Introduction
Hashimoto's thyroiditis (HT) is characterized by
high prevalence, hidden onset and slow progress. At present, the
relationship between vitamin D and HT has become a hot spot at home
and abroad, but the mechanism has not yet been clarified. Studies
have shown that there exist the upregulation of Th1 cytokines,
downregulation of Th2 cytokines, transformation and interaction
between TH1 and Th2 in HT (1,2).
Therefore, intervention in the differentiation and selection of Th
cell subsets may be helpful to the treatment of autoimmune
reaction. Vitamin D and its receptor-mediated biological effects
are involved in many pathophysiological processes, such as
regulation of calcium and phosphorus metabolism, immune regulation,
anti-inflammation, anti-infection, and tumor prevention.
25-Hydroxyvitamin D3 [25-(OH)D3] is an active
form of vitamin D. Studies have found that 25-(OH)D3
inhibits Th1 cell activity and regulates Th1/Th2 cell imbalance
(3,4) and is beneficial to the maintenance of
intact thyroid tissue morphology in rats (5,6).
Correcting the imbalance of Th1/Th2 and adjusting the immune
response in HT patients has important clinical research value
(7,8). Autophagy is a normal metabolic pathway
to maintain the amount of eukaryotic cells, which is mainly
mediated by lysosomes. Its dysfunction is related to tumor and
infection. Studies have shown that vitamin D/vitamin D receptor
(VDR) can influence the progression of autoimmune thyroid diseases
by regulating autophagy (9-14).
In the present study, the immune mechanism related to vitamin D and
HT was investigated. Human thyroid cells were selected to study the
effect of 25-(OH)D3 on autophagy, apoptosis and
proliferation of cells, which may provide a new treatment strategy
for clinical HT.
Materials and methods
Research participants
Fifty newly diagnosed HT patients (age range, 30-60
years) at The Second Affiliated Hospital Of Qiqihar Medical
University (Qiqihar, Heilongjiang, China) from February 2019 to
December 2019 were selected as the HT group. There were 15 males
and 35 females, with a mean age of 38.91±6.53 years.
The inclusion criteria of the HT group were as
follows: i) Patients with diffuse or local enlargement of thyroid
gland; ii) anti-thyroglobulin antibodies (TGAb) and thyroid
peroxidase antibody (TPOAb) were both positive; iii) patients
accompanied by hypothyroidism, serum TSH level was higher than
normal. Exclusion criteria were as follows: i) Pregnant, lactating
and menopausal women; ii) dysfunctional or exhausted heart, lung,
brain, liver, kidney or other important organs; iii) patients
suffering from other autoimmune endocrine diseases; iv) patients
with thyroid cancer; v) patients who previously used
immunoenhancers or immunosuppressants within three months; vi)
patients with a history of vitamin D replacement therapy in the
past 12 months.
Fifty healthy individuals (30-60 years) who met the
physical health standards at our hospital from February 2019 to
December 2019 were selected as the normal control (NC) group. There
were 17 males and 33 females, with a mean age of 39.57±10.04 years.
Exclusion criteria were as follows: Patients with abnormal thyroid
function; pregnant women, lactating women, menopausal women or
women with other endocrine diseases, autoimmune diseases or
systemic diseases (tumor, asthma or other allergic diseases, active
infections); patients with other endocrine diseases and autoimmune
diseases; patients who took drugs in the previous three months and
had a history of vitamin D replacement therapy in the past 12
months.
All subjects signed informed consent. The present
study was approved by the Ethics Committee of The Second Affiliated
Hospital Of Qiqihar Medical University (Qiqihar, Heilongjiang,
China) after examination.
Materials
Following informed consent of the patients, another
20 cases of thyroid adenoma with Hashimoto's thyroiditis were
collected as the case group, and 20 cases of normal tissues
adjacent to benign thyroid adenoma were collected as the control
group. Nthy-ori3-1 cells (normal cells of human thyroid follicular
epithelium) were purchased from the Wuhan Cell Bank of the Chinese
Academy of Sciences. The rabbit anti-β-actin antibody (ab179467),
rabbit anti-LC-3 antibody (ab48394), rabbit anti-mTOR antibody
(ab109268) and rabbit anti-caspase-3 antibody (ab197202) were all
purchased from Abcam. RPMI-1640 culture medium was purchased from
BD Biosciences. Fetal bovine serum (FBS) was purchased from Gibco
(Thermo Fisher Scientific, Inc.). The MTT cell proliferation kit
(cat. no. 4890-025-k) was purchased from Trevigen, USA.
25-(OH)D3 was purchased from Shanghai Yubo Biotechnology
Co., Ltd. (D455087). Double-distilled water (ddH2O) was
used to prepare 20, 40, 60, 80 and 100 mmol/l solutions.
Methods Determination of relevant
indexes
Levels of five free thyroid functions: Free
triiodothyronine (FT3), free thyroxine (FT4), anti-thyroglobulin
antibodies (TGAb), thyroid peroxidase antibodies (TPOAb), and the
third generation thyroid-stimulating hormone (s-TSH) were detected
by a full-time examiner through chemiluminescence immunoassay
analyzer (IMMULITE 2000; Siemens, Germany).
Determination of
25-(OH)D3
We collected venous blood from the patients on an
empty stomach in the morning, which was placed in a serum
separation glue test tube, and let stand at room temperature for 1
h. After centrifugation, we took the upper serum, and added it to
the 25(OH)D3 kit (ZCi Bio, cat. no. ZC-31637). We
applied the double antibody sandwich enzyme-linked immunosorbent
(ELISA) for detection, using a microplate reader (Shanghai Kehua
Bio-engineering Co., Ltd.). All procedures were strictly in
accordance with the specifications included in the kits.
