1. Introduction
Despite major advancements in primary and secondary
prevention strategies, coronary artery disease (CAD) remains a
major cause of morbidity and mortality worldwide (1-3).
Percutaneous coronary intervention (PCI), which was introduced as
an alternative means of coronary revascularization to coronary
artery bypass grafting surgery in 1979(4), is considered an effective and safe
treatment modality for suitable patients with acute or stable CAD
(1).
Patients with acute myocardial infarction (AMI) are
among the highest-risk patients undergoing PCI. The introduction of
stenting decreased the limitations of elastic recoil, restenosis
and flow-limiting dissections associated with plain old balloon
angioplasty (5). Due to their
improved safety and efficacy compared with first-generation
drug-eluting stents (DES) and bare-metal stents (BMS),
new-generation DES are currently recommended for PCI in patients
with AMI (1,3). However, in-stent restenosis (ISR),
increased risk of bleeding due to prolonged dual antiplatelet
therapy, as well as early and late stent thrombosis following
implantation (6-10).
Furthermore, late stent-related major adverse cardiovascular events
(MACEs) occur between 1-5 years after PCI, which presents a
challenge (11). For patients with
AMI, routine stenting is associated with an increased rate of acute
and subacute stent thrombosis compared with stable CAD, and the
1-year incidence of target lesion-related events remains high
(12,13). In addition, permanent vascular
implants impair coronary endothelial and vasomotor functions of the
coronary artery (14).
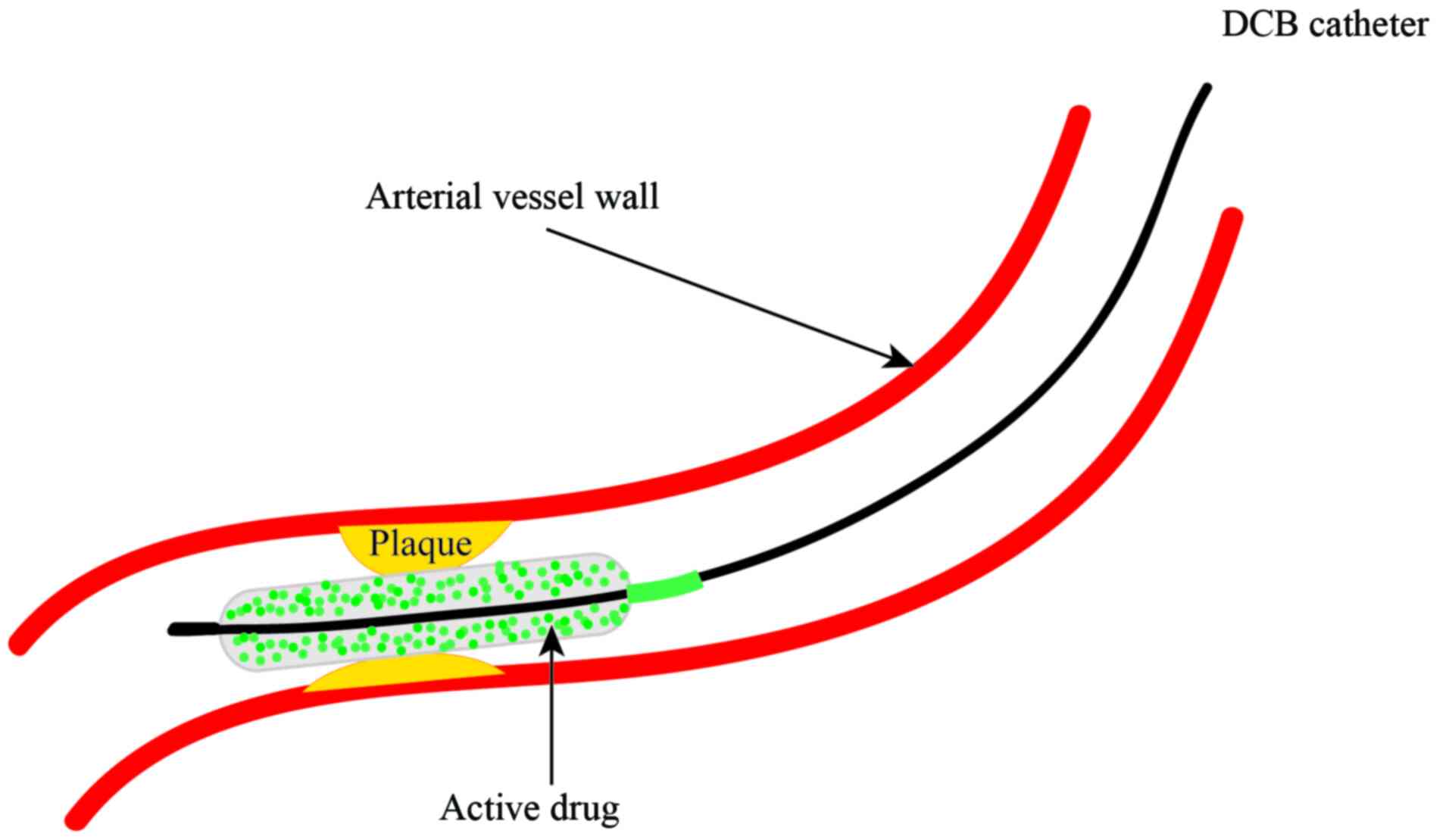
These limitations resulted in the development of
drug-coated balloons (DCBs). The rationale of DCB technology is
that a combination of balloons and drugs is used for the treatment
of coronary lesions to achieve lower rates of restenosis (Fig. 1) (15,16).
