Introduction
Gliomas account for 50% of primary brain tumours in
the central nervous system (1).
According to the malignancy of the tumour, the World Health
Organization (WHO) categorizes glioma into four grades, I-IV
(2). Patients incipiently remain
asymptomatic until obvious clinical symptoms appear, such as
headaches, seizures, nausea, sensory loss and aphasia (3). Standard treatment of glioma includes
surgical excision followed by adjuvant chemotherapy and/or
radiotherapy; however, the overall survival of patients diagnosed
with malignant glioma is poor due to a high risk of relapse
(4,5). Thus, an improved understanding of the
precise molecular mechanisms underlying glioma pathogenesis is
crucial to help provide novel therapeutic candidates for the
treatment of glioma.
Long non-coding RNAs (lncRNAs), which are
transcripts of >200 nucleotides without protein-coding ability,
have been demonstrated to serve an essential role in tumour
initiation and progression (6). For
example, small nucleolar RNA host gene 12 acts as a tumour promoter
by regulating cell proliferation, apoptosis, migration and
multidrug resistance in gastric, colorectal, non-small cell lung
and triple-negative breast cancer (7-10).
Metastasis associated lung adenocarcinoma transcript 1 acts as an
oncogene in different types of cancer, such as kidney carcinoma,
ovarian cancer and colorectal cancer, by regulating cell viability,
apoptosis, invasion, migration and epithelial-to-mesenchymal
transition (EMT) (11-13).
Recently, several studies have revealed that lncRNAs can affect the
initiation and progression of glioma (10-12).
For example, lncRNA H19 decreases chemoresistance to temozolomide
by suppressing EMT via regulation of the Wnt/β-catenin signalling
pathway in glioma cell lines (14).
lncRNA X-inactive specific transcript promotes tumorigenesis and
angiogenesis by sponging microRNA (miR)-429 in glioma cells
(15). P73 antisense RNA 1T acts as
a competing endogenous RNA to promote high mobility group proteins
B1 (HMGB1) expression via sponging the miR-142 promoter, and thus
functions as an oncogenic lncRNA by promoting proliferation and
invasion in glioma cells (16).
Small ubiquitin-like modifier 1 pseudogene 3
(SUMO1P3), a newly identified lncRNA, serves an oncogenic role in
bladder, breast and colon cancer, where it may be used as a
potential prognostic biomarker (17-19).
However, the effect of SUMO1P3 on glioma progression remains
unknown. Thus, the present study investigated the expression
pattern and function of SUMO1P3 in glioma and further analysed the
downstream molecular signalling pathway. The present study
demonstrated that SUMO1P3 may act as a tumour promoter in glioma,
and may be used as a novel diagnostic biomarker and therapeutic
target for glioma.
Materials and methods
Human tissues
Human brain tissue samples were collected from the
Department of Neurosurgery at The Third Affiliated Hospital of
Soochow University (Changzhou, China) during excision surgeries,
between June 2015 and September 2017. A total of 12 glioma tissues
(grade II, n=4; grade III, n=4 and grade IV, n=4) were obtained
from patients with glioma (age range, 24-64 years; median age, 47
years) and histologically confirmed by three independent
pathologists according to the 2016 WHO Classification of Tumors of
the Central Nervous System (20).
These patients were followed up by telephone every month for 12
months. A total of 10 tissue samples (n=10) with no tumour
complication were obtained from patients with cranial trauma (age
range, 21-65 years; median age, 45 years), who served as the
control. The tissues were immediately snap-frozen and stored in
liquid nitrogen until subsequent experimentation. The present study
was approved by the Research Ethics Board of the Third Affiliated
Hospital of Soochow University and written informed consent was
provided by patients or their guardians prior to the start of the
study.
Cell lines and culture
A total of three glioma cell lines, including U87,
LN229 and U251, and the human glial cell line, HEB, were purchased
from The Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences. The U87 cell line used in the present study
was authenticated by Shanghai VivaCell Biosciences Ltd., with STR
profiling (http://www.vivacell.com.cn/Helps/STRAuthentication.html)
as the U87 MG American Type Culture Collection (ATCC) version,
which was a glioblastoma of unknown origin. Cells were cultured in
Dulbecco's Modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) with 10% foetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.) at 37˚C with 5% CO2.
Cell transfection
Synthetic SUMO1P3 small interfering (si)RNA and a
scrambled non-targeting siRNA as negative control siRNA (si-NC)
were purchased from Shanghai GenePharma Co., Ltd. Cells were
transfected using Lipofectamine® 2000 transfection
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's instructions. Briefly, 10 µl siRNA (20 µmol/l)
was mixed with 150 µl Opti-Mem medium (Gibco; Thermo Fisher
Scientific, Inc.) in one tube. 5 µl Lipofectamine® 2000
reagent was mixed with 150 µl Opti-Mem medium in another tube. The
contents of the two tubes were mixed and incubated at room
temperature for 5 min. The mixture was subsequently added to the
cells in 6-well plates and the plates were incubated at 37˚C. After
24 h, the medium was replaced with DMEM with 10% FBS. The cells
were collected for subsequent experimentation following 24 h of
further culture. The sequences of these oligonucleotides were as
follows: siSUMO1P3-302, 5'-GGCGUUCCAAUGAAUUCAUTT-3'; siSUMO1P3-877,
5'-CUUAAUUCAAGCUACUCUTT-3'; siSUMO1P3-946,
5'-GAUAACUGAUAAGGAGAGATT-3'; and si-NC,
5'-UUCUCCGAACGUGUCACGUTT-3'. Cells were transfected using
Lipofectamine®™ 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from cells and human tissues
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
The purity and concentration of the RNA was evaluated with NanoDrop
2000 (Thermo Fisher Scientific, Inc.). Subsequently, 1 µg RNA was
used to synthesize cDNA using the PrimeScript™ RT Reagent kit
(Takara Bio, Inc.) according to the manufacturer's instructions.
