia, increased CRP, PCT, LDH and AST |
| Huang et al
(12) | China | 41 (13/28) | 30/11 | 49 | Retrospective | Increased PCT |
| Wang et al
(13) | China | 138 (36/102) | 75/63 | 52 | Retrospective | Leukocytosis,
lymphocytopenia, increased CRP, PCT, LDH and AST |
| Zhang et al
(14) | China | 140 (58/82) | 71/69 | 57 | Retrospective | Leukocytosis,
lymphocytopenia, increased CRP and PCT |
| Zhang et al
(15) | China | 95 (32/63) | 53/42 | 49 | Retrospective | Leukocytosis,
lymphocytopenia, increased CRP and AST |
Incidence of leukocytosis,
lymphocytopenia, increased PCT and CRP levels
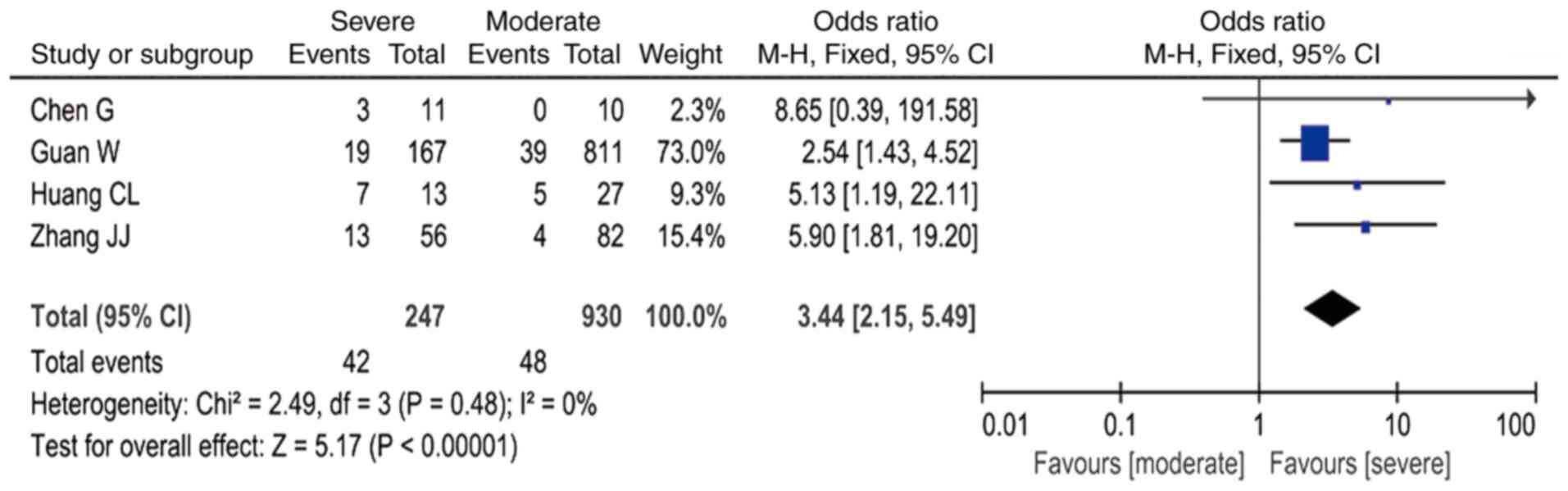
Through meta-analysis, it was established that,
among all the laboratory indicators in patients with severe
COVID-19, when compared with infected patients with moderate
disease, the presence of leukocytosis was associated with a ~3-fold
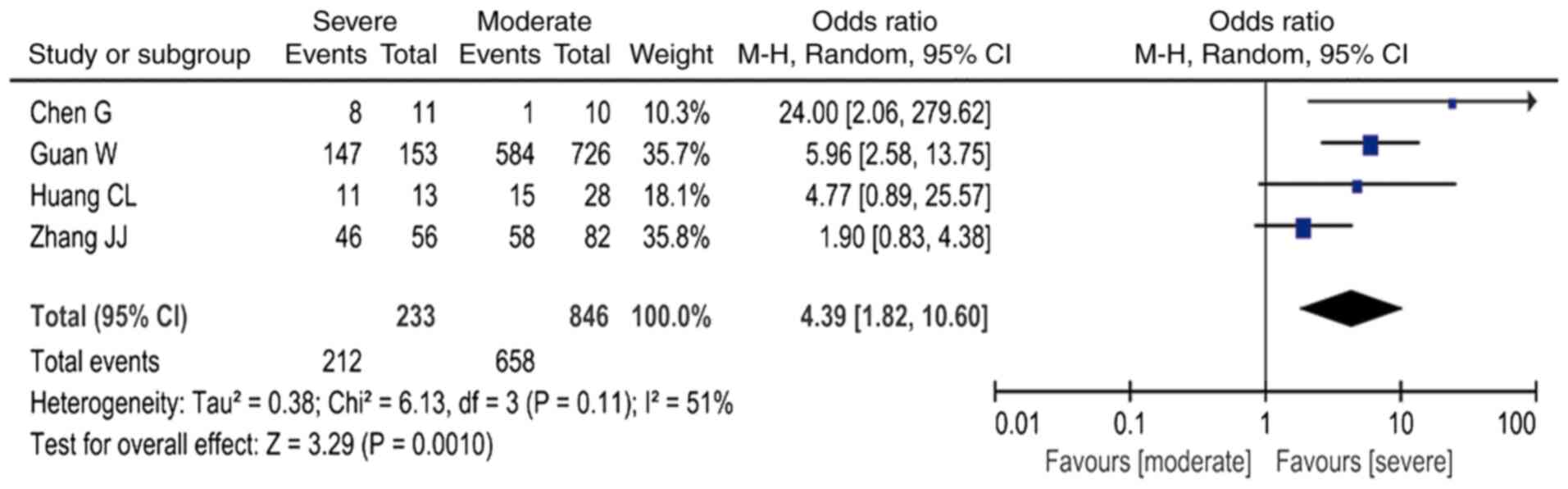
higher risk (OR=3.44; 95% CI: 2.15-5.49, I2=0%; Fig. 2); the presence of lymphocytopenia