Determination of the cytokines
The related cytokine kits purchased from Beijing
Keruimei Technology Co., Ltd. (Hu IL-2, 850.010.X; Hu IL-4,
950.020.X; Hu IL-6 CE, 950.030.X; Hu hs IL-10 kit, 850.880.X; Hu
IFN-γ kit CE, 950.000.X) and serum 25-(OH)D3 detection
kit (Shanghai Beyotime Biotechnology Co., Ltd., ZC-31637) were used
according to the manufacturers' instructions.
Cell culture
Nthy-ori3-1 cells (human thyroid follicular
epithelial normal cells) were purchased from Wuhan Cell Bank of the
Chinese Academy of Sciences. The cells were placed in RPMI-1640
culture medium containing 10% FBS at 37˚C with 5% CO2.
When the cells grew to a logarithmic growth stage, they were
inoculated into a 6-well plate. Then the culture continued for 24 h
with different concentrations of 25-(OH)D3 (0, 20, 40,
60, 80 and 100 mmol/l) for subsequent experimentation.
Effect of 25-(OH)D3 on the
expression of mTOR and LC3B-II in cells by western blot
analysis
Cells were digested with trypsin, washed with PBS,
centrifuged at 12,000 x g at 37˚C for 10 min, fully lysed with
lysis solution, and centrifuged to obtain the supernatant. The
protein concentration was determined by BCA assay. SDS-PAGE protein
loading buffer (5X) was prepared, and the protein was denatured by
boiling water bath for 10 min. Cooling, loading, and
electrophoresis were performed respectively. Proteins (10 µl) were
transferred to PVDF membranes, with the current of 15-20 mA
overnight. Commassie blue staining solution was used for rapid
staining. PAGE membrane was rinsed, blocking solution was added and
incubated overnight on a shaking table at room temperature. Then
the blocking solution was removed, and the primary antibody (1:500)
was added and incubated overnight at 4˚C on a shaking table. The
sample was then washed, goat anti-rabbit secondary antibody (HRP
labeled, 1:6,000), was added and incubation was carried out at 37˚C
for 1 h. The secondary antibody was recovered and washed 3 times.
BeyoECL Plus (Beyotime Biotechnology, Inc.) was used to detect
protein; pressing plate and rinsing were performed. Protein
expression was represented by absorbance of target
protein/absorbance of internal reference (GADPH), using ImageJ
v1.8.0 software (National Institutes of Health, Bethesda).
Detection of apoptosis as detected by
Annexin V-PE/7-AAD double staining method
25-(OH)D3 was added to treat the cells
for 24 h, and then the cells were collected. Binding buffer (100
µl) was then added to each well, and 5 µl Annexin Ⅴ-PE was added,
and incubation was carried out at 4˚C in the dark for 15 min. 7-AAD
(10 µl) was added and incubation was carried out for 5 min in the
dark, and 400 µl binding buffer was added. Then flow cytometry was
used, in which Annexin Ⅴ-PE was detected through FL1 channel and
7-AAD was detected through FL3 channel.
Cell proliferation as determined by
MTT assay
The cells in the logarithmic growth phase were added
to 96-well plates, at 5x103 cells per well and 4
multiple wells in each group. The cells were cultured in an
incubator for 2 h. 25-(OH)D3 at different concentrations
was added to each well and cultured for 24 h. The 96-well plate was
taken out, 20 µl MTT (5 mg/ml) was added to each experimental well
and incubated at room temperature for 4 h. The supernatant was
removed from each well, and 150 µl DMSO was added to each well. The
absorbance (A) of each well at 490 nm was measured, and the cell
growth inhibition rate was calculated as: (1-A value of the case
group/A value of the control group) x100%.
Statistical analysis
SPSS 20.0 (IBM Corp.) was used for data analysis.
The data are expressed as mean ± standard deviation (SD), and the
comparison between the two groups was conducted by normality test
and variance homogeneity test. The t-test was used when the
criteria were met. Analysis of variance (ANOVA) was used for
multi-group comparisons. Pearson analysis and multiple stepwise
regression analysis were used to analyze the correlation of each
molecule. GraphPad Prism 5.0 (GraphPad Software, Inc.) was used for
figure analysis. A difference was deemed statistically significant
at P<0.05.
Results
Comparison of general data and five
free thyroid functions between the HT and NC group
As documented in Table
I, statistical analysis showed that there was no statistical
difference in age, sex and body mass index (BMI) between the two
groups (P>0.05). The level of s-TSH in the case group was
significantly higher than that in the control group (P<0.01).
The levels of FT3 and FT4 in the HT group were significantly lower
than those in the NC group. Rank sum test analysis showed that
there was a significant difference between the two groups
(P<0.01). The levels of TPOAb and TGAb in the HT group were
significantly higher than those in the NC group, and rank sum test
analysis showed that there were significant differences between the
two groups (P<0.01).
 | Table IComparison of the general data and
five free thyroid functions between the HT and NC group. |
Table I
Comparison of the general data and
five free thyroid functions between the HT and NC group.
