Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory
autoimmune disease characterized by pannus formation and the
progressive destruction of articular cartilage and bone (1,2). RA
has high morbidity and disability rates and is often accompanied by
cardiovascular and other systemic complications, which seriously
affect the quality of life of patients (3,4). It
was previously reported that the abnormal proliferation and
apoptosis of RA synovial fibroblasts (RASFs) served a key role in
RA progression (5). RASFs generally
affect the biological activity of synovial cells, eventually
leading to synovitis and progressive bone destruction (6). Therefore, studying the molecular
mechanisms that affect the proliferation of RASFs may provide a
novel target for RA treatment.
MicroRNAs (miRNAs/miRs) are a class of non-coding
RNAs of ~22 nucleotides in length that are ubiquitous in almost all
species. miRNAs inhibit gene translation by binding to the target
gene 3'-untranslated region (3'-UTR), thereby regulating gene
expression at the post-transcriptional level (7). miRNAs are involved in the regulation
of numerous cell biological activities, including cell
proliferation, apoptosis, cell division and the immune response
(8). miRNAs were also discovered to
serve important roles in numerous types of diseases, including
cancer, diabetes, cardiovascular diseases and cognitive disorders
(9). In addition, as negative
regulators of gene regulation, miRNAs were demonstrated to affect
the progression of various types of orthopedic disease, such as
ankylosing spondylitis, osteoarthritis, osteoporosis and RA
(10). High-throughput sequencing
technology previously identified 50 upregulated and 35
downregulated miRNAs in RASFs (11,12).
Related studies reported that several miRNAs were abnormally
expressed during RA development, such as miR-155(13), miR-21(14), miR-150-5p (15) and miR-23b (16). Notably, the expression levels of
miR-23a-5p were reported to be downregulated in RASFs (12). To date, miR-23a-5p was found to be
abnormally expressed in pancreatic ductal adenocarcinoma (PDAC)
cells (17), acute myeloid leukemia
cells (18) and renal cell
carcinoma (RCC) cells and tissues (19). Accumulating evidence has suggested
that the miR-23a-5p/ATP-binding cassette transporter A1/G1 axis may
promote plaque stability and macrophage-derived foam cell
formation, which eventually inhibits atherosclerosis progression
(20). Furthermore,
osteoclast-derived miR-23a-5p-containing exosomes efficiently
suppressed osteogenic differentiation, indicating that miR-23a-5p
in exosomes may serve a critical role in the process of bone
remodeling (21). However, the
biological effects and underlying mechanisms of miR-23a-5p in RA
pathogenesis remain to be investigated.
The present study first investigated the expression
levels of miR-23a-5p in the plasma of patients with RA.
Furthermore, the biological functions and underlying mechanism of
miR-23a-5p on the proliferation, migration, apoptosis and
inflammatory cytokine secretion of RASFs were determined.
Specifically, the regulatory effect of miR-23a-5p on toll-like
receptor (TLR) 4, which is important for immune and inflammatory
responses (22,23), was investigated.
Materials and methods
Patient studies
Patients with RA (n=20, 9 males and 11 females; mean
age, 48.95±15.23 years) were clinically diagnosed at Southwest
Medical University Affiliated Hospital (Luzhou, China) from March
2018 to March 2019. All enrolled patients met the RA classification
diagnostic criteria established by the American College of
Rheumatology/European League Against Rheumatism (24). A standard score of ≥6 points can be
diagnosed as RA. All patients were diagnosed with RA for the first
time and had not previously received any RA-related treatment. The
exclusion criteria for the RA patients were as follows: i) The
patients were suffering from autoimmune liver disease, ankylosing
spondylitis, autoimmune hemolytic anemia, Sjogren's syndrome and
other autoimmune diseases; the patient is suffering from acute
coronary syndrome, hypertensive disease, cerebral infarction and
other cardiovascular and cerebrovascular diseases; ii) the patients
were suffering from endocrine diseases, such as diabetes mellitus
and hyperthyroidism; iii) the patients were suffering from
infectious diseases, such as hepatitis B and epidemic encephalitis;
iv) the patients had cancer or other chronic diseases; v) the
patients had abnormal liver and kidney function; vi) the patients
had a history of smoking and/or alcoholism; and vii) the patients
had recently taken anticoagulant drugs or immunosuppressive agents.
A total of 5 ml venous blood was collected from each subject in the
morning following fasting. After leaving to stand for 30 min, blood
samples were centrifuged at 4˚C at 1,000 x g for 10 min.
Subsequently, 200 µl serum (non-hemolytic state) was added to a
tube and preserved at -80˚C until further analysis. The present
study was approved by the Ethics Committee of Southwest Medical
University Affiliated Hospital (Luzhou, China) and written informed
consent was obtained from all participants prior to the study.
Cell culture
Human fibroblast-like synoviocytes (MH7A cells) and
normal synovial fibroblasts (HFLS) were purchased from the American
Type Culture Collection. The cells were cultured in high-glucose
DMEM (HyClone; Cytiva) supplemented with 10% FBS (HyClone; Cytiva)
and 1% penicillin/streptomycin (Beijing Solarbio Science &
Technology Co., Ltd.), and maintained in a humidified incubator
with 5% CO2 and 37˚C. Where indicated, MH7A cells were
treated with human TNF-α (R&D Systems, Inc.) at 20 ng/ml for 12
h at 37˚C.
Cell transfection
The transient transfection of pcDNA-TLR4 and
pcDNA-negative control (NC) plasmids (both from Shanghai GenePharma
Co., Ltd.), miR-23a-5p mimic and NC mimic (both from Guangzhou
RiboBio Co., Ltd.) or small interfering RNA (siRNA/si)-TLR4 and
si-NC (both from Guangzhou RiboBio Co., Ltd.) was performed in
6-well plates using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. Briefly, MH7A cells were seeded in a 6-well plate at a
concentration of 2x105 cells/well, and siRNA
transfection was performed when the cells grew to 40-60%
confluence. All molecules were transfected at a concentration of 50
nM. After transfection, the culture plate was placed in a constant
temperature incubator at 37˚C and 5% CO2 for 48 h. The
sequences of transfected RNA oligonucleotides were as follows:
miR-23a-5p mimic forward, 5'-GGGGUUCCUGGGGAUGGGAUUU-3' and reverse,
5'-AAAUCCCAUCCCCAGGAACCCC-3'; NC mimic forward,
5'-UUCUCCGAACGUGUCACGUdTdT-3' and reverse,
5'-ACGUGACACGUUCGGAGAAdTdT-3'; si-TLR4 forward,
5'-GGGCUUAGAACAACUAGAATT-3' and reverse,
5'-UUCUAGUUGUUCUAAGCCCTT-3'; si-NC forward,
5'-CCCUUGUCGUGAAUUUACUTT-3' and reverse,
5'-AGUAAAUUCACGACAAGGGTT-3'.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from MH7A cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). qPCR was subsequently performed using a SYBR Premix Ex Taq™
II kit (Takara Bio, Inc.) according to the manufacturer's protocol.
The following thermocycling conditions were used for the qPCR:
Initial denaturation at 95˚C for 10 min; followed by 40 cycles of
95˚C for 5 sec, 60˚C for 30 sec and 70˚C for 60 sec. The following
primer pairs were used for the qPCR (Takara Bio, Inc.): IL-6
forward, 5'-CCTGACCCAACCACAAATGC-3' and reverse,
5'-ATCTGAGGTGCCCATGCTAC-3'; IL-1β forward,
5'-CCTGTCCTGCGTGTTGAAAGA-3' and reverse,
5'-GGGAACTGGGCAGACTCAAA-3'; IL-10 forward,
5'-GAGATGCCTTCAGCAGAGTGAAGA-3' and reverse,
5'-AGGCTTGGCAACCCAGGTAAC-3'; TLR4 forward,
5'-AGAACCTGGACCTGAGCTTTAATC-3' and reverse,
5'-GAGGTGGCTTAGGCTCTGATATG-3' and β-actin forward,
5'-CATGTACGTTGCTATCCAGGC-3' and reverse,
5'-CTCCTTAATGTCACGCCACGAT-3'. The relative gene expression levels
were determined using the 2-∆∆Cq method (25) on ABI 7500 PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.).