was associated with a ~4-fold higher risk (OR=4.39; 95% CI:
1.82-10.60, I2=51%; Fig.
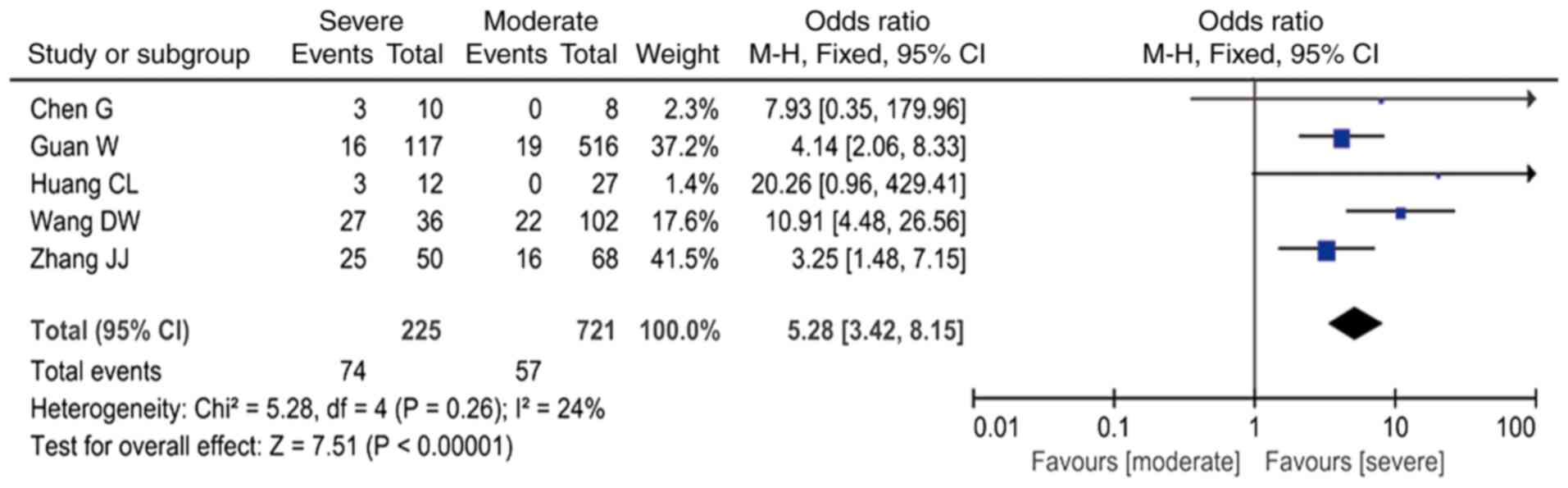
3); increased PCT was associated with an ~5-fold higher risk
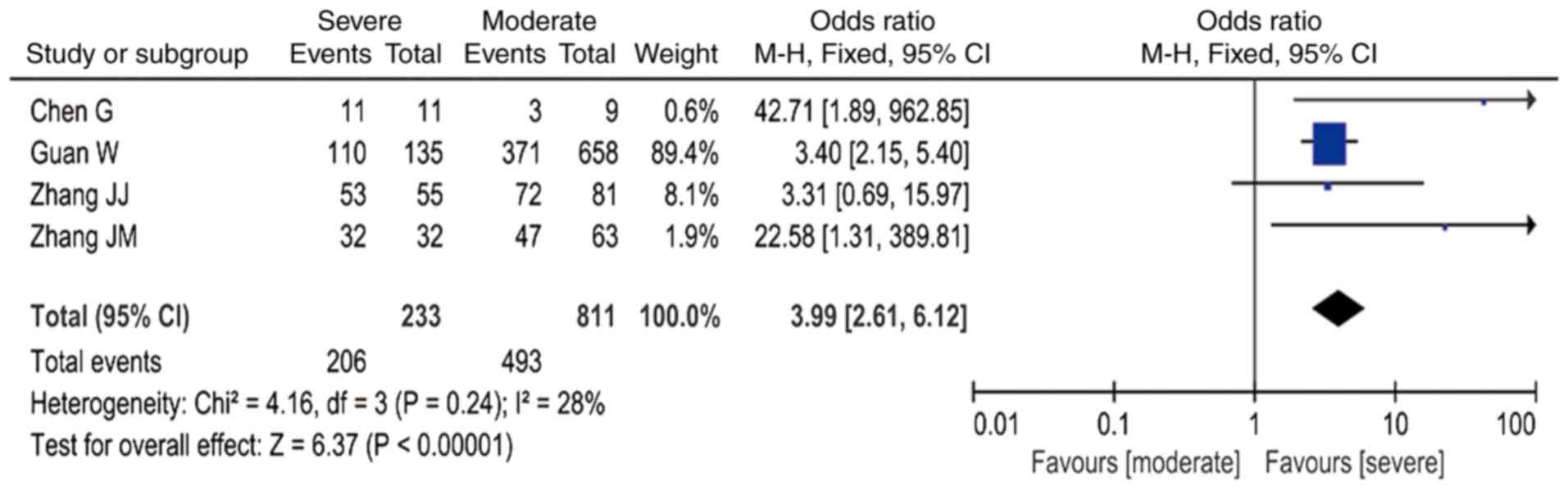
(OR=5.28; 95% CI: 3.42-8.15 I2=24%; Fig. 4); and increased CRP was associated
with a ~4-fold higher risk (OR=3.99; 95% CI: 2.61-6.12,
I2=28%; Fig. 5 and
Table III).
 | Table IIIMeta-analysis results. |
Table III
Meta-analysis results.