qPCR was subsequently performed using SYBR Green PCR Master mix
(Takara Bio, Inc.) on the ABI 7500 system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The thermocycling conditions were
as follows: 10 min at 95˚C, followed by 40 cycles for 10 sec at
95˚C and 40 sec at 60˚C. GAPDH served as the endogenous control for
SUMO1P3 and the relative gene expression was calculated using the
2-ΔΔCq method (21). The
following primer sequences were used for qPCR: SUMO1P3 forward,
5'-ACTGGGAATGGAGGAAGA-3' and reverse, 5'-TGAGAAAGGATTGAGGGAAAAG-3';
GAPDH forward, 5'-GGAGCGAGATCCCTCCAAAAT-3' and reverse,
5'-GGCTGTTGTCATACTTCTCATGG-3'.
Western blotting
Proteins were extracted from cells using RIPA buffer
(Beyotime Institute of Biotechnology) and the protein concentration
was determined using BCA assays. Subsequently, proteins (30
µg/lane) were separated on 10% SDS-PAGE gels and then transferred
onto PVDF membranes. After blocking with 10% non-fat milk for 1 h
at room temperature, the membranes were incubated with antibodies
against N-cadherin (1:1,500; cat. no. 13116), E-cadherin (1:1,000;
cat. no. 14472), β-catenin (1:1,000; cat. no. 8480), cyclin D1
(1:1,000; cat. no. 2978) and β-actin (1:2,500; cat. no. 3700)
overnight at 4˚C. Subsequently, the membranes were incubated with a
secondary horseradish peroxidase (HRP)-labelled goat anti-mouse IgG
(1:3,000; cat. no. A0216) or HRP-labelled goat anti-rabbit IgG
(1:3,000; cat. no. A0208) for 1 h at room temperature. All the
primary antibodies were purchased from Cell Signalling Technology,
Inc., and the secondary antibodies were purchased from Beyotime
Institute of Biotechnology. Protein bands were visualized using the
ECL Advanced Western Blot Detection kit (Thermo Fisher Scientific,
Inc.) on the Bio-Rad ChemiDoc™ Touch (Bio-Rad Laboratories, Inc.)
and subsequently quantified using Image Lab software (version 5.2;
Bio-Rad Laboratories, Inc.).
Wound healing assay
Transfected cells were seeded into 6-well plates at
a density of 50,000 cells/well. When the cell confluence reached
~85%, scratches were performed and the cells were incubated in
serum-free medium. The plates were observed under a IX71 light
microscope (magnification, x100; Olympus Corporation) at 0 and 48 h
after the scratch, and the distance was measured using ImageJ
software (National Institutes of Health).
Cell proliferation assay
Cell proliferation was assessed via the Cell
Counting Kit-8 (CCK-8) assay (Beyotime Institute of Biotechnology)
according to the manufacturer's instructions. Transfected cells
were seeded into 96-well plates at a density of 3,000 cells/well.
At the indicated time points (0, 24, 48 and 72 h), 10 µl CCK-8
regent was added into each well, and the plates were incubated at
37˚C for 2 h. The absorbance was measured at a wavelength of 450 nm
using BioTek Elx800 (BioTek Instruments, Inc.).
Migration and invasion assays
As previously described, Transwell plates were used
to assess cell migration and invasion in vitro (22,23).
For cell invasion assays, the chambers (Corning, Inc.) were
precoated with Matrigel (BD Biosciences) at 37˚C for 5 h. The
chambers were observed in six randomly selected fields under a IX71
light microscope (magnification, x200; Olympus Corporation). The
number of migrated cells in every field was counted.
Cell cycle analysis
Transfected cells were collected, fixed in 70%
ethanol solution for 6 h at 4˚C. Cells were then washed with PBS
and subsequently incubated with PI and RNase from a cell cycle and
apoptosis analysis kit (cat. no. C1052; Beyotime Institute of
Biotechnology) for 30 min at 37˚C according to the manufacturer's
instructions. The cell cycle was analysed on a Guava EasyCyte
6HT-2L flow cytometer (EMD Millipore), and assessed using the
ModFit LT software (Version 3.1; Verity Software House, Inc.).
Statistical analysis
All experiments were performed independently in
triplicates, and data were presented as the mean ± standard error
of the mean. Statistical analysis was performed using GraphPad
Prism (version 8.3.0; GraphPad Software, Inc.) and the SPSS
statistical software package (version 22.0; IBM Corp.). Unpaired
Student's t-test was performed for comparisons between two groups.
One-way ANOVA followed by Tukey's post-hoc test was used for
multiple comparisons. Kaplan-Meier analysis and log-rank test were
used for survival analysis of patients with glioma with low and
high expression levels of SUMO1P3. P<0.05 was considered to
indicate a statistically significant difference.
