Introduction
Cavernous transformation of the portal vein (CTPV),
also known as portal cavernoma, is a relatively rare condition
resulting from chronic portal vein thrombosis and/or occlusion,
which leads to the formation of numerous periportal or intrahepatic
venous collaterals (1). Since it
was first reported in 1869, CTPV remains a disease with insidious
clinical presentation and is a common cause of portal hypertension
(2).
Since various factors causing chronic portal vein
thrombosis and/or occlusion can lead to CTPV, its etiology is not
clear. CTPV is an uncommon finding in adults whose cause usually
cannot be identified (3). In some
patients, CTPV may be associated with congenital anomalies or
neonatal umbilical vein sepsis (4).
Additionally, secondary portal vein thrombosis with CTPV may often
be accompanied by cirrhosis (5).
The clinical outcomes differ between the CTPV patients with or
without liver cirrhosis (6).
However, CTPV patients without cirrhosis often present with
characteristic imaging features, such as hypertrophy of the caudate
lobe, mimic chronic liver disease and cirrhosis (7). Therefore, differential diagnosis of
different types of CTPV may be difficult, and a significant number
of cases may need to undergo invasive liver biopsy (8).
Transient elastography (TE) is a simple non-invasive
method used to assess liver stiffness (LS) (9). Although the diagnostic value of LS
measurements (LSM) has been well evaluated in liver fibrosis and
cirrhosis in chronic liver diseases, such as chronic hepatitis B,
hepatitis C and non-alcoholic steatohepatitis (9), its role in patients with CTPV remains
to be identified. Since LSM has a high diagnostic value in the
prediction of cirrhosis, the present study hypothesized that LSM
may be a valuable non-invasive tool for the differential diagnosis
of CTPV patients with or without cirrhosis. Therefore, the present
study aimed to evaluate the diagnostic power of LSM in predicting
cirrhosis in patients with CTPV.
Patients and methods
Patients
Consecutive patients diagnosed with CTPV and
simultaneously underwent liver biopsy were retrospectively
recruited in Zhongshan Hospital, Fudan University between January
2013 and December 2017. The study protocol conformed to the ethical
guidelines of the 1975 Declaration of Helsinki and was approved by
the Institutional Review Board of the Zhongshan Hospital, Fudan
University. Written informed consent was obtained from all
patients. All recruited patients underwent contrast-enhanced
64-slice spiral computed tomography (CT) and TE. CT scans were
independently reviewed by two radiologists. Patients who met any of
the following criteria were excluded from the study: History of a
variceal bleed in the previous 6 weeks; jaundice; malignant tumor
or any previous shunt surgery. Patients with poor CT image quality
or missing data were also excluded.
Two control groups were included in the study:
Patients with chronic hepatitis B (CHB)-related cirrhosis and
healthy volunteers. Retrospective liver TE data from age-matched
cirrhosis patients with histologically proven CHB (n=34) were
recorded between January 2013 and December 2017. All patients
provided written consent to undergo TE examination and permit the
inclusion of their data in future studies without disclosing their
identity. Similarly, age-matched healthy volunteers (n=20) were
also included in the study, after obtaining informed consent.
Laboratory examination, imaging and
upper gastrointestinal endoscopy
Blood tests included serum bilirubin, alanine
transaminase (ALT), aspartate transaminase (AST), alkaline
phosphatase (ALP), γ-glutamyl transpeptidase (GGT), serum albumin,
platelet count, international normalized ratio (INR) and relevant
tests for evaluation of the etiology of the liver disease (markers
for hepatitis B and hepatitis C viruses). CTPV was diagnosed based
on contrast-enhanced CT and ultrasound for the abdomen (1). In the CTPV and cirrhosis groups, the
endoscopic findings were recorded and subsequently graded according
to the criteria proposed at the Baveno V Consensus Conference
(10). Endoscopic examinations were
independently performed by two experienced endoscopists with good
agreement in grading esophageal varices (κ=0.96).
LSM by transient elastography
LSM was assessed for each patient using the
FibroScan@ apparatus (Echosens) with an M-probe
following an overnight fast. Measurements were performed according
to the Liver Stiffness Study Group ‘Elastica’ of the Italian
Association for the Study of the Liver (11). At least 10 successful measurements
for each patient were evaluated. Only LSs with a success rate of
>60% and interquartile range of <30% were considered reliable
(12,13). The physicians who undertook all the
examinations had prior experience with at least 1,000 TE
procedures.
Liver histology and quantification of
fibrosis
Ultrasonography-guided percutaneous liver biopsies
were performed for all patients in the CTPV and cirrhosis groups.
