Introduction
Asthma is one of the major chronic and
non-communicable syndromes in both children and adults, whereby
~334 million people suffer from this disease worldwide (1). The pathophysiological process of
asthma involves several types of cells and cellular components,
among which airway remodeling is a well-known characteristic caused
by recurrent injury and repair processes initiated by chronic
inflammation (2). As a chronic
inflammatory disorder of the respiratory air passages, asthma may
initially be reversible but progresses into an irreversible state
due to airway remodeling (3).
Moreover, the prevalence of asthma has been increasing worldwide
with a yearly rate of 1.8%, and 300 million people affected are in
mainland China, causing a substantial economic burden (4,5);
therefore, understanding the precise pathophysiology of asthma is
paramount to achieve optimal management.
Recent research has focused on the identification of
candidate pharmacologically active ingredients in natural herbs to
prevent and treat asthma (6-9).
Glycyrrhizic acid (GA), also known as glycyrrhizin, is one of the
most important bioactive constituents isolated from the traditional
Chinese herb Glycyrrhiza uralensis, which has been
demonstrated to display multiple pharmacological activities, such
as anticancer, antiviral and anti-inflammatory effects (10-12).
In addition, the number of studies focusing on the protective
effects of GA against asthma have increased; however, its
underlying molecular mechanism involved in asthma remains unknown
(13,14).
Research has reported that targeting the
transforming growth factor-β1 (TGF-β1)/Smad signaling pathway may
provide a novel therapeutic method for asthma airway remodeling
(15). TGF-1 is considered the main
regulator of airway remodeling in asthma and directly affects the
deposition of collagen in the airway wall (16). The Smad protein, an important member
of the TGF-β signal transduction system, is central to the signal
transduction pathway (17,18). However, whether GA attenuates asthma
symptoms through the TGF-β1/Smad signaling pathway remains unclear.
Thus, the present study aimed to investigate the function of GA on
the TGF-1/Smad signaling pathway in airway inflammation and
remodeling in ovalbumin (OVA)-induced asthma mouse models, in order
to provide a theoretical background for asthma management.
Materials and methods
Experimental animals
A total of 50 healthy female BALB/c mice (20-22 g)
aged 10-12 weeks were obtained from Hunan SJA Laboratory Animal
Co., Ltd. (Changsha, China) and housed in the Laboratory Animal
Center of Medical College of Hunan Normal University. All mice were
fed in an animal house at a relative humidity of 40-60%,
temperature of 22-26˚C and sufficient light. In addition, the mice
had free access to food and water in a controlled environment
(sterile food and drinking water in accordance with room pressure
standard). All animal experiments were approved by the local Ethics
Committee of Beijing University of Chinese Medicine and performed
in accordance with the Guide for the Care and Use of Laboratory
Animals (19).
Animal grouping and model
establishment
A total of 50 female mice were randomly assigned
into five groups (10 mice/group), as follows: Blank group, asthma
group, GA group (80 mg/kg; cat. no. 1295888; Sigma-Aldrich; Merck
KGaA), dexamethasone group (2 mg/kg; cat. no. D1756; Sigma-Aldrich;
Merck KGaA) and the GA + TGF-β1 group. During the course of asthma
sensitization, the mice in the asthma, GA, dexamethasone and GA +
TGF-β1 groups were intraperitoneally injected with 0.2 ml
sensitizing liquid supplemented with 200 µg/ml OVA (Sigma-Aldrich;
Merck KGaA) and 20 mg/ml aluminum hydroxide suspension (Chengdu
Kelong Chemical Co., Ltd.), at days 0, 7 and 14. In addition, mice
in the GA + TGF-β1 group were intraperitoneally injected with
exogenous recombinant TGF-β1 (0.1 µg/rat; 100-21C; PeproTech,
Inc.). Mice in the blank group were simultaneously given
intraperitoneal injections of the same amount of normal saline
solution (0.7%) as the control group. After the mice were in phase
excitation from the 14th day, the OVA solution (0.2 ml) was
delivered into the noses of mice in the asthma, GA, dexamethasone
and GA + TGF-β1 groups to construct a chronic asthma model, and the
blank group received a continuous nasal drip with the same amount
of normal saline. The excitation period lasted for 6 days.
In the excitation phase, mice were anesthetized by
intraperitoneal injection with 250 mg/kg tert butyl alcohol (cat.
no. 471712; Sigma-Aldrich, Merck KGaA) and tribromethanol (cat. no.
T48402; Sigma-Aldrich, Merck KGaA) mixture (diluted with PBS, x40).
The OVA solution was delivered to the nose of mice using a 50 µl
micropump (Shanghai Elab Scientific Instruments). At the time of
excitation, mice in the GA and dexamethasone groups were
intraperitoneally injected with GA and dexamethasone 30 min before
the nasal drip according to 80 and 2 mg/kg standards (20), respectively. The general conditions
of the mice in each group, including their appetites, fur, mental
conditions, weight changes and airway pathological changes were
observed and recorded every day.
Preparation for lung tissue
slices
All mice were anesthetized by injection with 250
mg/kg tert butyl alcohol (cat. no. 471712; Sigma-Aldrich, Merck
KGaA) and tribromethanol (cat. no. T48402; Sigma-Aldrich, Merck
KGaA) mixture ~24 h after model establishment. Subsequently, their
eyeballs were fully exposed, and blood was collected via orbital
blood sampling and transferred into a sodium citrate
anticoagulation tube. The tube was centrifuged for 10 min at room
temperature at 1,000 x g prior to collection of the supernatant and
storage at -20˚C.
The mice were sacrificed by cervical vertebrae
dislocation, and the thoracic cavity was immediately opened to
expose the lung tissues. Normal saline (0.7%) was injected into the
lung tissues for perfusion and rinsed using 10 ml injection syringe
until the lung tissues blanched. The right lung tissues were
rapidly extracted and placed in liquid nitrogen for storage,
whereas the left lung tissues were harvested and fixed in 4%
paraformaldehyde for 24 h (room temperature) to prepare paraffin
sections. The tissue sections were subsequently subjected to
hematoxylin and eosin (H&E) and Masson staining.
H&E staining
The paraffin-embedded tissue samples were cut into
5-µm-thick sections and deparaffinized in xylene Ⅰ and xylene II
for 15 min each (room temperature), with an ethanol wash (two
times, 5 min each) at room temperature. The tissue sections were
subsequently rehydrated in a descending ethanol series (95, 80 and
70%; 5 min each) and washed two times with deionized water. The
paraffin sections were dewaxed, stained with hematoxylin for 10 min
(room temperature), washed in running water for 5 min followed by
differentiation in 1% hydrochloric acid ethanol solution for 30
sec, and re-washed in running water. Tissue sections were
subsequently stained with 1% eosin for 30 sec at room temperature
and washed in running water, prior to rehydration in an ethanol
series (95, 95, 100 and 100%; 1 min each) and cleared in xylene
(two times, 3 min each). Tissue sections were sealed with a neutral
resin seal sheet for observation under a fluorescence microscope
(DM3000 and DM3000 LED; Leica Microsystems GmbH).