Results
SUMO1P3 expression is upregulated in
glioma and high SUMO1P3 expression is associated with a poor
prognosis
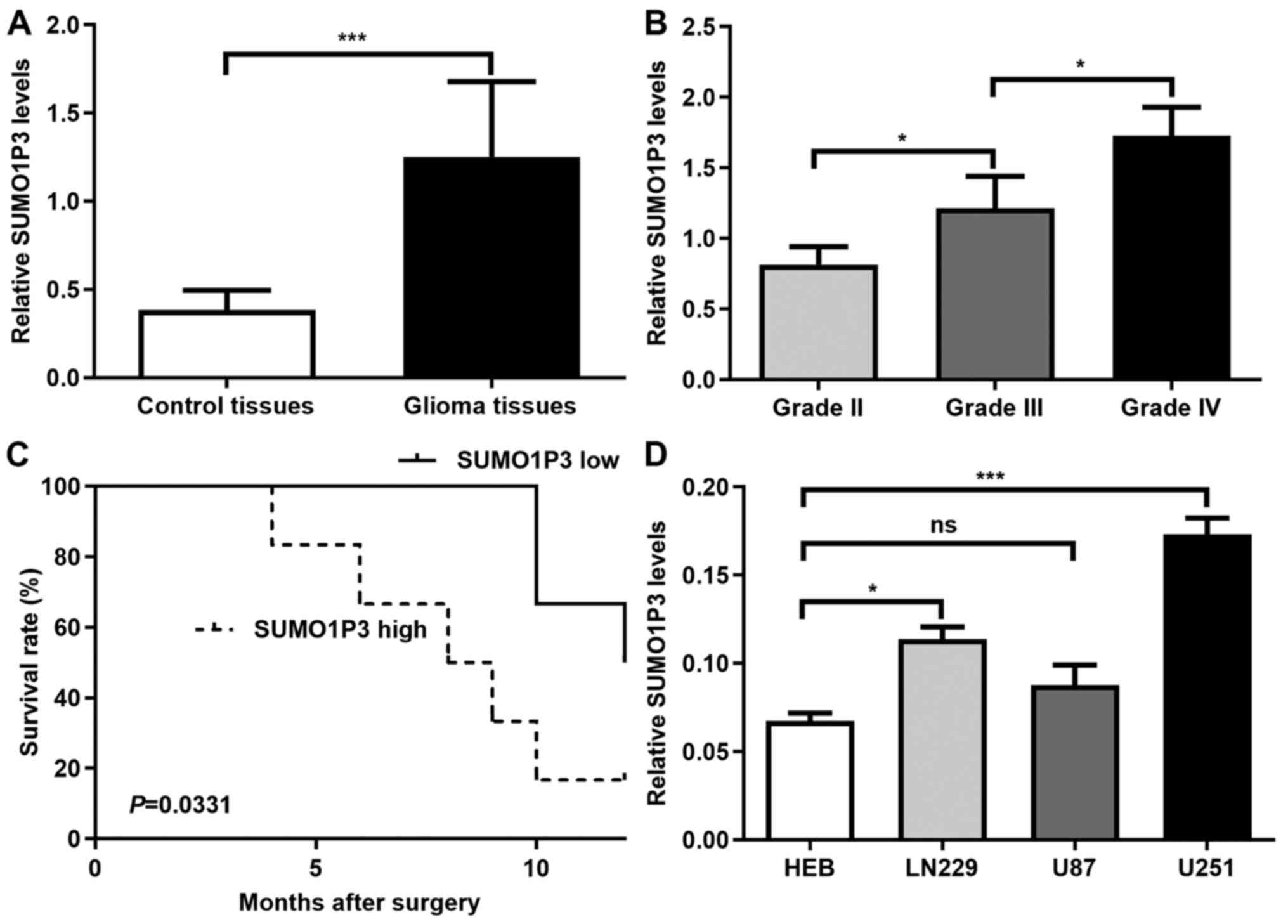
SUMO1P3 expression was assessed in 12 glioma and 10
control tissues. The clinical parameters of the tissue donors are
described in Table I. As presented
in Fig. 1A, SUMO1P3 expression was
significantly higher in glioma tissues compared with in control
tissues. The association between SUMO1P3 expression and glioma
malignancy was also analysed. As presented in Fig. 1B, SUMO1P3 expression increased with
increasing glioma grade. The present study further investigated
whether SUMO1P3 was a prognostic factor for glioma. The median
expression value of SUMO1P3 expression was used as a cut-off value
to divide all 12 glioma patients into two groups. Patients with
SUMO1P3 levels lower than the cut-off value were placed in the low
expression group and patients with higher SUMO1P3 expression were
assigned to the high expression group. Kaplan-Meier survival
analysis indicated that high SUMO1P3 expression was associated with
a poorer overall survival in patients with glioma compared with low
SUMO1P3 expression (Fig. 1C).
Furthermore, SUMO1P3 expression was assessed in 3 glioma cell
lines. The results demonstrated that SUMO1P3 expression was
upregulated in U251 and LN229 glioma cells compared with in HEB
cells, however there was no significant difference between U87 and
HEB cells (Fig. 1D). Thus, U251 and
LN229 glioma cells were selected for subsequent experiments.
Overall, the present results suggested that SUMO1P3 may be a
prognostic biomarker for glioma and may act as a tumour promoter in
glioma.
 | Table IClinical parameters of tissue
donors. |
Table I
Clinical parameters of tissue
donors.