Liver biopsy samples >15 mm in length and with ≥10 portal tracts
were considered eligible. The fibrosis staging (F) and inflammatory
activity (A) were determined by one pathologist, blinded to
patients' clinical characteristics, according to the METAVIR system
(14).
Measurement of hepatic vein pressure
gradient (HVPG)
Following an overnight fast, HVPG was measured using
a standard procedure (15-17).
HVPG is defined as the difference between the wedged hepatic venous
pressure and free hepatic venous pressure. Radiologists were
blinded to clinical data.
Statistical analysis
All statistical analyses were performed using SPSS
software version 20.0 (IBM Corp.). Continuous variables are
summarized as the mean ± SD or median and interquartile range and
categorical variables as frequency and percentage. Unpaired
Student's t-test and Mann-Whitney U test were applied for
comparison between two groups. Differences between the groups were
analyzed by one-way ANOVA and Kruskal-Wallis test, followed by
Bonferroni's post hoc test for multiple comparisons. χ2
and Fisher's exact tests were applied to compare categorical
variables. The area under the receiver operating characteristic
curve (AUROC) was used to evaluate the diagnostic values of each
significant parameter. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
In the present study, 20 patients (9 males, 11
females) with CTPV were retrospectively enrolled between January
2013 and December 2017. The mean age was 49.0±3.6 years. Among 20
patients, CTPV etiology was associated with hepatitis B (n=10),
alcohol consumption (n=1) and autoimmune hepatitis (n=1), while 8
patients were cryptogenic. All patients exhibited varying degrees
of esophageal varices confirmed via upper gastrointestinal
endoscopy, while 12 subjects (60%) had a history of hemorrhage
(Table I).
 | Table IClinical characteristics of the
patients. |
Table I
Clinical characteristics of the
patients.
| Characteristic | Patients with CTPV
(n=20) | Patients with
cirrhosis (n=34) | P-valuea | Healthy controls
(n=20) | P-valueb |
|---|
| Age (years) | 49.0±13.6 | 51.7±9.9 | 0.453 | 47.5±13.5 | 0.728 |
| Sex (M:F) | 11:9 | 26:8 | 0.006 | 12:8 | 0.343 |
| Hemoglobin (g/l) | 99.0 (80.8,
120.3) | 102.0 (80.8,
122.0) | 0.680 | 153.5 (141.8,
164) | <0.001 |
| Platelet
(109/l) | 90.0 (77.3,
104.3) | 82.5 (70.8,
98.5) | 0.258 | 188.0 (150.1,
212.5) | <0.001 |
| White blood count
(109/l) | 2.9 (2.1, 3.7) | 2.3 (1.8, 3.5) | 0.441 | 5.5 (4.5, 6.7) | <0.001 |
| Total bilirubin
(µmol/l) | 14.3 (10.3,
17.8) | 18.5 (10.4,
26.7) | 0.081 | 14.4 (10.3,
19.4) | 0.779 |
| Albumin (g/l) | 38.0 (35.0,
41.8) | 36.0 (32.8,
38.3) | 0.053 | 48.3 (46.3,
50.0) | <0.001 |
| ALT (IU/l) | 21.0 (14.0,
30.3) | 19.5 (14.8,
31.0) | 0.865 | 21.3 (14.5,
31.5) | 0.860 |
| AST (IU/l) | 39.0 (29.0,
46.0) | 26.0 (19.8,
45.0) | 0.011 | 22.5 (18.0,
27.0) | <0.001 |
| ALP (IU/l) | 72.0 (52.5,
92.3) | 85.0 (72.5,
119.8) | 0.030 | 45.5 (42.3,
55.8) | <0.001 |
| GGT (IU/l) | 51.0 (29.3,
78.5) | 32.5 (17.8,
70.0) | 0.452 | 24.0 (20.3,
32.8) | 0.002 |
| INR | 1.12 (1.00,
1.26) | 1.14 (1.04,
1.22) | 0.865 | 1.03 (0.98,
1.07) | 0.021 |
| Hemorrhage history,
n (%) | 12 (60%) | 12 (35%) | 0.095 | - | - |
| Esophageal varices
grade | 3 (2, 3.75) | 3 (2, 4) | 0.422 | - | - |
| Gastric varices, n
(%) | 15 (75%) | 23 (68%) | 0.759 | - | - |
| HVPG (mmHg) | 12.0 (6.5,
18.8) | 15.0 (10.8,
18.0) | 0.319 | - | - |
| METAVIR fibrosis
score (n) | | | <0.001 | | - |
|
F0-1 | 7 | 0 | | - | - |
|
F2-3 | 1 | 0 | | - | - |
|
F4 | 12 | 34 | | - | - |
| LS (kPa) | 12.5 (6.8,
21.5) | 21.0 (15.5,
27.2) | 0.017 | 4.9 (4.0, 5.8) | <0.001 |
In addition, 34 CHB-related cirrhosis patients (F4;
liver biopsy proven) and 20 healthy volunteers were enrolled in the
same period. All healthy subjects were asymptomatic and were
screened to rule out underlying liver diseases determined by normal
abdominal ultrasonography, normal liver function tests and negative
serology results for hepatitis B and C. The baseline
characteristics of 20 patients with CTPV and control groups are
shown in Table I. There were no
statistically significant differences in age among the three
groups.
