Introduction
Growth hormone deficiency (GHD) is caused by a
pituitary problem and is a condition in which the body does not
produce enough GH. Idiopathic short stature (ISS) refers to short
stature of unknown origin. The term ISS is defined as the absence
of a dysfunction in the GH/insulin like growth factor (IGF) axis,
or other identifiable disorder of the endocrine, genetic or organ
systems in short children (1). GH
stimulation tests remain the gold standard for determining the
ability of a child to produce adequate amounts of GH (2). However, the tolerance of children and
their families to the GH stimulation test is low because it
requires frequent blood sampling. In addition, there are a series
of adverse reactions and risks associated with the GH stimulation
test, which makes diagnosis difficult (3). Since the treatment guidelines for
children with GHD and ISS are different, and the dose of GH
required for children with GHD differs compared with that required
for children with ISS, it is necessary to distinguish between the
two causes of short stature before treatment (4). The pituitary gland diseases should
also be excluded before treatment; therefore, MRI examination is
necessary. Furthermore, neuroimaging has become an important part
of the diagnosis of GHD in children (5).
Previous studies have demonstrated that it is
feasible and necessary to distinguish children with ISS from those
with GHD; these previous studies have focused on the 3D volume of
the pituitary gland. Han et al (6) studied the pituitary glands of 23
children with ISS, 32 children with GHD and 75 normal children.
Compared with the normal children, 65.6% of the children with GHD
and 34.8% of the children with ISS had smaller pituitary gland
volumes. Kessler et al (7)
reported that the average pituitary volume of children with GHD was
230.8±89.6 mm3, for children with ISS it was 286.8±108.2
mm3 and in the healthy group it was 343.7±145.9
mm3 (P<0.001). However, to the best of our knowledge,
no previous study has focused on the MRI textures of the pituitary
gland. The present study aimed to develop predictive models using
clinical parameters and MRI texture features, in order to
distinguish between children with GHD and ISS.
Materials and methods
Children selection
The present retrospective analysis was approved by
the ethical review board of Children's Hospital of Hebei Province
(Shijiazhuang, China) and the need for informed consent was waived.
Patients were retrospectively selected from hospital records
between December 2018 and February 2019. Inclusion criteria were as
follows: i) Children whose height was <2 standard deviations for
the same age and sex; ii) children with subnormal growth velocity
for ≤6 months; iii) children that underwent GH stimulation test;
iv) children whose pituitary gland MRI was normal. The exclusion
criteria included: i) Children that were or are using GH; ii)
children that had intracranial abnormalities, such as malignant
tumors, hypothalamic hamartoma or Arnold Chiari malformation; ii)
children that had chronic liver and kidney diseases, skeletal
diseases, congenital heart disease, thyroid hormone axis
abnormalities and chromosomal abnormalities; iv) children whose MRI
scans had artifacts or could not be outlined in the sagittal
plane.
GHD was defined as peak serum GH levels <10 ng/ml
in the GH stimulation tests, and ISS was defined as serum GH levels
≥10 ng/ml in the GH stimulation tests.
GH stimulation tests
For GH stimulation tests, the children fasted after
8 p.m. and blood was collected from the median cubital vein in the
arm for basic GH tests in bed the next morning. The dosage of
arginine hydrochloride was 0.5 g/kg, which was diluted with water
for injection to a total of 200 ml and infused intravenously within
30 min. At 30, 60, 90 and 120 min after administration, blood was
collected from the other arm to measure serum GH which was tested
by the laboratory physician in Department of Laboratory Medicine,
Children's Hospital of Hebei Province.