DCBs have emerged as a novel application in PCI, and a DCB strategy
has already exhibited successful therapeutic potential for ISR
(17-20)
and small vessel disease (21-23).
DCBs are a class I indication to treat ISR, as described in the
2018 ESC/EACTS guidelines on myocardial revascularization (1); however, their role in AMI remains
unclear. The present review discusses the current studies for the
treatment of AMI with DCBs.
2. DCBs
The concept of DCBs has been extensively studied
(15,24,25).
DCBs are semi-compliant balloons covered with an antiproliferative
drug, which is in direct contact with the vessel wall and inhibits
the proliferation of smooth muscle cells (26). DCBs are a novel treatment strategy
for CAD, based on the fast delivery of antiproliferative drugs into
the vessel wall following single balloon inflation, which fulfills
the concept of ‘leaving nothing behind’ (27,28).
Although sirolimus and its analogues have been investigated in
DCBs, limited data are available for their use in CAD (29-31).
Paclitaxel-coated balloons, which contain a typical dose of 2.0-3.5
mg/mm2 of paclitaxel on the balloon surface, remain a
popular choice for coronary intervention (16). Paclitaxel is a highly lipophilic
antiproliferative drug that can permeate through the vessel wall.
In addition, it is relatively selective for smooth muscle cells,
and its cytotoxicity persists for at least 14 days (26). Following inflation of the balloon
for 30 sec, 16% of the drug is transferred into the vessel intima
to exert a sufficient antiproliferative effect (32). Rapid tissue release makes
paclitaxel-coated balloons attractive for use in DCBs. Table I lists currently available DCBs for
coronary use, worldwide.
 | Table ICurrently approved drug-coated
balloons. |
Table I
Currently approved drug-coated
balloons.
| Device | Company | Drug | Dose,
µg/mm2 |
|---|
| Agent | Boston
Scientific | Paclitaxel | 2.0 |
| Elutax SV | Aachen Resonance | Paclitaxel | 2.2 |
| Danubio | Minvasys | Paclitaxel | 2.5 |
| Dior I and II | Eurocor | Paclitaxel | 3.0 |
| SeQuent Please
Neo | Braun
Melsungen | Paclitaxel | 3.0 |
| Pantera Lux | Biotronik | Paclitaxel | 3.0 |
| Restore | Cardionovum | Paclitaxel | 3.0 |
| Essential | iVascular | Paclitaxel | 3.0 |
| IN. PACT
Falcon | Medtronic | Paclitaxel | 3.5 |
| Elutax | Aachen
Resonance | Paclitaxel | 2.2 |
| Virtue | Caliber
Therapeutics | Sirolimus | 4.0 |
| Selution | M.A. Med
Alliance | Sirolimus | 4.0 |
| Magictouch | Concept Medical
Research | Sirolimus | 4.0 |
3. Clinical trials of DCBs for AMI
ST-segment elevation MI (STEMI)
Primary percutaneous coronary intervention (PPCI) is
the most effective reperfusion strategy for STEMI, and stenting has
been demonstrated to decrease the incidence of repeat
revascularization (33,34). However, intervention stent treatment
is associated with an increased rate of thrombotic complications,
as well as ISR in the long-term (10,13).