Comparison of LS, HVPG and serum
parameters in CTPV and control groups
Compared with patients with CHB-related cirrhosis,
AST and ALP levels were significantly higher in patients with CTPV.
Hemoglobin, platelet, white blood count and albumin levels were
significantly lower, while AST, ALP, GGT and INR levels were
significantly higher in patients with CTPV compared with healthy
volunteers (Table I).
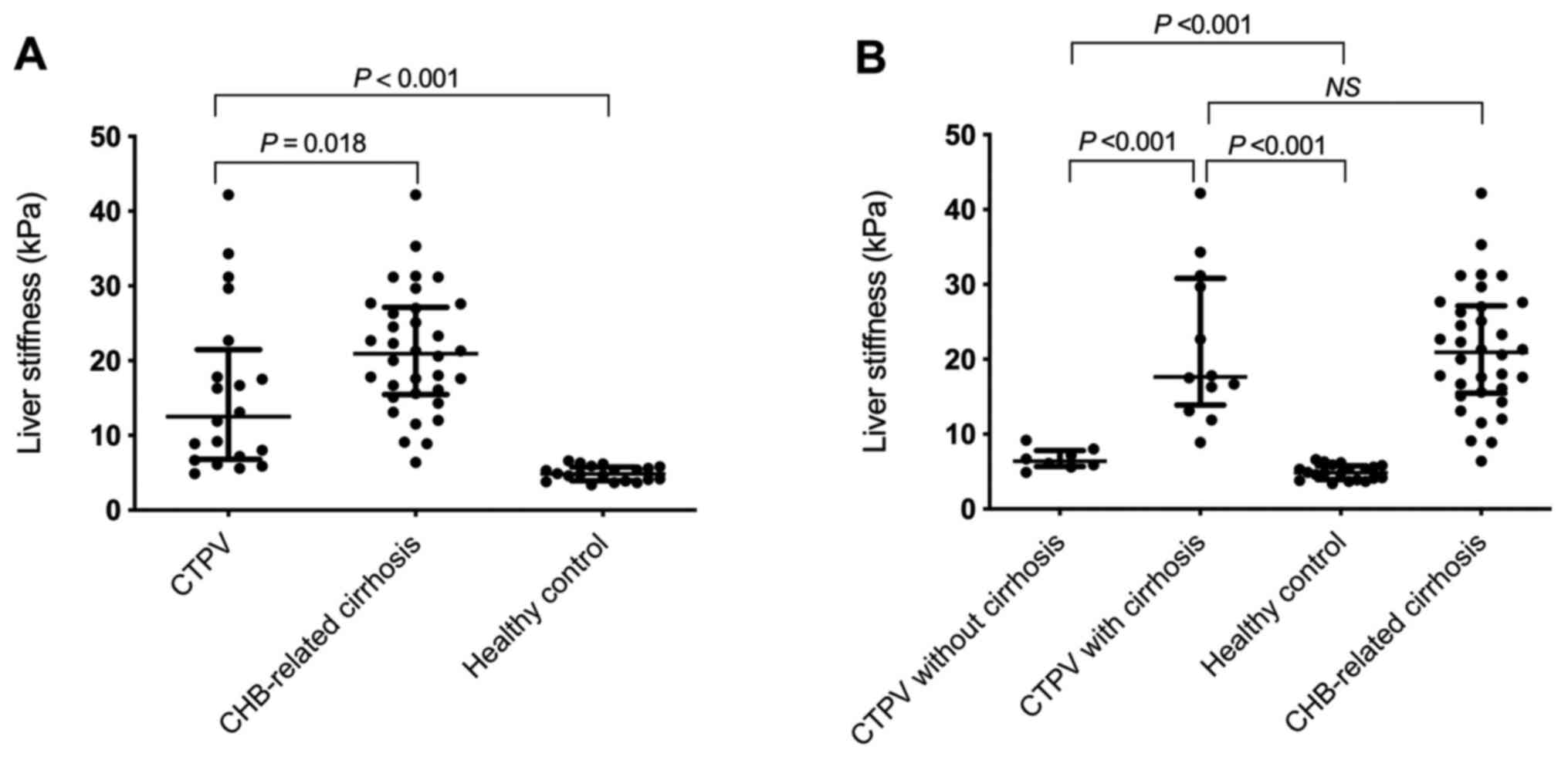
LS values in the CTPV group (12.5 kPa; range,
6.8-21.5 kPa) were significantly lower compared with the
CHB-related cirrhosis group (21.0 kPa; range, 15.5-27.2 kPa;
P=0.018), however, they were significantly higher compared with
healthy volunteers (4.9 kPa; range, 4.0-5.8 kPa; P<0.001;
Table I and Fig. 1A).