Masson staining
The paraffin-embedded tissue samples were cut into
5-µm thick sections and deparaffinized in xylene Ⅰ and xylene II
for 15 min each, with an ethanol wash (two times, 5 min each) at
room temperature. The tissue sections were subsequently rehydrated
in a descending an ethanol series (95, 80 and 70%, 5 min each),
with repeated cleaning cycles. Tissue sections were subsequently
stained with Masson trichrome stain (Beijing Solarbio Science &
Technology Co., Ltd.) for 5-10 min (room temperature) and rinsed
with 2% acetic acid solution, prior to differentiation in 1%
molybdophosphoric acid aqueous solution for 3-5 min. Aniline
solution was subsequently added to the tissue sections for 5 min,
followed by a final rinse with 0.2% glacial acetic acid solution.
Tissue sections were rehydrated in an ethanol series (95, 95, 100
and 100%; 1 min each) and cleared in xylene (two times, 3 min
each), prior to sealing with a neutral resin for observation using
an optical microscope (20X objective). The thickness of the airway
wall (Wat, µm2), smooth muscle layer (Wam,
µm2), thickness of the inside airway wall (Wai,
µm2), collagen deposition under the basement membrane
(Wcol, µm2) and basement membrane perimeter (Pbm) were
measured using Image-Pro Plus 6.0 (IPP 6.0) software (Media
Cybernetics, Inc.).
Periodic acid-Schiff (PAS)
staining
Goblet cell hyperplasia was assessed via PAS
staining. The paraffin-embedded lung tissues (5-µm-thick) were
deparaffinized in xylene Ⅰ and II for 20 min, and rehydrated in
ethyl alcohol Ⅰ, ethyl alcohol II and 75% alcohol for 5 min each,
prior to rinsing with tap water for 2 min. Tissue sections were
subsequently treated with periodic acid for 15 min and rinsed with
running water for 2 min, prior to staining with Coleman's Schiff
stain at room temperature in the dark for 30 min. Sections were
counterstained with hematoxylin and differentiated in 1%
hydrochloric acid ethanol (rinsed after each step). Tissue sections
were rehydrated in ethyl alcohol Ⅰ, ethyl alcohol II and ethyl
alcohol III (5 min each), cleared in xylene Ⅰ and II (5 min each)
and sealed in neutral resin prior to observation under an optical
microscope (20X objective). The PAS-positive area (APAS+) and
basement membrane perimeter (Pbm) was determined using IPP 6.0
software. The degree of goblet cell hyperplasia was represented as
APAS+/Pbm (µm2/µm).
Enzyme-linked immunosorbent assay
(ELISA)
ELISA was used to detect inflammatory factors in
serum of rats. The levels of inflammatory cytokines interleukin
(IL)-4, IL-5, IL-13 and IL-17 in mouse serum in each group were
determined using the following Mouse ELISA kits: IL-4 (cat. no.
BMS613), IL-5 (cat. no. EMIL5ALPHA), IL-13 (cat. no. BMS6015) and
IL-17 (cat. no. BMS6001; all purchased from Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's
protocols. The absorbance value (OD value) of each well was
measured at a wavelength of 450 nm, using a multimode microplate
(Synergy 2; BioTek Instruments, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from lung tissues using
TRIzol® reagent (Thermo Fisher Scientific, Inc.), and
the purity and concentration of RNA were detected using a multimode
microplate (BioTek Instruments, Inc.). Following quantification,
total RNA was reverse transcribed into cDNA using a Path-ID™
Multiplex One-Step RT-PCR kit (cat. no. 4442136; Applied
Biosystems; Thermo Fisher Scientific, Inc.). qPCR with SYBR-Green
detection was subsequently performed using a CFX Connect
fluorescence quantitative PCR detection system (Bio-Rad
Laboratories, Inc.). The primer sequences are presented in Table I. The following thermocycling
conditions were used for qPCR: Initial denaturation at 95˚C for 10
min, denaturation at 95˚C for 10 sec, annealing at 60˚C for 20 sec
and a final extension at 72˚C for 34 sec for 40 total cycles.
Relative mRNA levels were quantified using the 2-ΔΔCq
method (21) and normalized to the
internal reference gene GAPDH.
 | Table IPrimer sequences used for
quantitative PCR. |
Table I
Primer sequences used for
quantitative PCR.
| Primer | Sequence
(5'-3') |
|---|
| TGF-β1 | F:
GTGTGGAGCAACATGTGGAACTCTA |
| | R:
TTGGTTCAGCCACTGCCGTA |
| SMAD2 | F:
CCACTACCAGAGGGTGGAGA |
| | R:
TAACTGGCTGCAATCCAAG |
| SMAD7 | F:
AGGCATTCCTCGGAAGTCAA |
| | R:
TGGACAGTCTGCAGTTGGTTTG |
| GAPDH | F:
GTCGATGGCTAGTCGTAGCATCGAT |
| | R:
TGCTAGCTGGCATGCCCGATCGATC |
Western blotting
The right lung tissue of mice was ground into a
powder under liquid nitrogen and subsequently lysed using lysis
buffer for 30 min on ice. Cells were centrifuged at 7,500 x g for
10 min at 4˚C. The supernatant was collected, and total protein was
quantified using a bicinchoninic acid assay (Vazyme Biotech Co.,
Ltd.) and 30 µg protein/lane was separated via SDS-PAGE on a 10%
gel. The separated proteins were subsequently electroblotted onto
polyvinylidene difluoride membranes (EMD Millipore) and blocked
with 5% nonfat milk at room temperature for 1 h. The membranes were
incubated with primary antibodies against: Rabbit anti-mouse TGF-β1
(cat. no. 3711S; 1:1,000; Cell Signaling Technology, Inc.), Smad2
(cat. no. 5339S; 1:1,000; Cell Signaling Technology, Inc.) and
Smad7 (cat. no. ab216428; 1:1,000; Abcam) overnight at 4˚C.
Membranes were washed three times with TBST (10 min/wash).
Following the primary incubation, membranes were incubated with
horseradish peroxidase labeled goat anti-rabbit IgG secondary
antibody (1:5,000; cat. no. CW0103S; Beijing ComWin Biotech Co.,
Ltd.) at room temperature for 1 h. Membranes were re-washed three
time with TBST (10 min/wash), and protein bands were visualized
using a chemiluminescence imaging system (Tanon Science and
Technology Co., Ltd.) and normalized to the internal reference gene
GAPDH.