Thus, avoiding permanent implants may be favorable to prevent
stent-associated acute and long-term complications in patients with
STEMI. A DCB strategy may be attractive because it provides a
homogeneous delivery of the antiproliferative drug and also
suppresses endothelial inflammation (35,36).
Currently, five studies have been performed to
investigate the DCB strategy in patients with STEMI (Table II). Ho et al (37) investigated the feasibility of using
DCBs in patients undergoing PPCI by treating 89 patients with STEMI
with 89 coronary lesions with DCBs, among which 56% of patients
underwent thrombus aspiration and 4% of patients received bailout
stenting. At 30 days follow-up, four deaths were reported; however,
no patients experienced abrupt closure of the infarct-related
artery, target vessel revascularization (TVR), target-vessel-MI or
target lesion thrombosis. In this study, the authors recommended
thrombus aspiration for visible thrombus and sufficient
predilatation prior to DCB angioplasty to enable better contact,
prolong balloon inflation and provide preliminary experiences with
DCBs in PPCI. However, as the number of patients in the study was
relatively small and it was a single-center registry, further
studies with longer follow-up are required to confirm these
preliminary findings.
 | Table IIClinical trials of DCBs for the
treatment of ST-segment elevation myocardial infarction. |
Table II
Clinical trials of DCBs for the
treatment of ST-segment elevation myocardial infarction.
| Trial | Year | DCB type | Control group | Sample size, n | Clinical follow-up,
months | Angiographic
follow-up, months | Primary
endpoint | Secondary
endpoint | (Refs.) |
|---|
| DEB-AMI | 2012 | DIOR II | DCB + BMS or
DES | 50/50/50 | 6 | 6 | LLL | ISR, MACE (cardiac
death, MI and TVR) | (40) |
| PAPPA | 2014 | Pantera Lux | None | 100 | 12 | None | Cardiac death,
recurrent MI, TLR | The need for
additional stenting, stent thrombosis and major bleeding | (38) |
| Ho et
al | 2015 | SeQuent Please | None | 89 | 1 | None | Death, TVR,
recurrent MI or ST | NR | (37) |
| Gobic et
al | 2017 | SeQuent Please | DES | 41/37 | 6 | 6 | LLL, MACE (major
bleeding, MI, TLR and cardiac death) | NR | (39) |
| REVELATION | 2019 | Pantera Lux | DES | 60/60 | 9 | 9 | FFR value | LLL, MACE (cardiac
death, recurrent MI and TLR) and major bleeding. | (42) |
The PAPPA study (38) prospectively enrolled 100 patients
with STEMI and evaluated the safety and feasibility of a DCB-only
strategy in PPCI. In this study, 59 patients were treated with a
DCB angioplasty, while bailout stenting was performed in 41
patients due to type C to F dissection or residual stenosis
>50%. 1-year clinical follow-up was completed in 98% of
patients, and five MACEs were reported. A total of two patients
died from cardiac death, and three patients underwent target lesion
revascularization (TLR). To the best of our knowledge, this was the
first study that exhibited good 1-year clinical results of a
DCB-only strategy in the setting of primary PCI; however, its major
limitations include its observational nature, single-arm and
single-center design, and the lack of long-term follow-up.
Gobic et al (39) also compared the clinical and
angiographic outcomes in patients with STEMI treated with DCB-only
strategy vs. DES implantation during PPCI. A total of 75 patients
with STEMI were randomized into DCB or DES groups, and the study
endpoints were MACEs and late lumen loss (LLL) after 6-months
follow-up. After 1 month, two patients in each group experienced
reinfarction. At 6 months, MACEs were only reported in 5.4% of
patients in the DES group; LLL was 0.10±0.19 mm in the DES group
and -0.09±0.09 mm in the DCB group (P<0.05). This study
demonstrated that the DCB-only strategy had good clinical and
angiographic outcomes after a 6-months follow-up period. However,
the limitations of this study included a small sample size,
relatively short follow-up period and the lack of prior research on
the topic.
The DEB-AMI trial (40), which compared intravascular imaging
and clinical outcomes of patients with STEMI treated with BMS, DES
or DCB plus BMS, demonstrated that the combination of DCB and BMS
was not superior to BMS alone and inferior to paclitaxel-eluting
stents, in terms of LLL and binary restenosis at 6-months
follow-up. In the non-randomized fourth arm of the DEB-AMI study,
Nijhoff et al (41) reported
that DCB-only treatment resulted in a similar LLL and binary
restenosis rate compared with treatment with BMS alone or DCB plus
BMS; however, significantly higher LLL and binary restenosis rate
were achieved compared with the DES group, which may be due to the
DCB compound used in the study (DIOR GmbH, Bonn, Germany). Notably,
no statistically significant differences in MACEs, death, TLR, TVR,
myocardial infarction and stent thrombosis rates were observed
between DEB-only and DES treatments at 6-months follow-up. Thus,
DEB-only treatment remains a potential alternative during PPCI in
patients with contraindications to DES.