Western blotting
Whole MH7A cell lysates were prepared using RIPA
buffer (1% Triton X-100, 150 mmol/l NaCl, 1 mmol/l EGTA, 50 mmol/l
Tris-HCl, 0.1% SDS, 1% sodium deoxycholate and PMSF; Cell Signaling
Technology, Inc.). The concentration of protein was determined by a
BCA kit (Sigma-Aldrich; Merck KGaA). Total protein (30 µg/sample)
was separated via 10% SDS-PAGE. The separated proteins were
subsequently transferred onto nitrocellulose membranes (EMD
Millipore) and blocked with 5% non-fat milk at room temperature for
1 h. Subsequently, the membranes were incubated with the following
primary antibodies: Anti-Bax (1:500; cat. no. ab53154; Abcam),
anti-Bcl-2 (1:500; cat. no. ab196495; Abcam), anti-caspase-3
(1:500; cat. no. ab4051; Abcam), anti-cleaved-caspase-3 (1:50; cat.
no. ab2302; Abcam), anti-matrix metalloproteinase (MMP) 2 (1:500;
cat. no. ab97779; Abcam), anti-MMP9 (1:1,000; cat. no. ab38898;
Abcam), anti-proliferating cell nuclear antigen (PCNA; 1:500; cat.
no. ab18197; Abcam), anti-NF-κBp65 (1:500; cat. no. ab16502;
Abcam), anti-phosphorylated (p)-NF-κBp65 (1:2,000; cat. no.
ab86299; Abcam), anti-TLR4 (1:500; cat. no. ab13556; Abcam),
anti-VEGFB (1:1,000; cat. no. 2463; Cell Signaling Technology,
Inc.) and anti-β-actin (1:1,000; cat. no. ab8227; Abcam). Following
primary antibody incubation, the membranes were incubated with an
HRP-conjugated goat anti-rabbit IgG secondary antibody (1:5,000;
cat. no. BA1054; Boster Biological Technology). Protein bands were
visualized using ECL reagent (Affinity Biosciences) on a gel
imaging system (Bio-Rad Laboratories, Inc.) and analyzed using
Image Pro Plus 6.0 software (Media Cybernetics, Inc.). β-actin was
used as the internal loading control.
ELISA
A MH7A cell suspension was prepared and centrifuged
for 10 min at 800 x g at 4˚C, then stored at -20˚C until subsequent
analysis. The secretory levels of IL-6 (cat. no. ZC-32466), IL-1β
(cat. no. ZC-32420) and IL-10 (cat. no. ZC-32403) were analyzed
using their corresponding ELISA kits (Shanghai Zhuo Cai Biological
Technology Co., Ltd; http://www.zcibio.com/) according to the
manufacturers' instructions.
Dual luciferase reporter assay
The binding site of miR-23a-5p and TLR4 mRNA 3'-UTR
was predicted using bioinformatics online software, including
TargetScan version 7.1 (http://www.targetscan.org/) and miRDB version 6.0
(http://mirdb.org/). The fragment of the TLR4 3'-UTR
containing the predicted miR-23a-5p binding site was generated by
PCR using specific primers. The resulting PCR amplicon was cloned
into a pmirGLO Dual-Luciferase miRNA target expression vector
(Promega Corporation) containing the luciferase coding sequence.
Cells were seeded into 96-well plates, cultured to 50-70%
confluence/well and co-transfected with the TLR4 3'-UTR-wild-type
(wt) 1 or TLR4 3'-UTR-wt2 construct and miR-23a-5p mimic or NC
mimic. Cells were transfected using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). Following 24 h of
transfection, the relative luciferase activity was measured using a
Dual Luciferase Reporter Assay system (Promega Corporation)
according to the manufacturer's protocol. Firefly luciferase
activity was normalized to that of Renilla luciferase.
Cell viability assay
MH7A cell viability was monitored using a Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.) assay
according to the manufacturer's protocol. CCK-8 solution was added
to each well and incubated at 37˚C for 1.5 h. Cell viability was
measured using an ELISA microplate reader at a wavelength of 450
nm.
Colony formation assay
MH7A cells were seeded into 3.5-cm cell culture
dishes (1x103 cells/dish) and incubated at 37˚C with 5%
CO2 for 2 weeks. Following incubation, MH7A cells were
fixed with 20% methanol for 10 min at room temperature and then
stained with 0.1% crystal violet for 5 min at room temperature. The
number of colonies (>50 cells) were counted using an inverted
microscope (Olympus Corporation).
Flow cytometric analysis of
apoptosis
The apoptosis of MH7A cells was analyzed using an
Annexin V-FITC/propidium iodide (PI) flow cytometry kit (Becton,
Dickinson and Company) according to the manufacturer's protocol.
Briefly, transfected MH7A cells in 6-well plates were washed with
PBS (Invitrogen; Thermo Fisher Scientific, Inc.) and adjusted to a
density of 1x106 cells/ml. The cells were subsequently
resuspended in 100 µl binding buffer, 5 µl Annexin V-FITC and 5 µl
PI and incubated in the dark at 4˚C for 15 min. Apoptotic cells
were then analyzed by a BD FACSCelesta™ flow cytometer (Becton,
Dickinson and Company) and FlowJo software version 7.6.1 (FlowJo
LLC).
Transwell assay
A total of 1x105 MH7A cells/ml were
resuspended in DMEM and 200 µl cell suspension/well was plated into
the upper chambers of 24-well Transwell plates. The lower chambers
were filled with 600 µl DMEM supplemented with 10% FBS (HyClone;
Cytiva). Following incubation at 37˚C for 48 h, MH7A cells were
fixed with 4% paraformaldehyde for 20 min and stained with 0.1%
crystal violet for 15 min (both at room temperature). An inverted
microscope (Olympus Corporation) was used to observe the number of
cells in five randomly selected fields of view (magnification,
x100).
Statistical analysis
Data are presented as the mean ± SD. Statistical
analysis was performed using SPSS 20.0 (IBM Corp.). Statistical
differences between groups were determined using one-way ANOVA with
Tukey's post hoc test of means and unpaired Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
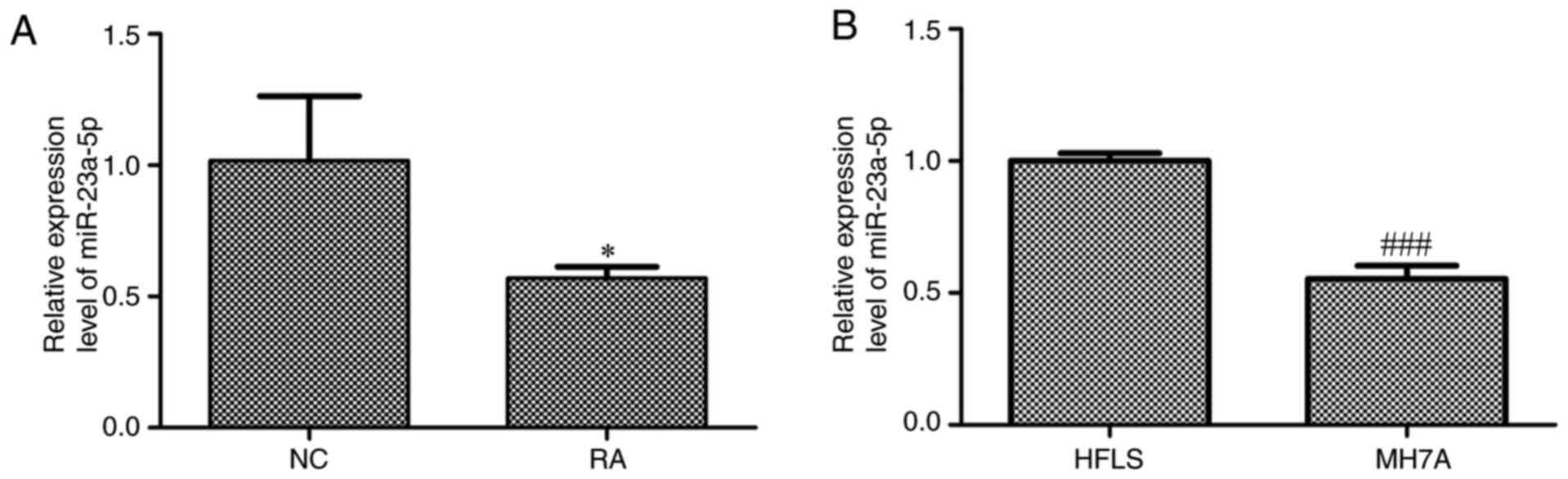
Expression levels of miR-23a-5p are
downregulated in the plasma from patients with RA and MH7A
cells
The expression levels of miR-23a-5p in the plasma of
patients with RA and MH7A cells were investigated. The results
revealed that miR-23a-5p expression levels were significantly
downregulated in the plasma of patients with RA compared with
normal controls (Fig. 1A).
