Introduction
Aconitum soongoricum Stapf. is a type of
perennial herb belonging to Ranunculaceae Aconitum, which is
mainly distributed in Northern Xinjiang, China. Aconitum
soongoricum Stapf. is abundantly produced in the Xinyuan,
Nileke County, Altay in Yili, Xinjiang, in mountains and grassland
slopes at an altitude of 1,800-2,600 meters (1-3).
Its toxic root is used for medicinal purposes, including expelling
wind and cold, relieving pain and swelling, and clearing meridians
and collaterals (4). Aconitum
soongoricum Stapf. has unique advantages in the treatment of
rheumatic diseases, which is worthy of investigation (5,6).
Aconitum soongoricum Stapf. has been shown to
be related to the standard Radix Aconiti in molecular and
chemical features, and the total alkaloid content is as high as
0.8%. Aconitum soongoricum Stapf. has been used as a folk
medicine by Kazakh herders, and no reports of associated adverse
reactions have been reported. Pharmacological studies have
demonstrated that Aconitum or its related alkaloids have
analgesic, anti-inflammatory, antitumor, anti-arrhythmia and
cardiotonic effects. Furthermore, the alkaloids in radix aconite,
aconitine, mesaconitine and hypaconitine all have strong
anti-inflammatory activities (7,8). In
the present study, the chemical constituents of Aconitum
soongoricum Stapf. were investigated, and the total alkaloid
contents were analyzed. A total of 4 alkaloids were obtained
through the silica gel column chromatography and low-pressure
column chromatography, which were subjected to EI-MS, IR,
1H-NMR, and 13C-NMR spectral analyses, and
identified as i) Aconitine, ii) songorine (Aconitum soongoricum
Stapf.), iii) 16, 17-dihydro-12β, 16β-epoxynapelline, and iv)
12-epi-napelline and 12-epi-dehydronapeline, respectively. Some of
these alkaloids have been demonstrated to have anti-rheumatic
effects (9). Therefore, further
in-depth investigation is required to identify the unknown
components in the Aconitum soongoricum Stapf.
Our previous study demonstrated that the raw and
processed products of Aconitum soongoricum Stapf. may
decline the serum levels of IL-1β, IL-2 and TNF-α in CIA and AA
rats. The associated mechanisms underlying the anti-inflammatory
effects require further in-depth studies (10).
Materials and methods
Sample sources
Chinese herb samples were harvested from Nileke
County, Yili, Xinjiang, in August 2012, which were identified as
the dry roots of Aconitum soongoricum Stapf. by Yonghe Li
(the chief Traditional Chinese Medicine pharmacist in the Fourth
Clinical Medical College of Xinjiang Medical University).
Extraction and separation of
alkaloids
The extraction and separation of alkaloids were
performed as previously described (11,12).
In brief, 10 kg dry roots of Aconitum soongoricum Stapf.
were crushed (through a 1x1 inch 20-mesh sieve) and extracted using
95% ethanol (cat. no. 130105; Xi'an Chemical Reagent Factory) at
25˚C and this was repeated 3 times (27 l ethanol each time). The
percolation extraction method was used (13). The dichloromethane extract was
concentrated by a rotary evaporator at 40˚C to remove the
dichloromethane solvent, and the saturated n-butanol extract was
concentrated by a rotary evaporator at 50˚C to remove the n-butanol
solvent. The extract was concentrated by thin-film evaporation, and
then dissolved with 5.5 l 2% HCl and extracted using petroleum
ether (a total of 8 l; cat. no. 20131105; Tianjin Hongyan Reagent
Factory). The remaining aqueous solution was adjusted to pH=4 with
ammonia solution, which was then subjected to the extraction with
dichloromethane three times (6 l each time; cat. no. 20131128;
Tianjin Chemical Reagent Co., Ltd.). The dichloromethane extract
was gathered, and the portion with pH=4 was obtained. The remaining
aqueous solution was adjusted to pH=8 with ammonia solution, which
was subjected to the extraction with dichloromethane three times (8
l each time), and the dichloromethane phase was obtained (pH=8).
The aqueous layer was adjusted to pH=11 with 10% NaOH and extracted
using dichloromethane three times (6 l each time) to obtain the
portion with pH=11. The aqueous layer was then extracted with
saturated n-butanol (2 l), to obtain the n-butanol portion. The
portions obtained were separated by the repeated silica gel,
Sephadex LH-20 gel column (Amersham Pharmacia Biotech AB) and
recrystallization. The fractions were concentrated, and the solvent
was removed, prior to the column purification.
Elution and identification of
compounds 1-5 Compound 1
The pH=4 portion was eluted with petroleum
ether-ethyl acetate-diethylamine (cat. no. 20131213; Tianjin
Hongyan Reagent Factory; 8:2:0.5, V/V/V; flow rate, 20 ml/min; 1 l
eluate was collected each time), and the eluates from the 18th to
23th washing rounds were collected, gathered, crystallized and
filtered, to obtain the Compound 1 (1.5449 g; solid colorless
crystal), which was identified as a single compound by the thin
layer chromatography (TLC; with the Rf value of 0.2), as previously
described (14). The potassium
bismuth iodide was used as the chromogenic agent for the TLC
method.
Compound 2
The portion of pH=8 was eluted with petroleum
ether-ethyl acetate-diethylamine (10:1:0.5; flow rate, 20 ml/min; 1
l elute was collected each time), and the elutes from the 6th to
11th rounds were collected, crystallized and filtered, to obtain
Compound 2 (1.3345 g), which was determined to be a single compound
according to the TLC (with an Rf value of 0.37).
Compound 3
The pH=8 portion was eluted with petroleum
ether-ethyl acetate-diethylamine (10:1:0.5; flow rate, 20 ml/min; 1
l elute was collected each time), and the elutes from the 1st to
3rd rounds were collected, which were then subjected to the
Sephadex LH-20 gel column, followed by eluting with
chloroform-petroleum ether-methanol (5:5:1; at a flow rate of 5
ml/min). A total of 15 ml elute was collected each time, and the
elute from the 7th eluting round was collected, crystallized,
filtered and re-crystallized with acetone, to obtain Compound 3
(100 mg), identified as a single compound based on the TLC (with an
Rf value of 0.52).
Compound 4
The pH=8 portion was eluted with petroleum
ether-ethyl acetate-diethylamine (7:3:0.5; flow rate, 20 ml/min; 1
l elute was collected each time), and the elutes from the 4th to
9th rounds were collected, crystallized and filtered, to obtain
Compound 4 (1.2910 g), identified as a single compound by TLC (with
an Rf value of 0.6).
Compound 5
The pH=4 portion was eluted with petroleum
ether-ethyl acetate-diethylamine (10:1:0.5; flow rate, 20 ml/min; 1
l elute was collected each time), and the elutes from the 3rd to
7th rounds were collected, subjected to the silica gel H column
chromatography, followed by the petroleum ether-ethyl
acetate-diethylamine (20:1:0.5) elution and pressurization. A total
of 50 ml elute was collected each time, and the elute from the 5th
round was collected, concentrated, crystallized and filtered, to
obtain Compound 5 (103 mg), identified as a single compound by TLC
(with an Rf value of 0.82).
Structural identification
The obtained solid substance was subjected to the
alkaloid physicochemical identification reaction and melting point
(mp) measurement, and the molecular formula of the compound was
obtained by ESI-MS as described previously (15). The reports on the diterpene
alkaloids were retrieved from the literature, and the structural
identification and analysis of the compounds were performed using
1H-NMR and 13C-NMR spectroscopy as described
previously (16) (INOVA-600 and 400
model superconducting nuclear magnetic resonance instrument; Varian
Medical Systems).
For the chromatographic conditions and system
suitability test, the XBridgeTM-C18 column (250x4.6 mm, 5 µm;
Waters Corporation) was used, with an octadecylsilane bonded silica
filler. The methanol-water-chloroform-triethylamine (volume ratio
of 67:33:2:0.1) was used as the mobile phase, and isocratic elution
was performed. The conditions were set as follows: The flow rate,
0.8 ml/min; detection wavelength, 235 nm; column temperature, 40˚C;
injection volume, 10 µl.