| Items | HT group (n=50) | NC group (n=50) | t/χ2 | P-value |
|---|
| Age (years) | 38.91±6.53 | 39.57±10.04 | 0.399 | 0.691 |
| Sex (M/F) | 15/35 | 16/34 | 0.047 | 0.829 |
| BMI
(kg/m2) | 29.17±4.89 | 28.93±3.09 | 0.293 | 0.770 |
| s-TSH (mIU/l) | 10.09±3.81 | 2.31±1.32 | 13.643 | <0.01a |
| FT3 (pmol/l) | 5.09±1.02 | 6.38±1.29 | 5.547 |
<0.01a |
| FT4 (pmol/l) | 12.09±7.02 | 17.02±1.07 | 4.909 |
<0.01a |
| TPOAb (IU/ml) | 801.01±400.23 | 9.02±3.18 | 13.975 |
<0.01a |
| TGAb (IU/ml) | 203.71±109.32 | 16.09±2.46 | 12.164 |
<0.01a |
Comparison of serum
25-(OH)D3 and Th1 and Th2 cytokines between the HT and
NC group
As documented in Table
II, serum 25-(OH)D3 level in the HT group
(19.52±3.89 ng/ml) was higher than that in the control group
(16.28±3.94 ng/ml) (P<0.01). Serum interferon (IFN)-γ and
interleukin (IL)-2 levels in the HT group were higher than those in
the NC group (P<0.01), while serum IL-10 levels in the HT group
were lower than those in the NC group (P<0.01). There was no
significant difference in serum IL-4 and IL-6 levels between the
two groups (P>0.05). This suggested that the serum
25-(OH)D3 level in patients with HT may be involved in
disease progression by upregulating IFN-γ and IL-2 levels and
downregulating IL-10 levels.
 | Table IIComparison of serum
25-(OH)D3 and T-helper cytokines between the HT and NC
group. |
Table II
Comparison of serum
25-(OH)D3 and T-helper cytokines between the HT and NC
group.
| Indicators | HT group
(n=50) | NC group
(n=50) | t | P-value |
|---|
|
25-(OH)D3 (ng/ml) |
19.52±3.89b | 16.28±3.94 | 3.793 |
<0.01b |
| IL-2 (pg/ml) |
4.09±1.24a | 2.18±1.13 | 8.050 |
<0.01b |
| IL-4 (pg/ml) | 2.13±1.20 | 2.29±1.32 | 0.634 | 0.528 |
| IL-6 (pg/ml) | 1.65±0.19 | 1.59±0.32 | 1.140 | 0.257 |
| IL-10 (pg/ml) |
1.99±0.23b | 2.98±0.52 | 12.311 |
<0.01b |
| IFN-γ (pg/ml) |
12.87±1.63b | 3.89±2.01 | 24.536 |
<0.01b |
Correlation analysis in the HT
group
As documented in Table
III, the level of 25-(OH)D3 in the HT group was
negatively correlated with IL-2 (r=-0.602) and IFN-γ (r=-0.605),
and positively correlated with IL-4 (r=0.507). It was negatively
correlated with FT3 (r=-0.407), s-TSH (r=-0.517), TPOAb (r=-0.701)
and TGAb (r=-0.515), and it had no significant correlation with
IL-6 and IL-10. 25-(OH)D3 was positively correlated with
FT4 levels (r=-0.515). s-TSH was positively correlated with IFN-γ
(r=0.627) and IL-2 (r=0.481), but not correlated with IL-4, IL-6
and IL-10. TPOAb was negatively correlated with
25-(OH)D3 (r=-0.701), positively correlated with IFN-γ
(r=0.663) and IL-2 (r=0.385), but not significantly correlated with
IL-4, IL-6 and IL-10. TGAb was negatively correlated with
25-(OH)D3 (r=-0.515), positively correlated with IFN-γ
(r=0.396), but not significantly correlated with IL-2, IL-4, IL-6
and IL-10.
 | Table IIICorrelation of indexes in the HT
group. |
Table III
Correlation of indexes in the HT
group.
| Item |
25-(OH)D3 | s-TSH | TPOAb | TGAb | FT4 | FT3 |
|---|
| IL-2 | r=-0.602 | r=-0.481 | r=0.385 | NS | r=-0.495 | r=0.368 |
| |
P<0.001b |
P=0.006a |
P=0.031a | |
P=0.004b |
P=0.039a |
| IL-4 | r=0.507 | NS | NS | NS | NS | NS |
| |
P=0.002b | | | | | |
| IL-6 | NS | NS | NS | NS | NS | NS |
| IL-10 | NS | NS | NS | NS | NS | NS |
| IFN-γ | r=0.605 | r=0.627 | r=0.663 | r=0.396 | r=0.533 | r=0.516 |
| |
P<0.001b |
P<0.001b |
P<0.001b |
P=0.024a |
P<0.001b |
P=0.002a |
|
25-(OH)D3 | 1 | r=-0.517 | r=-0.701 | r=-0.515 | r=0.665 | r=-0.407 |
| | |
P=0.002a |
P<0.001b |
P=0.003a |
P<0.001b |
P=0.022a |
Multiple stepwise regression
analysis
s-TSH in the case group was taken as dependent
variable. IFN-γ and IL-2 in the case group were taken as
independent variables. The results showed that s-TSH was
significantly affected by IFN-γ (P<0.01), while IL-2 did not
enter the regression equation.
TPOAb in the HT group was taken as a dependent
variable. IFN-γ and IL-2 in the case group were taken as
independent variables. The result showed that TPOAb was
significantly affected by IFN-γ (P<0.01), while IL-2 did not
enter the regression equation.