Similarly, the expression levels of miR-23a-5p were significantly
downregulated in MH7A cells compared with HFLS (Fig. 1B).
Pro-apoptotic effects of miR-23a-5p on
TNF-α-stimulated MH7A cells
The effects of miR-23a-5p on TNF-α-induced MH7A cell
development was subsequently determined using miR-23a-5p mimics
(Fig. 2A). MH7A cells were
transfected with miR-23a-5p mimic or NC mimic and then stimulated
with TNF-α. As shown in Fig. 2B,
MH7A cell viability was significantly increased by TNF-α compared
with the untreated control group, while transfection with
miR-23a-5p mimic significantly inhibited TNF-α-induced increases in
MH7A cell viability (Fig. 2B). In
addition, colony formation assay results revealed that miR-23a-5p
overexpression significantly blocked the increase in MH7A
proliferation induced by TNF-α (Fig.
2C). By contrast, the apoptotic rate was gradually decreased
following TNF-α stimulation, while transfection with miR-23a-5p
mimic significantly reversed the apoptosis of TNF-α-induced MH7A
cells (Fig. 2D). miR-23a-5p
overexpression-induced apoptosis was also confirmed using western
blotting. TNF-α stimulation also significantly downregulated Bax
and cleaved-caspase-3 expression levels while significantly
upregulating the expression levels of Bcl-2 in MH7A cells; these
findings were reversed following miR-23a-5p overexpression
(Fig. 2E). Furthermore,
transfection with miR-23a-5p mimic significantly inhibited
TNF-α-induced cell migration (Fig.
2F). The expression levels of four cell growth-related proteins
(PCNA, MMP2, MMP9 and VEGFB) were also significantly upregulated in
response to TNF-α stimulation. Meanwhile, transfection with
miR-23a-5p mimic significantly weakened this trend (Fig. 2G).
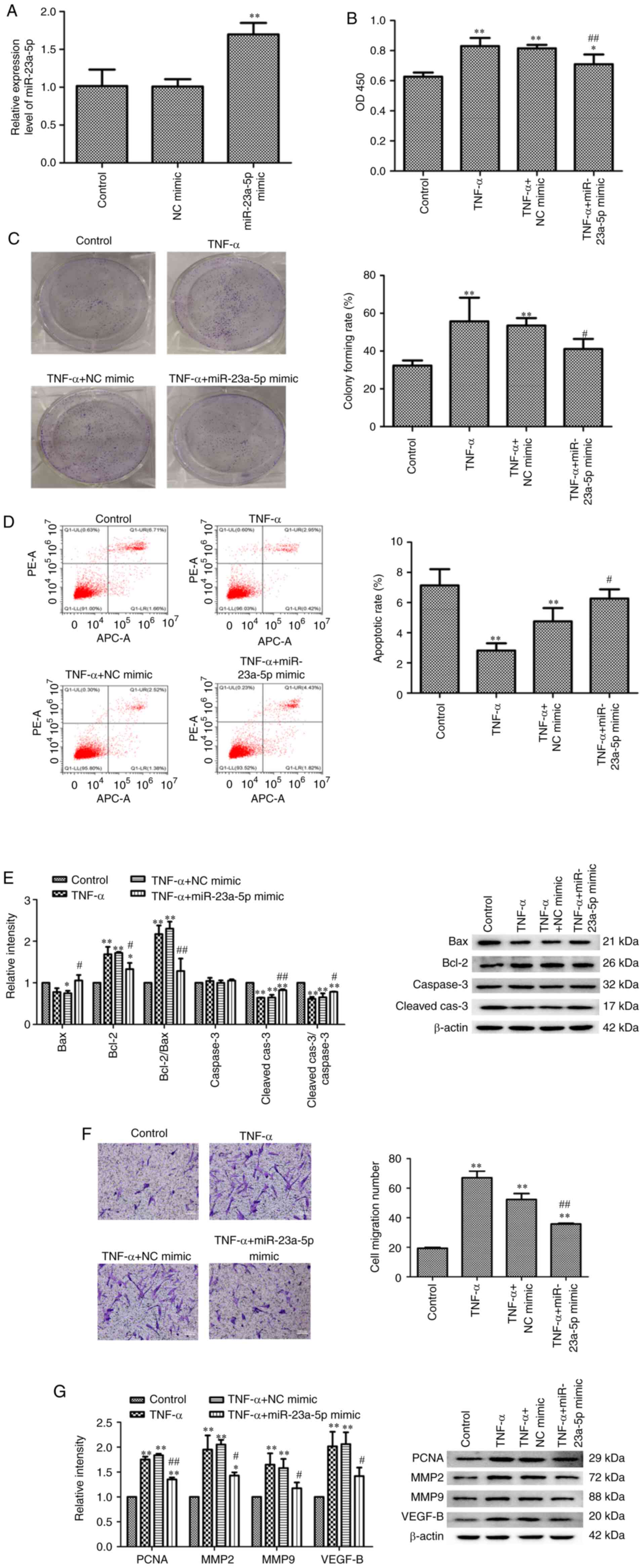 | Figure 2Pro-apoptotic effects of miR-23a-5p
on TNF-α-stimulated MH7A cells. (A) miR-23a-5p expression levels
were analyzed using reverse transcription-quantitative PCR.
Proliferation of MH7A cells was evaluated using (B) Cell Counting
Kit-8 and (C) colony formation assays. (D) Apoptosis of MH7A cells
was assessed using Annexin V-FITC/PI staining flow cytometry. (E)
Bax, Bcl-2, caspase3 and cleaved caspase-3 expression levels were
analyzed using western blotting. β-actin was used as the loading
control. (F) Migratory ability of MH7A cells was evaluated using a
Transwell assay (magnification, x100). (G) PCNA, MMP2, MMP9 and
VEGF-B expression levels were analyzed using western blotting.