Study cells and grouping
Rheumatoid arthritis HFLS-RA fibroblast-like
synoviocytes (suitable for the experiments) (17-19)
were derived from Bena Culture Collection (BNCC340356). These cells
were cultured using high-glucose Dulbecco's modified Eagle's medium
(12800-017; Gibco; Thermo Fisher Scientific, Inc.), containing 10%
FBS (FND500; Shanghai ExCell Biology, Inc.), supplemented with 1%
penicillin-streptomycin double antibodies (10,000 U; SC30010;
Gibco; Thermo Fisher Scientific, Inc.) in a 37˚C, 5% CO2
incubator, with saturated humidity. The HFLS-RA cells at a
confluence of 90% were subjected to the following treatments: i)
The blank group, including the normal cultured cells; ii) the
lipopolysaccharide (LPS) intervention group, in which the cells
were treated with medium containing 100 ng/ml LPS for 26 h; iii)
the Leflunomide + LPS intervention group, in which the cells were
treated with medium containing 100 ng/ml LPS for 2 h, followed by
treatment together with 150 µg/ml leflunomide for another 24 h; iv)
the Junggar aconitine + LPS intervention group, in which the cells
were treated with medium containing 100 ng/ml LPS for 2 h, followed
by treatment together with 350 µg/ml Junggar aconitine for a
further 24 h; v) the benzoylaconine + LPS intervention group, in
which the cells were treated with medium containing 100 ng/ml LPS
for 2 h, followed by treatment together with 1,000 µg/ml
benzoylaconine for a further 24 h; and vi) the aconine + LPS
intervention group, in which the cells were treated with medium
containing 100 ng/ml LPS for 2 h, followed by treatment together
with 500 µg/ml aconine for a further 24 h. All treatments were
performed at 37˚C.
Compound preparation
To prepare the songorine stock solution (prepared by
Traditional Chinese Medicine Pharmacy Laboratory and Traditional
Chinese Medicine Processing Research Laboratory, Xinjiang Medical
University, Urumqi, China ), a total of 100 mg substrate was
weighed and dissolved in 500 µl DMSO (D2650; Sigma-Aldrich; Merck
KGaA), to obtain a final concentration of 200 mg/ml. To prepare the
benzoylaconine stock solution (A0631; Chengdu Must Bio-Technology
Co., Ltd.), a total of 20 mg benzoylaconine was weighed and
dissolved in 100 µl DMSO, to obtain a final concentration of 200
mg/ml. To prepare the aconitine stock solution (MUST-14012802;
Chengdu Must Bio-Technology Co., Ltd.), a total of 100 mg drug was
weighed and dissolved in 100 µl DMSO to obtain a final
concentration of 200 mg/ml.
Cell Counting kit-8 (CCK-8) assay
Cells were seeded onto the 96-well plate, at a
density of 5x104 cells/ml. After 8 days, the culture
medium was replaced with 10% CCK-8 solution (Beyotime Institute of
Biotechnology), and the cells were incubated at 37˚C for 1 h. Next,
the optical density (OD) was read on the xMarkTM microplate reader
(Bio-Rad Laboratories, Inc.) at an absorbance of 450 nm, and the
growth curve was plotted accordingly. The inhibition rate was
calculated using the following formulation: Inhibition
rate=(ODblank control-ODsample)/(ODblank
control-ODreagent control) x100%. The
IC50 value was calculated by the probit analysis using
SPSS 19.0 software (IBM Corp.).
HFLS-RA cell viability
Cells were seeded onto a 96-well plate, at a density
of 5x104 cells/ml, and cultured in a 37˚C, 5%
CO2 incubator for 24 h. Following adhering, the cells
were incubated with 100 µl songorine at indicated concentrations
(0, 100, 300, 500, 700 and 900 µg/ml), 100 µl benzoylaconine at
indicated concentrations (0, 500, 1,000, 1,500, 2,000 and 3,000
µg/ml), or 100 µl aconitine at indicated concentrations (0, 100,
500, 1,000, 1,500 and 2,000 µg/ml). After 24, 48 and 72 h, the
culture medium was discarded, and the cells were treated with 100
µl 10% CCK-8 solution for at 37˚C for 1.5 h. Next, the OD was read
at 450 nm using a microplate reader. Cell images were captured
using a fluorescence inverted microscope (magnification, x200;
Eclipse TS100-F; Nikon).
ELISA
The contents of IL-6 (EH004-48; Shanghai ExCell
Biology, Inc.), IL-1β (EH001-48; Shanghai ExCell Biology, Inc.),
TNF-α (EH009-48l; Shanghai ExCell Biology, Inc.) and PG-E2
(CSB-E07965h; Wuhan Huamei Bioengineering Co., Ltd.) in the culture
supernatant were measured using ELISA kits. Cells were seeded onto
the 96-well plate at a density of 5x104 cells/ml, and
cultured in a 37˚C, 5% CO2 incubator for 24 h. Drug
intervention was performed following cell adhering and, at 2 h
before intervention, the cells were treated with 100 ng/ml
lipopolysaccharides (LPS; L6529-1MG; Sigma-Aldrich; Merck KGaA) at
37˚C for 24 h. The LPS treatment was to simulate an in vitro
inflammatory model and the cells would produce an inflammatory
reaction following LPS treatment (20). Next, the cells were treated with 150
µg/ml leflunomide, 350 µg/ml songorine, 1,000 µg/ml benzoylaconine
and 500 µg/ml aconitine at 37˚C for 24 h. ELISA was performed with
commercially available kits (IL-6 kit, EH004-48, Shanghai ExCell
Biology, Inc.; IL-1β kit, EH001-48, Shanghai ExCell Biology, Inc.;
TNF-α kit, EH009-48, Shanghai ExCell Biology, Inc.; and PG-E2 kit,
CSB-E07965h, CUSABIO), according to the manufacturer's
protocols.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The cDNA was
obtained using the TransScript One-Step gDNA Removal and cDNA
Synthesis SuperMix (AT311; TransGen Biotech Co., Ltd.) for 25˚C for
10 min, 42˚C for 30 min and 85˚C for 5 sec. RT-qPCR was performed
using the QuantiNava SYBR-Green kit (208054; Kaijie). Primer
sequences were as follows: HIF-1α forward,
5'-CACCACAGGACAGTACAGGAT-3' and reverse,
5'-CGTGCTGAATAATACCACTCACA-3'; VEGFA forward,
5'-CAGAAGGAGGAGGGCAGAATC-3' and reverse,
5'-GGTCTCGATTGGATGGCAGT-3'; TLR4 forward,
5'-AGACCTGTCCCTGAACCCTAT-3' and reverse,
5'-CGATGGACTTCTAAACCAGCCA-3'; and β-actin forward,
5'-ACAGAGCCTCGCCTTTGCC-3' and reverse, 5'-GAGGATGCCTCTCTTGCTCTG-3'.
The PCR system consisted of 5 µl SYBR Select mix, 0.05 µl Rox, 0.7
µl primer each, 1 µl cDNA and 10 µl ddH2O. The reaction
conditions were as follows: 94˚C for 30 sec; 94˚C for 5 sec, for a
total of 40 cycles; followed by 60˚C for 34 sec. Target gene
expression levels were calculated using the 2-ΔΔCT
method (21).
Western blot analysis
Cells were collected and lysed using RIPA lysis
buffer (Boster Biological Technology). Protein concentrations were
determined using the BSA method. A total of 30 mg protein per lane
was separated by 10% SDS-PAGE, which was then electronically
transferred onto polyvinylidene difluoride membranes. Following
blocking with 5% skimmed milk at room temperature for 1 h, the
membrane was incubated with primary antibodies against HIF-1α
(1:1,000 dilution; cat. no. ab113642; Abcam), VEGFA (1:1,000
dilution; cat. no. ab1316; Abcam), TLR4 (1:1,000 dilution; cat. no.
ab13867; Abcam) and β-actin (1:800 dilution; cat. no. D110024;
Sangon Biotech) at 4˚C overnight. Next, the membranes were
incubated with the HRP-conjugated pierce goat anti-rabbit IgG
(1:10,000 dilution; cat. no. 31460; Thermo Fisher Scientific, Inc.)
or pierce goat anti-mouse IgG (1:10,000 dilution; cat. no. 31430;
Thermo Fisher Scientific, Inc.) at room temperature for 1 h. The
protein bands were detected using the SuperSignal West Prico
Chemiluminescent Substrate (34080; Thermo Fisher Scientific, Inc.),
and the images were captured and analyzed using Chemi Analysis
software.