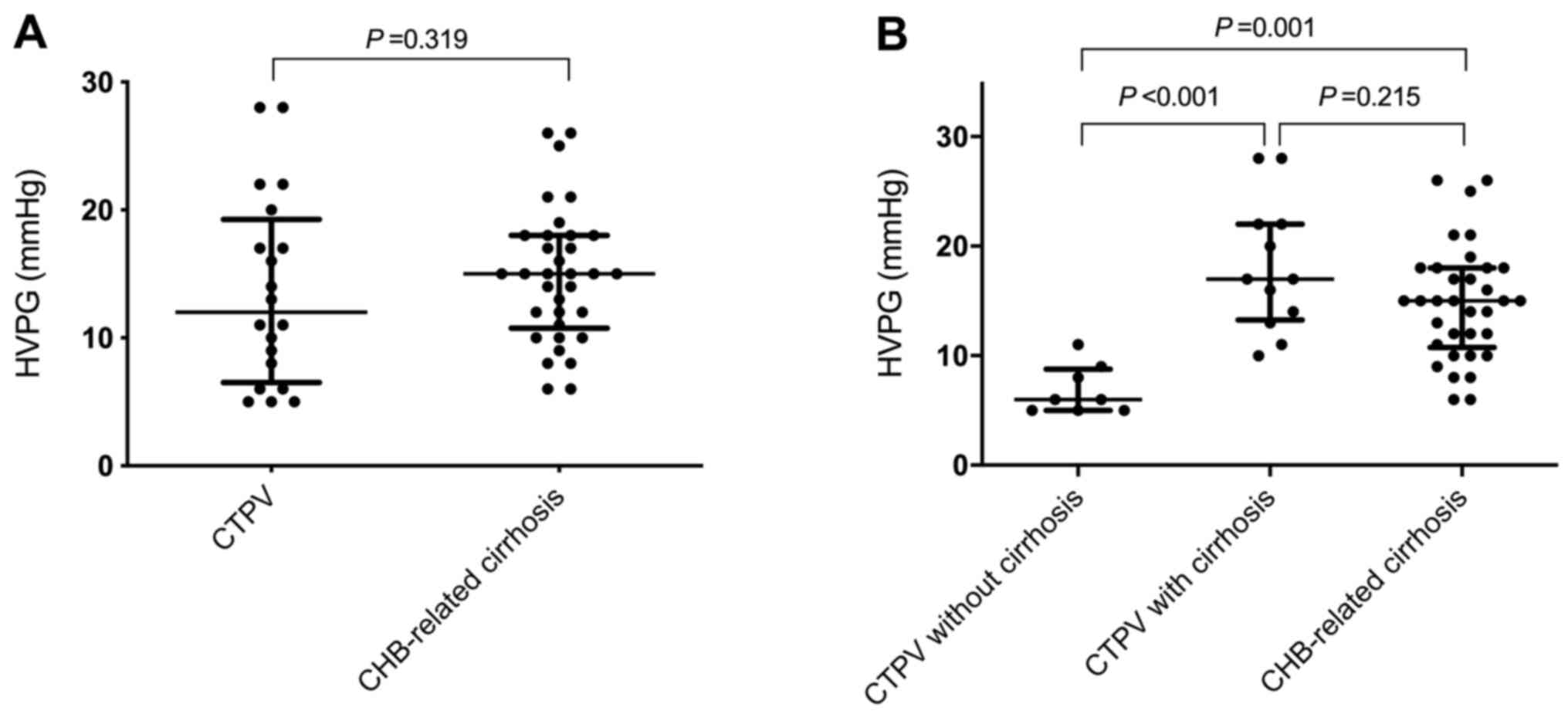
There was no statistically significant difference in
HVPG values between the CTPV (12.0 mmHg; range, 6.5-18.8 mmHg) and
CHB-related cirrhosis groups (15.0 mmHg; range, 10.8-18.0 mmHg;
P=0.319; Table I and Fig. 2A). However, HVPG values in CTPV
patients with cirrhosis (17.0 mmHg; range, 13.1-22.0 mmHg) were
significantly increased compared with CTPV patients without
cirrhosis (6.0 mmHg; range, 5.0-8.8 mmHg; P<0.001; Table II and Fig. 2B).