β-actin was used as the loading control. *P<0.05 and
**P<0.01 vs. control. #P<0.05 and
##P<0.01 vs. TNF-α + NC mimic. miR, microRNA; PI,
propidium iodide; PCNA, proliferating cell nuclear antigen; MMP,
matrix metalloproteinase; NC, negative control; Cleaved-cas3,
cleaved caspase-3. |
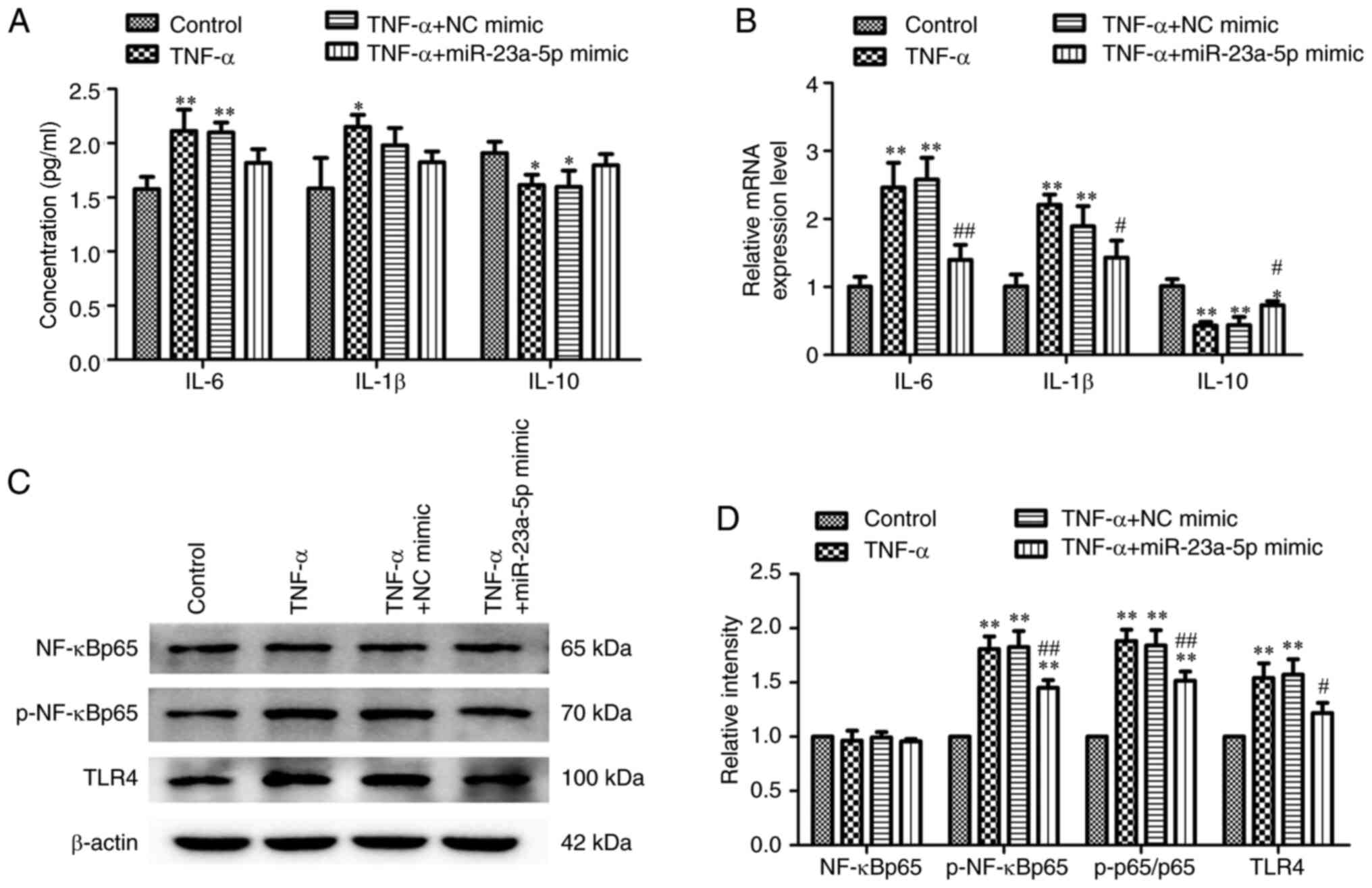
miR-23a-5p reverses TNF-α-induced
inflammatory injury and TLR4/NF-κB signaling activation in MH7A
cells
The effects of miR-23a-5p on inflammatory injury
following TNF-α stimulation was subsequently investigated. Briefly,
MH7A cells were stimulated with TNF-α following transfection with
miR-23a-5p mimic or NC mimic and the levels of cellular cytokines
were determined. Transfection of cells with miR-23a-5p mimic prior
to TNF-α stimulation gradually decreased TNA-α-induced IL-6 and
IL-1β concentrations and reversed the TNF-α-induced reduction in
IL-10 concentration (Fig. 3A).
Similarly, as shown in Fig. 3B, the
TNF-α-increased mRNA expression levels of IL-6, IL-1β and IL-10
were significantly blocked by miR-23a-5p mimic transfection. The
TLR4/NF-κB signaling pathway is considered to be closely related to
inflammation (26). Thus, the
changes in the activity of the TLR4/NF-κB signaling pathway in
response to miR-23a-5p transfection and TNF-α treatment were
investigated. Western blotting data in Fig. 3C and D revealed that TLR4 expression levels and
the phosphorylation levels of NF-κBp65 were both significantly
upregulated following TNF-α stimulation compared with controls.
Conversely, miR-23a-5p overexpression significantly inhibited TLR4
and p-NF-κBp65 levels, even following TNF-α stimulation (Fig. 3C and D).
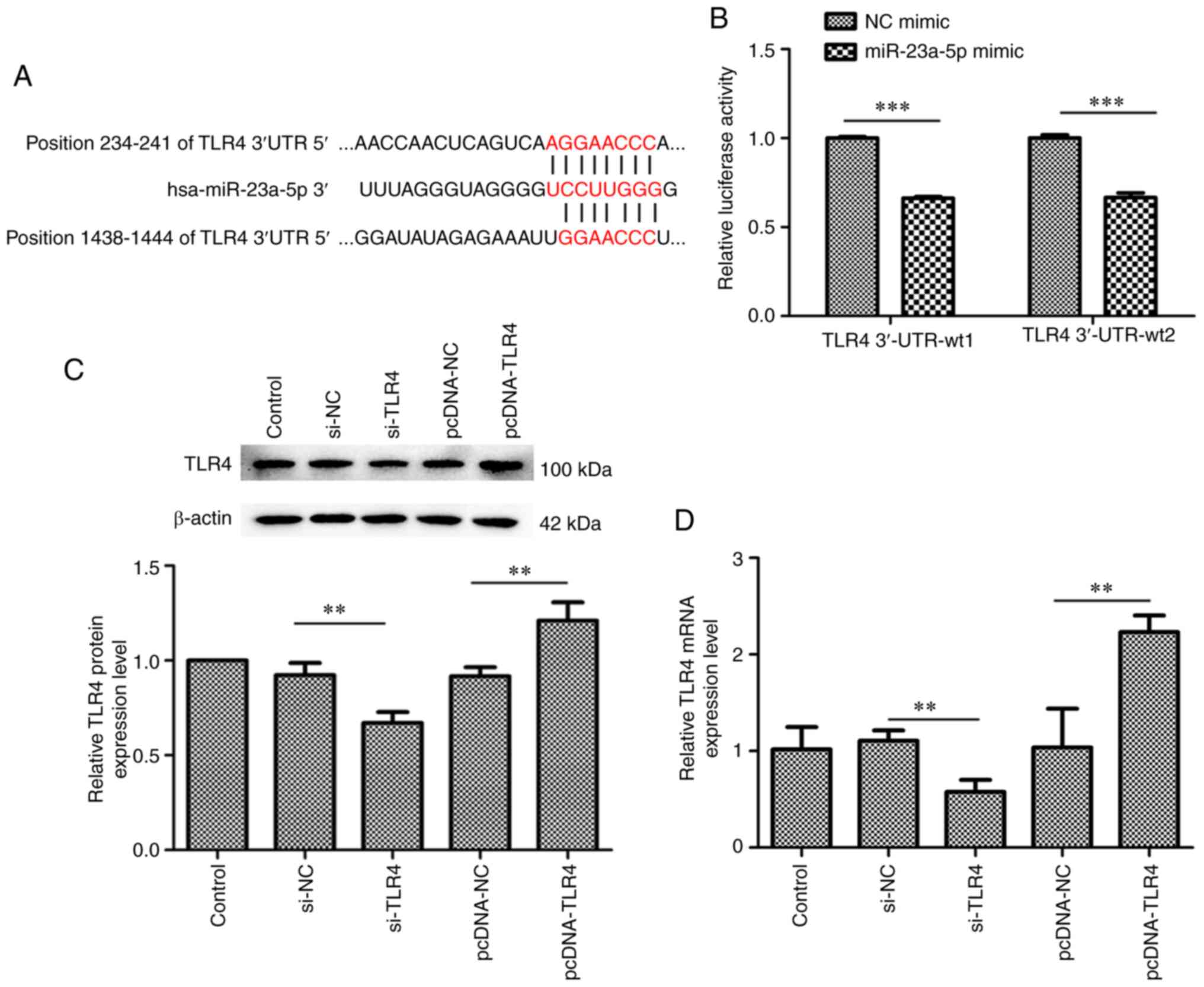
TLR4 is a target gene of
miR-23a-5p
The underlying mechanisms of the pro-apoptotic and
anti-inflammatory effects of miR-23a-5p were subsequently
determined. Bioinformatics analysis predicted that TLR4 was a
target gene of miR-23a-5p (Fig.