Statistical analysis
Data are expressed as the mean ± standard deviation.
SPSS 19.0 software (IBM Corp.) was used for statistical analysis.
Experiments were performed in triplicates. One-way analysis of
variance, followed by Tukey's test, was performed for group
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Identification and characterization of
obtained compounds
Compound 1 was a colorless solitary crystal. The
1H-NMR (400 MHz, CDCl3) indicated 1 nitrogen ethyl group
(δH: 1.10, 3H, t, J = 7.0 Hz); and 4 methoxy groups (δH: 3.11,
3.27, 3.34, 3.75, s, each 3H; Table
I). The chemical shift in the 13C-NMR spectroscopy
was virtually the same as the features for aconitine according to a
previous study (22) (Table II). Based on the ESI/MS, the
molecular ion peak m/z was found at 646 [M+H]+.
According to the nitrogen rule, it was inferred that the compound
contained an odd number of nitrogen atoms. The 13C-NMR
showed 34 carbon signals. Accordingly, the molecular formula was
inferred as C34H47NO11, with an
unsaturation degree of 12. NMR suggested that it may be a
C19-aconitine-type diterpene alkaloid. The compound and
aconitine reference substance developed the same color spots at the
same positions in various TLC developments. The compound structure
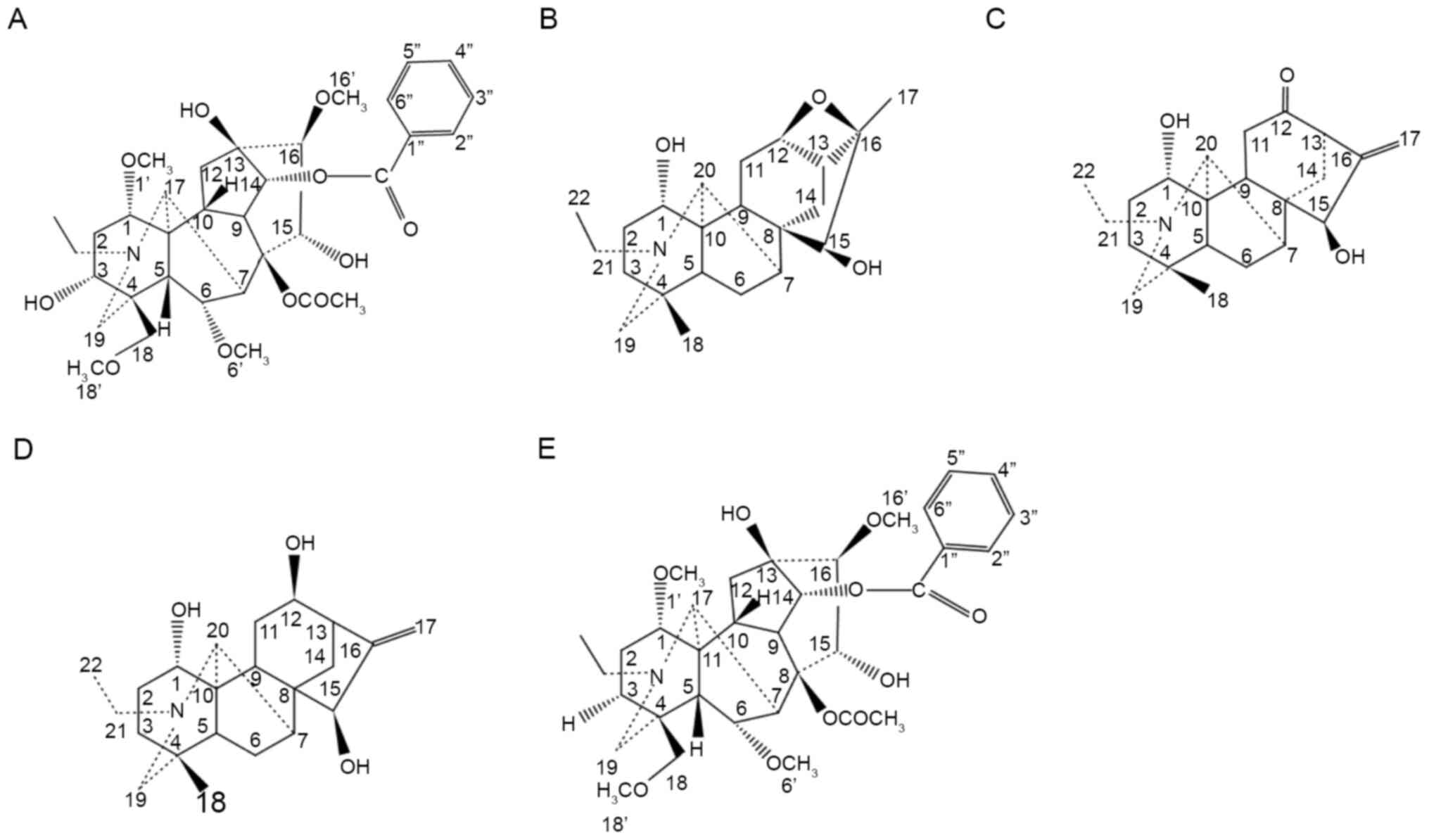
is presented in Fig. 1A.
 | Table IThe 1H-NMR data of
compounds 1-5. |
Table I
The 1H-NMR data of
compounds 1-5.
| Compound | |
1H-NMR |
|---|
| 1 | Aconitine | (400 MHz, CDCl3) δ
ppm: 1.10 (t, J=7.0 Hz, 3H, NCH2CH3), 1.39 (s, 3H, COCH3), 3.11,
3.27, 3.34, 3.75 (s, each 3H, OCH3), 4.04 (d, J=6.5 Hz, 1H, 6-βH),
4.48 (s, 1H), 4.88 (d, J=4.8 Hz, 1H, 14-αH), 8.03 (d, J=7.6 Hz, 2H,
Ar-H), 7.66-7.39 (m, 3H, Ar-H) |
| 2 | Songorine | (400 MHz, CDCl3)
δppm: 0.77 (s, 4H, 18-CH3), 1.07 (t, J=6.8 Hz, 3H, NCH2CH3), 3.10
(d, J=2.4 Hz, 1H), 3.34 (dd, J=17.1, 11.7 Hz, 2H), 3.45 (s, 1H,
15-H), 3.85 (s, 1H, 1-βH), 4.36 (d, J=8.1 Hz, 1H, 15-αH), 5.20,
5.30 (s, 2H, 17-CH2) |
| 3 | 16, 17-dihydro-12β,
16β-epoxynapelline | (400 MHz, CDCl3) δ
ppm: 0.74 (3H, s, 18-CH3), 1.04 (3H, t, J=8Hz, 22-H), 1.38 (3H, s,
17-CH3), 1.78 (1H, d, J=6Hz, 14α-H), 2.71 (1H, dd, J=4, 8Hz, 13-H),
3.42 (1H, brs, 20-H), 3.47 (1H, s, 15-H), 3.88 (1H, dd, J=16.3,
9.4Hz, 1-H), 4.83 (1H, dd, J=7.7, 3.9Hz, 12-H) |
| 4 |
12-epi-napellin | (400 MHz, CDCl3) δ
ppm: 0.77 (3H, s, 18-CH3), 1.09 (3H, t, J=4 Hz, NCH2CH3), 3.91 (1H,
br, s, 1-βH), 4.20 (1H, dd, J=15.9, 7.2Hz, 12-αH), 5.13, 5.34 (2H,
s, 17-CH2) |
| 5 | Deoxyaconitine | (500 MHz, CDCl3)
δppm: 1.09 (3H, t, J=7 Hz, NCH2CH3), 1.39 (3H, s, COCH3), 3.18,
3.29, 3.31, 3.76 (each 3H, s, 4x-OCH3), 4.39 (1H, d, J=3Hz,
15-αOH), 4.48 (1H, dd, J=3, 5.5Hz, 15-βH), 4.89 (1H, d, J=5Hz,
14-βH), 7.42-8.08 (5H, m, Ar-H) |
 | Table IIComparison of 13C NMR data
between compound 1 and aconitine (100 MHz). |
Table II
Comparison of 13C NMR data
between compound 1 and aconitine (100 MHz).