 | Table IICharacteristics of CTPV patients with
or without cirrhosis. |
Table II
Characteristics of CTPV patients with
or without cirrhosis.
| | Patients with
CTPV | |
|---|
| Characteristic | Cirrhosis
(n=12) | Non-cirrhosis
(n=8) | P-value |
|---|
| Age (years) | 57.4±7.9 | 36.4±9.9 | <0.001 |
| Sex (M:F) | 7:5 | 4:4 | 0.714 |
| Hemoglobin
(g/l) | 98.0 (80.8,
120.3) | 99.0 (76.5,
120.3) | 0.938 |
| Platelet
(109/l) | 90.0 (79.5,
98.0) | 95.0 (76.0,
105.0) | 0.908 |
| White blood count
(109/l) | 2.7 (2.1, 4.2) | 3.0 (1.5, 3.3) | 0.877 |
| Total bilirubin
(µmol/l) | 13.5 (10.3,
17.4) | 14.9 (10.8,
20.0) | 0.521 |
| Albumin (g/l) | 35.0 (35.0,
40.5) | 39.5 (38.0,
42.8) | 0.035 |
| ALT (IU/l) | 24.0 (14.0,
35.5) | 17.0 (14.0,
28.8) | 0.485 |
| AST (IU/l) | 44.0 (29.0,
49.0) | 36.5 (30.3,
42.0) | 0.561 |
| ALP (IU/l) | 77.0 (58.0,
97.3) | 67.5 (43.5,
80.8) | 0.164 |
| GGT (IU/l) | 57.5 (34.5,
90.3) | 36.0 (21.0,
54.0) | 0.217 |
| INR | 1.17 (1.00,
1.26) | 1.06 (0.99,
1.25) | 0.727 |
| HBsAg positive, n
(%) | 9 (75%) | 1 (13%) | 0.020 |
| Hemorrhage history,
n (%) | 7 (58%) | 5 (63%) | 0.852 |
| Esophageal varices
grade | 3 (2, 4) | 3 (2, 4) | 0.748 |
| Gastric varices, n
(%) | 8 (67%) | 7 (88%) | 0.603 |
| HVPG (mmHg) | 17.0 (13.1,
22.0) | 6.0 (5.0, 8.8) | <0.001 |
| LS (kPa) | 17.7 (13.9,
30.8) | 6.4 (5.7, 7.8) | <0.001 |
Association between LSM and cirrhosis
in patients with CTPV
In the CTPV group, liver biopsy showed preserved
acinar architecture and normal hepatocytes in 7 (35%) patients. A
total of 7 patients exhibited no or mild signs of fibrosis (F0-1),
while 1 patient showed significant fibrosis (F2-3) and the
remaining 12 patients were diagnosed with cirrhosis (F4; Table I). Based on histological evaluation,
patients with CTPV were divided into two different subgroups:
Cirrhosis and non-cirrhosis groups. The demographic and clinical
characteristics of CTPV patients with or without cirrhosis are
presented in Table II. Patients
with CTPV in the cirrhosis group were significantly older (57.4±7.9
vs. 36.4±9.9 years; P<0.001) and were characterized by increased
hepatitis B surface antigen positivity compared with the
non-cirrhosis group. In addition, the clinical outcome of CTPV is
differs between patients with cirrhosis and patients without
cirrhosis. However, the imaging manifestations of these two types
of CTPV may be similar (Fig. S1).
Therefore, differential diagnosis is difficult and liver biopsy is
occasionally required.