| Clinicopathological
parameters | Glioma (n=12) | Control (n=10) |
|---|
| Sex | | |
|
Male | 6 | 5 |
|
Female | 6 | 5 |
| Age, years | | |
|
<50 | 6 | 5 |
|
≥50 | 6 | 5 |
| WHO grade | | |
|
II | 4 | NA |
|
III | 4 | NA |
|
IV | 4 | NA |
| Follow-up | | |
|
Alive | 4 | NA |
|
Dead | 8 | NA |
|
Mean
survival time, months | 9.8±5.9 | NA |
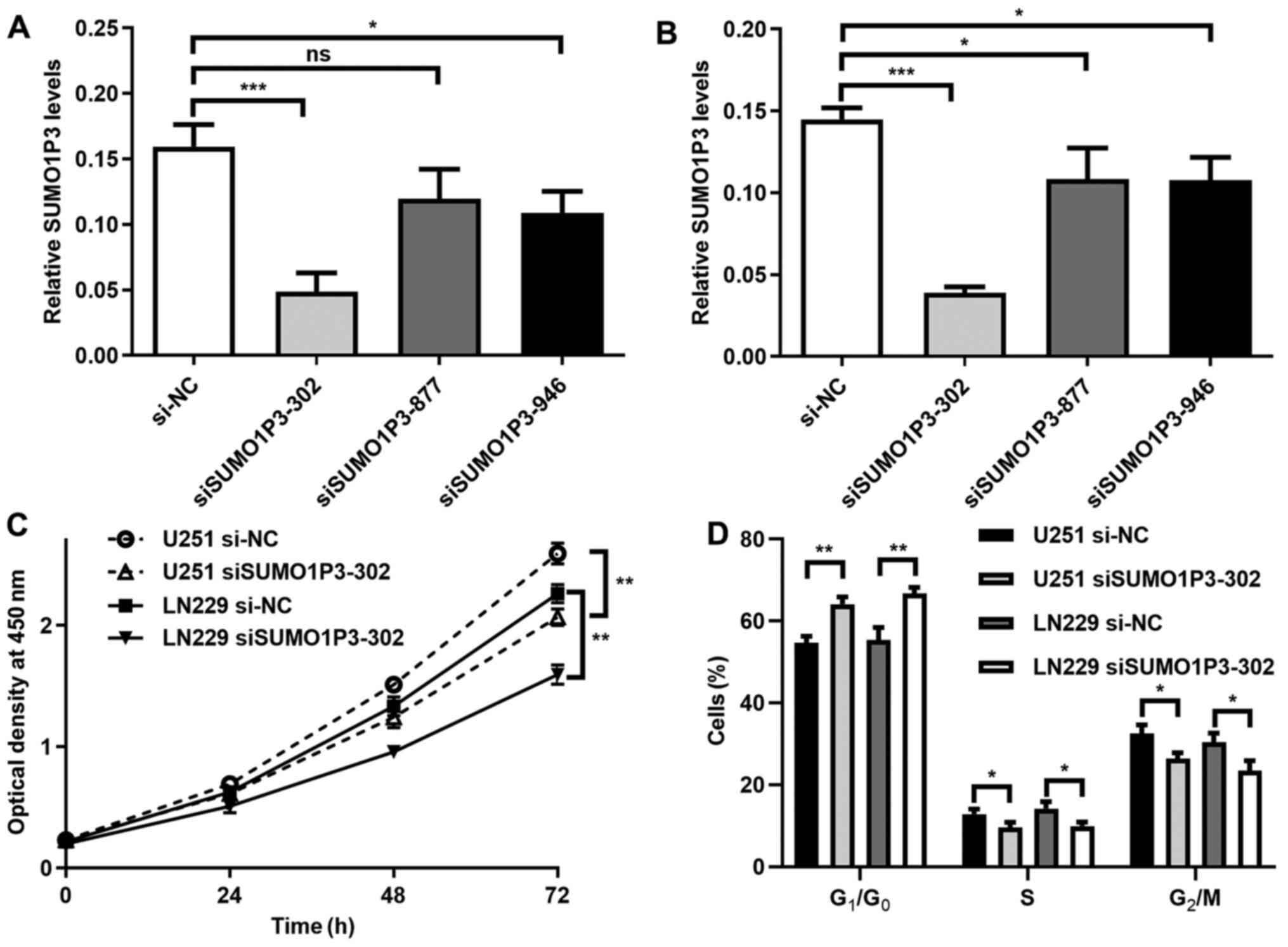
SUMO1P3-knockdown suppresses cell
proliferation and cell cycle in vitro
To investigate the function of SUMO1P3 in glioma,
its expression was suppressed via transfection with siRNA in glioma
cell lines. As presented in Fig. 2A
and B, SUMO1P3 expression was
significantly decreased following transfection with siSUMO1P3-302
in U251 and LN229 cells. The relative expression level in the
siSUMO1P3-302 group was 30% of the si-NC group, thus siSUMO1P3-302
was selected for subsequent experimentation. The results of the
cell proliferation assay demonstrated that SUMO1P3-knockdown
significantly supressed U251 and LN229 cell proliferation (Fig. 2C). The influence of SUMO1P3 on cell
cycle distribution was subsequently assessed. As presented in
Figs. 2D and S1, SUMO1P3-knockdown resulted in the
accumulation of cells in G1 phase, with 11.24 and 13.46%
more cells in G1/G0 phase in the U251 and
LN229 glioma cell lines compared with the si-NC groups. Meanwhile,
there was a significant decrease in cells in S and G2/M
phases in the siSUMO1P3-302 groups compared with cells in the si-NC
groups. Collectively, the current results suggested that
SUMO1P3-knockdown suppressed cell proliferation in glioma via
G0/G1 cell cycle arrest.