Recently, the REVELATION trial (42) was published, which was performed in
patients with STEMI to assess the efficacy and safety of a DCB
treatment strategy vs. DES treatment in PPCI. In this prospective
randomized trial, 120 patients were treated with either DCB
(Pantera Lux, Biotronik, Berlin, Germany) or DES (Orsiro,
Biotronik, Bülach, Switzerland or Xience, Abbott) in a 1:1 ratio,
and the primary endpoint was fractional flow reverse (FFR) at 9
months. At 9-months follow-up, the mean FFR value was 0.92±0.05 in
the DCB group vs. 0.91±0.06 in the DES group (P=0.27). Furthermore,
no significant differences in LLL and clinical outcomes were
observed between the DCB and DES groups. The DCB-only strategy was
non-inferior to DES treatment, and exhibited improved safety and
feasibility. However, this study had several limitations, including
a small sample size, relatively short follow-up period and it was a
single-center study.
Non-ST elevation MI (NSTEMI)
Only one study (PEPCAD NSTEMI) has compared the
clinical outcomes of patients with NSTEMI treated with DCBs or
stent (43). In this study, 210
patients with NSTEMI were enrolled, the primary endpoint was target
lesion failure (TLF), and second endpoints included MACEs and
individual clinical endpoints. During a follow-up of 9.2±0.7
months, DCB was determined to be superior to stents in terms of
TLF, and no significant difference in the rates of death, MI and
TLR was observed between DCB and stent treatments. DES are regarded
as the standard of care in most settings of acute coronary syndrome
(3,33), and the PEPCAD NSTEMI trial was the
first to demonstrate the safety and efficacy of DCBs for patients
with NSTEMI. However, DCBs in this setting require further
investigation in the form of larger randomized trials.
4. Limitations and perspectives
DCBs appear to be a feasible and attractive
treatment strategy for patients with AMI. However, DCB technology
is not without limitations. DCBs are associated with increased risk
of persistent residual stenosis and acute dissection, which may
require bailout stenting (44,45).
Furthermore, DCBs require optimal lesion preparation before the
apposition of the drug-coating surface to the lesion endothelium,
and in the presence of angiographic thrombus, DCBs may be
unsuitable due to inhibition of drug delivery to the vessel wall
(37,42,46).
In addition, in patients who had DES-ISR, DCBs may be associated
with higher TLR compared with DES (18).
Limited data are available for the use of DCBs in
AMI (Table III) and current
studies have several limitations. First, the number of patients
included in the studies have been relatively small. Secondly, the
follow-up periods have been relatively short, whereby the longest
follow-up period was only 12 months. Thus, the long-term safety and
efficacy of DCBs in AMI remain unknown. Thirdly, despite half of
the studies being randomized controlled trials (RCTs), only one
study is a multi-center study. Finally, only paclitaxel-coated
balloons were used in these studies, and no trails have
investigated the safety and efficacy of other DCBs in AMI, such as
sirolimus-coated.
 | Table IIIMain outcomes of each study. |
Table III
Main outcomes of each study.
| Study, year | DCB/competitor | Patients, n | Follow-up,
months | Primary
endpoint | Main results | (Refs.) |
|---|
| DEB-AMI, 2012 | DIOR II/DIOR II +
BMS/ Taxus Liberte | 150 | 6 | LLL | LLL, 0.51±0.59 for
DEB vs. 0.21±0.32 mm for DES (P<0.01); MACE, 17.5 for DEB vs.
4.1% for DES (P=0.68) | (40) |
| PAPPA, 2014 | Pantera Lux | 100 | 12 | Cardiac death,
recurrent MI and TLR | MACE, 5%; TLR,
3% | (38) |
| Ho et al,
2015 | SeQuent Please | 89 | 1 | Death, TVR,
recurrent MI or ST | Mortality, 4.5%;
TVR, 0.0% | (37) |
| Gobic et al,
2017 | SeQuent
Please/Biomime | 78 | 6 | LLL, MACE (major
bleeding, MI, TLR and cardiac death) | LLL, -0.09±0.09 vs.