LS values in CTPV patients with cirrhosis (17.7 kPa;
range, 13.9-30.8 kPa) were significantly higher compared with the
CTPV non-cirrhosis group (6.4 kPa; range, 5.7-7.8 kPa; P<0.001;
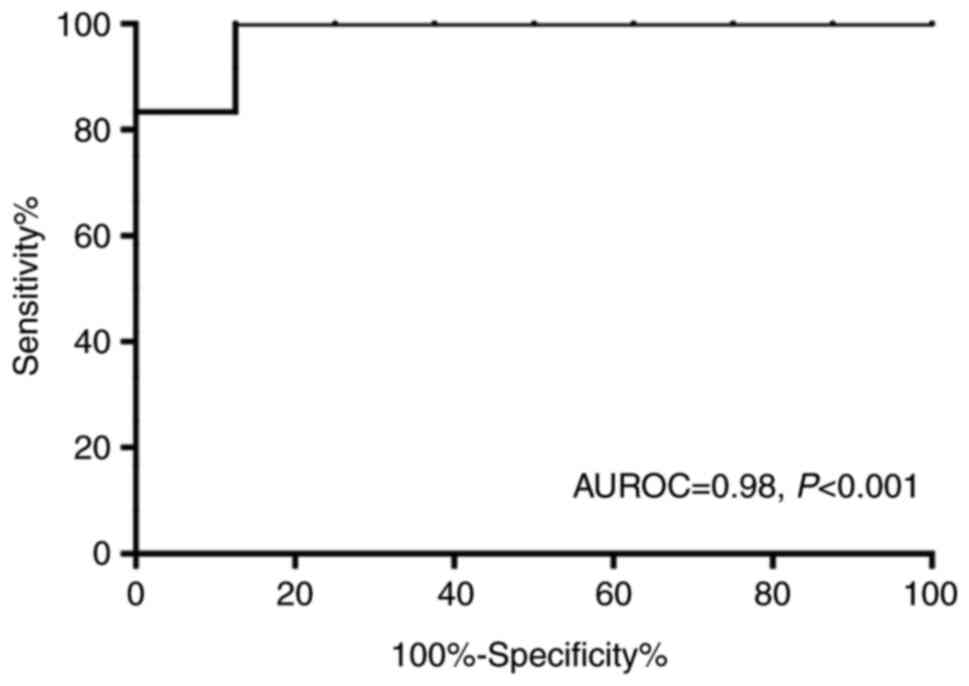
Table II and Fig. 1B). Furthermore, the AUROC value
evaluating the diagnostic value of LSM in cirrhosis (F=4) was 0.98
(Fig. 3). The LSM cut-off value was
set at 9.0 kPa, and the sensitivity and specificity rates for the
detection of cirrhosis were 92 and 88%, respectively (data not
shown).
Additionally, there was no statistically significant
difference in LS values between CTPV patients with cirrhosis (17.7
kPa; range, 13.9-30.8 kPa) and patients with CHB-related cirrhosis
(21.0 kPa; range, 15.5-27.2 kPa; P=1.000; Fig. 1B). However, LS values in CTPV
patients without cirrhosis (6.4 kPa; range, 5.7-7.8 kPa) were
significantly higher compared with those of healthy volunteers (4.9
kPa; range, 4.0-5.8 kPa; P<0.001; Fig. 1B).
Discussion
The present study aimed to evaluate the diagnostic
potential of the non-invasive method, LS, for predicting cirrhosis
in patients with CTPV. The results demonstrated that LS values were
higher in patients with CTPV compared with healthy controls. In
addition, LS values were significantly increased in CTPV patients
with cirrhosis compared with those without cirrhosis. These
findings indicated that CTPV patients with cirrhosis may exhibit a
poorer prognosis compared with those without cirrhosis. In addition
to the complications that may be caused by portal hypertension,
CTVP-cirrhotic patients may also suffer from cirrhosis-related
complications. Therefore, LSM may be used for the differential
diagnosis of CTPV patients with or without cirrhosis.
To the best of our knowledge, this is the first
study to evaluate the clinical application of LSM via TE in
patients with CTPV. In the present study, the AUROC value,
evaluating the diagnostic value of LS in cirrhosis, was 0.98, while
the sensitivity and specificity rates for the detection of
cirrhosis were 92 and 88%, respectively, with a cut-off value of
9.0 kPa. However, the cut-off value used in this study was
significantly lower than that applied for the diagnosis of
cirrhosis in other chronic liver diseases. Varying cut-offs have
been proposed for the diagnosis of cirrhosis according to the
etiology of liver disease, ranging from 9.7 kPa in hepatitis B to
22.7 kPa in alcoholic liver disease (18). According to the recent American
Gastroenterological Association Institute Technical Review on the
Role of Elastography in Chronic Liver Diseases (2017), the cut-offs
proposed for the diagnosis of cirrhosis were 12.5, 11 and 12.5 kPa
for hepatitis C, hepatitis B and alcoholic liver disease,
respectively (19). However, most
of these cut-off values have been defined in a single population
using ROC curves to maximize sensitivity and specificity and have
not been applied to a validation cohort (18). Therefore, the differences between
cut-offs may be associated with differences in cirrhosis prevalence
in the studied populations, known as spectrum bias (18).
A limited number of studies have evaluated LSM in
patients with extrahepatic portal vein obstruction (EHPVO). EHPVO
is another disorder of the portal venous system, which is
characterized by occlusion of the portal vein, resulting in the
formation of collateral vessels (20). EHPVO leads to pre-hepatic portal
hypertension, while some patients may be accompanied by CTPV
(21). Sharma et al
(22) demonstrated a statistically
significant difference (P=0.001) in LS values via TE between
patients with EHPVO (6.7 kPa) and healthy volunteers (4.6 kPa).