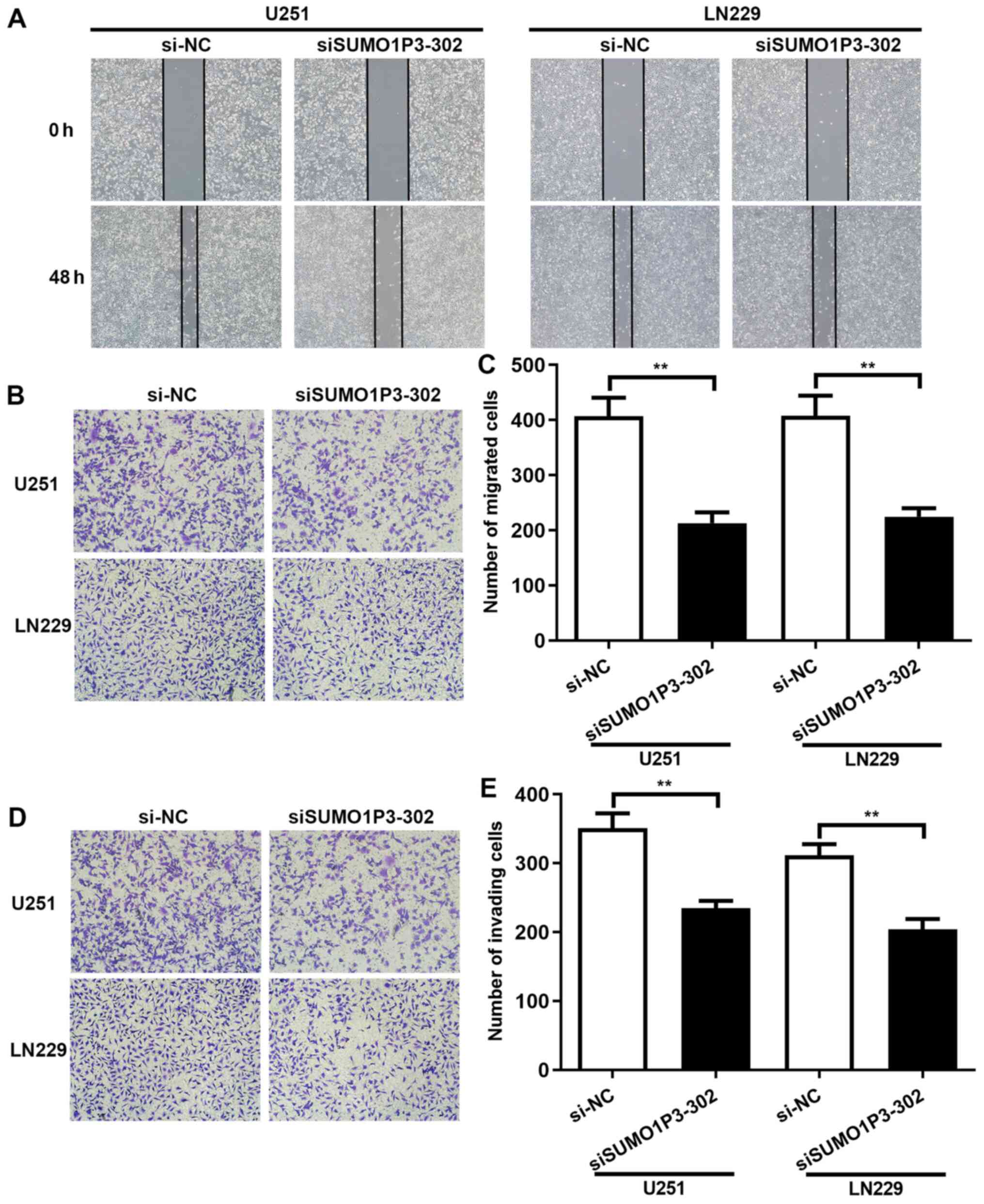
SUMO1P3-knockdown represses cell
migration and invasion in vitro
To assess the role of SUMO1P3 in cell migration and
invasion, wound healing and Transwell assays were performed. The
results of the wound healing assay demonstrated that
SUMO1P3-knockdown significantly decreased the speed of wound
closure in U251 and LN229 cells. Statistical analysis has been
performed and the results showed that the relative wound closure
percentage of U251 and LN229 cells at 48 h was 23.38 and 27.79% in
the siSUMO1P3-302 group, respectively, and 44.87 and 39.21% in the
si-NC group, respectively (Fig.
3A). The role of SUMO1P3 on cell migration and invasion was
also investigated. The results of the Transwell assay demonstrated
that the number of migrated U251 and LN229 cells in the
siSUMO1P3-302 group was 213 and 225 at 48 h, significantly lower
than the 407 and 408 cells in the si-NC group, respectively
(Fig. 3B and C). In accordance with the results of cell
migration, SUMO1P3-knockdown significantly supressed the invasive
ability of U251 and LN229 cells (Fig.
3D and E). Overall, the present
results suggested that SUMO1P3-knockdown supressed the migratory
and invasive abilities of U251 and LN229 cells.