4A). To confirm that miR-23a-5p regulated TLR4 expression
levels by directly binding to the TLR4 3'-UTR, a dual luciferase
reporter assay was performed. As shown in Fig. 4B, the relative luciferase activity
was significantly decreased in cells co-transfected with miR-23a-5p
mimic and TLR4 3'-UTR-wt1 or TLR4 3'-UTR-wt2 (Fig. 4B). Thus, a pcDNA-TLR4 plasmid and
TLR4 siRNA were constructed for use in subsequent experiments
(Fig. 4C and D). Both at the protein and mRNA level, the
expression of TLR4 was increased by pcDNA-TLR4 plasmid transfection
and was decreased by TLR4 siRNA transfection in MH7A cells
(Fig. 4C and D).
miR-23a-5p overexpression inhibits
TNF-α-induced-MH7A cell survival via targeting TLR4
The results demonstrated that miR-23a-5p mimic
reversed the TNF-α-induced protective effects over cell damage, as
evidenced by the decreased cell viability (Fig. 5A), increased apoptotic rate
(Fig. 5B) and the aberrant
expression levels of proteins associated with cell survival
(Fig. 5C). As expected,
transfection with pcDNA-TLR4 plasmid inhibited miR-23a-5p
mimic-induced cell damage (Fig.
5A-C). Furthermore, the migration of TNF-α-stimulated MH7A
cells was inhibited following miR-23a-5p overexpression. This
effect was further strengthened following transfection with
pcDNA-TLR4, which was evidenced through the upregulated expression
levels of PCNA, MMP2, MMP9 and VEGFB (Fig. 5D and E).
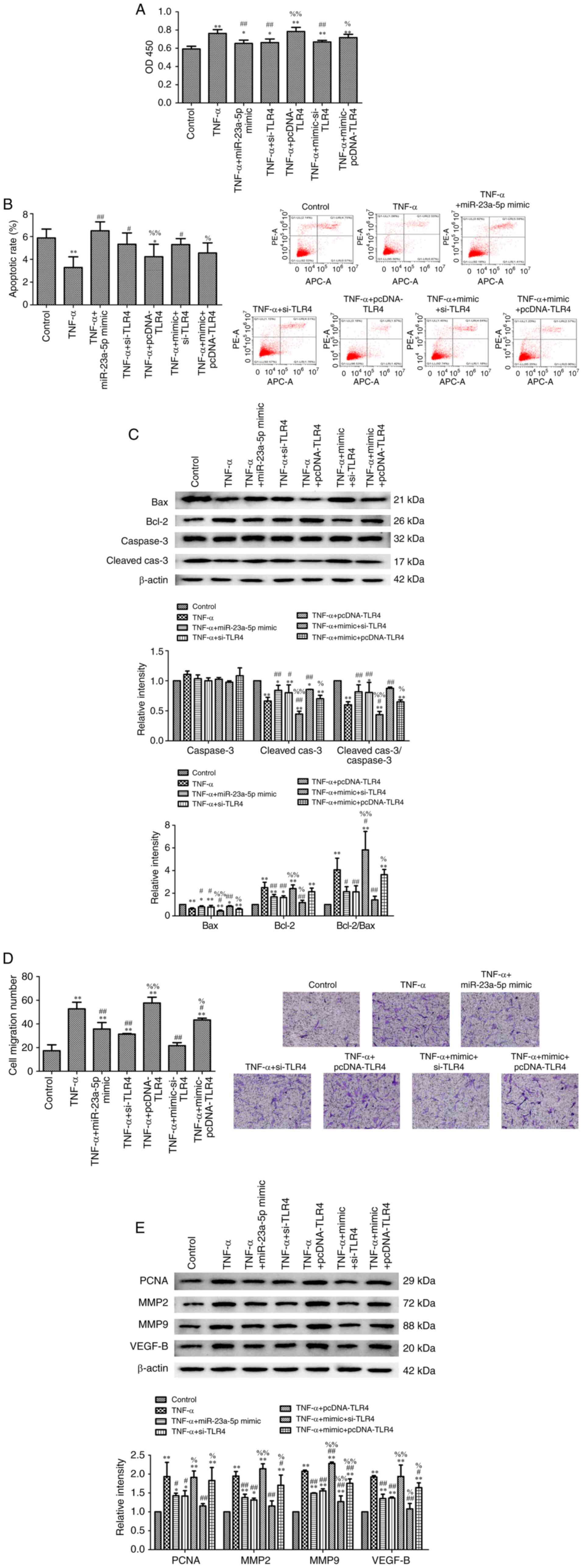 | Figure 5miR-23a-5p overexpression inhibits
TNF-α-induced-MH7A cell survival by targeting TLR4. (A)
Proliferation of MH7A cells was evaluated using a Cell Counting
Kit-8 assay. (B) Apoptosis of MH7A cells was measured using an
Annexin V-FITC/PI staining kit. (C) Bax, Bcl-2, caspase-3 and
cleaved caspase-3 expression levels were determined using western
blotting. β-actin was used as the loading control. (D) Migratory
ability of MH7A cells was evaluated using a Transwell assay
(magnification, x100). (E) PCNA, MMP2, MMP9 and VEGF-B expression
levels were determined using western blotting. β-actin was used as
the loading control. *P<0.05 and
**P<0.01 vs. control. #P<0.05 and
##P<0.01 vs. TNF-α. %P<0.05 and
%%P<0.01 vs. TNF-α + miR-23a-5p mimic. miR, microRNA;
TLR, toll-like receptor 4; PI, propidium iodide; PCNA,
proliferating cell nuclear antigen; MMP, matrix metalloproteinase;
si, small interfering RNA; Cleaved-cas3, cleaved caspase-3. |
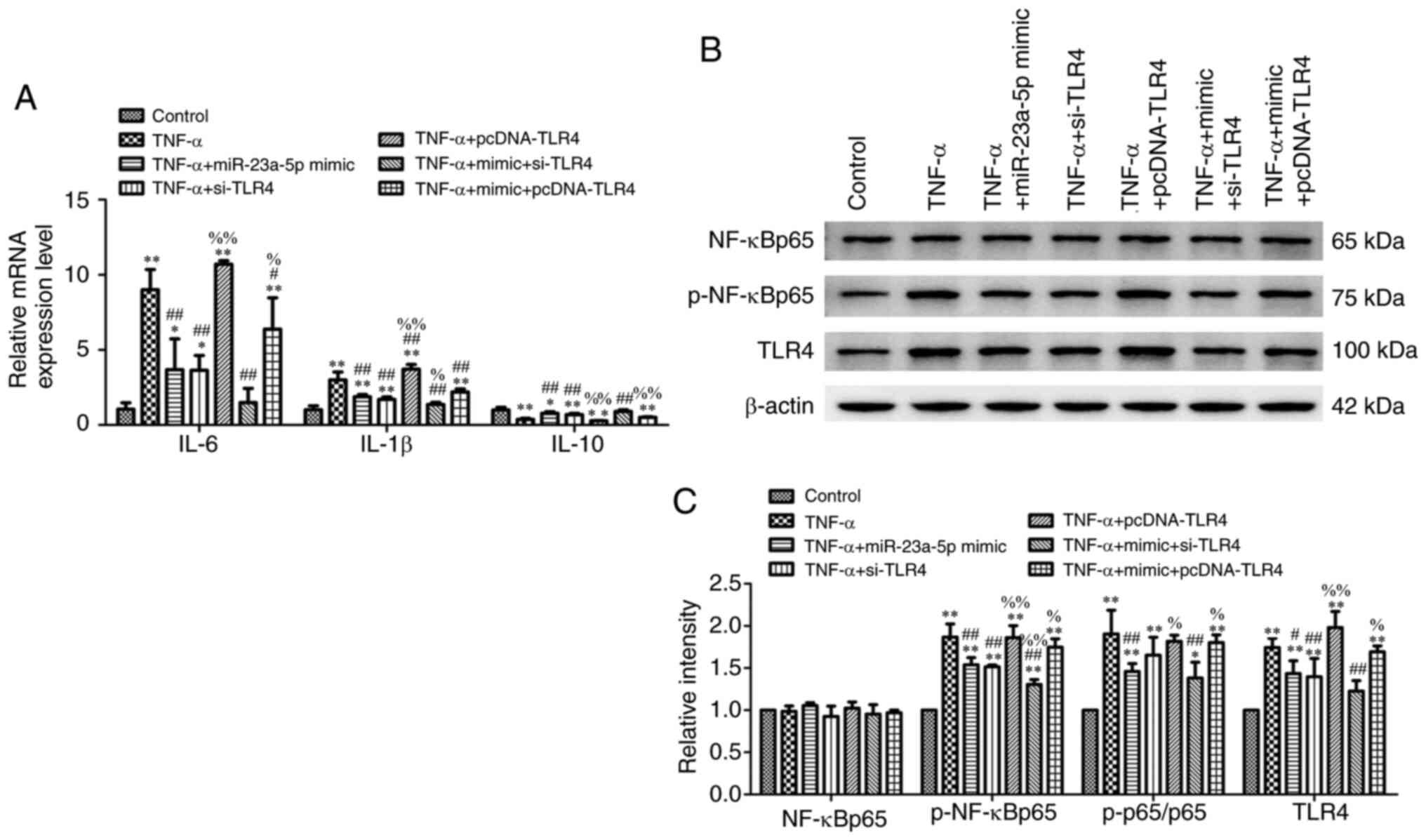
miR-23a-5p overexpression prevents
TNF-α-induced inflammatory injury and TLR4/NF-κB signaling
activation by targeting TLR4
Further experiments revealed that transfection of
cells with miR-23a-5p mimic significantly ameliorated TNF-α-induced
cell inflammation, which was evidenced through the downregulated
mRNA expression levels of IL-6 and IL-1β and upregulated mRNA
expression levels of IL-10 (Fig.