However, a recent study in EHPVO showed that LS values via 2D shear
wave elastography were similar to normal liver (23). Unlike CTPV, EHPVO is rarely
accompanied by cirrhosis, while histological examination revealed
that the architectural pattern of the liver is preserved (21). Among 20 CTPV patients enrolled in
the study, 8 non-cirrhotic patients were also diagnosed with EHPVO
according to the expanding consensus in portal hypertension of the
European Association for the Study of the Liver (24). LS values using TE in CTPV patients
with EHPVO (6.4 kPa; range, 5.7-7.8 kPa) were significantly lower
compared with patients without EHPVO (17.7 kPa; range, 13.9-30.8
kPa; P<0.001). However, further studies including more available
data from patients are required to evaluate the clinical efficacy
of LSM in CTPV.
Consistent with the results obtained by Sharma et
al (22), the median LS value
of CTPV patients without cirrhosis was 6.4 kPa (range, 5.7-7.8
kPa), which was significantly higher compared with age-matched
healthy controls. The increased LS values may be associated with
deprivation of portal blood to the liver, which in turn may
influence the functions of the hepatic parenchyma (22,25).
Additionally, hepatic hemodynamic changes may also explain this
finding. A recent case report suggested that the false-positive
results obtained using TE, namely portal vein thrombosis, may be
caused by hepatic arterial buffer response. Although four biopsies
were performed with no evidence of cirrhosis, the patient exhibited
increased LS values, indicating cirrhosis. Therefore, the authors
speculated that this observation could be caused by compensatory
arterial buffer response to the portal vein obstruction in the
hepatic vasculature and by arterial flow, which in turn could lead
to increased elastography grade of the liver (26).
However, there were some limitations of the present
study. Firstly, due to the retrospective nature of the study and
the small number of patients with CTPV, a further large-scale,
prospective study is required to evaluate the aforementioned
results. In addition, the thresholds for evaluating sensitivity and
specificity were determined on the basis of the present study
population, therefore, the diagnostic performance of LSM via TE
could be overestimated due to spectrum bias.
In conclusion, the present study demonstrated that
patients with CTPV exhibited higher LS values compared with healthy
controls. Furthermore, CTPV patients with cirrhosis had higher LS
values compared with those without cirrhosis. Therefore, LSM may be
used for the differential diagnosis of CTPV patients with or
without cirrhosis. However, further validation studies are
required.
Supplementary Material
Multi-slice spiral CT portal
venography in CTPV patients with or without cirrhosis. Patient 1,
CTPV patient with cirrhosis; LS, 12.9 kPa. Patient 2, CTPV patient
without cirrhosis; LS, 6.1 kPa. CT, computed tomography; LS, liver
stiffness; CTPV, cavernous transformation of the portal vein.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China (grant no. 81500457), the National Science and
Technology Major Project of China (grant no. 2017ZX10203202003002),
the Xiamen Medical and Health Guidance Project (grant no.
3502Z20199177) and the Xiamen Superior Subspecialty Construction
Project (grant no. 2018).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SW and WJ conceived the study and organized the
manuscript. YS and WM analyzed the data and wrote the manuscript.
LL collected clinical data. YH analyzed and interpreted the patient
data. YH, YS AND LL contributed to manuscript revision. All authors
read and approved the final manuscript.
Ethics approval and consent participate
The study protocol conformed to the ethical
guidelines of the 1975 Declaration of Helsinki and was approved by
the Institutional Review Board of the Zhongshan Hospital, Fudan
University (approval no. B2013-068). Written informed consent was
obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Elsayes KM, Shaaban AM, Rothan SM, Javadi
S, Madrazo BL, Castillo RP, Casillas VJ and Menias CO: A
comprehensive approach to hepatic vascular disease. Radiographics.
37:813–836. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kuy S, Dua A, Rieland J and Cronin DN II:
Cavernous transformation of the portal vein. J Vasc Surg.
63(529)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yonem O and Bayraktar Y: Is portal vein
cavernous transformation a component of congenital hepatic
fibrosis? World J Gastroenterol. 13:1928–1929. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bayraktar Y, Tuncer ZS, Kabukçu A,
Uzunalimoğlu B and Ayhan A: Pregnancy complicated by congenital
hepatic fibrosis with cavernous transformation of the portal vein:
A case report. Am J Obstet Gynecol. 177:459–461. 1997.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Francoz C, Belghiti J, Vilgrain V,
Sommacale D, Paradis V, Condat B, Denninger MH, Sauvanet A, Valla D
and Durand F: Splanchnic vein thrombosis in candidates for liver
transplantation: Usefulness of screening and anticoagulation. Gut.