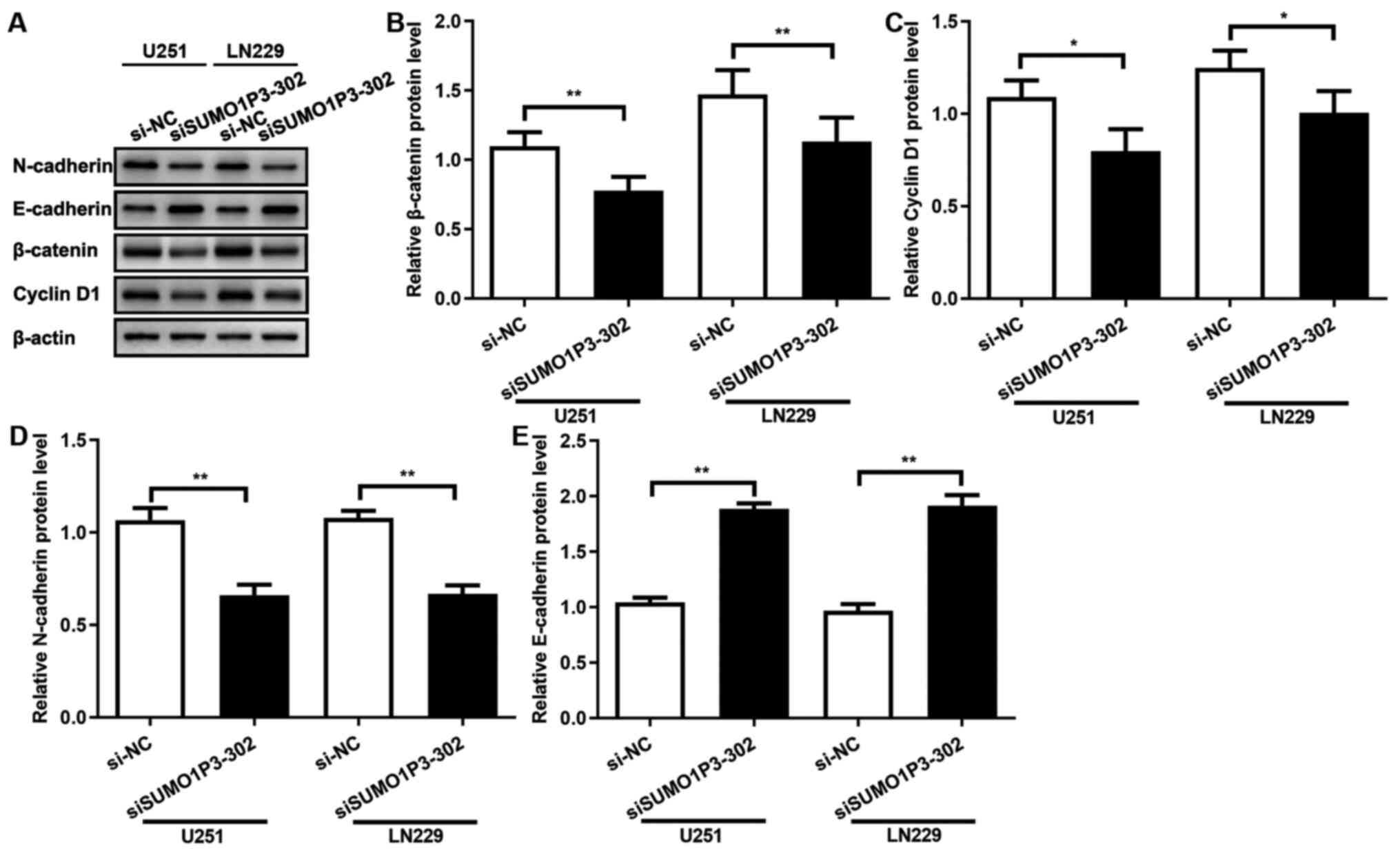
SUMO1P3 acts as a tumour promoter via
regulating the expression levels of β-catenin, cyclin-D1,
N-cadherin and E-cadherin
The results of the present study demonstrated that
SUMO1P3 acted as a tumour promoter in glioma cells by regulating
cell proliferation, migration and invasion, whereas the underlying
molecular mechanism remains unknown. β-catenin protein expression
was assessed following transfection with siRNA, and the results
demonstrated that SUMO1P3-knockdown significantly supressed
β-catenin expression in both cell lines (Fig. 4A and B). Cyclin-D1 expression was also assessed,
which is associated with the cell cycle and a downstream signalling
protein of β-catenin (24). As
presented in Fig. 4A and C, SUMO1P3-knockdown significantly
supressed cyclin-D1 expression. The expression levels of proteins
associated with migration and invasion were subsequently assessed.
As presented in Fig. 4A, D and E,
SUMO1P3-knockdown significantly supressed N-cadherin expression and
significantly increased E-cadherin expression. Overall, the current
results suggested that SUMO1P3 regulated cell proliferation, cell
cycle, migration and invasion by regulating the expression levels
of β-catenin, cyclin-D1, N-cadherin and E-cadherin.
Discussion
Previous studies have reported that lncRNAs are
aberrantly expressed in cancer and may serve a crucial role during
tumour progression (6,13,16,25).
To the best of our knowledge, Mei et al (26) has been the first to report that
SUMO1P3 expression is upregulated in gastric cancer tissues and may
serve as a potential biomarker for the diagnosis of gastric cancer.
Since then, multiples studies have demonstrated the expression
pattern and function of SUMO1P3 in different types of tumour.
SUMO1P3 expression is upregulated in bladder cancer tissues, and
SUMO1P3-knockdown inhibits cell proliferation and migration, while
inducing apoptosis in bladder cancer cells (17). Additionally, SUMO1P3 expedites the
malignant behaviours of colon cancer in vivo, such as
growth, metastasis and angiogenesis, by regulating the expression
levels of cyclin D1, vimentin, vascular endothelial growth factor A
and E-cadherin (19). A recent
study on breast cancer has suggested that SUMO1P3 acts as an
oncogenic lncRNA by targeting miR-320a (18), identified as a tumour suppressor in
different types of cancer, such as gastric cancer, non-small cell
lung cancer and colorectal cancer (27-29).
miR-320a supresses β-catenin expression by directly targeting the
3'-untranslated region of β-catenin mRNA in prostate cancer cells
(30). In addition, SUMO1P3
promotes hepatocellular carcinoma progression through enhancing the
Wnt/β-catenin signalling pathway by sponging miR-320a (31). Thus, it is possible that
SUMO1P3-knockdown represses β-catenin expression by targeting
miR-320a in glioma.