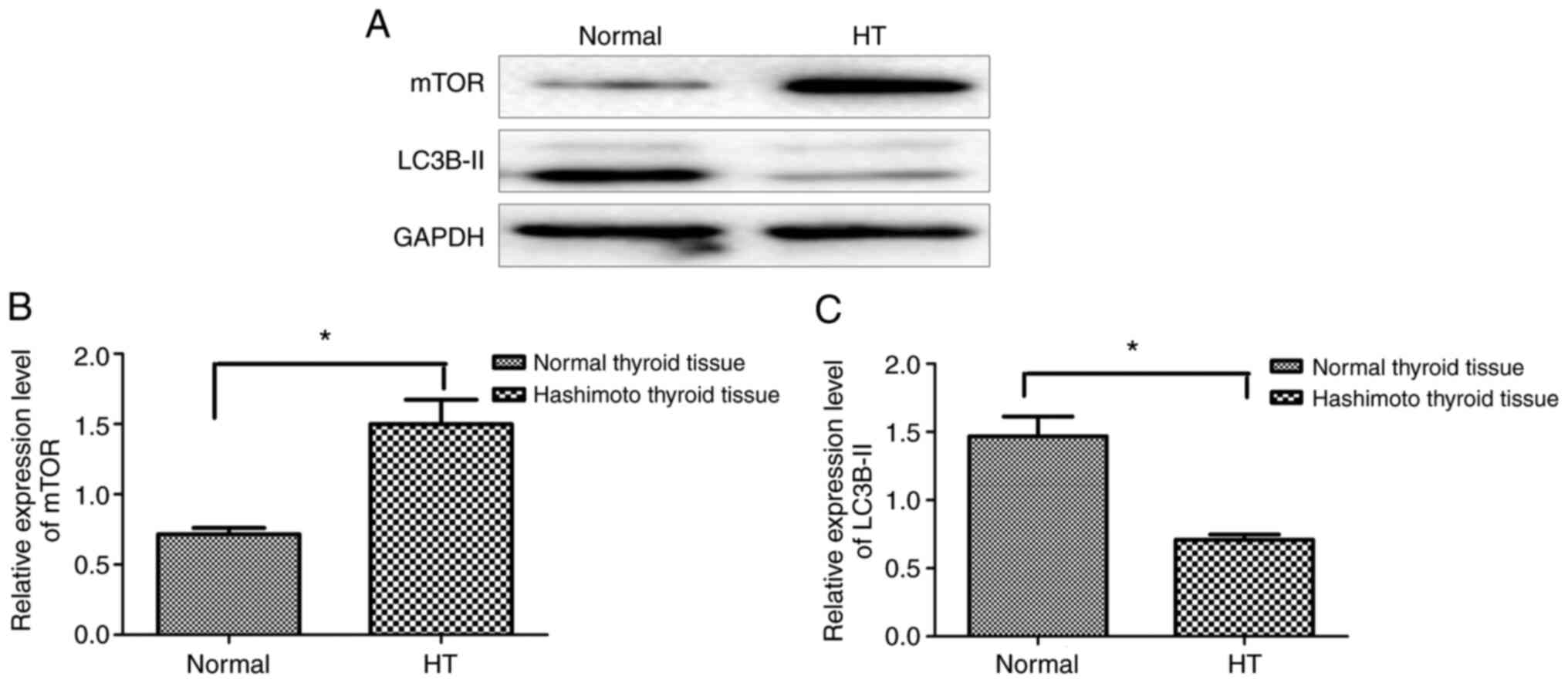
Expression level of autophagy-related
proteins in HT tissues
Western blotting showed that the expression level of
mTOR protein in the HT tissues was significantly higher than that
in the normal thyroid tissues (Fig.
1A and B) (P<0.05), while
LC3B-II protein in the HT tissues was lower than that in normal
thyroid tissues (Fig. 1A and
C, P<0.05). This suggests that
the level of autophagy-related proteins in HT tissues is lower than
that in normal tissues.
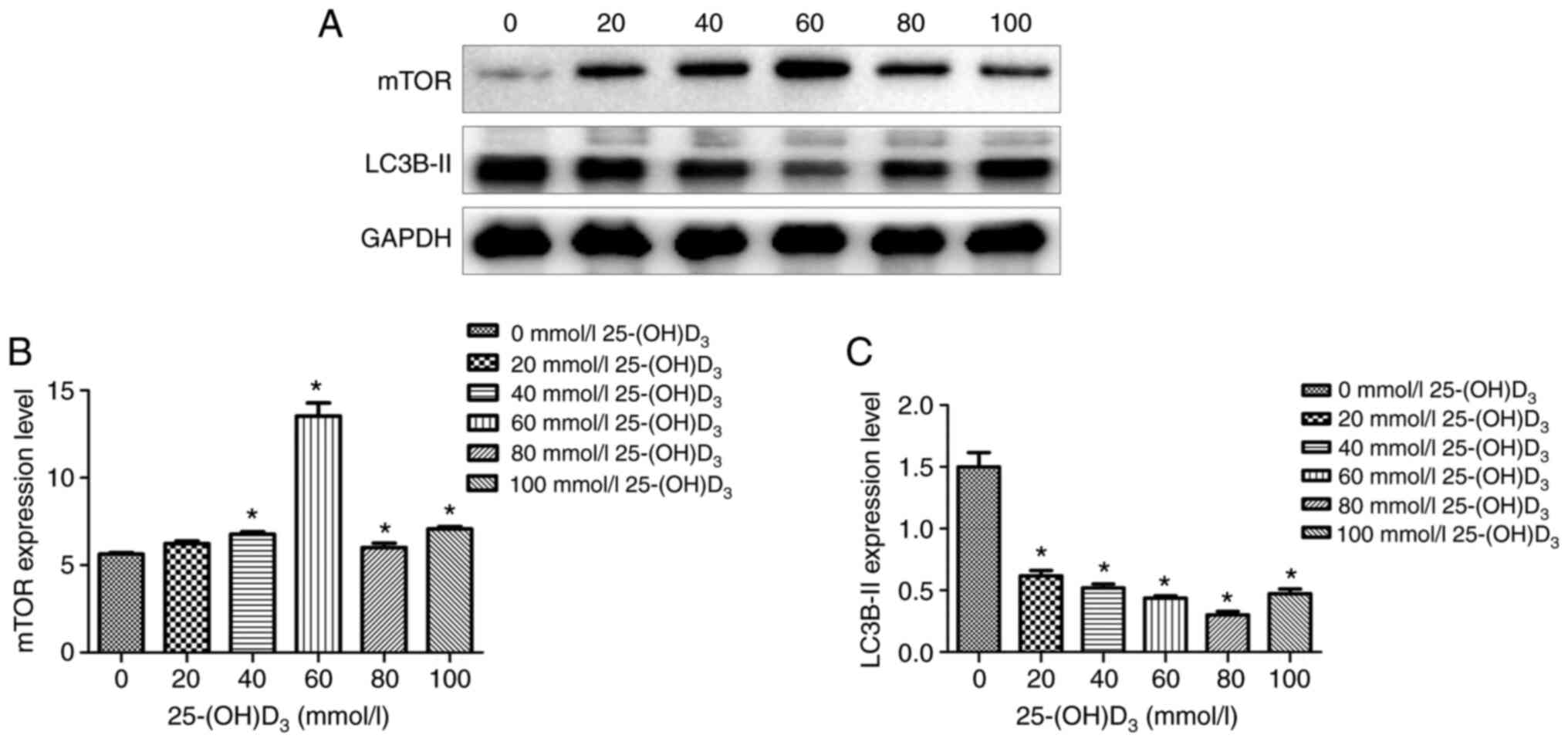
Effects of different concentrations of
25-(OH)D3 on the autophagy of thyroid cells
Nthy-ori3-1 cells were treated with
25-(OH)D3 at different concentrations for 24 h. The
expression levels of autophagy proteins, mTOR and LC3B, in each
group were detected by western blot analysis. The results of WB
