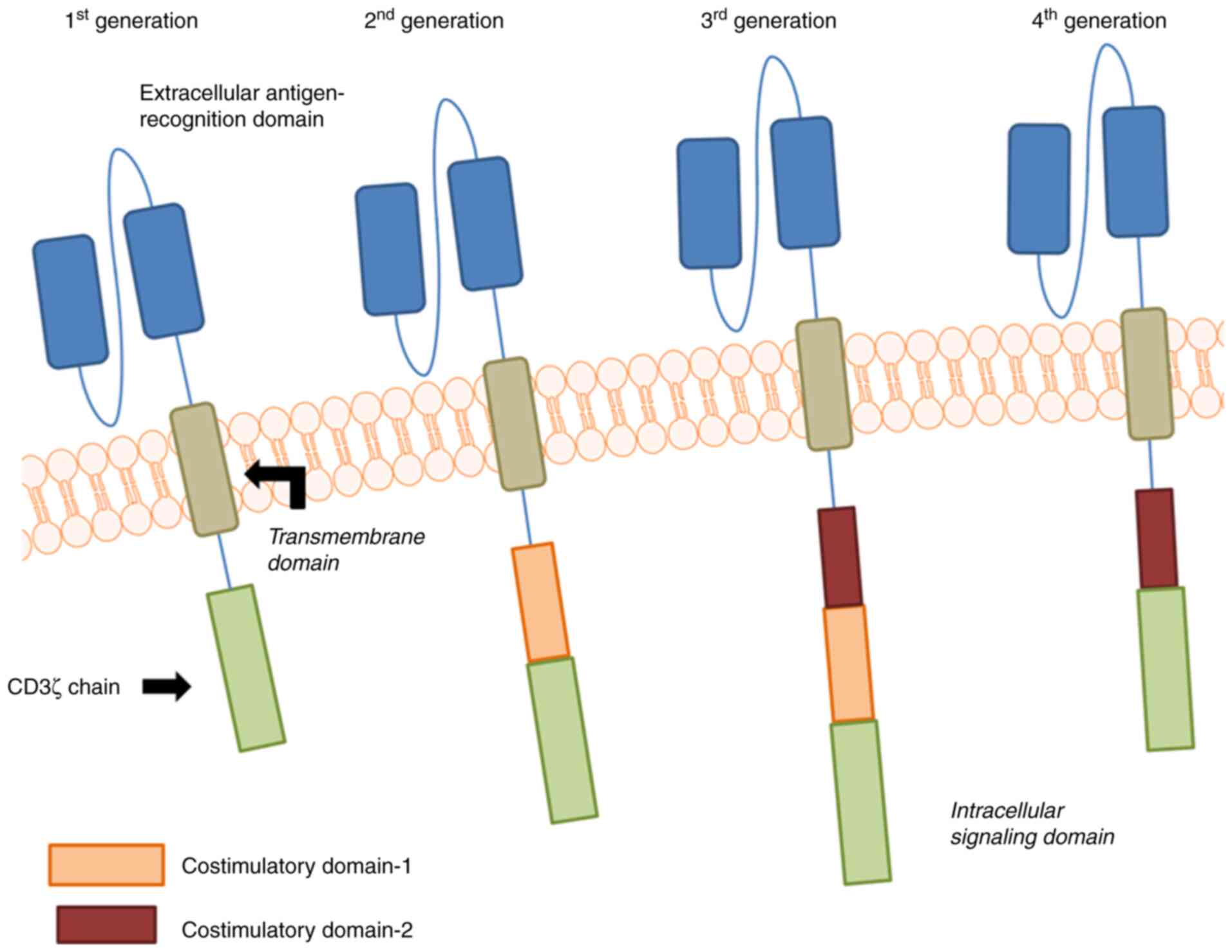
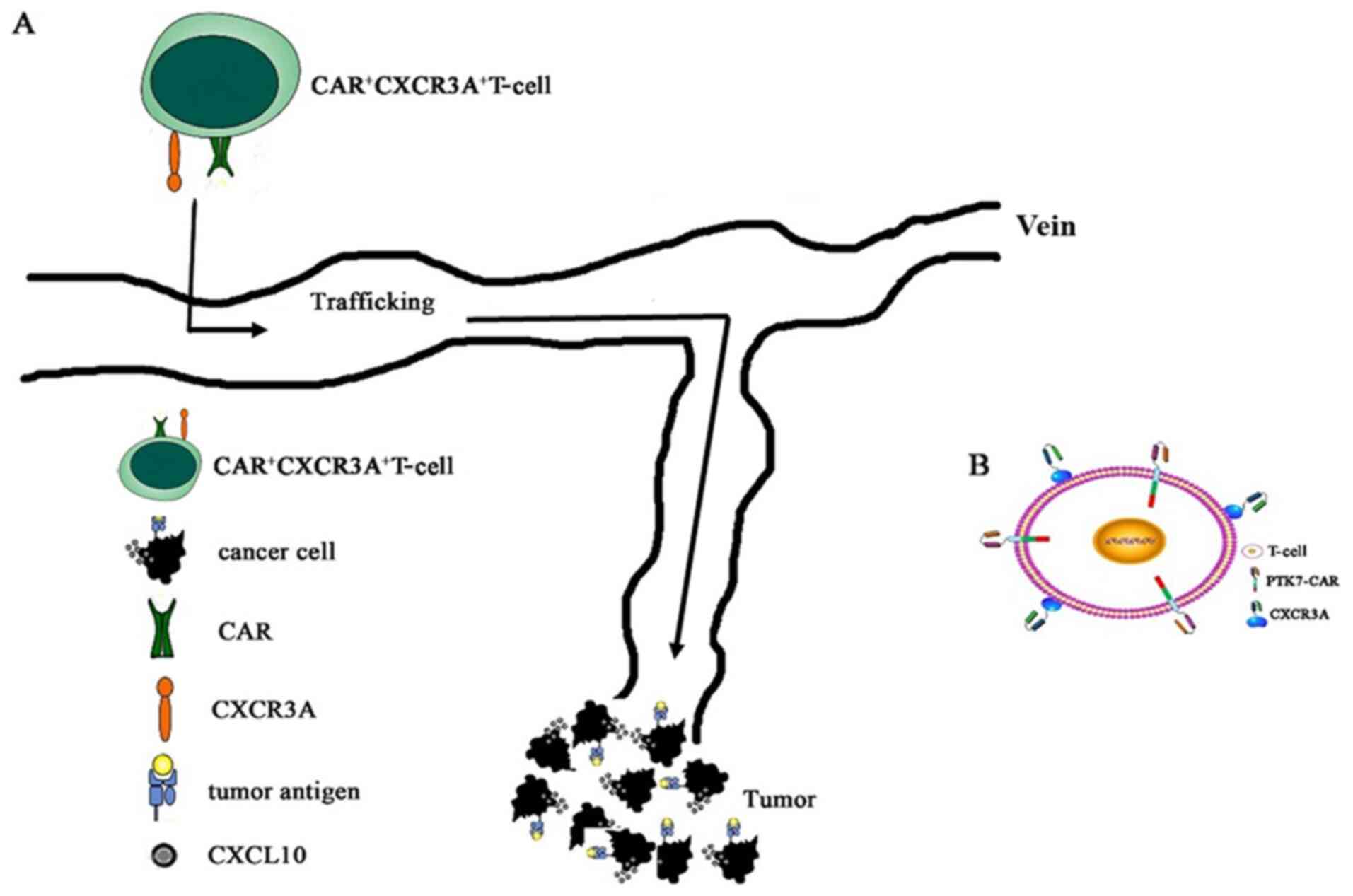
|
1
|
Panagopoulou TI and Rafiq QA: CAR-T
immunotherapies: Biotechnological strategies to improve safety,
efficacy and clinical outcome through CAR engineering. Biotechnol
Adv. 37(107411)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Couzin-Frankel J: Breakthrough of the year
2013. Cancer immunotherapy. Science. 342:1432–1433. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tan S, Li D and Zhu X: Cancer
immunotherapy: Pros, cons and beyond. Biomed Pharmacother.
124(109821)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Peng M, Mo Y, Wang Y, Wu P, Zhang Y, Xiong
F, Guo C, Wu X, Li Y, Li X, et al: Neoantigen vaccine: An emerging
tumor immunotherapy. Mol Cancer. 18(128)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yáñez L, Sánchez-Escamilla M and Perales
MA: CAR T cell toxicity: Current management and future directions.
HemaSphere. 3(e186)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ma CC, Wang ZL, Xu T, He ZY and Wei YQ:
The approved gene therapy drugs worldwide: from 1998 to 2019.