Statistical analysis
Every experiment was replicated thrice. Statistical
analysis was performed using SPSS 17.0 software (SPSS, Inc.) and
data are presented as the mean ± standard deviation. t-test was
used for comparison between two groups and one-way ANOVA was used
for comparisons among multiple groups. Homogeneous data were
analyzed using the least significant difference test (three groups)
or Tukey's post hoc test (more than three groups). Nonhomogeneous
data were analyzed using Kruskal-Wallis and Dunn's post hoc tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Establishment of chronic asthma mouse
models
Following sensitization and excitation by OVA, mice
in the asthma, GA and dexamethasone groups developed the following
symptoms: Shortness of breath, irritability, incontinence, rapid
breathing, irregular breathing rhythm, visibly trembling limbs,
cyanosis, cough and decreased activity. Conversely, no obvious
symptoms were observed among mice in the blank group. Notably, mice
in the GA and dexamethasone groups did not vary to a great extent;
however, mice in the asthma group had severe symptoms compared with
those in the GA and dexamethasone groups. No mice died during this
experiment.
The results of H&E staining are presented in
Fig. S1A, which exhibited no
remarkable airway inflammation and lung morphology changes in the
blank group. However, mice in the asthma group had a significantly
thicker airway wall and airway smooth muscle, a broadened strata
submucosa, mucosal epithelial hyperplasia and heightened mucus
secretion (P<0.01; Fig. S1A and
B). In addition, mice in each
group were scored in compliance with the scoring directive
(Table II) (22), which revealed notable airway
inflammation, inflammatory cell infiltration and airway remolding
in mice with OVA-induced asthma compared with mice in the blank
group (P<0.01; Fig. S1B and
C).
 | Table IIStandard of inflammation score. |
Table II
Standard of inflammation score.
| Score | Airway inflammatory
cells | Alveolar
inflammation |
|---|
| 0 | No cells | No infection |
| 1 | A few cells | A few macrophages
in alveoli |
| 2 | A ring of 1 cell
layer deep | Slight thickening
of alveolar walls, more macrophages and eosinophils in alveoli |
| 3 | A ring of 2-4 cells
deep | Significant
thickening of alveolar walls, multinucleated giant cells and
eosinophils in 30-50% of alveoli involved |
| 4 | A ring of >4
cells deep | Significant
thickening of alveolar walls, multinucleated giant cells and
eosinophils in >50% of alveoli involved |
| 5 | - | Alveolar
consolidation |
Masson staining stained the collagen fiber blue, the
muscle fiber cytoplasm red and the nucleus blue/brown. Following
Masson staining of the lung tissues, the degree of airway wall
thickness (Wat, µm2), smooth muscle layer (Wam,
µm2), thickness of the inside airway wall (Wai,
µm2) and collagen deposition under the basement membrane
(Wcol, µm2) were measured and standardized to the
perimeter of the basement membrane (Pbm). The results demonstrated
that mice in the asthma group exhibited effectively enhanced
deposition of airway collagen fiber in addition to elevated
Wat/Pbm, Wam/Pbm, Wai/Pbm and Wcol/Pbm, compared with mice in the
blank group (P<0.01; Fig. S1A
and D).
PAS staining was performed to detect goblet cells,
and the results demonstrated that there were no PAS-positive goblet
cells in the blank group. Conversely, a large number of
PAS-positive goblet cells were observed in the airway epithelium of
asthmatic mice, causing excessive secretion of airway mucus
(Fig. S1A). The PAS-positive area
(APAS+/Pbm) of the membrane length (µm) was
quantitatively measured and the results demonstrated that
APAS+/Pbm significantly increased in OVA-exposed mice
compared with the blank group (P<0.01; Fig. S1E). Taken together, these results
exhibited successful establishment of OVA-induced chronic asthma
models.
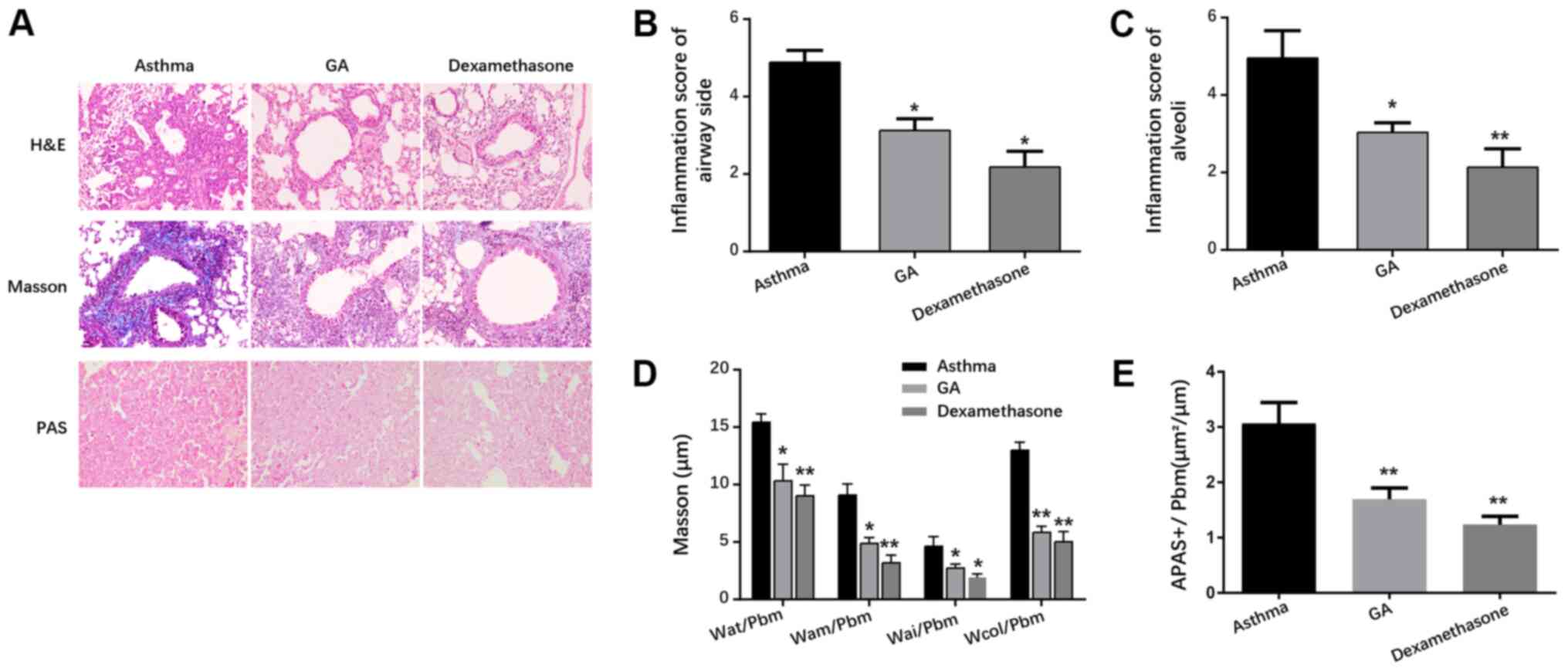
GA attenuates lesions in airways and
airway collagen deposition in mice with chronic asthma
As presented in Fig.