| C | Aconitine (6) | Compound 1 |
|---|
| 1 | 82.00 | 82.38 |
| 2 | 33.10 | 33.63 |
| 3 | 71.00 | 71.55 |
| 4 | 43.10 | 43.14 |
| 5 | 46.30 | 46.85 |
| 6 | 83.20 | 83.38 |
| 7 | 44.60 | 44.70 |
| 8 | 91.70 | 92.06 |
| 9 | 44.00 | 44.21 |
| 10 | 40.70 | 40.90 |
| 11 | 49.90 | 50.00 |
| 12 | 35.60 | 35.83 |
| 13 | 73.90 | 74.05 |
| 14 | 78.70 | 78.92 |
| 15 | 78.60 | 78.85 |
| 16 | 89.80 | 89.97 |
| 17 | 61.10 | 61.15 |
| 18 | 76.50 | 76.69 |
| 19 | 47.20 | 46.86 |
| 20 | 48.10 | 48.93 |
| 21 | 13.00 | 13.35 |
| 1' | 55.80 | 55.94 |
| 6' | 58.00 | 58.00 |
| 16' | 61.10 | 61.02 |
| 18' | 59.00 | 59.13 |
|
COCH3 | 172.30 | 172.43 |
|
COCH3 | 21.30 | 21.45 |
| ArCO | 166.00 | 166.08 |
| 1'' | 129.60 | 129.78 |
| 2'' | 129.50 | 129.61 |
| 3'' | 128.60 | 128.65 |
| 4'' | 133.20 | 133.29 |
Compound 2 was a colorless solitary crystal. Its
1H-NMR data are presented in Table I. The chemical shift in the
13C-NMR spectroscopy was virtually the same as the
features for songorine in a previous study (23) (Table
III). Based on the ESI/MS, the molecular ion peak m/z was
observed at 358 [M+H]+. According to the nitrogen rule,
it was inferred that the compound contained an odd number of
nitrogen atoms. The 13C-NMR showed 22 carbon signals.
Accordingly, the molecular formula was inferred as
C22H31NO3, with an unsaturation
degree of 8. NMR suggested that it may be a
C20-napelline-type diterpene alkaloid. Therefore, the
compound was identified as songorine. The compound structure is
presented in Fig. 1B.
 | Table IIIComparison of 13C NMR data
between compound 2 and songorine (100 MHz). |
Table III
Comparison of 13C NMR data
between compound 2 and songorine (100 MHz).
| C | Songorine (7) | Compound 2 |
|---|
| 1 | 70.40 | 70.37 |
| 2 | 31.50 | 31.66 |
| 3 | 31.90 | 32.24 |
| 4 | 34.10 | 34.11 |
| 5 | 49.10 | 49.19 |
| 6 | 23.60 | 23.23 |
| 7 | 43.70 | 43.51 |
| 8 | 49.90 | 50.03 |
| 9 | 35.10 | 35.21 |
| 10 | 52.10 | 52.42 |
| 11 | 37.20 | 37.33 |
| 12 | 209.00 | 209.9 |
| 13 | 53.60 | 53.79 |
| 14 | 38.00 | 38.13 |
| 15 | 77.30 | 77.22 |
| 16 | 150.90 | 151.08 |
| 17 | 111.60 | 111.42 |
| 18 | 26.00 | 26.07 |
| 19 | 57.20 | 57.41 |
| 20 | 66.00 | 66.01 |
|
N-CH2CH3 | 50.80 | 50.91 |
|
N-CH2CH3 | 13.50 | 13.63 |
Compound 3 was a colorless amorphous powder. The
1H-NMR (400 MHz, CDCl3) indicated 1 nitrogen ethyl group
(δH: 1.04, 3H, t, J = 8 Hz), 1 CH group (δH: 3.42, 1H, brs) and 2
CH3 groups (δH: 0.74, 1.38, s, each 3H; Table I). The chemical shift in the
13C-NMR spectroscopy was virtually the same as the
features for 16, 17-dihydro-12β, 16β-epoxynapelline according to a
previous study (24) (Table IV). Based on the ESI/MS, the
molecular ion peak m/z was observed at 360 [M+H]+.
According to the nitrogen rule, it was inferred that the compound
contained an odd number of nitrogen atoms. The 13C-NMR
showed 22 carbon signals. Accordingly, the molecular formula was
inferred as C22H33NO, with an unsaturation
degree of 7. NMR suggested that it may be a
C20-napelline-type diterpene alkaloid. Therefore, the
compound was identified as 16, 17-dihydro-12β, 16β-epoxynapelline.
The compound structure is presented in Fig. 1C.
 | Table IVComparison of 13C NMR data
between compound 3 and 16,17-dihydro-12β, 16β-epoxynapelline (100
MHz). |
Table IV
Comparison of 13C NMR data
between compound 3 and 16,17-dihydro-12β, 16β-epoxynapelline (100
MHz).
| C | 16,17-dihydro-12β,
16β-epoxynapelline (8) | Compound 3 |
|---|
| 1 | 70.90 | 70.86 |
| 2 | 32.10 | 31.97 |
| 3 | 38.00 | 38.22 |
| 4 | 33.80 | 33.88 |
| 5 | 51.30 | 51.29 |
| 6 | 22.50 | 22.52 |
| 7 | 43.40 | 43.39 |
| 8 | 49.20 | 49.22 |
| 9 | 38.10 | 38.03 |
| 10 | 51.40 | 51.53 |
| 11 | 26.00 | 26.00 |
| 12 | 77.40 | 77.51 |
| 13 | 38.50 | 38.55 |
| 14 | 28.70 | 28.76 |
| 15 | 79.70 | 79.73 |
| 16 | 89.20 | 89.33 |
| 17 | 21.80 | 21.84 |
| 18 | 25.90 | 26.00 |
| 19 | 57.30 | 57.28 |
| 20 | 66.40 | 66.54 |
| 21 | 50.90 | 50.79 |
| 22 | 13.60 | 13.61 |
Compound 4 was a colorless amorphous powder. The
1H-NMR (400 MHz, CDCl3) indicated 1 nitrogen ethyl group
(δH: 1.09, 3H, t, J=4 Hz), 1 CH group (δH: 3.91, 1H, brs) and 1 CH3
group (δH: 0.77, s, 3H; Table I).
The chemical shift in the 13C-NMR spectroscopy was
virtually the same as the features for 12-epi-napelline according
to a previous study (25) (Table V). Based on the ESI/MS, the
molecular ion peak m/z was observed at 360 [M+H]+.
According to the nitrogen rule, it was inferred that the compound
contained an odd number of nitrogen atoms. The 13C-NMR
showed 22 carbon signals. Accordingly, the molecular formula was
inferred as C22H33NO, with an unsaturation
degree of 7. NMR suggested that it may be a
C20-napelline-type diterpene alkaloid. Therefore, the
compound was identified as 12-epi-napelline. The compound structure
is presented in Fig. 1D.
 | Table VComparison of 13C NMR data
between compound 4 and 12-epi-napelline (100 MHz). |
Table V
Comparison of 13C NMR data
between compound 4 and 12-epi-napelline (100 MHz).
| C | 12-epi-napelline
(6) | Compound 4 |
|---|
| 1 | 67.20 | 67.10 |
| 2 | 29.70 | 29.57 |
| 3 | 31.70 | 31.60 |
| 4 | 33.80 | 33.77 |
| 5 | 48.80 | 48.58 |
| 6 | 23.60 | 23.58 |
| 7 | 44.00 | 43.84 |
| 8 | 51.10 | 51.04 |
| 9 | 37.20 | 37.02 |
| 10 | 52.60 | 52.54 |
| 11 | 32.70 | 32.64 |
| 12 | 70.00 | 69.89 |
| 13 | 44.00 | 43.88 |
| 14 | 36.30 | 36.05 |
| 15 | 77.00 | 77.23 |
| 16 | 155.00 | 155.00 |
| 17 | 111.40 | 111.55 |
| 18 | 26.30 | 26.40 |
| 19 | 58.30 | 58.29 |
| 20 | 66.20 | 66.33 |
| 21 | 50.90 | 50.93 |
| 22 | 13.30 | 13.47 |
Compound 5 was a colorless amorphous powder, with an
mp of 169-170˚C, C34H47NO10,
ESI/MS ([M+H]+, m/z 630), 1H-NMR (500 MHz,
CDCl3) δppm: 1.09 (3 H each, t, J=7 Hz,
NCH2CH3), 1.39 (3 H each, s,
COCH3), 3.18, 3.29, 3.31, 3.76 (3 H each, s,
4x-OCH3), 4.39 (1 H each, d, J=3 Hz, 15-αOH),
4.48 (1 H each, dd, J=3, 5.5 Hz, 15-βH), 4.89 (1 H each, d,
J=5 Hz, 14-βH), 7.42-8.08 (5 H each, m, Ar-H; Table I).