Biotechnol Adv. 40(107502)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mullard A: FDA approves first CAR T
therapy. Nat Rev Drug Discov. 16(669)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
No authors listed. FDA approves second CAR
T-cell therapy. Cancer Discov. 8:5–6. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Huang X and Yang Y: Driving an improved
CAR for cancer immunotherapy. J Clin Invest. 126:2795–2798.
2016.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Huang R, Li X, He Y, Zhu W, Gao L, Liu Y,
Gao L, Wen Q, Zhong JF, Zhang C, et al: Recent advances in CAR-T
cell engineering. J Hematol Oncol. 13(86)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jackson HJ, Rafiq S and Brentjens RJ:
Driving CAR T-cells forward. Nat Rev Clin Oncol. 13:370–383.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Depil S, Duchateau P, Grupp SA, Mufti G
and Poirot L: ‘Off-the-shelf’ allogeneic CAR T cells: Development
and challenges. Nat Rev Drug Discov. 19:185–199. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schmidts A and Maus MV: Making CAR T cells
a solid option for solid tumors. Front Immunol.
9(2593)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chmielewski M and Abken H: TRUCKs: The
fourth generation of CARs. Expert Opin Biol Ther. 15:1145–1154.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li D, Li X, Zhou WL, Huang Y, Liang X,
Jiang L, Yang X, Sun J, Li Z, Han WD, et al: Genetically engineered
T cells for cancer immunotherapy. Signal Transduct Target Ther.
4(35)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Schuster SJ, Bishop MR, Tam CS, Waller EK,
Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin
JR, et al: JULIET Investigators: Tisagenlecleucel in adult relapsed
or refractory diffuse large B-cell lymphoma. N Engl J Med.
380:45–56. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yamada S, Kaneko MK, Sayama Y, Asano T,
Sano M, Yanaka M, Nakamura T, Okamoto S, Handa S, Komatsu Y, et al:
Development of novel mouse monoclonal antibodies against human
CD19. Monoclon Antib Immunodiagn Immunother. 39:45–50.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Schubert ML, Hückelhoven A, Hoffmann JM,
Schmitt A, Wuchter P, Sellner L, Hofmann S, Ho AD, Dreger P and
Schmitt M: Chimeric antigen receptor T cell therapy targeting
CD19-positive leukemia and lymphoma in the context of stem cell
transplantation. Hum Gene Ther. 27:758–771. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Iovino L and Shadman M: Novel therapies in
chronic lymphocytic leukemia: A rapidly changing landscape. Curr
Treat Options Oncol. 21(24)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Porter DL, Hwang WT, Frey NV, Lacey SF,
Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, et al:
Chimeric antigen receptor T cells persist and induce sustained
remissions in relapsed refractory chronic lymphocytic leukemia. Sci
Transl Med. 7(303ra139)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fry TJ, Shah NN, Orentas RJ,
Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S,
Delbrook C, Yates B, et al: CD22-targeted CAR T cells induce
remission in B-ALL that is naive or resistant to CD19-targeted CAR
immunotherapy. Nat Med. 24:20–28. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Liu J, Tan X, Ma YY, Liu Y, Gao L, Gao L,
Kong P, Peng XG, Zhang X and Zhang C: Study on the prognostic value
of aberrant antigen in patients with acute B lymphocytic leukemia.
Clin Lymphoma Myeloma Leuk. 19:e349–e358. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Qin H, Edwards JP, Zaritskaya L, Gupta A,
Mu CJ, Fry TJ, Hilbert DM and LaFleur DW: Chimeric antigen
receptors incorporating D domains targeting CD123 direct potent
mono- and bi-specific antitumor activity of T cells. Mol Ther.
27:1262–1274. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Borot F, Wang H, Ma Y, Jafarov T, Raza A,
Ali AM and Mukherjee S: Gene-edited stem cells enable CD33-directed
immune therapy for myeloid malignancies. Proc Natl Acad Sci USA.
116:11978–11987. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xu J, Chen LJ, Yang SS, Sun Y, Wu W, Liu
YF, Xu J, Zhuang Y, Zhang W, Weng XQ, et al: Exploratory trial of a
biepitopic CAR T-targeting B cell maturation antigen in
relapsed/refractory multiple myeloma. Proc Natl Acad Sci USA.
116:9543–9551. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
D'Agostino M and Raje N: Anti-BCMA CAR
T-cell therapy in multiple myeloma: Can we do better? Leukemia.