| Laboratory
results | OR, 95% CI | I2 |
|---|
| Leukocytosis | 3.44,
2.15-5.49 | 0% |
|
Lymphocytopenia | 4.39,
1.82-10.60 | 51% |
| Increased PCT | 5.28,
3.42-8.15 | 24% |
| Increased CRP | 3.99,
2.61-6.12 | 28% |
| Increased LDH | 8.33,
1.75-39.69 | 69% |
| Increased AST | 3.02,
2.13-4.26 | 0% |
Incidence of increased LDH and
AST
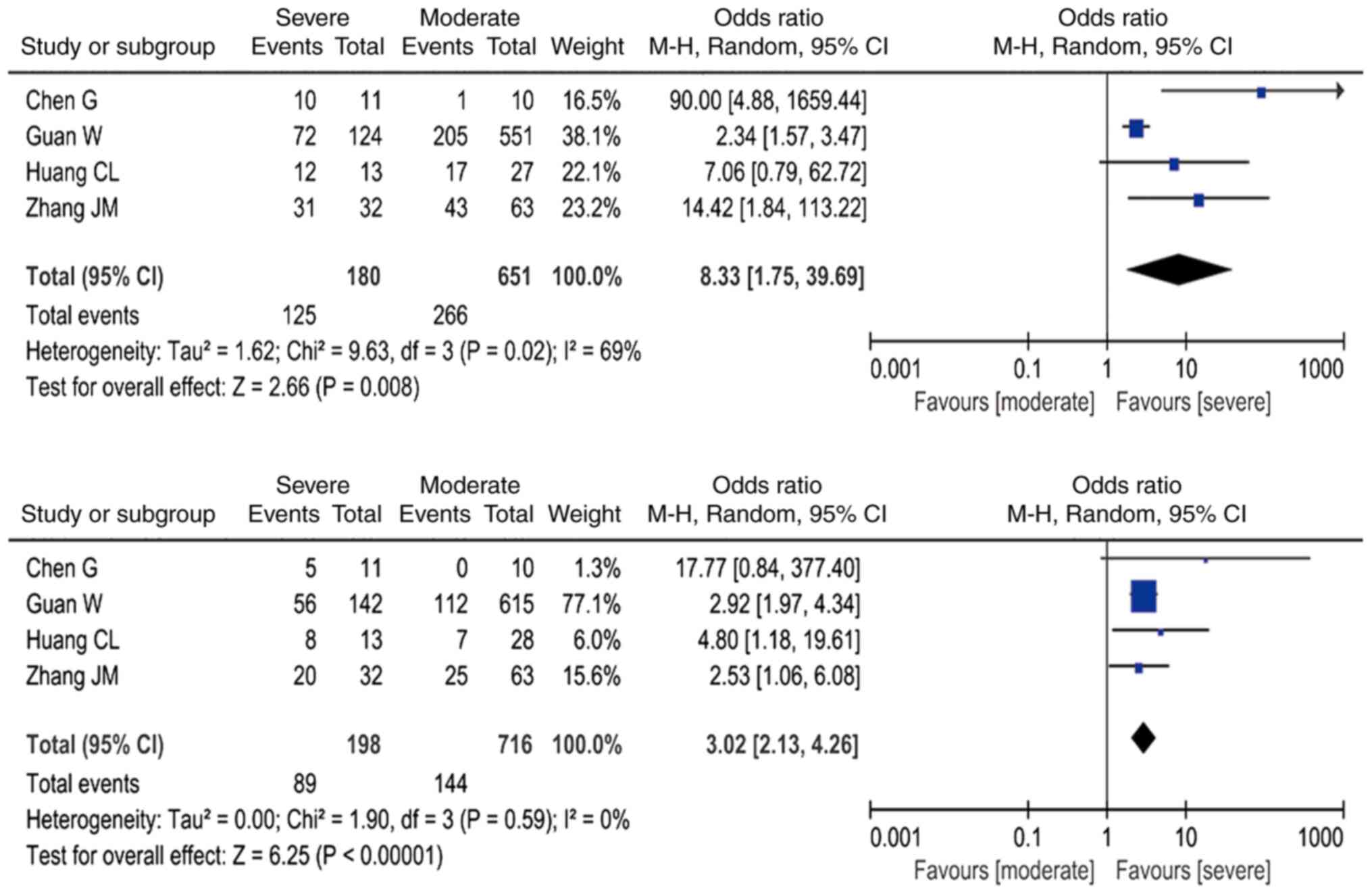
The meta-analysis also revealed that, compared with
patients with moderate COVID-19, the presence of increased AST
levels was associated with a ~3-fold higher risk of severe COVID-19
(OR=3.02; 95% CI: 2.13-4.26, I2=0%; Fig. 6A). Furthermore, the presence of
increased LDH levels was associated with an 8-fold higher risk of
severe COVID-19 (OR=8.33; 95% CI: 1.75-39.69), but this result must
be interpreted with caution due to the high heterogeneity
(I2=69%; Fig. 6B and
Table III).