The aforementioned data, as well as the results from
the 13C-NMR spectroscopy, were virtually the same as the
features for deoxyaconitine, according to a previous study
(24) (Table VI). Based on the ESI/MS, the
molecular ion peak m/z was observed at 630 [M+H]+.
According to the nitrogen rule, it was inferred that the compound
contained an odd number of nitrogen atoms. The 13C-NMR
showed 34 carbon signals. Accordingly, the molecular formula was
inferred as C34H47NO10, with an
unsaturation degree of 12. NMR suggested that it may be a
C20-aconitine-type diterpene alkaloid. Therefore, the
compound was identified as deoxyaconitine. The compound structure
is presented in Fig. 1E.
 | Table VIComparison of 13C NMR data
between compound 5 and deoxyaconitine (100 MHz). |
Table VI
Comparison of 13C NMR data
between compound 5 and deoxyaconitine (100 MHz).
| C | Deoxyaconitine
(9) | Compound 5 |
|---|
| 1 | 85.10 | 85.29 |
| 2 | 26.20 | 26.41 |
| 3 | 35.10 | 35.30 |
| 4 | 39.00 | 39.08 |
| 5 | 49.10 | 49.26 |
| 6 | 83.10 | 83.25 |
| 7 | 45.00 | 45.12 |
| 8 | 91.90 | 92.11 |
| 9 | 44.40 | 44.61 |
| 10 | 40.80 | 40.99 |
| 11 | 49.80 | 49.94 |
| 12 | 36.20 | 36.66 |
| 13 | 73.90 | 74.15 |
| 14 | 78.80 | 78.86 |
| 15 | 78.60 | 78.85 |
| 16 | 89.90 | 90.16 |
| 17 | 61.30 | 61.47 |
| 18 | 80.10 | 80.30 |
| 19 | 53.00 | 53.14 |
| 20 | 49.10 | 49.02 |
| 21 | 13.30 | 13.51 |
| 1' | 56.10 | 56.35 |
| 6' | 57.90 | 58.03 |
| 16' | 60.90 | 61.05 |
| 18' | 58.90 | 59.09 |
| COCH3 | 172.30 | 172.45 |
| COCH3 | 21.30 | 21.45 |
| ArCO | 166.00 | 166.18 |
| 1'' | 129.70 | 129.86 |
| 2'' | 129.50 | 129.64 |
| 3'' | 128.50 | 128.66 |
| 4'' | 133.10 | 133.27 |
Effects of compound interventions on
the growth of HFLS-RA cells
The proliferation of HFLA-RA cells was assessed
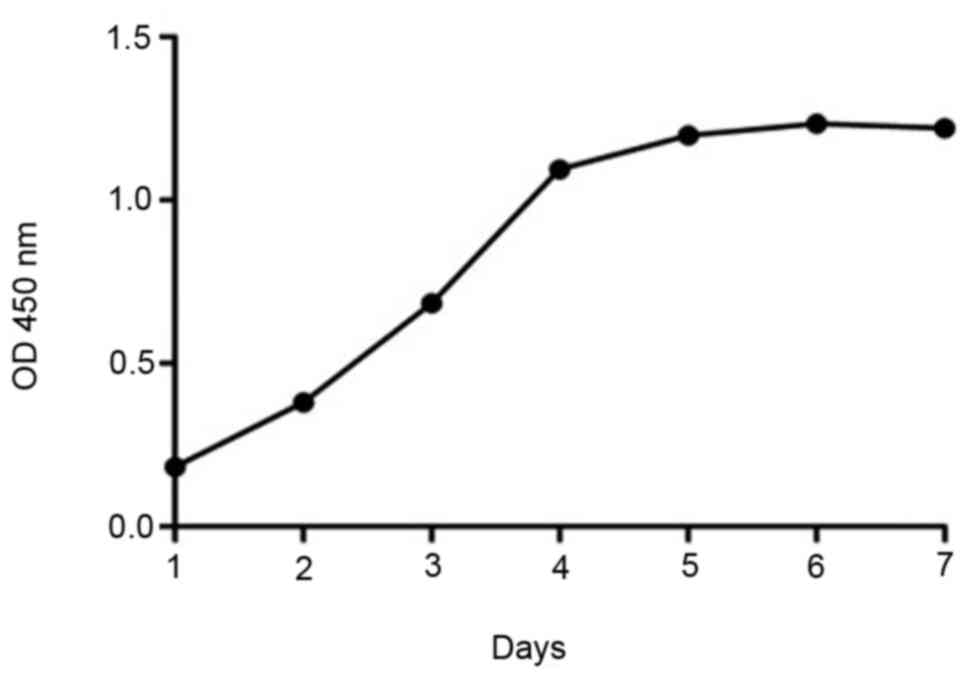
using the CCK-8 assay, and a growth curve was obtained. At 24 h
after seeding, the cells were in the latency phase, and at 48 h
they were in the logarithmic growth phase. Starting from the 4th
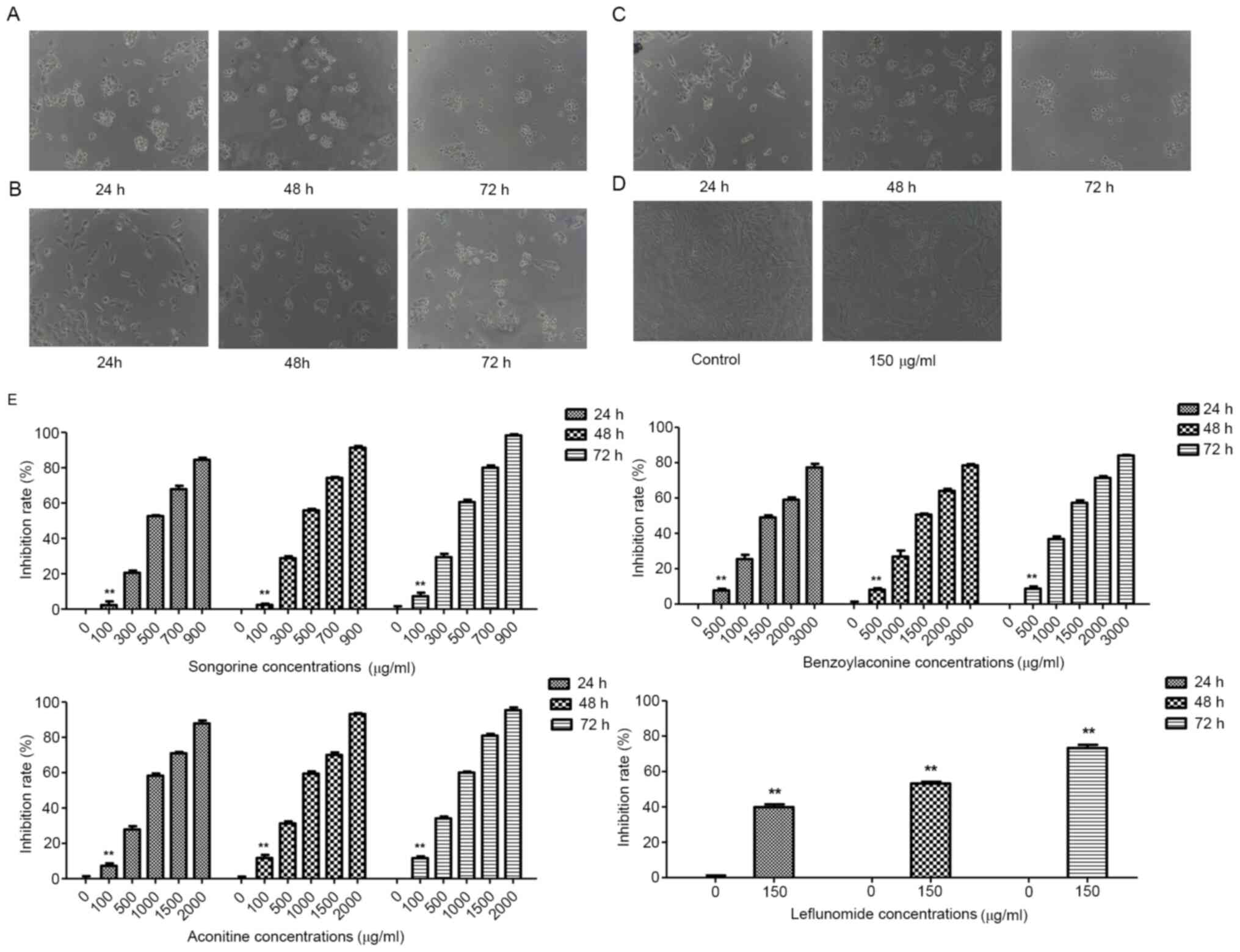
day, the growth platform began and it lasted until day 7 (Fig. 2). The effects of drug interventions
on the growth of HFLS-RA cells were then assessed (Fig. 3A-D). The results of the present
study demonstrated that, for the effects of songorine on the cell
proliferation, the IC50 values for the intervention for
24, 48 and 72 h were 491.4, 436.7 and 385.6 µg/ml, respectively
(Fig. 3E). Furthermore, the
IC50 values for the benzoylaconine intervention for 24,
48 and 72 h were 1,632.0, 1,552.6 and 1,332.5 µg/ml, respectively
(Fig. 3E). Furthermore, the
IC50 values for the aconitine intervention for 24, 48
and 72 h were 775.1, 679.9 and 609.9 µg/ml, respectively (Fig. 3E). These results suggested that
songorine, benzoylaconine and aconitine at different concentrations
may inhibit the proliferation of HFLS-RA cells, to different
extents.