1A, mice in the GA and dexamethasone groups exhibited modest
airway inflammation, and decreased airway wall and airway smooth
muscle layers compared with the asthma group. In addition, lesions
in all groups of mice were scored in compliance with the scoring
directive (Table II). The results
demonstrated that mice in the GA and dexamethasone groups had
mitigated airway inflammation, inflammatory cell infiltration and
airway remolding compared with the asthma group (P<0.05 and
P<0.01; Fig. 1B and C).
Masson staining demonstrated that mice in the GA and
dexamethasone groups exhibited elevated deposition of airway
collagen fiber, as well as mitigated Wat/Pbm, Wam/Pbm, Wai/Pbm and
Wcol/Pbm compared with mice in the asthma group (P<0.05;
Fig. 1A and P<0.01; Fig. 1D). PAS staining demonstrated that
the number of PAS-positive goblet cells significantly decreased in
the GA and dexamethasone groups compared with the asthma group,
suggesting that GA and dexamethasone decreased airway mucus
secretion in asthmatic mice (P<0.05; Fig. 1A and P<0.01; Fig. 1E).
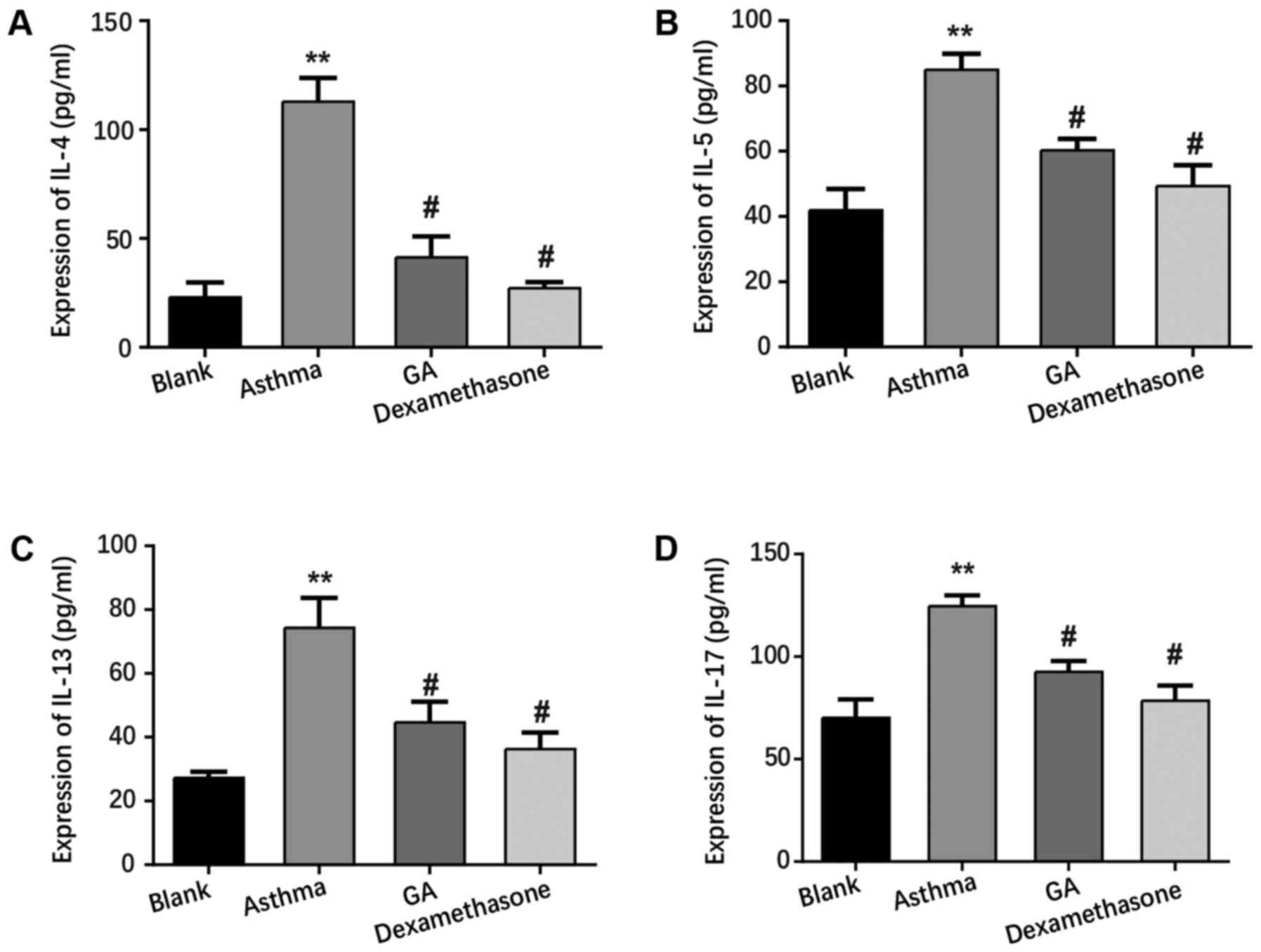
GA suppresses inflammation in mice
with chronic asthma
ELISA was performed to detect the serum levels of
inflammatory cytokines from each group. As presented in Fig. 2, the levels of IL-4, IL-5, IL-13 and
IL-17 significantly increased in the asthma group compared with the
blank group (P<0.01), while the GA and dexamethasone groups
exhibited lower levels of IL-4, IL-5, IL-13 and IL-17 compared with
the asthma group (P<0.05). Collectively, these results exhibit
the anti-inflammatory role of GA in mice with chronic asthma.