0.10±0.19 mm (P<0.05); MACE, 0.0 vs. 5.4% (P=0.29) | (39) |
| REVELATION,
2019 | Pantera Lux/Orsiro
or Xience | 120 | 9 | FFR value | FFR value,
0.92±0.05 vs. 0.91±0.06 mm (P=0.27); LLL, 0.05 (-0.40-0.20) vs.
0.00 mm (-0.16-0.10) (P=0.51) | (42) |
| PEPCAD NSTEMI,
2020 | SeQuent Please/BMS
or current gerenation DES | 210 | 9 | TLF | TLF, 3.8 vs. 6.6%
(p=0.53); MACE, 6.7 vs. 14.2% (P=0.11) | (43) |
Regarding the shortcomings of existing studies,
larger multi-center RCTs with longer follow-up periods are required
to evaluate the clinical use of paclitaxel or sirolimus-coated
balloons in patients with AMI. Currently, sirolimus and its
derivatives, such as everolimus, are successfully implemented in
stent technology and are the main drugs used in DES in clinical
practice (47-49).
A recent study reported promising results of sirolimus-coated
balloons ISR compared with paclitaxel-coated balloons (30). According to its success in stents
and recent evidence in DCBs, sirolimus and its derivatives may be
alternative drug coatings for AMI.
5. Conclusions
Stenting remains the standard reperfusion strategy
in most settings of AMI; however, ISR, stent thrombosis and
reinfarction present challenges. DCBs represent a safe and
effective method for the treatment of ISR and coronary small vessel
disease, and appear to be a promising strategy in the cases of AMI.
However, further studies are required to determine the long-term
benefits of DCBs compared with those of new-generation DES.
Acknowledgements
Thanks to the Jiaxing Institute of Arteriosclerotic
Diseases.
Funding
The present review was supported by the Key Medicine Disciplines
Co-construction Project of Jiaxing Municipal (grant no.
2019-ss-xxgbx).
Availability of data and materials
Not applicable.
Authors' contributions
HH drafted the initial manuscript and LS performed
the literature review. HH and LS confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Neumann FJ, Sousa-Uva M, Ahlsson A,
Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V,
Head SJ, et al: 2018 ESC/EACTS guidelines on myocardial
revascularization. Eur Heart J. 40:87–165. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Knuuti J, Wijns W, Saraste A, Capodanno D,
Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C,
Cuisset T, et al: 2019 ESC guidelines for the diagnosis and
management of chronic coronary syndromes. Eur Heart J. 41:407–477.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Collet JP, Thiele H, Barbato E, Barthelemy
O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T,
Folliguet T, et al: 2020 ESC guidelines for the management of acute
coronary syndromes in patients presenting without persistent
ST-segment elevation. Eur Heart J ehaa575, 2020.
|
|
4
|
Gruntzig AR, Senning A and Siegenthaler
WE: Nonoperative dilatation of coronary-artery stenosis:
Percutaneous transluminal coronary angioplasty. N Engl J Med.
301:61–68. 1979.PubMed/NCBI View Article : Google Scholar
|
|
5
|
McKavanagh P, Zawadowski G, Ahmed N and
Kutryk M: The evolution of coronary stents. Expert Rev Cardiovasc
Ther. 16:219–228. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Huang KN, Grandi SM, Filion KB and
Eisenberg MJ: Late and very late stent thrombosis in patients with
second-generation drug-eluting stents. Can J Cardiol. 29:1488–1494.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chang M and Park DW: Optimal duration of
dual antiplatelet therapy after implantation of drug-eluting
stents: Shorter or longer? Cardiol Ther. 3:1–12. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hassan AK, Bergheanu SC, Stijnen T, van
der Hoeven BL, Snoep JD, Plevier JW, Schalij MJ and Wouter Jukema
J: Late stent malapposition risk is higher after drug-eluting stent
compared with bare-metal stent implantation and associates with
late stent thrombosis. Eur Heart J. 31:1172–1180. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cassese S, Byrne RA, Tada T, Pinieck S,
Joner M, Ibrahim T, King LA, Fusaro M, Laugwitz KL and Kastrati A:
Incidence and predictors of restenosis after coronary stenting in
10 004 patients with surveillance angiography. Heart. 100:153–159.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Byrne RA, Joner M and Kastrati A: Stent
thrombosis and restenosis: What have we learned and where are we
going? The andreas gruntzig lecture ESC 2014. Eur Heart J.