Effects of compound interventions on
cytokine contents in culture supernatant
The effects of drug interventions on the cytokine
contents in culture supernatant were subsequently investigated. The
results demonstrated that, compared with the blank group, the
contents of IL-6, IL-1β, TNF-α and PGE-2 in the culture supernatant
were significantly increased by treatment with LPS (P<0.05).
Furthermore, compared with the LPS group, the contents of IL-6,
IL-1β, TNF-α and PGE-2 in the culture supernatant were
significantly declined in the leflunomide + LPS and the drug
intervention + LPS groups (P<0.01). Furthermore, the contents of
IL-6, IL-1β, TNF-α and PGE-2 in the culture supernatant in the
intervention + LPS groups were higher than that in the leflunomide
+ LPS group (P<0.01; Table
VII). These results suggested that, the drug interventions may
significantly improve the LPS-induced cellular inflammatory
responses.
 | Table VIIIntervention effects on the cytokine
content of the culture supernatant. |
Table VII
Intervention effects on the cytokine
content of the culture supernatant.
| Group | IL-6 (pg/ml) | IL-1β (pg/ml) | TNF-α (pg/ml) | PGE-2 (pg/ml) |
|---|
| Blank | 39.927±0.239 | 10.062±0.503 | 9.862±0.158 | 8.846±0.096 |
| LPS |
67.528±1.311a |
17.594±0.658a |
21.368±0.863a |
25.886±3.028a |
| Leflunomide +
LPS |
42.204±0.906b |
11.540±0.566a,b |
11.802±0.394a,b |
9.226±1.146b |
| Songorine+LPS |
47.510±1.759a-c |
12.910±0.482a-c |
13.878±0.360a-c |
10.966±0.846b |
| Benzoylaconine +
LPS |
51.746±1.098a-c,d |
13.641±0.722a-c |
13.836±0.566a-c |
14.185±1.225a-c |
| Aconitine +
LPS |
45.590±1.392a-c,e |
11.816±0.489a,b,e |
12.341±0.596a,b,d,e |
14.765±0.586a-d |
Effects of compound interventions on
the expression of HIF-1α, VEGFA and TLR4
The effects of drug interventions on the mRNA and
protein expression levels of HIF-1α, VEGFA and TLR4 were
investigated by RT-qPCR and Western blot analysis, respectively.
The results from the RT-qPCR and Western blot analysis demonstrated
that, compared with the blank group, the mRNA and protein
expression levels of HIF-1α, VEGFA and TLR4 in the cells were
significantly increased in the LPS group (P<0.05). Furthermore,
compared with the LPS group, all the mRNA and protein expression
levels of HIF-1α, VEGFA and TLR4 in the cells were significantly
decreased in the leflunomide + LPS and intervention + LPS groups
(P<0.01). Furthermore, compared with the leflunomide + LPS
group, the mRNA expression levels of HIF-1α, VEGFA and TLR4 were
higher in the intervention + LPS groups (P<0.01; Fig. 4; Table VIII).
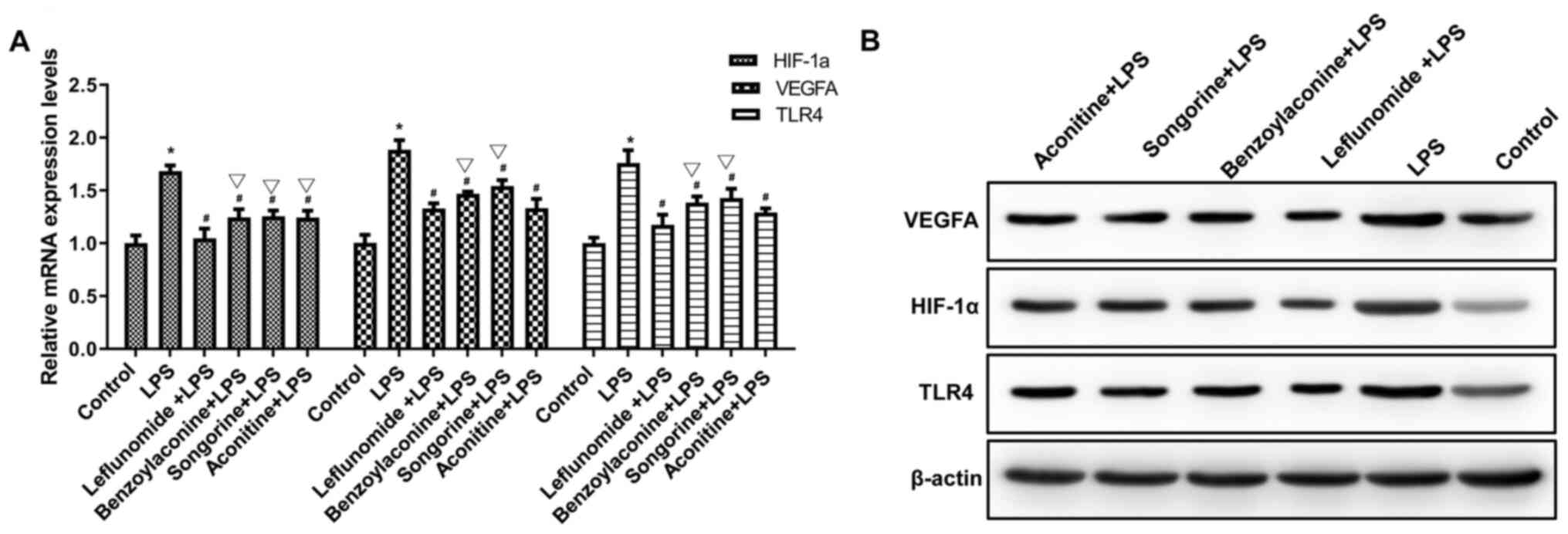 | Figure 4Effects of interventions on mRNA and
protein expression levels of TLR4, HIF-1α and VEGF. Cells were
treated with LPS, in combination with songorine, benzoylaconine,
aconitine and leflunomide. Next, the (A) mRNA and (B) protein
expression levels of TLR4, HIF-1α and VEGF were detected by Western
blot analysis. Experiments were performed in triplicate. One-way
analysis of variance, followed by Tukey's test, was performed for
group comparison. *P<0.01, compared with the control;
#P<0.01; compared with LPS; sP<0.01,
compared with leflunomide + LPS. LPS, lipopolysaccharide. |
 | Table VIIIEffect of interventions on HIF-1α,
VEGFA, and TLR4 protein expression levels. |
Table VIII
Effect of interventions on HIF-1α,
VEGFA, and TLR4 protein expression levels.
| Group | HIF-1α | VEGFA | TLR4 |
|---|
| Blank | 0.592±0.149 | 0.745±0.157 | 0.736±0.138 |
| LPS |
1.287±0.304a |
1.409±0.139a |
1.426±0.296a |
| Leflunomide +
LPS |
0.662±0.159b |
0.850±0.040b |
0.685±0.196b |
| Songorine +
LPS | 0.903±0.190 |
1.154±0.163a | 0.854±0.253 |
| Benzoylaconine +
LPS | 0.951±0.268 | 1.141±0.215 | 0.857±0.141 |
| Aconitine +
LPS | 0.852±0.208 |
1.164±0.116a | 0.845±0.213 |
Discussion
In the present study, five monomeric compounds were
isolated from the total alkaloids extracted from the roots of
Aconitum soongoricum Stapf.: i) Aconitine, ii) songorine,
iii) 12-epi-napelline, iv) 16, 17-dihydro-12β, 16β-epoxynapelline,
and v) deoxyaconitine. These components had certain pharmacological
activities. The aconitine and deoxyaconitine were first isolated
from Aconitum soongoricum Stapf., which were not only
C19-type, but also diester-type diterpene alkaloids.