6A). Compared with the TNF-α + miR-23a-5p mimic group, the mRNA
expression levels of IL-6 and IL-1β were all upregulated, while
IL-10 mRNA expression levels were downregulated in the TNF-α +
miR-23a-5p mimic + pcDNA-TLR4 group (Fig. 6A). By contrast, TLR4 knockdown
further strengthened the effects of the miR-23a-5p mimic by
downregulating IL-6 and IL-1β expression levels and upregulating
IL-10 expression levels (Fig. 6A).
The effects of TLR4 overexpression on the TLR4/NF-κB signaling
pathway were analyzed in MH7A cells stimulated with TNF-α and
co-transfected with miR-23a-5p mimic and pcDNA-TLR4 plasmid. The
downregulated expression levels of TLR4 and decreased
p-NF-κBp65/NF-κBp65 ratio induced by TNF-α and miR-23a-5p mimic
transfection were reversed in cells treated with TNF-α and
co-transfected with miR-23a-5p mimic and pcDNA-TLR4 simultaneously
(Fig. 6B and C). Meanwhile, TLR4 knockdown enhanced the
inhibitory effects of miR-23a-5p on TLR4 and p-NF-κBp65 levels
(Fig. 6B and C).
Discussion
In recent years, accumulating studies have reported
that miRNAs are closely associated with the pathological process of
RA. Previous studies have revealed that the expression levels of
mature miR-146a, miR-146a/b precursor, miR-23b and miR-26a-5p were
all highly upregulated in fibroblast-like synoviocytes (FLSs) from
patients with RA (16,27). In addition, miR-223 and miR-128-3p
expression levels were discovered to be upregulated in the T
lymphocytes of patients with RA (28,29).
Another previous study also reported that miR-126, miR-20a and
miR-613 expression levels were markedly downregulated in the
peripheral synovial tissues of patients with RA or RASFs (30-32).
The results of the present study revealed that the expression
levels of miR-23a-5p were significantly downregulated in the
peripheral blood plasma of patients with RA and RASFs. miR-23a-5p
is located on chromosome 19p13 and was identified to serve a role
in regulating the normal growth, differentiation and apoptosis of
cells, and has been shown to be widely involved in regulating
various physiological and pathological processes (33-35).
Previous studies have confirmed that miR-23a-5p may be a potential
biomarker for human systemic inflammatory response syndrome and
seizures (36,37). miR-23a-5p overexpression was found
to regulate the proliferation, migration, invasion and apoptosis of
PDAC, RCC and bladder cancer (17,19,38).
The results of the present study revealed that miR-23a-5p
overexpression could inhibit cell proliferation, invasion and
inflammation, as well as promote cell apoptosis in TNF-α-treated
RASFs. These results suggested that miR-23a-5p may serve a crucial
role in RA pathogenesis.
TNF-α, a physiological inflammatory mediator
produced by activated monocytes and macrophages, has been
identified to serve an important role in the pathogenesis of RA
(39). TNF-α was found to stimulate
RASF proliferation and the secretion of IL-6,
granulocyte-macrophage colony stimulating factor, MMPs,
prostaglandins and other effector molecules (40-42).
RA pathogenesis is related to the immune inflammatory response and
is accompanied by secretory changes to various pro- and
anti-inflammatory factors, such as TNF-α, IL-1β, IL-6, IL-10 and
IL-17 (5,43). A recent study reported that miR-206
promoted bone metabolism and the secretion of pro-inflammatory
cytokines, including IL-16 and IL-17, during the pathological
process of RA (44). In addition,
miR-451 inhibited the secretion of pro-inflammatory cytokines
TNF-α, IL-1β and IL-6 in synovial fibroblasts by downregulating the
expression levels of p38 MAPK protein (45). The data in the present study
revealed that TNF-α promoted the inflammation of MH7A cells, while
the overexpression of miR-23a-5p significantly inhibited the
secretion of pro-inflammatory factors, IL-6 and IL-1β, and
stimulated the secretion of the anti-inflammatory factor,
IL-10.
The invasion of RASFs into the cartilage and the
destruction of bone tissue are important characteristics of RA
(46). The erosive damage caused in
RA is attributed to MMPs, PCNA and VEGF, amongst other factors
(47). miR-221 silencing was
discovered to play a role in RA pathogenesis via downregulating the
expression levels of MMP3 and MMP9 and promoting the release of
inflammatory cytokines and chemokines in FLSs (48). As a sponge of miR-138, the long
non-coding RNA HOX transcript antisense RNA significantly decreased
the lipopolysaccharide-induced upregulation of PCNA, IL-1β and
TNF-α expression levels, thereby alleviating chondrocyte
proliferation, migration and inflammation (48). In addition, miR-143 and miR-145 were
found to modulate RASF susceptibility to TNF-α and
VEGF165 stimuli through downregulating insulin-like
growth factor binding protein 5 and semaphorin 3A expression
levels, respectively (49). In the
present study, TNF-α stimulation upregulated MMP2, MMP9, VEGFB and
PCNA expression levels, which were subsequently partially reversed
by miR-23a-5p overexpression.
Numerous miR-23a-5p target genes have been
identified, including activating transcription factor 3,
extracellular matrix protein 1, Runt-related transcription factor
2, insulin-like growth factor 2 and TLR2 (17,21,50,51).
Mechanistically, the results of the present study identified TLR4
as a direct target of miR-23a-5p in RASFs. TLR4, as a member of the
TLR family, is widely distributed in various cells, such as T
cells, B cells, lung macrophages, adipocytes and intestinal
epithelial cells (52-55).
The extracellular domain of the TLR4 structure can combine with the
myeloid differentiation-2/CD14 complex, identify
pathogen-associated molecular patterns and eventually activate
NF-κB signaling (56). The
TLR4/NF-κB pathway has been reported to promote the expression of
inflammatory factors and be involved in the inflammatory response
of RA (57,58). Previous studies have demonstrated
that miRNAs participated in the inflammatory damage of RA by
regulating the TLR4/NF-κB signaling pathway. For example, miR-146a
and miR-548a-3p expression levels were reported to mediate the
proliferation and inflammation of rheumatoid arthritis
fibroblast-like synoviocytes by downregulating the TLR4/NF-κB
signaling pathway (57,59). The findings of the present study
suggested that TLR4 expression may be epigenetically regulated as a
miR-23a-5p target and may be involved in the proliferation and
inflammation of MH7A cells. To address this hypothesis, the cell
viability, migratory ability and pro-inflammatory responses of MH7A
cells following TLR4 knockdown and overexpression were
investigated. The data demonstrated that TLR4 knockdown
strengthened miR-23a-5p mimic-induced reduction in cell viability
and migration in TNF-α-treated MH7A cells, which simultaneously
further inhibited NF-κB signaling activation and the production of
pro-inflammatory factors. Conversely, TLR4 overexpression reversed
the effects of miR-23a-5p overexpression on MH7A cells.