36:3320–3331. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Madhavan MV, Kirtane AJ, Redfors B,
Genereux P, Ben-Yehuda O, Palmerini T, Benedetto U, Biondi-Zoccai
G, Smits PC, von Birgelen C, et al: Stent-related adverse events
>1 year after percutaneous coronary intervention. J Am Coll
Cardiol. 75:590–604. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Loh JP, Pendyala LK, Kitabata H, Torguson
R, Omar A, Minha S, Chen F, Satler LF, Pichard AD and Waksman R:
Comparison of outcomes after percutaneous coronary intervention
among different coronary subsets (stable and unstable angina
pectoris and ST-segment and non-ST-segment myocardial infarction).
Am J Cardiol. 113:1794–1801. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gonzalo N, Barlis P, Serruys PW,
Garcia-Garcia HM, Onuma Y, Ligthart J and Regar E: Incomplete stent
apposition and delayed tissue coverage are more frequent in
drug-eluting stents implanted during primary percutaneous coronary
intervention for ST-segment elevation myocardial infarction than in
drug-eluting stents implanted for stable/unstable angina: Insights
from optical coherence tomography. JACC Cardiovasc Interv.
2:445–452. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hung MJ, Hsu KH, Chang NC, Tsimikas S and
Hung MY: Prevalence of coronary artery spasm after stent placement
and its association with inflammation. Int J Cardiol. 179:252–255.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ang H, Lin J, Huang YY, Chong TT, Cassese
S, Joner M and Foin N: Drug-Coated balloons: Technologies and
clinical applications. Curr Pharm Des. 24:381–396. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jeger RV, Eccleshall S, Wan Ahmad WA, Ge
J, Poerner TC, Shin ES, Alfonso F, Latib A, Ong PJ, Rissanen TT, et
al: Drug-coated balloons for coronary artery disease: Third report
of the international DCB consensus group. JACC Cardiovasc Interv.
13:1391–1402. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Scheller B, Hehrlein C, Bocksch W, Rutsch
W, Haghi D, Dietz U, Bohm M and Speck U: Treatment of coronary
in-stent restenosis with a paclitaxel-coated balloon catheter. N
Engl J Med. 355:2113–2124. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Giacoppo D, Alfonso F, Xu B, Claessen
BEPM, Adriaenssens T, Jensen C, Perez-Vizcayno MJ, Kang DY,
Degenhardt R, Pleva L, et al: Paclitaxel-coated balloon angioplasty
vs. drug-eluting stenting for the treatment of coronary in-stent
restenosis: A comprehensive, collaborative, individual patient data
meta-analysis of 10 randomized clinical trials (DAEDALUS study).
Eur Heart J. 41:3715–3728. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rittger H, Brachmann J, Sinha AM,
Waliszewski M, Ohlow M, Brugger A, Thiele H, Birkemeyer R, Kurowski
V, Breithardt OA, et al: A randomized, multicenter, single-blinded
trial comparing paclitaxel-coated balloon angioplasty with plain
balloon angioplasty in drug-eluting stent restenosis: The
PEPCAD-DES study. J Am Coll Cardiol. 59:1377–1382. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Scheller B, Clever YP, Kelsch B, Hehrlein
C, Bocksch W, Rutsch W, Haghi D, Dietz U, Speck U, Böhm M and
Cremers B: Long-term follow-up after treatment of coronary in-stent
restenosis with a paclitaxel-coated balloon catheter. JACC
Cardiovasc Interv. 5:323–330. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jeger RV, Farah A, Ohlow MA, Mangner N,
Mobius-Winkler S, Leibundgut G, Weilenmann D, Wohrle J, Richter S,
Schreiber M, et al: Drug-coated balloons for small coronary artery
disease (BASKET-SMALL 2): An open-label randomised non-inferiority
trial. Lancet. 392:849–856. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cortese B, Micheli A, Picchi A, Coppolaro
A, Bandinelli L, Severi S and Limbruno U: Paclitaxel-coated balloon
versus drug-eluting stent during PCI of small coronary vessels, a
prospective randomised clinical trial. The PICCOLETO study. Heart.
96:1291–1296. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Latib A, Colombo A, Castriota F, Micari A,
Cremonesi A, De Felice F, Marchese A, Tespili M, Presbitero P,
Sgueglia GA, et al: A randomized multicenter study comparing a
paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in
small coronary vessels: The BELLO (Balloon elution and late loss
optimization) study. J Am Coll Cardiol. 60:2473–2480.