These components represented the main analgesic and
anti-inflammatory active ingredients in the herb, but they were
also the main toxic components. In the present study, the
songorine, 16, 17-dihydro-12β, 16β-epoxynapelline, 12-epi-napelline
isolated from Aconitum soongoricum Stapf. were all
napelline-type C20 diterpene alkaloids. These alkaloids had
versatile pharmacological activities, including anti-arrhythmic
effects, relaxing peripheral blood vessels and inhibiting
tyrosinase activity. Furthermore, the toxicity of
C20-type diterpene alkaloids was smaller than the
C19 alkaloids. In particular, the toxicities of
songorine and 12-epi-napelline were lower than that of aconitine,
and the acute toxicity of songorine was much lower than that of
aconitine, with higher physiological activity. Therefore, these
components represented potential drugs for the treatment of the
aforementioned diseases by regulating the neurotransmitter levels
in the central nervous system. It is necessary to investigate the
anti-arrhythmic and peripheral relaxing effects of these three
C20napelline-type diterpene alkaloids in Aconitum
soongoricum Stapf.
The results demonstrated that songorine,
benzoylaconine and aconitine had anti-rheumatic activities in
vitro. The inhibiting rate of leflunomide (150 µg/ml) on the
cell proliferation within 24 h was <50%, and the optimal
intervention time was set at 24 h. The optimal intervention
concentrations for other drugs were obtained from the data
concerning the actual inhibiting rates from the CCK-8 assay: 350
µg/ml for songorine, 1,000 µg/ml for benzoylaconine and 500 µg/ml
for aconitine.
The results of the present study demonstrated that
the treatment of LPS significantly increased the contents of PGE-2,
IL-6, IL-1β and TNF-α in the culture supernatants and increased the
intracellular mRNA and protein expression levels of TLR4, HIF-1α
and VEGF. These results suggested that LPS may stimulate the
expression of inflammatory cytokines in HFLS-RA cells. Furthermore,
LPS is the exogenous ligand for TLR4, which may promote the
expression of TLR4, as well as the downstream HIF-1α and VEGF.
Furthermore, compared with the LPS group, the contents of PGE-2,
IL-6, IL-1β and TNF-α culture supernatant were significantly
decreased, and the mRNA and protein expression levels of TLR4,
HIF-1α and VEGF in the cells were significantly decreased, in the
leflunomide + LPS group. These results suggested that leflunomide
may significantly decrease the LPS-induced inflammatory responses.
Furthermore, compared with the LPS group, the co-treatments of LPS
together with songorine, benzoylaconine and aconitine had
significantly decreased contents of PGE-2, IL-6, IL-1β and TNF-α in
the culture supernatant, as well as significantly downregulated
mRNA and protein expression levels of TLR4, HIF-1α and VEGF in the
cells. These results suggested that these three components may also
significantly improve the LPS-induced intracellular inflammatory
responses. It is speculated that songorine, benzoylaconine and
aconitine in Aconitum soongoricum Stapf. may inhibit the
proliferation of HFLS-RA cells, which may involve the regulation of
the TLR4, HIF-1α and VEGFA pathways. Further studies are required
to investigate the underlying mechanisms for the anti-rheumatic
activity in Aconitum soongoricum Stapf.
The results from the ELISA demonstrated that,
compared with the blank group, the contents of IL-6, IL-1β, TNF-α
and PGE-2 in the culture supernatant were significantly increased
in the LPS group. Furthermore, compared with the LPS group, the
contents of IL-6, IL-1β, TNF-α and PGE-2 in the culture supernatant
were significantly lower in the leflunomide + LPS and intervention
+ LPS groups. The results from the RT-qPCR and Western blot
analysis demonstrated that, compared with the blank group, the mRNA
and protein expression levels of HIF-1α, VEGFA and TLR4 were
significantly increased in the LPS group. Furthermore, compared
with the LPS group, the mRNA and protein expression levels of
HIF-1α, VEGFA and TLR4 were significantly decreased in the
leflunomide + LPS and intervention + LPS groups. These results
suggested that the monomer components of songorine, benzoylaconine
and aconitine from Aconitum soongoricum Stapf. have certain
anti-rheumatic activities, which may inhibit the proliferation of
HFLS-RA cells. In these components, aconitine has the best
inhibiting effect. We hypothesized that the anti-rheumatic
mechanism may be through inhibiting the production of inflammatory
cytokines and downregulating the expression levels of HIF-1α, VEGF
and TLR4.
In the present study, five alkaloids from the
Aconitum soongoricum Stapf. were isolated, purified and
identified. Some of the alkaloids have been demonstrated to have
anti-rheumatic effects. However, the action mechanisms of the other
alkaloids required further investigation. Therefore, in the present
study, the isolated and purified songorine, benzoylaconine and
aconitine were tested separately, to investigate the anti-rheumatic
mechanisms and clarify the active constituents in the aconitum. In
further in-depth studies in the future, the isolated chemicals
would be used in combination, to provide evidence for the clinical
treatment of related diseases.
For the antitumor mechanism of aconite, current
studies are focused on their influence on the expression of
multidrug resistance gene, mdr. It has been demonstrated that, with
the treatment of aconitine alkaloids, the expression levels of ras
proto-oncogenes would be inhibited, which in turn affects the
Ras/Raf/MEK/MAPK signaling cascade and subsequently inhibits the
tumor cell proliferation (26).
However, due to the complexity of tumorigenesis and anticancer
mechanisms, the underlying mechanisms of these compounds inhibiting
tumor growth remain to be confirmed in the future. Furthermore, as
for the toxic effects of these compounds on other cell lines,
further studies are required.
The pharmacological effects and toxicity of the
compounds were investigated and analyzed based on the literature
review (27-29).
Zhao et al (27) used the
whole-cell patch clamp technique to study the effect of sogorine on
the inward current of rat brain cells induced by GABA, and their
results demonstrated that the compound may significantly inhibit
the current, with an IC50 value of 19.6 mmol/l.
Furthermore, it has been demonstrated that songorine has an
inhibitory effect on acetylcholinesterase, in a
concentration-dependent manner (28). Furthermore, the half-lethal dose of
songorine for the intravenous injection has been demonstrated to be
128 mg/kg, which is ~10,000 times that of aconitine (0.12 mg/kg)
and 5 times that of benzoyl aconitine (23 mg/kg) (29). Our previous study demonstrated that
the Junggar aconite heating boiled processed products were
superior to the other two types of processed products in terms of
acute toxicity and pharmacological activity (11). The effective dose of the heating
boiled processed products was 3.82 g/person/day, similar to the
clinical dosage for the herbicides based on the Chinese
Pharmacopoeia (30). Adverse
reactions were as follows: The association between aconitine
alkaloids and local anesthesia was investigated by quantitative
structure-activity relationship (31). It has been believed that the local
anesthetic effect results from the aryl ester group at the C-14
position, and that the activity of the aryl ester group at the C-4
position are relatively weak. For the Junggar aconite,
Aconitum Ranunculaceae, its main component is aconitine, a
highly toxic diester aconitine. The toxicological effects of
aconitine are mainly exerted by exciting and then paralyzing the
sensory nerves and central nervous system, paralyzing the
cholinergic nerves and respiratory center, leading to a series of
M- and N-like symptoms of cholinergic nerves, particularly for the
vagus nerve center of the medulla oblongata. Finally, the subject
would die due to the respiratory paralysis and central inhibition.