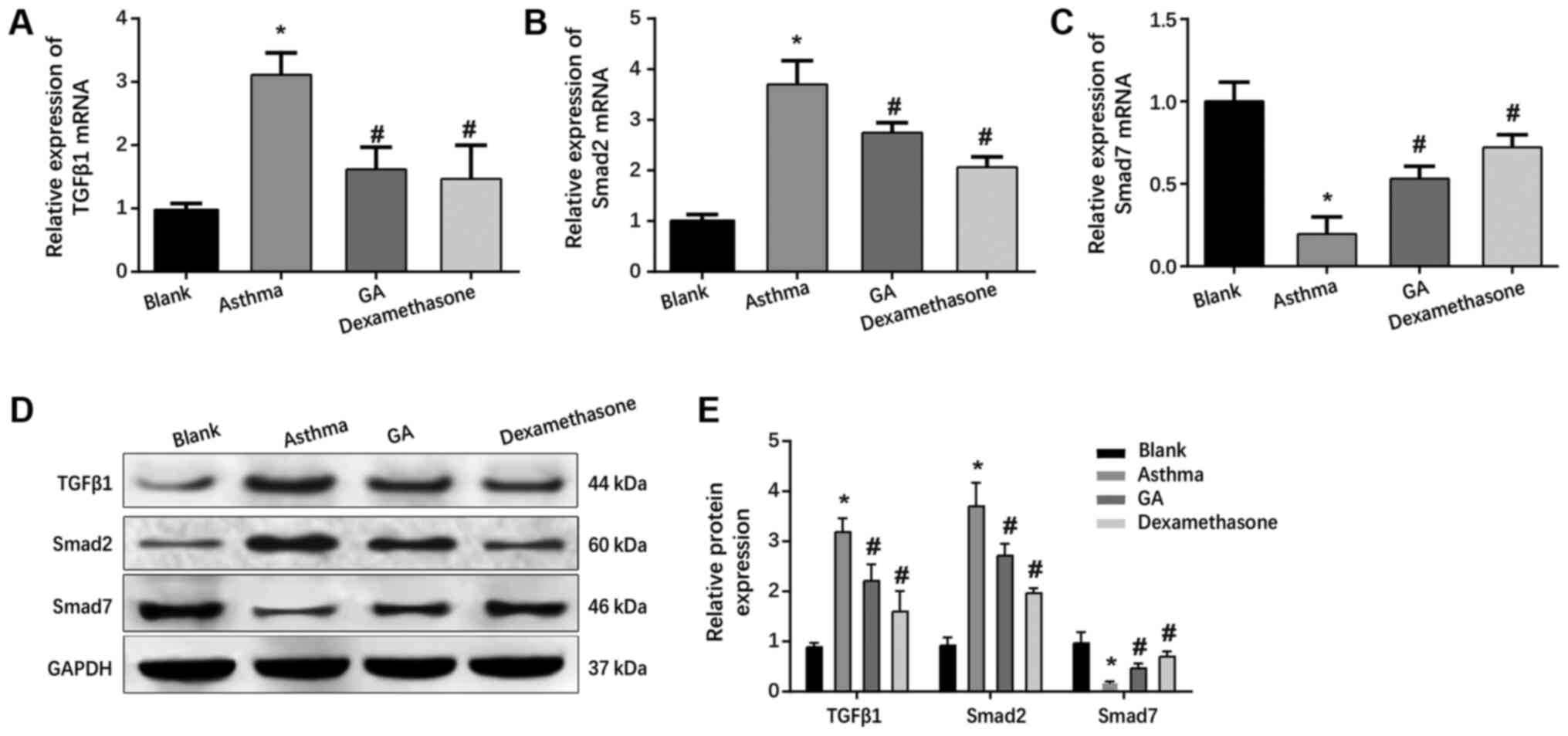
GA inhibits the TGF-β1/Smad signaling
pathway in OVA-challenged mice with asthma
mRNA and protein levels of TGF-β1, Smad2 and Smad7
in the lung tissues of mice in each group were assessed via western
blot and RT-qPCR analyses. The results demonstrated that both mRNA
and protein levels of TGF-β1 and Smad2 significantly increased in
the asthma, GA and dexamethasone groups (P<0.05; Fig. 3A, B,
D and E), while there was an obvious decrease in
Smad7 mRNA and protein levels compared with the blank group
(P<0.05; Fig. 3C-E).
The levels of TGF-β1 and Smad2 significantly
decreased (P<0.05; Fig. 3A,
B, D and E),
whereas Smad7 was strongly expressed in the GA and dexamethasone
groups (P<0.05; Fig. 3C-E)
compared with the asthma group. Taken together, these results
suggest that GA ameliorates airway inflammation and remodeling by
downregulating the TGF-β1/Smad signaling pathway in asthma.
Effects of exogenous recombinant
TGF-β1 on the regulation of GA in asthmatic mice
To further validate the association between GA and
the GF-β1/Smad signaling pathway on airway inflammation and
remodeling, mice with chronic asthma were established by
intraperitoneal injection of exogenous recombinant TGF-β1 and GA.
As presented in Fig. 4A, mice in
the GA + TGF-β1 group had aggravated airway inflammation, as well
as thickened airway walls and airway smooth muscle layers compared
with the GA group. Inflammation in all groups of mice was scored
(Table II), and the results
demonstrated that airway inflammation, inflammatory cell
infiltration and airway remolding were considerably exacerbated in
mice in the GA + TGF-β1 group compared with the GA group
(P<0.05; Fig. 4B and C).
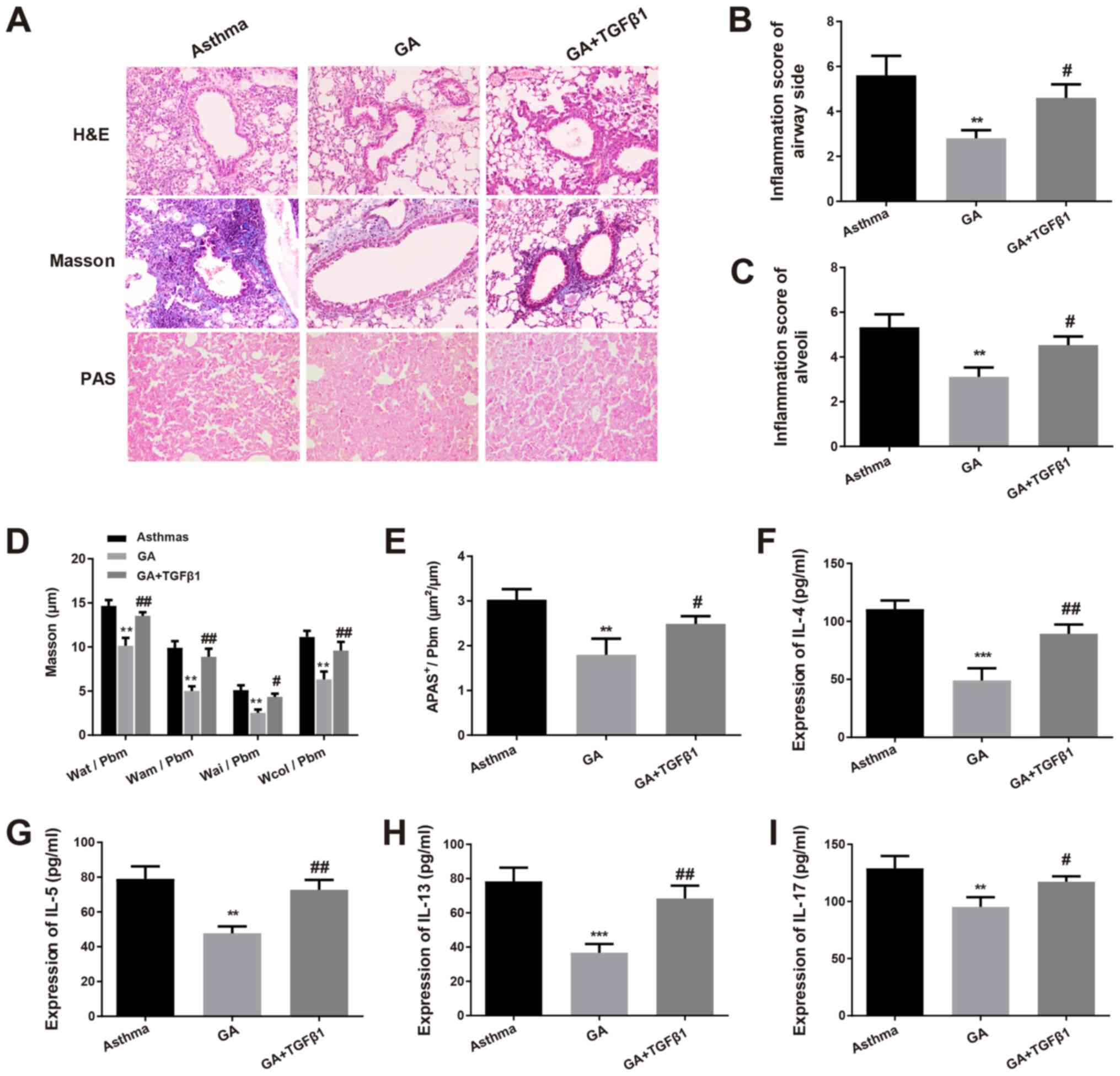 | Figure 4Effects of TGF-β1 in asthmatic mice.