Collectively, these data suggested that the knockdown of TLR4,
which was linked to upregulated miR-23a-5p expression levels, may
reduce the sensitivity of MH7A cells to TNF-α.
In conclusion, the findings of the present study
provided evidence to suggest that the miR-23a-5p/TLR4/NF-κB
signaling pathway may exert roles in the pathogenesis of RA and
serve as promising targets for RA diagnosis and treatment. However,
the role of miR-23a-5p in vivo in RA requires further
investigation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XB and CH conceived and designed the study; XB, CH
and LM performed the experiments; XB and LM analyzed the data; XB,
CH and LM confirmed the authenticity of the raw data; XB, CH and LM
wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Southwest Medical University Affiliated Hospital
(Luzhou, China) and written informed consent was obtained from all
participants prior to the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Picerno V, Ferro F, Adinolfi A, Valentini
E, Tani C and Alunno A: One year in review: The pathogenesis of
rheumatoid arthritis. Clin Exp Rheumatol. 33:551–558.
2015.PubMed/NCBI
|
|
2
|
Smolen JS, Aletaha D and McInnes IB:
Rheumatoid arthritis. Lancet. 388:2023–2038. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Syed Mohamed Suhail SM, Nur Atiqah I, Nur
Salimi Zakirah ZA, Lailatul Syazwani Z, Batis WW, Md Monoto EM,
Abdul Wahab A and Mohd Shahrir MS: Diagnostic performance of
anti-RA33 antibody as a serological marker for rheumatoid
arthritis. Malays J Pathol. 41:259–265. 2019.PubMed/NCBI
|
|
4
|
El-Maghraby HM, Rabie RA and Makram WK:
Correlation between relative expression of IL 17 and PERP in
rheumatoid arthritis patients and disease activity. Egypt J
Immunol. 26:19–29. 2019.PubMed/NCBI
|
|
5
|
Kim EK, Kwon JE, Lee SY, Lee EJ, Kim DS,
Moon SJ, Lee J, Kwok SK, Park SH and Cho ML: IL-17-mediated
mitochondrial dysfunction impairs apoptosis in rheumatoid arthritis
synovial fibroblasts through activation of autophagy. Cell Death
Dis. 8(e2565)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang S, Wang S, Li H, Zhu L and Wang Y:
Inhibition of the TGF-β/Smads signaling pathway attenuates
pulmonary fibrosis and induces anti-proliferative effect on
synovial fibroblasts in rheumatoid arthritis. Int J Clin Exp
Pathol. 12:1835–1845. 2019.PubMed/NCBI
|
|
7
|
Evangelatos G, Fragoulis GE, Koulouri V
and Lambrou GI: MicroRNAs in rheumatoid arthritis: From
pathogenesis to clinical impact. Autoimmun Rev.
18(102391)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Su Z, Yang Z, Xu Y, Chen Y and Yu Q:
MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget.
6:8474–8490. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
McAlinden A and Im GI: MicroRNAs in
orthopaedic research: Disease associations, potential therapeutic
applications, and perspectives. J Orthop Res. 36:33–51.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chen YJ, Chang WA, Wu LY, Hsu YL, Chen CH
and Kuo PL: Systematic analysis of differential expression profile
in rheumatoid arthritis chondrocytes using next-generation
sequencing and bioinformatics approaches. Int J Med Sci.
15:1129–1142. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tseng CC, Wu LY, Tsai WC, Ou TT, Wu CC,
Sung WY, Kuo PL and Yen JH: Differential expression profiles of the
transcriptome and miRNA interactome in synovial fibroblasts of
rheumatoid arthritis revealed by next generation sequencing.
Diagnostics (Basel). 9(98)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Su LC, Huang AF, Jia H, Liu Y and Xu WD:
Role of microRNA-155 in rheumatoid arthritis. Int J Rheum Dis.
20:1631–1637. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jin S, Chen H, Li Y, Zhong H, Sun W, Wang
J, Zhang T, Ma J, Yan S, Zhang J, et al: Maresin 1 improves the
Treg/Th17 imbalance in rheumatoid arthritis through miR-21. Ann
Rheum Dis. 77:1644–1652. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen Z, Wang H, Xia Y, Yan F and Lu Y:
Therapeutic potential of mesenchymal cell-derived
miRNA-150-5p-expressing exosomes in rheumatoid arthritis mediated
by the modulation of MMP14 and VEGF. J Immunol. 201:2472–2482.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu X, Ni S, Li C, Xu N, Chen W, Wu M, van
Wijnen AJ and Wang Y: Circulating microRNA-23b as a new biomarker
for rheumatoid arthritis. Gene. 712(143911)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Huang W, Huang Y, Gu J, Zhang J, Yang J,
Liu S, Xie C, Fan Y and Wang H: miR-23a-5p inhibits cell
proliferation and invasion in pancreatic ductal adenocarcinoma by
suppressing ECM1 expression. Am J Transl Res. 11:2983–2994.
2019.PubMed/NCBI
|
|
18
|
Ganesan S, Palani HK, Lakshmanan V,
Balasundaram N, Alex AA, David S, Venkatraman A, Korula A, George
B, Balasubramanian P, et al: Stromal cells downregulate miR-23a-5p
to activate protective autophagy in acute myeloid leukemia. Cell
Death Dis. 10(736)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Quan J, Jin L, Pan X, He T and Lai Y, Chen
P, Lin C, Yang S, Zeng H and Lai Y: Oncogenic miR-23a-5p is
associated with cellular function in RCC. Mol Med Rep.
16:2309–2317. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yang S, Ye ZM, Chen S, Luo XY, Chen SL,
Mao L, Li Y, Jin H, Yu C, Xiang FX, et al: MicroRNA-23a-5p promotes
atherosclerotic plaque progression and vulnerability by repressing
ATP-binding cassette transporter A1/G1 in macrophages. J Mol Cell
Cardiol. 123:139–149. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang JX, Xie P, Li YS, Wen T and Yang XC:
Osteoclast-derived miR-23a-5p-containing exosomes inhibit
osteogenic differentiation by regulating Runx2. Cell Signal.
70(109504)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cao C, Chai Y, Shou S, Wang J, Huang Y and
Ma T: Toll-like receptor 4 deficiency increases resistance in
sepsis-induced immune dysfunction. Int Immunopharmacol. 54:169–176.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rocha DM, Caldas AP, Oliveira LL, Bressan
J and Hermsdorff HH: Saturated fatty acids trigger TLR4-mediated
inflammatory response. Atherosclerosis. 244:211–215.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Aletaha D, Neogi T, Silman AJ, Funovits J,
Felson DT, Bingham CO III, Birnbaum NS, Burmester GR, Bykerk VP,
Cohen MD, et al: 2010 Rheumatoid arthritis classification criteria:
An American college of rheumatology/european league against
rheumatism collaborative initiative. Arthritis Rheum. 62:2569–2581.
2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kong L, Wang L, Zhao Q, Di G and Wu H:
Rhodojaponin II inhibits TNF-α-induced inflammatory cytokine
secretion in MH7A human rheumatoid arthritis fibroblast-like
synoviocytes. J Biochem Mol Toxicol. 34(e22551)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nakasa T, Miyaki S, Okubo A, Hashimoto M,
Nishida K, Ochi M and Asahara H: Expression of microRNA-146 in
rheumatoid arthritis synovial tissue. Arthritis Rheum.
58:1284–1292. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Xia Z, Meng F, Liu Y, Fang Y, Wu X, Zhang
C, Liu D and Li G: Decreased MiR-128-3p alleviates the progression
of rheumatoid arthritis by up-regulating the expression of TNFAIP3.
Biosci Rep. 38(BSR20180540)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fulci V, Scappucci G, Sebastiani GD,
Giannitti C, Franceschini D, Meloni F, Colombo T, Citarella F,
Barnaba V, Minisola G, et al: miR-223 is overexpressed in
T-lymphocytes of patients affected by rheumatoid arthritis. Hum
Immunol. 71:206–211. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gao J, Kong R, Zhou X, Ji L, Zhang J and
Zhao D: MiRNA-126 expression inhibits IL-23R mediated TNF-α or
IFN-γ production in fibroblast-like synoviocytes in a mice model of
collagen-induced rheumatoid arthritis. Apoptosis. 23:607–615.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wei XJ, Li XW, Lu JL, Long ZX, Liang JQ,
Wei SB, Lu CX and Lu WZ: MiR-20a regulates fibroblast-like
synoviocyte proliferation and apoptosis in rheumatoid arthritis.