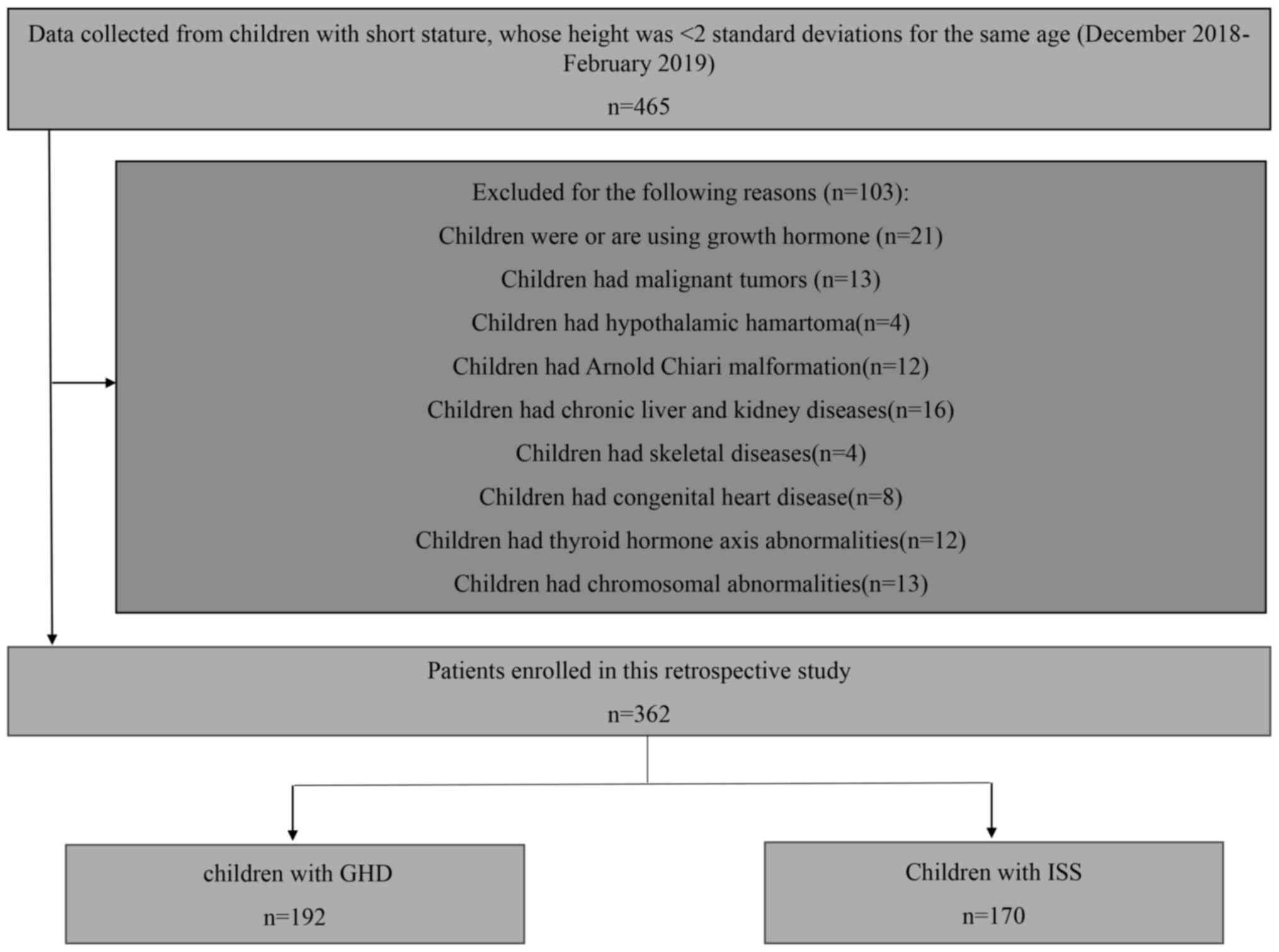
The recruitment pathway for the 362 children
included in the present study is shown in Fig. 1. All of the recruited patients were
children with short stature. The 362 children included 235 male
patients and 127 female patients, with an average age of 8.94 years
(range: 5-13 years). There were 190 children with GHD and 172
children with ISS.
MRI
MRI scans were performed on a Philips 1.5 Tesla MRI
system (Achieva 1.5 T; Philips Medical Systems B.V.) using an
8-channel head coil. T1WI sagittal (TR/TE, 550/15 msec; FOV,
140x140 mm2; reconstruction matrix, 256; slice
thickness, 3 mm; slice gap, 0 mm) images of the brain were
obtained.