(A) H&E, Masson and PAS staining (magnification, x200) were
performed to determine the pathological changes in the airways and
alveolar tissues of mice. (B) Inflammation of the airway and airway
side, as well as (C) pulmonary alveoli in mice of each group were
detected. (D) Masson staining was performed to determine the levels
of Wat/Pbm, Wam/Pbm, Wai/Pbm and Wcol/Pbm. (E) The PAS-positive
area per total area was measured. The serum levels of (F) IL-4, (G)
IL-5, (H) IL-13 and (I) IL-17 in mice of each group were detected
via the enzyme-linked immunosorbent assay. **P<0.01,
***P<0.001 vs. asthma group; #P<0.05,
##P<0.01 vs. GA group. TGF-β1, transforming growth
factor-β1; H&E, hematoxylin and eosin; PAS, periodic
acid-Schiff; IL, interleukin; GA, glycyrrhizic acid. |
Masson staining confirmed that there were decreased
deposition of airway collagen fiber coupled with increased Wat/Pbm,
Wam/Pbm, Wai/Pbm and Wcol/Pbm in the GA + TGF-β1 group compared
with the GA group (P<0.05 and P<0.01; Fig. 4A and D). PAS staining demonstrated that the
number of PAS-positive goblet cells significantly increased in the
GA + TGF-β1 group compared with the GA group, suggesting that
TGF-β1 increased airway mucus secretion in asthmatic mice
(P<0.05; Fig. 4A and E). In addition, ELISA was performed to
determine the serum levels of inflammatory cytokines in mice
treated with GA alone or with both TGF-β1 and GA. The results
demonstrated that the levels of IL-4, IL-5, IL-13 and IL-17
significantly increased in the GA + TGF-β1 group compared with the
GA group (P<0.05 and P<0.01; Fig.
4F-I). Collectively, these results suggest that the
anti-inflammatory role of GA in mice with chronic asthma occurs by
downregulating the TGF-β1/Smad signaling pathway.
Discussion
Immense efforts have been made for asthma therapy;
however, due to the heterogenicity and complexity of asthma,
effectively controlling asthma remains difficult (23). Previous studies have demonstrated
that GA ameliorates asthma and other human disease symptoms
including septic acute kidney injury, liver injury and lung injury
(14,24-26).
Thus, the present study aimed to investigate the therapeutic
effects of GA on airway inflammation and remodeling in asthmatic
mice and determine its underlying molecular mechanism in asthma.
Taken together, the results of the present study suggest that GA
may exert anti-asthmatic effects in mice by modulating the
TGF-β1/Smad signaling pathway.
H&E staining demonstrated an elevated number of
airway smooth muscle cells, and the smooth muscle layer was notably
thickened in mice following OVA treatment. Consistent with findings
of a previous report (27), Masson
staining indicated that intrapulmonary bronchial Wat/Pbm, Wam/Pbm,
Wai/Pbm and Wcol/Pbm increased in the OVA-sensitized group compared
with the blank group. Furthermore, PAS staining demonstrated that
there was a large number of PAS-positive goblet cells in the airway
epithelium of asthmatic mice. Thus, the present study successfully
established an asthma model in mice.
Currently, the anti-inflammatory role of GA has
received increasing attention (28,29).
In addition, it has been demonstrated that GA can alleviate the
symptoms of allergic asthma (30).
The results of the present study demonstrated that the ratios of
Wat/Pbm, Wam/Pbm, Wai/Pbm and Wcol/Pbm significantly decreased in
the GA and dexamethasone groups compared with those in the asthma
group. This suggests that GA may suppress increased thickness in
the airway wall and smooth muscle, yielding a positive effect on
improving airway remodeling. Similarly, the number of PAS-positive
goblet cells significantly decreased in the GA and dexamethasone
groups in contrast to the asthma group, suggesting that GA and
dexamethasone may decrease airway mucus secretion in asthmatic
mice. In addition, the levels of IL-4, IL-5, IL-13 and IL-17 were
significantly lower in the GA and dexamethasone groups compared
with the asthma group, indicating that GA may suppress the
inflammatory response in chronic asthma.
Increasing evidence have demonstrated that the
TGF-β1/Smad signaling pathway is considered one of the most
essential molecular mechanisms for airway remodeling in asthma
(15,31-33).
TGF-β1 is a profibrotic cytokine that is believed to exert a
critical role in chronic asthma (34). The Smad family of proteins is the
only proven substrate acted by the TGF-β1 receptor (35), and also the Smad protein has been
reported to modulate the intracellular mediators for TGF-β1
signaling (36). In addition,
previous studies have reported that TGF-β1 may have several
biological effects via the activation of downstream mediators, such
as Smad2, Smad3 and Smad4, and TGF-β1 is negatively regulated by
Smad7 expression (37-39).