Eur Rev Med Pharmacol Sci. 21:3886–3893. 2017.PubMed/NCBI
|
|
32
|
Liu L, Zuo Y, Xu Y, Zhang Z, Li Y and Pang
J: MiR-613 inhibits proliferation and invasion and induces
apoptosis of rheumatoid arthritis synovial fibroblasts by direct
down-regulation of DKK1. Cell Mol Biol Lett. 24(8)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kurkewich JL, Hansen J, Klopfenstein N,
Zhang H, Wood C, Boucher A, Hickman J, Muench DE, Grimes HL and
Dahl R: The miR-23a~27a~24-2 microRNA cluster buffers transcription
and signaling pathways during hematopoiesis. PLoS Genet.
13(e1006887)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang N, Tan HY, Feng YG, Zhang C, Chen F
and Feng Y: microRNA-23a in human cancer: Its roles, mechanisms and
therapeutic relevance. Cancers (Basel). 11(7)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Huang J, Jiang R, Chu X, Wang F, Sun X,
Wang Y and Pang L: Overexpression of microRNA-23a-5p induces
myocardial infarction by promoting cardiomyocyte apoptosis through
inhibited of PI3K/AKT signalling pathway. Cell Biochem Funct.
38:1047–1055. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Caserta S, Kern F, Cohen J, Drage S,
Newbury SF and Llewelyn MJ: Circulating plasma microRNAs can
differentiate human sepsis and systemic inflammatory response
syndrome (SIRS). Sci Rep. 6(28006)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Roncon P, Soukupovà M, Binaschi A,
Falcicchia C, Zucchini S, Ferracin M, Langley SR, Petretto E,
Johnson MR, Marucci G, et al: MicroRNA profiles in hippocampal
granule cells and plasma of rats with pilocarpine-induced
epilepsy-comparison with human epileptic samples. Sci Rep.
5(14143)2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li Y, Quan J, Pan X, Zhou J, He A, Lai Y
and Zhou G: Suppressing cell growth and inducing apoptosis by
inhibiting miR-23a-5p in human bladder cancer. Mol Med Rep.
18:5256–5260. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yamanaka H: TNF as a target of
inflammation in rheumatoid arthritis. Endocr Metab Immune Disord
Drug Targets. 15:129–134. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lee AS, Ellman MB, Yan D, Kroin JS, Cole
BJ, van Wijnen AJ and Im HJ: A current review of molecular
mechanisms regarding osteoarthritis and pain. Gene. 527:440–447.
2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Guo X, Wang S, Godwood A, Close D, Ryan
PC, Roskos LK and White WI: Pharmacodynamic biomarkers and
differential effects of TNF- and GM-CSF-targeting biologics in
rheumatoid arthritis. Int J Rheum Dis. 22:646–653. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wei ST, Sun YH, Zong SH and Xiang YB:
Serum levels of IL-6 and TNF-α may correlate with activity and
severity of rheumatoid arthritis. Med Sci Monit. 21:4030–4038.
2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu W, Zhang Y, Zhu W, Ma C, Ruan J, Long
H and Wang Y: Sinomenine inhibits the progression of rheumatoid
arthritis by regulating the secretion of inflammatory cytokines and
monocyte/macrophage subsets. Front Immunol. 9(2228)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
ElAtta AA, Ali Y, Bassyouni I and Talaat
R: Correlation of myomir-206 and proinflammatory cytokines (IL-16
and IL-17) in patients with rheumatoid arthritis. Reumatologia.
57:72–77. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wang ZC, Lu H, Zhou Q, Yu SM, Mao YL,
Zhang HJ, Zhang PC and Yan WJ: MiR-451 inhibits synovial
fibroblasts proliferation and inflammatory cytokines secretion in
rheumatoid arthritis through mediating p38MAPK signaling pathway.
Int J Clin Exp Pathol. 8:14562–14567. 2015.PubMed/NCBI
|
|
46
|
Li J, Song Q, Shao L, Zhang LL, Guo XH and
Mao YJ: MiR-124a inhibits proliferation and invasion of rheumatoid
arthritis synovial fibroblasts. Eur Rev Med Pharmacol Sci.
22:4581–4588. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Nagaoka A, Takizawa N, Takeuchi R, Inaba
Y, Saito I, Nagashima Y, Saito T and Aoki I: Possible involvement
of peptidylprolyl isomerase Pin1 in rheumatoid arthritis. Pathol
Int. 61:59–66. 2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yang S and Yang Y: Downregulation of
microRNA-221 decreases migration and invasion in fibroblast-like
synoviocytes in rheumatoid arthritis. Mol Med Rep. 12:2395–2401.
2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Hong BK, You S, Yoo SA, Park D, Hwang D,
Cho CS and Kim WU: MicroRNA-143 and -145 modulate the phenotype of
synovial fibroblasts in rheumatoid arthritis. Exp Mol Med.
49(e363)2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Gu X, Gao Y, Mu DG and Fu EQ: MiR-23a-5p
modulates mycobacterial survival and autophagy during mycobacterium
tuberculosis infection through TLR2/MyD88/NF-κB pathway by
targeting TLR2. Exp Cell Res. 354:71–77. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yang J, Li Y, Yu Z, Zhou Y, Tu J, Lou J
and Wang Y: Circular RNA Circ100084 functions as sponge of
miR-23a-5p to regulate IGF2 expression in hepatocellular carcinoma.
Mol Med Rep. 21:2395–2404. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Dubois Cauwelaert N, Baldwin SL, Orr MT,
Desbien AL, Gage E, Hofmeyer KA and Coler RN: Antigen presentation
by B cells guides programing of memory CD4(+) T-cell responses to a
TLR4-agonist containing vaccine in mice. Eur J Immunol.
46:2719–2729. 2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kato K, Hanss AD, Zemskova MA, Morgan NE,
Kim M, Knox KS, Lin Y, Lillehoj EP and Kim KC: Pseudomonas
aeruginosa increases MUC1 expression in macrophages through the
TLR4-p38 pathway. Biochem Biophys Res Commun. 492:231–235.
2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhang M, Wang C, Wu J, Ha X, Deng Y, Zhang
X, Wang J, Chen K, Feng J, Zhu J, et al: The effect and mechanism
of KLF7 in the TLR4/NF-κB/IL-6 inflammatory signal pathway of
adipocytes. Mediators Inflamm. 2018(1756494)2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Layunta E, Latorre E, Forcén R, Grasa L,
Castro M, Arias MA, Alcalde AI and Mesonero JE: NOD2 modulates
serotonin transporter and interacts with TLR2 and TLR4 in
intestinal epithelial cells. Cell Physiol Biochem. 47:1217–1229.
2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Rallabhandi P, Bell J, Boukhvalova MS,
Medvedev A, Lorenz E, Arditi M, Hemming VG, Blanco JCG, Segal DM
and Vogel SN: Analysis of TLR4 polymorphic variants: New insights
into TLR4/MD-2/CD14 stoichiometry, structure, and signaling. J
Immunol. 177:322–332. 2006.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Liu W, Wu YH, Zhang L, Xue B, Wang Y, Liu
B, Liu XY, Zuo F, Yang XY, Chen FY, et al: MicroRNA-146a suppresses
rheumatoid arthritis fibroblast-like synoviocytes proliferation and
inflammatory responses by inhibiting the TLR4/NF-kB signaling.
Oncotarget. 9:23944–23959. 2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Yan S, Wang P, Wang J, Yang J, Lu H, Jin
C, Cheng M and Xu D: Long non-coding RNA HIX003209 promotes
inflammation by sponging miR-6089 via TLR4/NF-κB signaling pathway
in rheumatoid arthritis. Front Immunol. 10(2218)2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Wang Y, Zheng F, Gao G, Yan S, Zhang L,
Wang L, Cai X, Wang X, Xu D and Wang J: MiR-548a-3p regulates
inflammatory response via TLR4/NF-κB signaling pathway in
rheumatoid arthritis. J Cell Biochem. 2018.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|