experiment showed that with the increase in 25-(OH)D3
concentration, the expression level of mTOR protein was
significantly increased (Fig. 2A
and B) and the expression level of
LC3B-II protein was significantly decreased, especially when the
concentration of 25-(OH)D3 was 60 mmol/l (Fig. 2A and C).
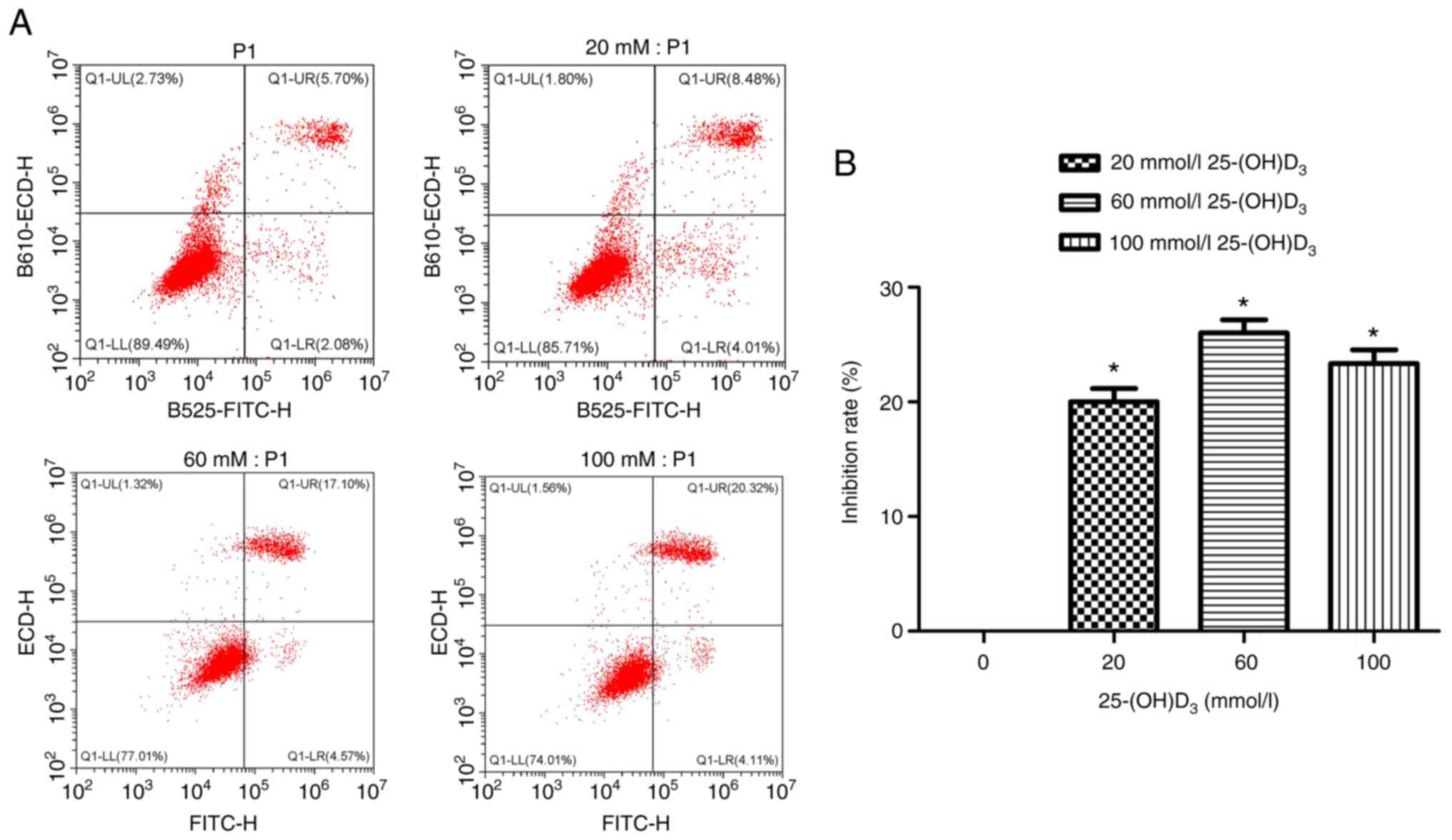
Effects of different concentrations of
25-(OH)D3 on apoptosis of Nthy-ori 3-1 cells
Nthy-ori 3-1 cells were treated with different
concentrations of 25-(OH)D3 (0, 20, 60, 100 mmol/l) for
24 h, and then the cells were collected to detect the apoptosis
rate. The results showed that the apoptosis rate was gradually but
significantly increased with the increase in 25-(OH)D3
concentration (P<0.05), especially when treated with 60 mmol/l
(Fig. 3).