The results of the present study demonstrated that TGF-β1 and Smad2
mRNA and protein levels notably increased in mouse lung tissues in
each group, while opposite effects were observed following GA
treatment. Furthermore, as an inhibitory Smad protein, Smad7 blocks
TGF-β signaling through a negative feedback loop (40). The present results showed that Smad7
exerted an inhibitory role in asthmatic mouse, and this inhibitory
effect was enhanced by GA treatment. Taken together, GA effectively
promoted Smad7 mRNA and protein expression, and downregulated the
levels of TGF-β1 and Smad2 in the lung tissues of mice with asthma.
Thus, the TGF-β1/Smad signaling pathway may be a novel therapeutic
approach for controlling asthma airway remodeling.
The present study also aimed to determine whether
the TGF-β1/Smad signaling pathway was implicated in the regulation
of GA on airway inflammation and remodeling. A previous study
reported that OVA-challenged mouse fibroblasts undergo
morphological alterations in response to exogenous TGF-β1
stimulation (41). In the present
study, exogenous TGF-β1 in combination with GA were
intraperitoneally injected into asthmatic mice to detect airway
inflammation and remodeling. As expected, exogenous TGF-β1
significantly increased airway inflammation, inflammatory cell
infiltration and airway remolding, accompanied by decreased
deposition of airway collagen fiber and increased Wat/Pbm, Wam/Pbm,
Wai/Pbm and Wcol/Pbm, which also resulted in the promotion of
airway mucus secretion and inflammatory cytokines in asthmatic
mice. Taken together, these results suggest that GA inhibits asthma
airway remodeling in mice with asthma at least partly via
inactivation of the TGF-β1/Smad signaling pathway.
In conclusion, the present study demonstrated that
GA is closely associated with chronic asthma development, and its
underlying molecular mechanism was investigated. To the best of our
knowledge, the present study was the first to demonstrate that GA
may suppress chronic asthma, at least in part via the inhibition of
TGF-β1 and Smad2, as well as upregulation of Smad7 expression in
mice with asthma. Collectively, the results of the present study
indicate that GA attenuates airway inflammation and remodeling in
asthma mainly through the TGF-β/Smad signaling pathway, suggesting
that GA may be used as a medical treatment for chronic asthma.
However, the present study was not without limitations. The present
findings had only been investigated in vitro and in
vivo, and more data are required to validate the results of our
study before those treatments yield benefits in the clinic.
Prospective studies will perform in vitro experiments and
deeper analysis to further substantiate the specific target cells
for GA treatment of asthma.
Supplementary Material
Mice with chronic asthma exhibit
severe lesions in the airways and more deposited airway collagen
fiber. (A) The pathological changes in the airways and alveolar
tissues of mice were determined via H&E, Masson and PAS
staining (magnification, x200). (B) Inflammation score of the
airways and airway side in mice of each group. (C) Inflammation
score of the pulmonary alveoli in mice of each group. (D) The
levels of Wat/Pbm, Wam/Pbm, Wai/Pbm and Wcol/Pbm were determined
via Masson staining. (E) The PAS-positive area per total area was
measured. **P<0.01 vs. blank group. H&E,
hematoxylin and eosin; PAS, periodic acid-Schiff.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and.or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZY and YF conceived the study and designed and
performed the experiments. ZY analyzed the data and wrote the
manuscript. YF provided critical materials and supervised the
study. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the local
Ethics Committee of Beijing University of Chinese Medicine
(approval no. A-20190821006) and performed in accordance with the
Guide for the Care and Use of Laboratory Animals.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Papi A, Brightling C, Pedersen SE and
Reddel HK: Asthma. Lancet. 391:783–800. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhu X, Li Q, Hu G, Wang J, Hu Q, Liu Z, Wu
G and Zhong Y: BMS345541 inhibits airway inflammation and
epithelialmesenchymal transition in airway remodeling of asthmatic
mice. Int J Mol Med. 42:1998–2008. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jo A, Lee SH, Kim DY, Hong SJ, Teng MN,
Kolliputi N, Lockey RF, Schleimer RP and Cho SH: Mast cell-derived
plasminogen activator inhibitor type 1 promotes airway inflammation
and remodeling in a murine model of asthma. J Allergy Clin Immunol.
142:294–297.e5. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gupta A, Chakraborty S and Agrawal A:
Molecular and Genomic Basis of Bronchial Asthma. In: Clinical
Molecular Medicine. Academic Press, pp353-366, 2020.
|
|
5
|
Wu P, Xu B, Shen A, He Z, Zhang CJ, Ming
WK and Shen K: The economic burden of medical treatment of children
with asthma in China. BMC Pediatr. 20(386)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bao Z, Zhang P, Yao Y, Lu G, Tong Z, Yan
B, Tu L, Yang G and Zhou J: Deguelin attenuates allergic airway
inflammation via inhibition of NF-κB pathway in mice. Int J Biol
Sci. 13:492–504. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cho IH, Choi YJ, Gong JH, Shin D, Kang MK
and Kang YH: Astragalin inhibits autophagy-associated airway
epithelial fibrosis. Respir Res. 16(51)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li Y, Wang H and Yang X: Effects of
catalpol on bronchial asthma and its relationship with cytokines. J
Cell Biochem. 120:8992–8998. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sung JE, Lee HA, Kim JE, Yun WB, An BS,
Yang SY, Kim DS, Lee CY, Lee HS, Bae CJ and Hwang DY:
Saponin-enriched extract of Asparagus cochinchinensis alleviates
airway inflammation and remodeling in ovalbumin-induced asthma
model. Int J Mol Med. 40:1365–1376. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fouladi S, Masjedi M, Ghasemi R, Hakemi MG
and Eskandari N: The in vitro impact of glycyrrhizic acid on
CD4+ T lymphocytes through OX40 receptor in the patients
with allergic rhinitis. Inflammation. 41:1690–1701. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Qu L, Chen C, He W, Chen Y, Li Y, Wen Y,
Zhou S, Jiang Y, Yang X, Zhang R and Shen L: Glycyrrhizic acid
ameliorates LPS-induced acute lung injury by regulating autophagy
through the PI3K/AKT/mTOR pathway. Am J Transl Res. 11:2042–2055.
2019.PubMed/NCBI
|
|
12
|
Su X, Wu L, Hu M, Dong W, Xu M and Zhang
P: Glycyrrhizic acid: A promising carrier material for anticancer
therapy. Biomed Pharmacother. 95:670–678. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gao L, Tang H, He H, Liu J, Mao J, Ji H,
Lin H and Wu T: Glycyrrhizic acid alleviates bleomycin-induced
pulmonary fibrosis in rats. Front Pharmacol. 6(215)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wu Q, Tang Y, Hu X, Wang Q, Lei W, Zhou L
and Huang J: Regulation of Th1/Th2 balance through OX40/OX40L
signalling by glycyrrhizic acid in a murine model of asthma.