34:21–34. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Brudno JN, Maric I, Hartman SD, Rose JJ,
Wang M, Lam N, Stetler-Stevenson M, Salem D, Yuan C, Pavletic S, et
al: T cells genetically modified to express an anti-B-cell
maturation antigen chimeric antigen receptor cause remissions of
poor-prognosis relapsed multiple myeloma. J Clin Oncol.
36:2267–2280. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Morandi F, Horenstein AL, Costa F,
Giuliani N, Pistoia V and Malavasi F: CD38: A target for
immunotherapeutic approaches in multiple myeloma. Front Immunol.
9(2722)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Vasuthasawat A, Yoo EM, Trinh KR,
Lichtenstein A, Timmerman JM and Morrison SL: Targeted
immunotherapy using anti-CD138-interferon α fusion proteins and
bortezomib results in synergistic protection against multiple
myeloma. MAbs. 8:1386–1397. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sun M, Shi H, Liu C, Liu J, Liu X and Sun
Y: Construction and evaluation of a novel humanized HER2-specific
chimeric receptor. Breast Cancer Res. 16(R61)2014.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Gao H, Li K, Tu H, Pan X, Jiang H, Shi B,
Kong J, Wang H, Yang S, Gu J, et al: Development of T cells
redirected to glypican-3 for the treatment of hepatocellular
carcinoma. Clin Cancer Res. 20:6418–6428. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li D, Li N, Zhang YF, Fu H, Feng M,
Schneider D, Su L, Wu X, Zhou J, Mackay S, et al: Persistent
polyfunctional chimeric antigen receptor T cells that target
glypican 3 eliminate orthotopic hepatocellular carcinomas in mice.
Gastroenterology. 158:2250–2265.e20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu Y, Di S, Shi B, Zhang H, Wang Y, Wu X,
Luo H, Wang H, Li Z and Jiang H: Armored inducible expression of
IL-12 enhances antitumor activity of glypican-3-targeted chimeric
antigen receptor-engineered T cells in hepatocellular carcinoma. J
Immunol. 203:198–207. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wei X, Lai Y, Li J, Qin L, Xu Y, Zhao R,
Li B, Lin S, Wang S, Wu Q, et al: PSCA and MUC1 in non-small-cell
lung cancer as targets of chimeric antigen receptor T cells.
OncoImmunology. 6(e1284722)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sotoudeh M, Shirvani SI, Merat S,
Ahmadbeigi N and Naderi M: MSLN (Mesothelin), ANTXR1 (TEM8), and
MUC3A are the potent antigenic targets for CAR T cell therapy of
gastric adenocarcinoma. J Cell Biochem. 120:5010–5017.
2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zeng C, Cheng J, Li T, Huang J, Li C,
Jiang L, Wang J, Chen L, Mao X, Zhu L, et al: Efficacy and toxicity
for CD22/CD19 chimeric antigen receptor T-cell therapy in patients
with relapsed/refractory aggressive B-cell lymphoma involving the
gastrointestinal tract. Cytotherapy. 22:166–171. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhu H, You Y, Shen Z and Shi L:
EGFRvIII-CAR-T cells with PD-1 knockout have improved anti-glioma
activity. Pathol Oncol Res. 26:2135–2141. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang BL, Li D, Gong YL, Huang Y, Qin DY,
Jiang L, Liang X, Yang X, Gou HF, Wang YS, et al: Preclinical
evaluation of chimeric antigen receptor-modified T cells specific
to epithelial cell adhesion molecule for treating colorectal
cancer. Hum Gene Ther. 30:402–412. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Liu K, Song G, Zhang X, Li Q, Zhao Y, Zhou
Y, Xiong R, Hu X, Tang Z and Feng G: PTK7 is a novel oncogenic
target for esophageal squamous cell carcinoma. World J Surg Oncol.
15(105)2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gutgarts V, Jain T, Zheng J, Maloy MA,
Ruiz JD, Pennisi M, Jaimes EA, Perales MA and Sathick J: Acute
kidney injury after CAR-T cell therapy: Low incidence and rapid
recovery. Biol Blood Marrow Transplant. 26:1071–1076.
2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Aldoss I, Khaled SK, Budde E and Stein AS:
Cytokine release syndrome with the novel treatments of acute
lymphoblastic leukemia: Pathophysiology, prevention, and treatment.