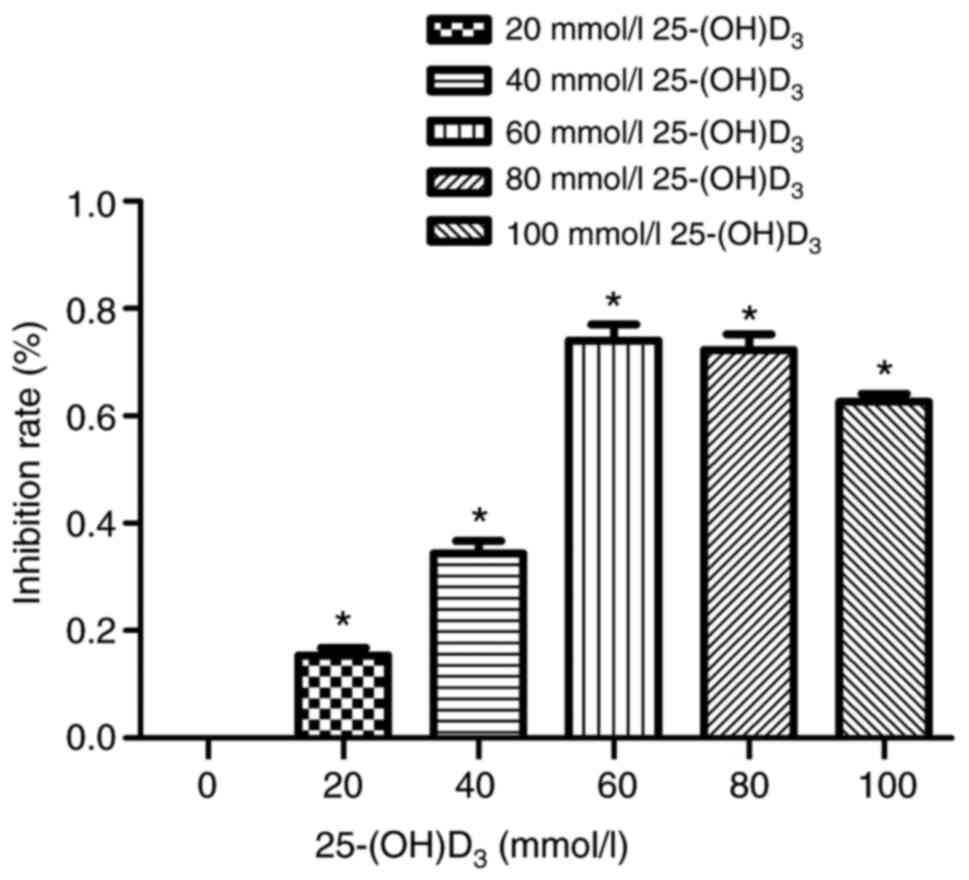
Effects of different concentrations of
25-(OH)D3 on the proliferation of Nthy-ori 3-1
cells
With the increase in the concentration of
25-(OH)D3, the cell proliferation ability was
significantly decreased (increased rate of inhibition), especially
when treated with 60 mmol/l. When treated with 80 and 100 mmol/l,
the proliferation capacity was not significantly decreased as the
inhibition rate decreased. We speculated that excessive
25-(OH)D3 may produce cytotoxicity when increased to
excessively high concentration (Fig.
4).
Discussion
Hashimoto's thyroiditis (HT) is a common autoimmune
thyroiditis (AIT). It has been reported that a variety of cytokines
are involved in the occurrence of the disease by alteration of
certain functions of immune-competent cells and thyroid follicular
epithelial cells. Studies have shown that Th1 and Th2 cytokines
play important roles in the occurrence and development of HT
(15-17).
It was found previously that serum IFN-γ is positively correlated
with TGAb and TPOAb, and IL-2 is positively correlated with TGAb
and TMAb (18). IFN-γ was found to
be highly expressed and IL-4 was lowly expressed in the serum of an
autoimmune thyroiditis model (19).
The positive expression of IFN-γ in HT patients is significantly
higher than that of IL-4, indicating that the thyroid tissue of HT
patients is dominated by Th1 cells that secrete IFN-γ, and Th1/Th2
cell imbalance shifts to the predominance of Th1 cell drift. The
imbalance of Th1/Th2 cell population activates the pathological
immune response of the thyroid, leading to a series of
pathophysiological changes (20).