Segmentation and feature
extraction
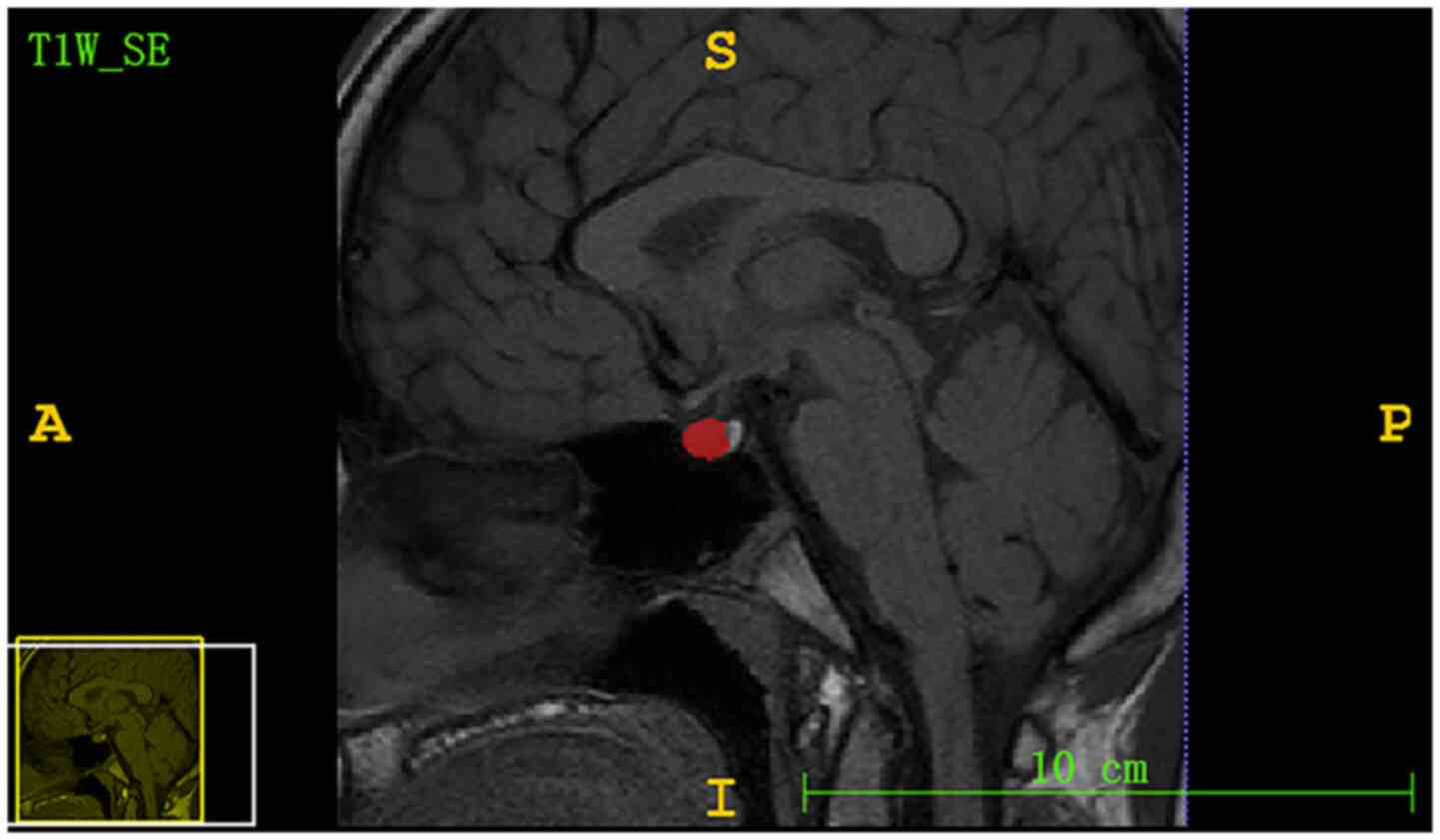
The region of interest (ROI) from the pituitary
gland was segmented by a radiologist with 6 years of experience
using ITK-SNAP software (version 3.6.0; www.itksnap.org). The ROI included the largest level
of the sagittal T1 non-enhanced sequence, which displayed the
pituitary stalk, as shown in Fig.
2. A total of 57 MRI textures (12 histogram features, nine form
factor features and 36 grey level co-occurrence matrix features)
were extracted from the pituitary gland ROI using Matlab R2014a
software (MathWorks) and C++ language was used to write
features.