54:691–697. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ma J, Yan Z, Luo J, Liu Q, Wang J and Qiu
S: Rational classification of portal vein thrombosis and its
clinical significance. PLoS One. 9(e112501)2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vilgrain V, Condat B, Bureau C, Hakimé A,
Plessier A, Cazals-Hatem D and Valla DC: Atrophy-hypertrophy
complex in patients with cavernous transformation of the portal
vein: CT evaluation. Radiology. 241:149–155. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Intagliata NM, Caldwell SH and Tripodi A:
Diagnosis, development, and treatment of portal vein thrombosis in
patients with and without cirrhosis. Gastroenterology.
156:1582–1599.e1. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Friedrich-Rust M, Poynard T and Castera L:
Critical comparison of elastography methods to assess chronic liver
disease. Nat Rev Gastroenterol Hepatol. 13:402–411. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
de Franchis R and Faculty BV: Revising
consensus in portal hypertension: Report of the Baveno V consensus
workshop on methodology of diagnosis and therapy in portal
hypertension. J Hepatol. 53:762–768. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bonino F, Arena U, Brunetto MR, Coco B,
Fraquelli M, Oliveri F, Pinzani M, Prati D, Rigamonti C, Vizzuti F,
et al: Liver stiffness, a non-invasive marker of liver disease: A
core study group report. Antivir Ther. 15 (Suppl 3):S69–S78.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Castera L, Forns X and Alberti A:
Non-invasive evaluation of liver fibrosis using transient
elastography. J Hepatol. 48:835–847. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kettaneh A, Marcellin P, Douvin C, Poupon
R, Ziol M, Beaugrand M and de Lédinghen V: Features associated with
success rate and performance of FibroScan measurements for the
diagnosis of cirrhosis in HCV patients: A prospective study of 935
patients. J Hepatol. 46:628–634. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bedossa P and Poynard T: An algorithm for
the grading of activity in chronic hepatitis C. The METAVIR
cooperative study group. Hepatology. 24:289–293. 1996.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bosch J, Abraldes JG, Berzigotti A and
García-Pagan JC: The clinical use of HVPG measurements in chronic
liver disease. Nat Rev Gastroenterol Hepatol. 6:573–582.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Groszmann RJ and Wongcharatrawee S: The
hepatic venous pressure gradient: Anything worth doing should be
done right. Hepatology. 39:280–282. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Colecchia A, Montrone L, Scaioli E,
Bacchi-Reggiani ML, Colli A, Casazza G, Schiumerini R, Turco L,
Biase AR, Mazzella G, et al: Measurement of spleen stiffness to
evaluate portal hypertension and the presence of esophageal varices
in patients with HCV-related cirrhosis. Gastroenterology.
143:646–654. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
European Association for Study of Liver;
Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH
clinical practice guidelines: Non-invasive tests for evaluation of
liver disease severity and prognosis. J Hepatol. 63:237–264.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Singh S, Muir AJ, Dieterich DT and
Falck-Ytter YT: American gastroenterological association institute
technical review on the role of elastography in chronic liver
diseases. Gastroenterology. 152:1544–1577. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sarin SK, Sollano JD, Chawla YK,
Amarapurkar D, Hamid S, Hashizume M, Jafri W, Kumar A, Kudo M,
Lesmana LA, et al: Consensus on extra-hepatic portal vein
obstruction. Liver Int. 26:512–519. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gauthier F: Recent concepts regarding
extra-hepatic portal hypertension. Semin Pediatr Surg. 14:216–225.
2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sharma P, Mishra SR, Kumar M, Sharma BC
and Sarin SK: Liver and spleen stiffness in patients with
extrahepatic portal vein obstruction. Radiology. 263:893–899.
2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Madhusudhan KS, Sharma R, Kilambi R,
Shylendran S, Shalimar Sahni P and Gupta AK: 2D shear wave
elastography of liver in patients with primary extrahepatic portal
vein obstruction. J Clin Exp Hepatol. 7:23–27. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
de Franchis R and Faculty B VI: Expanding
consensus in portal hypertension: Report of the Baveno VI consensus
workshop: Stratifying risk and individualizing care for portal
hypertension. J Hepatol. 63:743–752. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rangari M, Gupta R, Jain M, Malhotra V and
Sarin SK: Hepatic dysfunction in patients with extrahepatic portal
venous obstruction. Liver Int. 23:434–439. 2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Huang R, Gao ZH, Tang A, Sebastiani G and
Deschenes M: Transient elastography is an unreliable marker of
liver fibrosis in patients with portal vein thrombosis. Hepatology.
68:783–785. 2018.PubMed/NCBI View Article : Google Scholar
|