The expression levels of IFN-γ, IL-2, IL-4, IL-6 and
IL-10 in serum of HT group and control group were detected. It was
found that the levels of IFN-γ, IL-2 and IL-6 in the serum of the
HT group were higher than those of the control group, while the
levels of IL-4 and IL-10 were lower.
Vitamin D is an important vitamin necessary for the
human body. With the discovery of its vitamin D receptor (VDR) in
many immune organs and tissues, it was found that it plays an
important role in immune regulation. Studies have shown that HT
patients may have 25-(OH)D3 deficiency (21). It was previously found that the
level of 25-(OH)D3 was significantly correlated with the
levels of IL-2, IFN-γ and IL-4 in patients with HT hypothyroidism,
suggesting that 25-(OH)D3 could affect the immune
function of the body by affecting the secretion of Th1 and Th2
cytokines (22). The degree and
incidence of the disease can be reduced by adding
25-(OH)D3 to experimental autoimmune thyroiditis (AIT)
rats and giving 25-(OH)D3 before the onset of AIT. After
the application of 25-(OH)D3, the levels of IFN-γ and
IL-12 were found to be decreased, while the levels of IL-4 and
IL-10 were increased (23). There
are few reports concerning the correlation between
25-(OH)D3 and Th1/Th2 cytokines in patients with HT.
This study showed that the level of 25(-OH)D3 was
negatively correlated with the levels of IL-2, IFN-γ, IL-4, IL-6
and IL-10 in the serum of HT patients, which is consistent with
existing research results. It suggested that serum
25-(OH)D3 levels in HT patients may be involved in
disease progression by upregulation of IFN-γ and IL-2 levels and
downregulation of IL-10 levels.
Autophagy is a function that allows cells to
maintain cell activity by degrading and recycling harmful
substances such as damaged organelles through lysosomes. Mammalian
target of rapamycin (mTOR) is a conserved protein kinase that plays
a key role in coordinating the balance between cell growth and
autophagy. When cells lack nutrients, mTOR can be inhibited to
induce autophagy. mTOR and LC3B are two important autophagy-related
proteins. mTOR-mediated signal transduction acts on downstream
effectors, which can initiate transcription and translation of
related genes and regulate autophagy. Activation of the mTOR
signaling pathway can inhibit autophagy, and LC3B is an important
marker of autophagy. When autophagy is activated, cytoplasmic LC3B
(i.e., LC3B-I) changes into membrane LC3B (i.e., LC3B-II). Its
expression level can be used as an indicator to evaluate the level
of autophagy.
In the present study, the high expression of mTOR
protein and the low expression of LC3B-II protein in thyroid
tissues of patients with HT revealed that the level of autophagy in
HT tissues was lower than that in normal tissues. Nthy-ori3-1
thyroid cells were treated with different concentrations (0, 20,
40, 60, 80 and 100 mmol/l) of 25-(OH)D3 for 24 h.
Western blot results showed that with the increase in
concentration, the expression level of mTOR protein increased, and
the expression level of LC3B-II protein was decreased, especially
when the concentration of 25-(OH)D3 was 60 mmol/l. This
suggested that a certain concentration of 25-(OH)D3
inhibited the expression of autophagy-related protein LC3B-II by
raising mTOR. Consistently, the level of autophagy-related protein
LC3B-II in the thyroid tissue of HT patients was lower than that in
normal tissues, suggesting that in HT patients,
25-(OH)D3 induces abnormal autophagy in thyroid
epithelial cells and is related to HT. With the increase of
25-(OH)D3 concentration, the cell proliferation ability
obviously decreased. A certain concentration of
25-(OH)D3 can participate in the process of HT by
inhibiting autophagy and proliferation of thyroid epithelial
cells.
In conclusion, the present study explored the
relationship of Th1 and Th2 cytokine balance with
25-(OH)D3 in HT patients. Human HT were selected to
detect the effect of 25-(OH)D3 on autophagy and
proliferation of human HT. The mechanism of vitamin D on HT at
cellular level was discussed. This study provides a new direction
and idea for the prevention, control and treatment of Hashimoto's
thyroiditis.
Acknowledgements
Not applicable.
Funding
This work was supported by Qiqihar Science and Technology Plan
Project (SFGG-201940).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JH, YL, HL, CZ, JZ, XS and SZ conceived and designed
this study. JZ offered administrative support. JH, YL, HL, CZ, JZ,
XS and SZ dealt with the experimental materials for study. JH, YL,
HL, CZ, JZ and XS helped with data collection and summary. JH, YL,
HL, CZ, JZ, XS and SZ were responsible for data analysis and
interpretation. JH, YL and JZ wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Second Affiliated Hospital of Qiqihar Medical University.
Signed written informed consents were obtained from the
patients.
Patent consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sheng JG, Wang B, Cao KK and Zhang S:
Relationship of thyroid ultrasound elasticity contrast index with
serum autoantibody and Th1/Th2 cytokine levels in patients with
Hashimoto's thyroiditis. J Hainan Med Univ. 22:147–150. 2016.
|
|
2
|
Qin Q, Liu P, Liu L, Wang R, Yan N, Yang
J, Wang X, Pandey M and Zhang JA: The increased but non-predominant
expression of Th17- and Th1-specific cytokines in Hashimoto's
thyroiditis but not in Graves' disease. Braz J Med Biol Res.
45:1202–1208. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lu J, Qiu C, Ye L, Fan X and Ke M:
Observation of topological valley transport of sound in sonic
crystals. Sci Found China. 13(2)2007.
|
|
4
|
Chen B, Qu S, Li M, Ye L, Zhang S, Qin T
and Fan H: Effects of 1,25-dihydroxyvitamin D3 in an
ovalbumin-induced allergic rhinitis model. Int Immunopharmacol.