Curr Oncol Rep. 21(4)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Riegler LL, Jones GP and Lee DW: Current
approaches in the grading and management of cytokine release
syndrome after chimeric antigen receptor T-cell therapy. Ther Clin
Risk Manag. 15:323–335. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shimabukuro-Vornhagen A, Gödel P, Subklewe
M, Stemmler HJ, Schlößer HA, Schlaak M, Kochanek M, Böll B and von
Bergwelt-Baildon MS: Cytokine release syndrome. J Immunother
Cancer. 6(56)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chou CK and Turtle CJ: Assessment and
management of cytokine release syndrome and neurotoxicity following
CD19 CAR-T cell therapy. Expert Opin Biol Ther. 20:653–664.
2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Thakar MS, Kearl TJ and Malarkannan S:
Controlling cytokine release syndrome to harness the full potential
of CAR-based cellular therapy. Front Oncol. 9(1529)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Alvi RM, Frigault MJ, Fradley MG, Jain MD,
Mahmood SS, Awadalla M, Lee DH, Zlotoff DA, Zhang L, Drobni ZD, et
al: Cardiovascular events among adults treated with chimeric
antigen receptor T-cells (CAR-T). J Am Coll Cardiol. 74:3099–3108.
2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Liu D and Zhao J: Cytokine release
syndrome: Grading, modeling, and new therapy. J Hematol Oncol.
11(121)2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Gödel P, Shimabukuro-Vornhagen A and von
Bergwelt-Baildon M: Understanding cytokine release syndrome.
Intensive Care Med. 44:371–373. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Acharya UH, Dhawale T, Yun S, Jacobson CA,
Chavez JC, Ramos JD, Appelbaum J and Maloney DG: Management of
cytokine release syndrome and neurotoxicity in chimeric antigen
receptor (CAR) T cell therapy. Expert Rev Hematol. 12:195–205.
2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Gauthier J, Hirayama AV, Purushe J, Hay
KA, Lymp J, Li DH, Yeung CCS, Sheih A, Pender BS, Hawkins RM, et
al: Feasibility and efficacy of CD19-targeted CAR T cells with
concurrent ibrutinib for CLL after ibrutinib failure. Blood.
135:1650–1660. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Tu S, Zhou X, Guo Z, Huang R, Yue C, He Y,
Li M, Chen Y, Liu Y, Chang LJ, et al: CD19 and CD70 dual-target
chimeric antigen receptor T-cell therapy for the treatment of
relapsed and refractory primary central nervous system diffuse
large B-cell lymphoma. Front Oncol. 9(1350)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Shen D, Song H, Xu X, Xu W, Wang D, Liang
J, Fang M, Liao C, Chen X, Li S, et al: Chimeric antigen receptor T
cell therapy can be administered safely under the real-time
monitoring of Th1/Th2 cytokine pattern using the cytometric bead
array technology for relapsed and refractory acute lymphoblastic
leukemia in children. Pediatr Hematol Oncol. 37:288–299.
2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Cornillon J, Hadhoum N, Roth-Guepin G,
Quessar A, Platon L, Ouachée-Chardin M, Nicolas-Virelizier E,
Naudin J, Moreau AS, Masouridi-Levrat S, et al: Management of CAR-T
cell-related encephalopathy syndrome in adult and pediatric
patients: Recommendations of the French Society of Bone Marrow
transplantation and cellular Therapy (SFGM-TC). Bull Cancer.
107:S12–S17. 2020.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
54
|
Kennedy LB and Salama AKS: A review of
cancer immunotherapy toxicity. CA Cancer J Clin. 70:86–104.
2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Turtle CJ, Hay KA, Hanafi LA, Li D,
Cherian S, Chen X, Wood B, Lozanski A, Byrd JC, Heimfeld S, et al:
Durable molecular remissions in chronic lymphocytic leukemia
treated with CD19-specific chimeric antigen receptor-modified T
cells after failure of Ibrutinib. J Clin Oncol. 35:3010–3020.
2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Karschnia P, Jordan JT, Forst DA,
Arrillaga-Romany IC, Batchelor TT, Baehring JM, Clement NF,
Gonzalez Castro LN, Herlopian A, Maus MV, et al: Clinical
presentation, management, and biomarkers of neurotoxicity after
adoptive immunotherapy with CAR T cells. Blood. 133:2212–2221.