2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Waksman R and Pakala R: Drug-eluting
balloon: The comeback kid? Circ Cardiovasc Interv. 2:352–358.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wessely R: New drug-eluting stent
concepts. Nat Rev Cardiol. 7:194–203. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Axel DI, Kunert W, Goggelmann C, Oberhoff
M, Herdeg C, Kuttner A, Wild DH, Brehm BR, Riessen R, Köveker G and
Karsch KR: Paclitaxel inhibits arterial smooth muscle cell
proliferation and migration in vitro and in vivo using local drug
delivery. Circulation. 96:636–645. 1997.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yerasi C, Case BC, Forrestal BJ, Torguson
R, Weintraub WS, Garcia-Garcia HM and Waksman R: Drug-coated
balloon for de novo coronary artery disease: JACC state-of-the-art
review. J Am Coll Cardiol. 75:1061–1073. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cheng Y, Leon MB and Granada JF: An update
on the clinical use of drug-coated balloons in percutaneous
coronary interventions. Expert Opin Drug Deliv. 13:859–872.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Verheye S, Vrolix M, Kumsars I, Erglis A,
Sondore D, Agostoni P, Cornelis K, Janssens L, Maeng M, Slagboom T,
et al: The SABRE trial (Sirolimus angioplasty balloon for coronary
in-stent restenosis): Angiographic results and 1-year clinical
outcomes. JACC Cardiovasc Interv. 10:2029–2037. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ali RM, Abdul Kader MASK, Wan Ahmad WA,
Ong TK, Liew HB, Omar AF, Mahmood Zuhdi AS, Nuruddin AA, Schnorr B
and Scheller B: Treatment of coronary drug-eluting stent restenosis
by a sirolimus- or paclitaxel-coated balloon. JACC Cardiovasc
Interv. 12:558–566. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cortese B, Di Palma G and Latini R: Magic
Touch(R): Preliminary clinical evidence with a novel
sirolimus drug coated balloon. Minerva Cardioangiol. 66:508–517.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Scheller B, Speck U, Abramjuk C, Bernhardt
U, Bohm M and Nickenig G: Paclitaxel balloon coating, a novel
method for prevention and therapy of restenosis. Circulation.
110:810–814. 2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ibanez B, James S, Agewall S, Antunes MJ,
Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA,
Halvorsen S, et al: 2017 ESC Guidelines for the management of acute
myocardial infarction in patients presenting with ST-segment
elevation: The Task Force for the management of acute myocardial
infarction in patients presenting with ST-segment elevation of the
European society of cardiology (ESC). Eur Heart J. 39:119–177.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Alawami M, Sadler M, Kasargod C, Watson T,
Webster M and Ruygrok P: Outcomes of patients with ST elevation
myocardial infarction in the era of second-generation drug eluting
stents; five-year follow-up. N Z Med J. 132:34–41. 2019.PubMed/NCBI
|
|
35
|
Herdeg C, Oberhoff M, Baumbach A, Blattner
A, Axel DI, Schroder S, Heinle H and Karsch KR: Local paclitaxel
delivery for the prevention of restenosis: Biological effects and
efficacy in vivo. J Am Coll Cardiol. 35:1969–1976. 2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Oberhoff M, Kunert W, Herdeg C, Kuttner A,
Kranzhofer A, Horch B, Baumbach A and Karsch KR: Inhibition of
smooth muscle cell proliferation after local drug delivery of the
antimitotic drug paclitaxel using a porous balloon catheter. Basic
Res Cardiol. 96:275–282. 2001.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ho HH, Tan J, Ooi YW, Loh KK, Aung TH, Yin
NT, Sinaga DA, Jafary FH and Ong PJ: Preliminary experience with
drug-coated balloon angioplasty in primary percutaneous coronary
intervention. World J Cardiol. 7:311–314. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Vos NS, Dirksen MT, Vink MA, van Nooijen
FC, Amoroso G, Herrman JP, Kiemeneij F, Patterson MS, Slagboom T
and van der Schaaf RJ: Safety and feasibility of a
PAclitaxel-eluting balloon angioplasty in Primary Percutaneous
coronary intervention in Amsterdam (PAPPA): One-year clinical
outcome of a pilot study. EuroIntervention. 10:584–590.