Discussion
SARS-CoV-2, which has a single-strand,
positive-sense RNA genome, is a novel Betacoronavirus infecting
humans (16). SARS and MERS are
also notable exceptions among coronaviruses, as those that are
pathogenic to humans are generally associated with mild clinical
symptoms (17). Between December
2019 and May 2020, there was a near exponential growth in the
number of new cases of SARS-CoV-2 infection, reaching numerous
countries globally. The outbreak of COVID-19 has put health
authorities worldwide on high alert. At present, all confirmed
cases of COVID-19 are diagnosed based on i) clinical manifestations
and abnormal findings of chest X-ray or computed tomography and ii)
a positive result on the reverse transcription-PCR assay on nasal
and pharyngeal swab specimens (3,15).
Different clinical characteristics have been reported between
patients with moderate and severe COVID-19(10); however, characteristic differential
laboratory abnormalities have yet to be identified. Laboratory
diagnostics may contribute to assessing disease severity or
predicting prognosis (18). In the
present study, laboratory results were collected to investigate the
differences that may help distinguish patients with moderate from
those with severe COVID-19.
The present meta-analysis revealed that, when
compared with patients with moderate disease severity, the presence
of leukocytosis, lymphocytopenia, increased PCT and CRP levels was
associated with a 3-, 4-, 5- and 4-fold higher risk of severe
COVID-19, respectively. Lippi and Plebani (19) revealed that the white blood cell
count, CRP and PCT levels increased, whereas the lymphocyte count
decreased in patients with COVID-19. A further meta-analysis
demonstrated that lymphocytopenia and an increase in CRP levels
were more common among patients with COVID-19(6). Rodriguez-Morales et al
(20) also reported elevated levels
of inflammatory markers, such as CRP and LDH, in patients with
COVID-19. The results of the present study demonstrated that the
incidence of elevated inflammatory markers also differed between
moderate and severe COVID-19 cases. Thus, a higher risk of
occurrence of leukocytosis, lymphocytopenia, and increased PCT and
CRP levels in this study was correlated with the severity of
COVID-19. There remains a question whether the leukocytosis,
lymphocytopenia, increased PCT and increased CRP levels are the
results or causative factors of the COVID-19 infection, which
cannot be determined from our results. These factors may be
essential severity criteria, similar to a biomarker, as well as
possible targets for intervention to minimize the risk of severe
cases.
The mechanisms underlying leukocytosis,
lymphocytopenia, and increased PCT and CRP levels in severe cases
remain undetermined, but they may be associated with host immune
responses during virus infection. Airborne SARS-CoV-2 may enter the
peripheral blood from the lungs, leading to infection of
angiotensin-converting enzyme 2-expressing target cells, such as in
the lungs, heart, kidneys, gastrointestinal tract, and other
unknown target organs (21).
Stimulating the innate immune system by pathogen-associated
molecular patterns may trigger an antiviral response, leading to
activation of several signaling pathways and, ultimately,
transcription factors, such as nuclear factor-κB, activator protein
1, interferon response factor (IRF)3 and IRF7, accompanied by their
nuclear translocation. Simultaneously, the humoral immune response
also plays a protective role, as B cells or plasma cells produce
specific antibodies to help neutralize viruses, whereas the T-cell
responses are aimed at the recognition and killing of infected
cells (22,23).
During the host immune responses, the recruitment of
neutrophils occurs through chemotaxis of pro-inflammatory
cytokines, and lymphocyte reduction occurs via different
mechanisms, including i) apoptosis or impairment of lymphocytes and
ii) bone marrow suppression during a cytokine storm (22,23).