Respirology. 21:102–111. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu YD, Sun X, Zhang Y, Wu HJ, Wang H and
Yang R: Protocatechuic acid inhibits TGF-beta1-induced
proliferation and migration of human airway smooth muscle cells. J
Pharmacol Sci. 139:9–14. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ojiaku CA, Yoo EJ and Panettieri RA Jr:
Transforming growth factor β1 function in airway remodeling and
hyperresponsiveness. The missing link? Am J Respir Cell Mol Biol.
56:432–442. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Macias MJ, Martin-Malpartida P and
Massague J: Structural determinants of Smad function in TGF-β
signaling. Trends Biochem Sci. 40:296–308. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
van der Kraan PM: Differential role of
transforming growth factor-beta in an osteoarthritic or a healthy
joint. J Bone Metab. 25:65–72. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ogden BE, Pang William W, Agui T and Lee
BH: Laboratory animal laws, regulations, guidelines and standards
in China Mainland, Japan, and Korea. ILAR J. 57:301–311.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shi YX, Dai X, Wang LJ, et al: Effect of
ligustrazine on airway inflammation and airway remodeling in
asthmatic mice by regulating TGF-β1/Smad signaling pathway. Drugs
Clinic. 1:20–26. 2019.
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nakagami Y, Favoreto S Jr, Zhen G, Park
SW, Nguyenvu LT, Kuperman DA, Dolganov GM, Huang X, Boushey HA,
Avila PC and Erle DJ: The epithelial anion transporter pendrin is
induced by allergy and rhinovirus infection, regulates airway
surface liquid, and increases airway reactivity and inflammation in
an asthma model. J Immunol. 181:2203–2210. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Blasi F, Bettoncelli G, Canonica GW,
Centanni S, Crimi N, DiMaria G, Gasparini S, Gentili G, Girbino G,
Mereu C, et al: The management of asthma in the phenotype and
biomarker era: The proposal of a new diagnostic-therapeutic model.
J Asthma. 53:665–667. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liang B, Guo XL, Jin J, Ma YC and Feng ZQ:
Glycyrrhizic acid inhibits apoptosis and fibrosis in
carbon-tetrachloride-induced rat liver injury. World J
Gastroenterol. 21:5271–5280. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhao H, Liu Z, Shen H, Jin S and Zhang S:
Glycyrrhizic acid pretreatment prevents sepsis-induced acute kidney
injury via suppressing inflammation, apoptosis and oxidative
stress. Eur J Pharmacol. 781:92–99. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhao H, Zhao M, Wang Y, Li F and Zhang Z:
Glycyrrhizic acid prevents sepsis-induced acute lung injury and
mortality in rats. J Histochem Cytochem. 64:125–137.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tang X, Nian H, Li X, Yang Y, Wang X, Xu
L, Shi H, Yang X and Liu R: Effects of the combined extracts of
Herba epimedii and Fructus Ligustrilucidi on airway
remodeling in the asthmatic rats with the treatment of budesonide.
BMC Complement Altern Med. 17(380)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pang H, Huang T, Song J, Li D, Zhao Y and
Ma X: Inhibiting HMGB1 with glycyrrhizic acid protects brain injury
after DAI via its anti-inflammatory effect. Mediators Inflamm.
2016(4569521)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yu JY, Ha JY, Kim KM, Jung YS, Jung JC and
Oh S: Anti-inflammatory activities of licorice extract and its
active compounds, glycyrrhizic acid, liquiritin and liquiritigenin,
in BV2 cells and mice liver. Molecules. 20:13041–13054.
2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Fouladi S, Masjedi M, Ganjalikhani Hakemi
M and Eskandari N: The review of in vitro and in vivo studies over
the glycyrrhizic acid as natural remedy option for treatment of
allergic asthma. Iran J Allergy Asthma Immunol. 18:1–11.
2019.PubMed/NCBI
|
|
31
|
Wang C, Zheng M, Choi Y, Jiang J, Li L, Li
J, Xu C, Xian Z, Li Y, Piao H, et al: Cryptotanshinone attenuates
airway remodeling by inhibiting crosstalk between tumor necrosis
factor-like weak inducer of apoptosis and transforming growth
factor beta 1 signaling pathways in asthma. Front Pharmacol.
10(1338)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lee HY, Kim IK, Yoon HK, Kwon SS, Rhee CK
and Lee SY: Inhibitory effects of resveratrol on airway remodeling
by transforming growth factor-beta/Smad signaling pathway in
chronic asthma model. Allergy Asthma Immunol Res. 9:25–34.
2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Qu ZH, Yang ZC, Chen L, Lv ZD, Yi MJ and
Ran N: Inhibition airway remodeling and transforming growth
factor-β1/Smad signaling pathway by astragalus extract in asthmatic
mice. Int J Mol Med. 29:564–568. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jeon WY, Shin IS, Shin HK, Jin SE and Lee
MY: Aqueous extract of Gumiganghwal-tang, a traditional herbal
medicine, reduces pulmonary fibrosis by transforming growth
factor-β1/Smad signaling pathway in murine model of chronic asthma.
PLoS One. 11(e0164833)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Feng Y, Yang C, Yang W and Jiang T: Effect
of dexamethasone on TGF-β1/Smad3 signalling pathway in airway
remodelling model of asthmatic rats. J Coll Physicians Surg Pak.
29:537–540. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang J, Li HY, Wang HS and Su ZB:
MicroRNA-485 modulates the TGF-β/Smads signaling pathway in chronic
asthmatic mice by targeting Smurf2. Cell Physiol Biochem.
51:692–710. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen L, Yang T, Lu DW, Zhao H, Feng YL,
Chen H, Chen DQ, Vaziri ND and Zhao YY: Central role of
dysregulation of TGF-β/Smad in CKD progression and potential
targets of its treatment. Biomed Pharmacother. 101:670–681.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hu HH, Chen DQ, Wang YN, Feng YL, Cao G,
Vaziri ND and Zhao YY: New insights into TGF-β/Smad signaling in
tissue fibrosis. Chem Biol Interact. 292:76–83. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Walton KL, Johnson KE and Harrison CA:
Targeting TGF-β mediated SMAD signaling for the prevention of
fibrosis. Front Pharmacol. 8(461)2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Briones-Orta MA, Tecalco-Cruz AC,
Sosa-Garrocho M, Caligaris C and Macias-Silva M: Inhibitory Smad7:
Emerging roles in health and disease. Curr Mol Pharmacol.
4:141–153. 2011.PubMed/NCBI
|
|
41
|
Sugiura H, Liu X, Duan F, Kawasaki S, Togo
S, Kamio K, Wang XQ, Mao L, Ahn Y, Ertl RF, et al: Cultured lung
fibroblasts from ovalbumin-challenged ‘asthmatic’ mice differ
functionally from normal. Am J Respir Cell Mol Biol. 37:424–430.
2007.PubMed/NCBI View Article : Google Scholar
|