The main toxic effects of aconitine are based on the serious
damages to the nervous and cardiovascular systems, inhibiting
breathing and inducing arrhythmias.
In conclusion, the monomer components were isolated
from Aconitum soongoricum Stapf., and the specific structure
and physiological activities were investigated. The results of the
present study may contribute toward improving the evaluation index
of drug processing technology, standardizing the processing
technology and quality control. However, further in-depth studies
are required to analyze the toxicological activities of these
monomer components of Aconitum soongoricum Stapf., as well
as the hydrolysis mechanism and pathway during the drug
processing.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 81460603) and ‘Tianshan Xuesong’
Autonomous Region Science and Technology Innovation Leaders (grant
no. 2017XS12).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FZ conceived and designed the experiments; LZ and MS
performed the experiments; JZ and MY analyzed the data; LZ wrote
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hao DC, Ge GB, Xiao PG, Wang P and Yang L:
Drug metabolism and pharmacokinetic diversity of ranunculaceae
medicinal compounds. Curr Drug Metab. 16:294–321. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hao DC, Xiao PG, Ma HY, Peng Y and He CN:
Mining chemodiversity from biodiversity: Pharmacophylogeny of
medicinal plants of Ranunculaceae. Chin J Nat Med. 13:507–520.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wu M and Wei Y: Ethnobotany of Aconitum in
Xinjiang. Chin Wild Plant Resources. 23:29–30. 2004.(In
Chinese).
|
|
4
|
Mei H, Zhao F, Juan LI, Jun LU and Nie J:
The study on acute toxicity of Xinjiang aconitum leucostomum
worosch and its processed products. J Xinjiang Med Univ. 7:918–921.
2013.(In Chinese).
|
|
5
|
Malik J, Tauchen J, Landa P, Kutil Z,
Marsik P, Kloucek P, Havlik J and Kokoska L: In vitro
antiinflammatory and antioxidant potential of root extracts from
Ranunculaceae species. S Afr J Bot. 109:128–137. 2017.
|
|
6
|
Wu JJ, Zhu YF, Guo ZZ, Lou YM, He SG, Guan
Y, Zhu LJ, Liu ZQ, Lu LL and Liu L: Aconitum alkaloids, the major
components of Aconitum species, affect expression of multidrug
resistance-associated protein 2 and breast cancer resistance
protein by activating the Nrf2-mediated signalling pathway.
Phytomedicine. 44:87–97. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li J, Xu GQ and Zhao FC: Resources surveys
of aconitum plants in Cinjiang province(II). Lishizhen Med Materia
Med Res. 12:2888–2889. 2011.(In Chinese).
|
|
8
|
Liu SM, Nie JH, Pan R and Zhao FC:
Determination of the content of total alkaloid in Xinjiang genus
Aconitum. J Xinjiang Med Univ. 2:193–196. 2012.
|
|
9
|
Khader SZA, Ahmed SSZ, Arunachalam T,
Nayaka S, Balasubramanian SK, SyedAmeen ST and Ponnusamy P: Radical
scavenging potential, antiinflammatory and antiarthritic activity
of isolated isomer Methyl-γ-Orsellinate and roccellatol from
Roccella montagnei Bel. Bulletin Faculty Pharmacy Cairo Uni.
56:39–45. 2018.
|
|
10
|
Lü S, Wang Q, Li G, Sun S, Guo Y and Kuang
H: The treatment of rheumatoid arthritis using Chinese medicinal
plants: From pharmacology to potential molecular mechanisms. J
Ethnopharmacol. 176:177–206. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jiang T, Xuan YH, Fu L, et al: Content
changes of four alkaloids in the aconite of Junggar in the
processing process. Chin Traditional Med. 38:2641–2646. 2016.
|
|
12
|
Siyi M, Lin YY, Zhang J and Zhao YC:
Screening of anti-EGFR active ingredients in Aconite from Junggar,
Xinjiang based on cell membrane chromatography. Chin Pharmacist.
21:766–771. 2018.
|
|
13
|
Wang F, Zhao J, Zhao F and Nie J: Study on
the chemical constituents of diphtheria aconite. China Pharmacy.
9:1233–1235. 2015.
|
|
14
|
Zhong Y and Shu R: TLC identification of
flavonoids and alkaloids from Chinese medicinal herbs. Chinese Folk
Medicine. 17:9–11. 2017.
|
|
15
|
Liao SG, Li YT, Zhang LJ, Wang Z, Chen TX,
Huang Y, Li J, Wang AM, Li YJ, Lan YY and Wang YL:
UPLC-PDA-ESI-MS/MS analysis of compounds extracted by cardiac h9c2
cell from Polygonum orientale. Phytochem Anal. 24:25–35.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Djabrouhou N and Guermouche MH:
Development of a stability-indicating HPLC method of etifoxine with
characterization of degradation products by LC-MS/TOF, 1H and 13C
NMR. J Pharm Biomed Anal. 100:11–20. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ma JD, Jing J, Wang JW, Yan T, Li QH, Mo
YQ, Zheng DH, Gao JL, Nguyen KA and Dai L: A novel function of
artesunate on inhibiting migration and invasion of fibroblast-like
synoviocytes from rheumatoid arthritis patients. Arthritis Res
Ther. 21(153)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wen X, Chen X, Liang X, Zhao H, Li Y, Sun
X and Lu J: The small molecule NSM00191 specifically represses the
TNF-α/NF-κB axis in foot and ankle rheumatoid arthritis. Int J Biol
Sci. 14:1732–1744. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Matsumoto Y, Ichihara H, Hino M,
Umebayashi M and Ueoka R: Therapeutic effects of hybrid liposomes
without drugs for rheumatoid arthritis. Drug Deliv. 22:619–626.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhou L, Li L, Wang Y, Gao Q and Geng YQ:
Effects of RANKL on the proliferation and apoptosis of
fibroblast-like synoviocytes in rheumatoid arthritis through
regulating the NF-κB signaling pathway. Eur Rev Med Pharmacol Sci.
23:9215–9221. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li ZB, Lv GH and Chen DL: A study on the
chemical constituents of alkaloids in radix aconiti agrestis. Nat
Product Res. 19:9–14. 1997.
|
|
23
|
Xie H, Wei X and Wei B: Studies on the
alkaloid constituents from Aconitum karakolicum Rap. J00A0Tropical
Subtropical Botany. 5:57–59. 1997.
|
|
24
|
Zhang F, Peng SL, Liao X, Yu KB and Ding
LS: Three new diterpene alkaloids from the roots of Aconitum
nagarum var. lasiandrum. Planta Med. 71:1073–1076. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fuente GDL, Reina M, Valencia E and
Rodríguez-Ojeda A: The diterpenoid alkaloids from Aconitum
napellus. Heterocycles. 27:1109–1113. 1988.
|
|
26
|
Rao CL and Peng C: Study on the effect of
aconitic alkaloid on RAS gene expression and its molecular
mechanism of antitumor activity. Modern Preventive Med.
37:1098–1100, 1103. 2010.
|
|
27
|
Zhao XY, Wang Y, Li Y, Chen XQ, Yang HH,
Yue JM and Hu GY: Songorine, a diterpenoid alkaloid of the genus
Aconitum, is a novel GABA(A) receptor antagonist in rat brain.
Neurosci Lett. 337:33–36. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yang X, Wan L, He X and Song G: Inhibits
acetylcholinesterase activity. Anh Agricultural Sci.
36(3499)2008.
|
|
29
|
Li R, Feng F and Liu J: Research progress
on structure-activity relationship of C20 diterpenoid alkaloids.
Strait Pharmaceutical J. 25:1–4. 2013.
|
|
30
|
National Pharmacopoeia Commission.
Pharmacopoeia of the People's Republic of China, Part One. Beijing:
China Medical Science and Technology Press, 39, 2015.
|
|
31
|
Polishchuk P: Interpretation of
quantitative structure-activity relationship models: Past, present,
and future. J Chem Inf Model. 57:2618–2639. 2017.PubMed/NCBI View Article : Google Scholar
|