Accumulating evidence suggests that the immune system is impaired
during the period of disease that allows the development of viral
hyperinflammation (10,24). A limited number of studies revealed
that severe COVID-19 cases had a relatively distinct profile of
decreased memory T cells and cytotoxic CD8+ T cells
(24). It was also demonstrated
that severe COVID-19 may induce a cytokine storm and lymphocyte
damage, as well as suppression of interferon-γ production (24). The numbers of T cells and B cells
were further reduced, while the levels of inflammatory cytokines
continued to increase in patients with severe disease (21). Based on the aforementioned results,
leukocytosis, lymphocytopenia, and increased PCT and CRP levels may
serve as predictive biomarkers for COVID-19 severity.
The results of the present study also demonstrated
that, when compared with patients with moderate symptoms, increased
LDH and AST levels were more common in patients with severe
COVID-19, suggesting that abnormalities in liver function may be
present in severe cases; however, Zhang et al (25) did not consider the impairment of
liver function to be a prominent characteristic of COVID-19, or to
have serious clinical implications. Severe COVID-19 may induce a
cytokine storm, and the activated immunity and excessive
inflammation are always accompanied by liver tissue injury and
liver dysfunction (26); therefore,
the liver function of patients with COVID-19 should be carefully
monitored, particularly in severe cases.
There were several limitations to the present
meta-analysis: i) There were differences in the standards of
laboratory results, which depends on the laboratory instruments and
testing methods; ii) the number of studies available for inclusion
in the sensitivity analysis was limited, resulting in data with
considerable heterogeneity that may be inaccurate; and iii) all
studies included in the present analysis are retrospective, and the
majority of the patients are Chinese. Additional studies of high
quality, over a broader geographic scope, should be included to
ensure proper clinical intervention.
In conclusion, the incidence of leukocytosis,
lymphocytopenia, and elevated CRP, PCT, AST and LDH levels, was
increased among patients with severe COVID-19 when compared with
patients with moderately severe disease. Serial white blood cell
count, lymphocyte count, CRP, PCT, LDH and AST measurements may
prove crucial for predicting the progression towards a more severe
form of the disease. Inflammatory markers and liver function
parameters should be closely monitored in patients with COVID-19,
particularly those with severe disease.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Ningbo Medical Science and
Technology Plan (grant no. 2016A31).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
Study design: YH and YZ; data collection: YH, LM and
YZ; data analysis and writing of the manuscript: YH, LM and YZ. All
the authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization. Coronavirus
disease 2019 (COVID-19): Situation report,73. Available from:
https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/.
Accessed, April 2, 2020.
|
|
2
|
Li Q, Guan X, Wu P, Wang X, Zhou L, Tong
Y, Ren R, Leung KSM, Lau EHY, Wong JY, et al: Early transmission
dynamics in Wuhan, China, of novel coronavirus-infected pneumonia.
N Engl J Med. 382:1199–1207. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
The diagnosis and treatment of Coronavirus
disease 2019 (version 7). Available from: http://www.cac.gov.cn/.
|
|
4
|
Sun P, Qie S, Liu Z, Ren J, Li K and Xi J:
Clinical characteristics of hospitalized patients with SARS-CoV-2
infection: A single arm meta-analysis. J Med Virol. 92:612–617.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo
Q, Ji R, Wang H, Wang Y and Zhou Y: Prevalence of comorbidities in
the novel Wuhan coronavirus (COVID-19) infection: A systematic
review and meta-analysis. Int J Infect Dis.
4023:S1201–9712(20)30136-3. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y,
Huang TB, Zhang HY, Sun WM and Wang YP: 2019 novel coronavirus
patients' clinical characteristics, discharge rate, and fatality
rate of meta-analysis. J Med Virol. 577–583. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
National Health Commission & State
Administration of Traditional Chinese Medicin. Diagnosis and
Treatment Protocol for COVID-19 (trial version 7). Updated:
2020-03-29. Available from: http://en.nhc.gov.cn/2020-03/29/c_78469.htm.
|
|
8
|
Farrah K, Young K, Tunis MC and Zhao L:
Risk of bias tools in systematic reviews of health interventions:
An analysis of PROSPERO-registered protocols. Syst Rev.