47:182–189. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Akhtar E, Mily A, Haq A, Al-Mahmud A,
El-Arifeen S, Hel Baqui A, Roth DE and Raqib R: Prenatal high-dose
vitamin D3 supplementation has balanced effects on cord blood Th1
and Th2 responses. Nutr J. 15(75)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wen HY, Luo J, Li XF, Wei DD and Liu Y:
1,25-Dihydroxyvitamin D3 modulates T cell differentiation and
impacts on the production of cytokines from Chinese Han patients
with early rheumatoid arthritis. Immunol Res. 67:48–57.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Safdari V, Alijani E, Nemati M and
Jafarzadeh A: Imbalances in T cell-related transcription factors
among patients with Hashimoto's thyroiditis. Sultan Qaboos Univ Med
J. 17:e174–e180. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Guo Y, Zynat J, Xing S, Xin L, Li S,
Mammat N, Chen Y, Zhao L, Zhao H and Wang X: Immunological changes
of T helper cells in flow cytometer-sorted CD4+ T cells
from patients with Hashimoto's thyroiditis. Exp Ther Med.
15:3596–3602. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ikemoto Y, Kuroda K, Nakagawa K, Ochiai A,
Ozaki R, Murakami K, Jinushi M, Matsumoto A, Sugiyama R and Takeda
S: Vitamin D regulates maternal T-helper cytokine production in
infertile women. Nutrients. 10(902)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chung BH, Kim BM, Doh KC, Cho ML, Kim KW
and Yang CW: Protective effect of 1α,25-dihydroxyvitamin D3 on
effector CD4+ T cell induced injury in human renal
proximal tubular epithelial cells. PLoS One.
12(e0172536)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sheikh V, Kasapoglu P, Zamani A, Basiri Z,
Tahamoli-Roudsari A and Alahgholi-Hajibehzad M: Vitamin D3 inhibits
the proliferation of T helper cells, downregulate CD4+ T
cell cytokines and upregulate inhibitory markers. Hum Immunol.
79:439–445. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Song JH, Park E, Kim MS, Cho KM, Park SH,
Lee A, Song J, Kim HJ, Koh JT and Kim TS: L-Asparaginase-mediated
downregulation of c-Myc promotes
1,25(OH)2D3-induced myeloid differentiation
in acute myeloid leukemia cells. Int J Cancer. 140:2364–2374.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhao M, Duan XH, Wu ZZ, Gao CC, Wang N and
Zheng ZH: Severe vitamin D deficiency affects the expression of
autophagy related genes in PBMCs and T-cell subsets in active
systemic lupus erythematosus. Am J Clin Exp Immunol. 6:43–51.
2017.PubMed/NCBI
|
|
14
|
Abu EL, Maaty MA and Wölfl S: Vitamin D as
a novel regulator of tumor metabolism: Insights on potential
mechanisms and implications for anti-cancer therapy. Int J Mol Sci.
18(2184)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Upadhyay R, Dua B, Sharma B, Natrajan M,
Jain AK, Kithiganahalli Narayanaswamy B and Joshi B: Transcription
factors STAT-4, STAT-6 and CREB regulate Th1/Th2 response in
leprosy patients: Effect of M. leprae antigens. BMC Infect Dis.
19(52)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Abebe F: Synergy between Th1 and Th2
responses during mycobacterium tuberculosis infection: A review of
current understanding. Int Rev Immunol. 38:172–179. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xue M, Xie J, Liu L, Huang Y, Guo F, Xu J,
Yang Y and Qiu H: Early and dynamic alterations of Th2/Th1 in
previously immunocompetent patients with community-acquired severe
sepsis: A prospective observational study. J Transl Med.
17(57)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zeng R, Lyu Y, Zhang G, Shou T, Wang K,
Niu H and Yan X: Positive effect of RORγt on the prognosis of
thyroid papillary carcinoma patients combined with Hashimoto's
thyroiditis. Am J Transl Res. 10:3011–3024. 2018.PubMed/NCBI
|
|
19
|
Zhang K, Wang Y, Ma W, Hu Z and Zhao P:
Genistein improves thyroid function in Hashimoto's thyroiditis
patients through regulating Th1 cytokines. Immunobiology.
222:183–187. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cui Z, Wang Z, Liu X, Cai Y, Xu X and Yang
T: Establishment of clinical diagnosis model of Graves' disease and
Hashimoto's thyroiditis. J Transl Med. 17(11)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Helvaci N, Oguz SH, Kabacam S, Karabulut
E, Akbiyik F, Alikasifoglu M and Gurlek A: Clock gene PERIOD3
polymorphism is associated with susceptibility to Graves' disease
but not to Hashimoto's thyroiditis. Chronobiol Int. 36:1343–1350.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li Y, Xu L, Zhou YH, Ouyang XY and Cao T:
Combination of periodontal, orthodontic and endodontic therapy in
upper anterior teeth with hopeless prognosis and long-time
follow-up: A case report. Beijing Da Xue Xue Bao Yi Xue Ban.
49:740–744. 2017.PubMed/NCBI(In Chinese).
|
|
23
|
Sheng L, Turner AG, Tarulli GA, Barratt K,
Kremer R, Morris HA, Callen DF and Anderson PH: Abstract P4-05-02:
Conditional inactivation of the 25-hydroxyvitamin D-24-hydroxylase
(Cyp24a1) in the mouse mammary epithelium alters mammary gland
development. Cancer Res. 77 (Suppl 4)(P4-05-02)2017.
|