Cyclin D1 is a downstream molecule of β-catenin
(24). Cyclin D1 mediates the
progression from G1 to S phase, thus slowing the
proliferation of cancer cells (32). In the present study,
SUMO1P3-knockdown supressed proliferation in glioma cells probably
via cell cycle arrest caused by suppression of cyclin D1. β-catenin
and E-cadherin are epithelial cell markers, and N-cadherin is a
mesenchymal phenotype marker during the EMT process (33). Increasing evidence suggests that an
E-cadherin to N-cadherin shift is mainly involved in EMT and serves
a key role in glioma progression and invasion (34). According to the present results, it
may be speculated that SUMO1P3-knockdown may supress cell migration
and invasion by regulating the expression levels of β-catenin,
N-cadherin and E-cadherin. However, the current study presents some
limitations. Firstly, only 12 glioma tissues and 10 control tissues
were used in the present study. The number of patients was
relatively small, therefore further studies using more human
tissues should be performed to confirm the relationship between
SUMO1P3 expression and glioma malignancy. Furthermore, control
tissues were obtained from patients with severe acute traumatic
brain injury during a needed surgery, thus theses tissues were not
healthy tissues. Despite consent for the collection of tissues was
provided by the patients or their guardians, it would have been
unethical for healthy tissue to be used. A recent study has
reported that SUMO1P3 promotes glioma cell proliferation,
migration, and invasion via the Wnt/β-catenin pathway (35). The results and conclusions are
consistent with the present study, except for the expression levels
of SUMO1P3 in U87 glioma cell line. Lou et al (35) demonstrated high SUMO1P3 expression
in U87 cells, whereas the present study identified there was no
significant difference between U87 and HEB cells. Since there are
two versions of U87 cell line, one is the original glioblastoma
cell line established in the University of Uppsala (https://web.expasy.org/cellosaurus/CVCL_GP63) and the
other is the U87 MG ATCC version, which is most probably a
glioblastoma but whose origin is unknown (https://web.expasy.org/cellosaurus/CVCL_0022),
there are differences in the behaviours of these cell lines. The
present study confirmed the U87 cell line used was the ATCC version
by STR profiling. Regarding Lou et al have not clarified the
version of U87 cell line used, it is possible that they used the
original glioblastoma cell line established in the University of
Uppsala.
In conclusion, the present study revealed the
expression pattern of SUMO1P3 and its tumorigenic function in
glioma tissues and cells. SUMO1P3-knockdown was demonstrated to
suppress cell proliferation, cell cycle, migration and invasion by
regulating the expression levels of β-catenin, cyclin-D1,
N-cadherin and E-cadherin in U251 and LN229 glioma cells. Overall,
the current results suggest that SUMO1P3 may act as a novel
diagnostic biomarker and therapeutic target for glioma.
Supplementary Material
Original flow cytometry data of the
cell cycle analysis. Representative results of the cell cycle
analysis in SUMO1P3-knockdown cells.
Acknowledgements
Not applicable.
Funding
The present study was funded by Changzhou Municipal Commissions
of Health and Family Planning Major Scientific and Technological
Project (grant no. ZD201620), Changzhou Municipal Commission of
Health and Family Planning Youth Talent Scientific and
Technological Project (grant no. QN201807) and Funding from Young
Talent Development Plan of Changzhou Health Commission (grant no.
CZQM2020042).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DD and JC collaborated to design the study,
performed the cell cycle assay, analysed the data, drafted the
initial manuscript and confirmed the authenticity of the raw data.
YM performed RT-qPCR, western blotting and the wound healing
assays. LX performed cell proliferation, migration and invasion
assays. NS acquired and analysed the data of patients, collected
the human tissues and helped in revising the paper. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Board of the Third Affiliated Hospital of Soochow University
(grant no. 2020031; Changzhou, China). Written informed consent was
provided by all patients or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Millaer KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bielle F: Building diagnoses with four
layers: WHO 2016 classification of CNS tumors. Rev Neurol (Paris).
172:253–255. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Boussiotis VA and Charest A:
Immunotherapies for malignant glioma. Oncogene. 37:1121–1141.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: The avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Stupp R, Taillibert S, Kanner A, Read W,
Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K,
et al: Effect of tumor-treating fields plus maintenance
temozolomide vs maintenance temozolomide alone on survival in
patients with glioblastoma: A randomized clinical trial. JAMA.
318:2306–2316. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang JZ, Xu CL, Wu H and Shen SJ: LncRNA
SNHG12 promotes cell growth and inhibits cell apoptosis in
colorectal cancer cells. Braz J Med Biol Res.
50(e6079)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang O, Yang F, Liu Y, Lv L, Ma R, Chen C,
Wang J, Tan Q, Cheng Y, Xia E, et al: C-MYC-induced upregulation of
lncRNA SNHG12 regulates cell proliferation, apoptosis and migration
in triple-negative breast cancer. Am J Transl Res. 9:533–545.
2017.PubMed/NCBI
|
|
9
|
Wang P, Chen D, Ma H and Li Y: LncRNA
SNHG12 contributes to multidrug resistance through activating the
MAPK/Slug pathway by sponging miR-181a in non-small cell lung
cancer. Oncotarget. 8:84086–84101. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang H and Lu W: LncRNA SNHG12 regulates
gastric cancer progression by acting as a molecular sponge of
miR320. Mol Med Rep. 17:2743–2749. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gordon MA, Babbs B, Cochrane DR, Bitler BG
and Richer JK: The long non-coding RNA MALAT1 promotes ovarian
cancer progression by regulating RBFOX2-mediated alternative
splicing. Mol Carcinog. 58:196–205. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Xu Y, Zhang X, Hu X, Zhou W, Zhang P,
Zhang J, Yang S and Liu Y: The effects of lncRNA MALAT1 on
proliferation, invasion and migration in colorectal cancer through
regulating SOX9. Mol Med. 24(52)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ye Y, Zhang F, Chen Q, Huang Z and Li M:
LncRNA MALAT1 modified progression of clear cell kidney carcinoma
(KIRC) by regulation of miR-194-5p/ACVR2B signaling. Mol Carcinog.