2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gobic D, Tomulic V, Lulic D, Zidan D,
Brusich S, Jakljevic T and Zaputovic L: Drug-coated balloon versus
drug-eluting stent in primary percutaneous coronary intervention: A
feasibility study. Am J Med Sci. 354:553–560. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Belkacemi A, Agostoni P, Nathoe HM,
Voskuil M, Shao C, Van Belle E, Wildbergh T, Politi L, Doevendans
PA, Sangiorgi GM and Stella PR: First results of the DEB-AMI (drug
eluting balloon in acute ST-segment elevation myocardial
infarction) trial: A multicenter randomized comparison of
drug-eluting balloon plus bare-metal stent versus bare-metal stent
versus drug-eluting stent in primary percutaneous coronary
intervention with 6-month angiographic, intravascular, functional,
and clinical outcomes. J Am Coll Cardiol. 59:2327–2337.
2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Nijhoff F, Agostoni P, Belkacemi A, Nathoe
HM, Voskuil M, Samim M, Doevendans PA and Stella PR: Primary
percutaneous coronary intervention by drug-eluting balloon
angioplasty: The nonrandomized fourth arm of the DEB-AMI
(drug-eluting balloon in ST-segment elevation myocardial
infarction) trial. Catheter Cardiovasc Interv. 86 (Suppl
1):S34–S44. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Vos NS, Fagel ND, Amoroso G, Herrman JR,
Patterson MS, Piers LH, van der Schaaf RJ, Slagboom T and Vink MA:
Paclitaxel-coated balloon angioplasty versus drug-eluting stent in
acute myocardial infarction: The REVELATION randomized trial. JACC
Cardiovasc Interv. 12:1691–1699. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Scheller B, Ohlow MA, Ewen S, Kische S,
Rudolph TK, Clever YP, Wagner A, Richter S, El-Garhy M, Böhm M, et
al: Bare metal or drug-eluting stent versus drug-coated balloon in
non-ST-elevation myocardial infarction: The randomised PEPCAD
NSTEMI trial. EuroIntervention. 15:1527–1533. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Liu Y, Zhang YJ, Deng LX, Yin ZY, Hu T,
Wang Q, Li Y, Li JY, Guo WY, Mou FJ and Tao L: 12-Month clinical
results of drug-coated balloons for de novo coronary lesion in
vessels exceeding 3.0 mm. Int J Cardiovasc Imaging. 35:579–586.
2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Venetsanos D, Lawesson SS, Panayi G, Todt
T, Berglund U, Swahn E and Alfredsson J: Long-term efficacy of drug
coated balloons compared with new generation drug-eluting stents
for the treatment of de novo coronary artery lesions. Catheter
Cardiovasc Interv. 92:E317–E326. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Megaly M, Buda KG, Xenogiannis I, Vemmou
E, Nikolakopoulos I, Saad M, Rinfret S, Abbott JD, Aronow HD,
Garcia S, et al: Systematic review and meta-analysis of short-term
outcomes with drug-coated balloons vs. stenting in acute myocardial
infarction. Cardiovasc Interv Ther: Oct 10, 2020 (Epub ahead of
print).
|
|
47
|
Claessen BE, Henriques JP and Dangas GD:
Clinical studies with sirolimus, zotarolimus, everolimus, and
biolimus A9 drug-eluting stent systems. Curr Pharm Des.
16:4012–4024. 2010.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Navarese EP, Tandjung K, Claessen B,
Andreotti F, Kowalewski M, Kandzari DE, Kereiakes DJ, Waksman R,
Mauri L, Meredith IT, et al: Safety and efficacy outcomes of first
and second generation durable polymer drug eluting stents and
biodegradable polymer biolimus eluting stents in clinical practice:
Comprehensive network meta-analysis. BMJ. 347(f6530)2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Sabate M, Raber L, Heg D, Brugaletta S,
Kelbaek H, Cequier A, Ostojic M, Iniguez A, Tuller D, Serra A, et
al: Comparison of newer-generation drug-eluting with bare-metal
stents in patients with acute ST-segment elevation myocardial
infarction: A pooled analysis of the EXAMINATION (clinical
Evaluation of the Xience-V stent in Acute Myocardial INfArcTION)
and COMFORTABLE-AMI (Comparison of Biolimus Eluted From an Erodible
Stent Coating With Bare Metal Stents in Acute ST-Elevation
Myocardial Infarction) trials. JACC Cardiovasc Interv. 7:55–63.
2014.PubMed/NCBI View Article : Google Scholar
|