2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Santomasso BD, Park JH, Salloum D, Riviere
I, Flynn J, Mead E, Halton E, Wang X, Senechal B, Purdon T, et al:
Clinical and biological correlates of neurotoxicity associated with
CAR T-cell therapy in patients with B-cell acute lymphoblastic
leukemia. Cancer Discov. 8:958–971. 2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lee DW, Kochenderfer JN, Stetler-Stevenson
M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M,
Shah NN, et al: T cells expressing CD19 chimeric antigen receptors
for acute lymphoblastic leukaemia in children and young adults: A
phase 1 dose-escalation trial. Lancet. 385:517–528. 2015.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Oved JH, Barrett DM and Teachey DT:
Cellular therapy: Immune-related complications. Immunol Rev.
290:114–126. 2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Gupta A and Moore JA: Tumor lysis
syndrome. JAMA Oncol. 4(895)2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Feldmann A, Arndt C, Koristka S, Berndt N,
Bergmann R and Bachmann MP: Conventional CARs versus modular CARs.
Cancer Immunol Immunother. 68:1713–1719. 2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Neelapu SS: Managing the toxicities of CAR
T-cell therapy. Hematol Oncol. 37 (Suppl 1):48–52. 2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Xia AL, Wang XC, Lu YJ, Lu XJ and Sun B:
Chimeric-antigen receptor T (CAR-T) cell therapy for solid tumors:
Challenges and opportunities. Oncotarget. 8:90521–90531.
2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Greenbaum U, Yalniz FF, Srour SA, Rezvani
K, Singh H, Olson A, Blumenschein G Jr, Hong DS, Shpall EJ and
Kebriaei P: Chimeric antigen receptor therapy: How are we driving
in solid tumors? Biol Blood Marrow Transplant. 26:1759–1769.
2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Katz SC, Burga RA, McCormack E, Wang LJ,
Mooring W, Point GR, Khare PD, Thorn M, Ma Q, Stainken BF, et al:
Phase I hepatic immunotherapy for metastases study of
intra-arterial chimeric antigen receptor-modified T-cell therapy
for CEA+ liver metastases. Clin Cancer Res.
21:3149–3159. 2015.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Adachi K, Kano Y, Nagai T, Okuyama N,
Sakoda Y and Tamada K: IL-7 and CCL19 expression in CAR-T cells
improves immune cell infiltration and CAR-T cell survival in the
tumor. Nat Biotechnol. 36:346–351. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Alanio C, Barreira da Silva R, Michonneau
D, Bousso P, Ingersoll MA and Albert ML: CXCR3/CXCL10 Axis Shapes
Tissue Distribution of Memory Phenotype CD8+ T Cells in
Nonimmunized Mice. J Immunol. 200:139–146. 2018.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Liu L, Bi E, Ma X, Xiong W, Qian J, Ye L,
Su P, Wang Q, Xiao L, Yang M, et al: Enhanced CAR-T activity
against established tumors by polarizing human T cells to secrete
interleukin-9. Nat Commun. 11(5902)2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Knochelmann HM, Smith AS, Dwyer CJ, Wyatt
MM, Mehrotra S and Paulos CM: CAR T Cells in solid tumors:
Blueprints for building effective therapies. Front Immunol.
9(1740)2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Morgan MA and Schambach A: Engineering
CAR-T cells for improved function against solid tumors. Front
Immunol. 9(2493)2018.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Yu S, Yi M, Qin S and Wu K: Next
generation chimeric antigen receptor T cells: Safety strategies to
overcome toxicity. Mol Cancer. 18(125)2019.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Akbari P, Huijbers EJM, Themeli M,
Griffioen AW and van Beijnum JR: The tumor vasculature an
attractive CAR T cell target in solid tumors. Angiogenesis.
22:473–475. 2019.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Morgan RA, Yang JC, Kitano M, Dudley ME,
Laurencot CM and Rosenberg SA: Case report of a serious adverse
event following the administration of T cells transduced with a
chimeric antigen receptor recognizing ERBB2. Mol Ther. 18:843–851.
2010.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Gargett T and Brown MP: The inducible
caspase-9 suicide gene system as a ‘safety switch’ to limit
on-target, off-tumor toxicities of chimeric antigen receptor T
cells. Front Pharmacol. 5(235)2014.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Kloss CC, Condomines M, Cartellieri M,
Bachmann M and Sadelain M: Combinatorial antigen recognition with
balanced signaling promotes selective tumor eradication by
engineered T cells. Nat Biotechnol. 31:71–75. 2013.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Liu D, Zhao J and Song Y: Engineering
switchable and programmable universal CARs for CAR T therapy. J
Hematol Oncol. 12(69)2019.PubMed/NCBI View Article : Google Scholar
|