58:279–292. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Jia L, Tian Y, Chen Y and Zhang G: The
silencing of LncRNA-H19 decreases chemoresistance of human glioma
cells to temozolomide by suppressing epithelial-mesenchymal
transition via the Wnt/β-Catenin pathway. Onco Targets Therapy.
11:313–321. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cheng Z, Li Z, Ma K, Li X, Tian N, Duan J,
Xiao X and Wang Y: Long non-coding RNA XIST promotes glioma
tumorigenicity and angiogenesis by acting as a molecular sponge of
miR-429. J Cancer. 8:4106–4116. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang R, Jin H and Lou F: The long
non-coding RNA TP73-AS1 interacted with miR-142 to modulate brain
glioma growth through HMGB1/RAGE pathway. J Cell Biochem.
119:3007–3016. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhan Y, Liu Y, Wang C, Lin J, Chen M, Chen
X, Zhuang C, Liu L, Xu W, Zhou Q, et al: Increased expression of
SUMO1P3 predicts poor prognosis and promotes tumor growth and
metastasis in bladder cancer. Oncotarget. 7:16038–16048.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu J, Song Z, Feng C, Lu Y, Zhou Y, Lin Y
and Dong C: The long non-coding RNA SUMO1P3 facilitates breast
cancer progression by negatively regulating miR-320a. Am J Transl
Res. 9:5594–5602. 2017.PubMed/NCBI
|
|
19
|
Zhang LM, Wang P, Liu XM and Zhang YJ:
LncRNA SUMO1P3 drives colon cancer growth, metastasis and
angiogenesis. Am J Transl Res. 9:5461–5472. 2017.PubMed/NCBI
|
|
20
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
Classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Deng D, Luo K, Liu H, Nie X, Xue L, Wang
R, Xu Y, Cui J, Shao N and Zhi F: p62 acts as an oncogene and is
targeted by miR-124-3p in glioma. Cancer Cell Int.
19(280)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Deng D, Xue L, Shao N, Qu H, Wang Q, Wang
S, Xia X, Yang Y and Zhi F: miR-137 acts as a tumor suppressor in
astrocytoma by targeting RASGRF1. Tumour Biol. 37:3331–3340.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chattopadhyay S, Chaklader M and Law S:
Aberrant Wnt signaling pathway in the hematopoietic stem/progenitor
compartment in experimental leukemic animal. J Cell Commun Signal.
13:39–52. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sun Y, Zheng ZP, Li H, Zhang HQ and Ma FQ:
ANRIL is associated with the survival rate of patients with
colorectal cancer, and affects cell migration and invasion in
vitro. Mol Med Rep. 14:1714–1720. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mei D, Song H, Wang K, Lou Y, Sun W, Liu
Z, Ding X and Guo J: Up-regulation of SUMO1 pseudogene 3 (SUMO1P3)
in gastric cancer and its clinical association. Med Oncol.
30(709)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ge X, Cui H, Zhou Y, Yin D, Feng Y, Xin Q,
Xu X, Liu W, Liu S and Zhang Q: miR-320a modulates cell growth and
chemosensitivity via regulating ADAM10 in gastric cancer. Mol Med
Rep. 16:9664–9670. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhao W, Sun Q, Yu Z, Mao S, Jin Y, Li J,
Jiang Z, Zhang Y, Chen M, Chen P, et al: MiR-320a-3p/ELF3 axis
regulates cell metastasis and invasion in non-small cell lung
cancer via PI3K/Akt pathway. Gene. 670:31–37. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhao H, Dong T, Zhou H, Wang L, Huang A,
Feng B, Quan Y, Jin R, Zhang W, Sun J, et al: miR-320a suppresses
colorectal cancer progression by targeting Rac1. Carcinogenesis.
35:886–895. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hsieh IS, Chang KC, Tsai YT, Ke JY, Lu PJ,
Lee KH, Yeh SD, Hong TM and Chen YL: MicroRNA-320 suppresses the
stem cell-like characteristics of prostate cancer cells by
downregulating the Wnt/beta-catenin signaling pathway.
Carcinogenesis. 34:530–538. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wu S, Chen S, Lin N and Yang J: Long
non-coding RNA SUMO1P3 promotes hepatocellular carcinoma
progression through activating Wnt/β-catenin signalling pathway by
targeting miR-320a. J Cell Mol Med. 24:3108–3116. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Fu M, Wang C, Li Z, Sakamaki T and Pestell
RG: Minireview: Cyclin D1: Normal and abnormal functions.
Endocrinology. 145:5439–5447. 2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tam WL and Weinberg RA: The epigenetics of
epithelial-mesenchymal plasticity in cancer. Nat Med. 19:1438–1449.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Noh MG, Oh SJ, Ahn EJ, Kim YJ, Jung TY,
Jung S, Kim KK, Lee JH, Lee KH and Moon KS: Prognostic significance
of E-cadherin and N-cadherin expression in Gliomas. BMC cancer.
17(583)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lou JY, Luo J, Yang SC, Ding GF, Liao W,
Zhou RX, Qiu CZ and Chen JM: Long non-coding RNA SUMO1P3 promotes
glioma progression via the Wnt/β-catenin pathway. Eur Rev Med
Pharmacol Sci. 24:9571–9580. 2020.PubMed/NCBI View Article : Google Scholar
|