8(280)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Slim K, Nini E, Forestier D, Kwiatkowski
F, Panis Y and Chipponi J: Methodological index for non-randomized
studies (minors): Development and validation of a new instrument.
ANZ J Surg. 73:712–716. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen G, Wu D, Guo W, Cao Y, Huang D, Wang
H, Wang T, Zhang X, Chen H, Yu H, et al: Clinical and immunological
features of severe and moderate coronavirus disease 2019. J Clin
Invest. 130:2620–2629. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He
JX, Liu L, Shan H, Lei CL, Hui DSC, et al: Clinical characteristics
of coronavirus disease 2019 in China. N Engl J Med. 382:1708–1720.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu
Y, Zhang L, Fan G, Xu J, Gu X, et al: Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China. Lancet.
395:497–506. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J,
Wang B, Xiang H, Cheng Z, Xiong Y, et al: Clinical characteristics
of 138 hospitalized patients with 2019 novel coronavirus-infected
pneumonia in Wuhan, China. JAMA. 323:1061–1069. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang JJ, Dong X, Cao YY, Yuan YD, Yang
YB, Yan YQ, Akdis CA and Gao YD: Clinical characteristics of 140
patients infected with SARS-CoV-2 in Wuhan, China. Allergy.
75:1730–1741. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang G, Zhang J, Wang B, Zhu X, Wang Q
and Qiu S: Analysis of clinical characteristics and laboratory
findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan,
China: A retrospective analysis. Respir Res. 21(74)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lu R, Zhao X, Li J, Niu P, Yang B, Wu H,
Wang W, Song H, Huang B, Zhu N, et al: Genomic characterisation and
epidemiology of 2019 novel coronavirus: Implications for virus
origins and receptor binding. Lancet. 395:565–574. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou
J, Liu W, Bi Y and Gao GF: Epidemiology, genetic recombination, and
pathogenesis of coronaviruses. Trends Microbiol. 24:490–502.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lippi G and Plebani M: A modern and
pragmatic defnition of laboratory medicine. Clin Chem Lab Med.
58(1171)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lippi G and Plebani M: Laboratory
abnormalities in patients with COVID-2019 infection. Clin Chem Lab
Med. 58:1131–1134. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rodriguez-Morales AJ, Cardona-Ospina JA,
Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y,
Escalera-Antezana JP, Alvarado-Arnez LE, Bonilla-Aldana DK,
Franco-Paredes C, Henao-Martinez AF, et al: Clinical, laboratory
and imaging features of COVID-19: A systematic review and
meta-analysis. Travel Med Infect Dis. 34(101623)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lin L, Lu L, Cao W and Li T: Hypothesis
for potential pathogenesis of SARS-CoV-2 infection-a review of
immune changes in patients with viral pneumonia. Emerg Microbes
Infect. 9:727–732. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rokni M, Ghasemi V and Tavakoli Z: Immune
responses and pathogenesis of SARS-CoV-2 during an outbreak in
Iran: Comparison with SARS and MERS. Rev Med Virol.
30(e2107)2020.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Azkur AK, Akdis M, Azkur D, Sokolowska M,
van de Veen W, Brüggen MC, O'Mahony L, Gao Y, Nadeau K and Akdis
CA: Immune response to SARS-CoV-2 and mechanisms of
immunopathological changes in COVID-19. Allergy. 75:1564–1581.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao
Y, Xie C, Ma K, Shang K, Wang W and Tian DS: Dysregulation of
immune response in patients with coronavirus 2019 (COVID-19) in
Wuhan, China. Clin Infect Dis. 71:762–768. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang Y, Zheng L, Liu L, Zhao M, Xiao J
and Zhao Q: Liver impairment in COVID-19 patients: A retrospective
analysis of 115 cases from a single centre in Wuhan city, China.
Liver Int. 40:2095–2103. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Possamai LA, Thursz MR, Wendon JA and
Antoniades CG: Modulation of monocyte/macrophage function: A
therapeutic strategy in the treatment of acute liver failure. J
Hepatol. 61:439–445. 2014.PubMed/NCBI View Article : Google Scholar
|