To assess the intra- and interobserver
reproducibility of MRI texture features, A total of 50 children
were randomly selected from the 362 children for pituitary gland
segmentation 1 month later by the same radiologist and another
radiologist with 10 years of experience in diagnosis. They both had
no information or knowledge of the clinical and laboratory data.
Subsequently, an intragroup consistency analysis was performed on
the 50 images drawn by the same radiologist, and inter-group
consistency analysis was performed on the 50 images drawn by the
two radiologists.
The quantitative features available for analysis in
this study were 57 MRI textures, age, IGF-1, IGF binding protein
(IGFBP)-3, serum calcium and serum alanine aminotransferase (ALT).
All the blood examinations were routine procedures performed by the
laboratory in the Children's Hospital of Hebei Province. The
qualitative feature was sex.
Statistical analysis
Continuous variables are presented as mean ± SD,
whereas categorical variables are presented as counts. The
differences in continuous variables were analyzed through unpaired
Student's t-test, including 57 MRI textures, age, IGF-1, IGFBP-3,
serum calcium and serum ALT. Differences in categorical variables
were analyzed with the χ2 test, including sex. P<0.05
was considered to indicate a statistically significant difference.
Intraobserver and interobserver consistency was evaluated by
intraclass correlation coefficient (ICC) using the absolute
agreement method. The property of the model was determined by
applying the receiver operating characteristic (ROC) curve
analysis. The accuracy, sensitivity, specificity, positive
predictive value (PPV) and negative predictive value (NPV) were
calculated at a cutoff value (the maximum value of Youden index) to
evaluate the efficiency of the predictive model. Statistical
analysis was performed using SPSS software version 21.0 (IBM
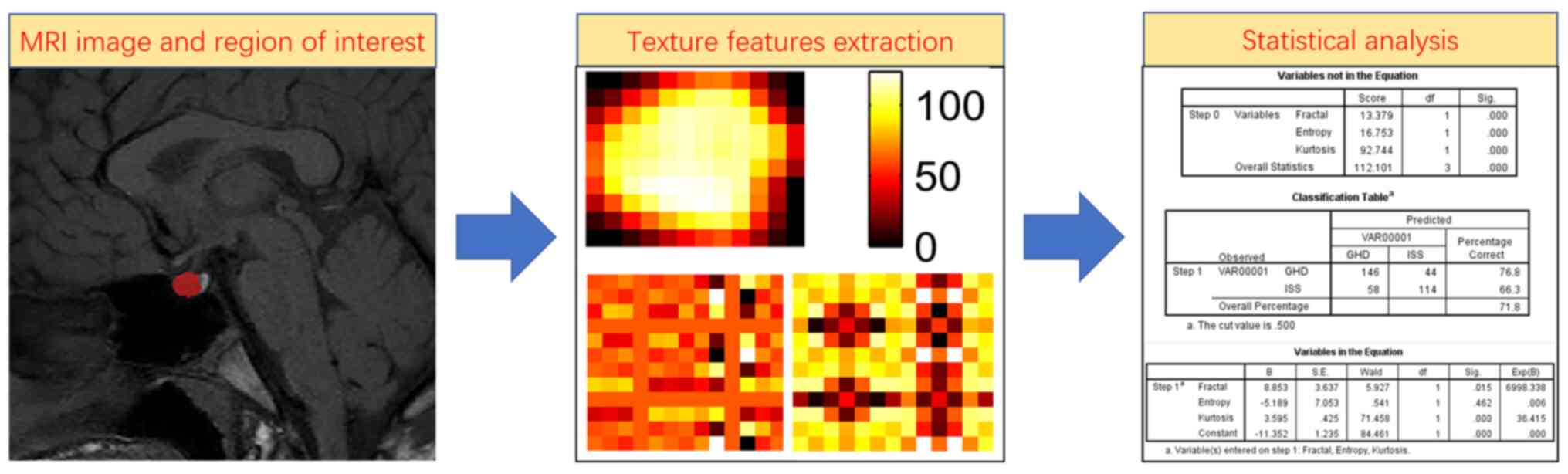
Corp.). The flow chart of the analysis is shown in Fig. 3.
Results
Characteristics of the children
recruited
The characteristics of all of the children recruited
are detailed in Table I. There were
362 children with short stature recruited in the present study.
 | Table IClinical features of children with GHD
and ISS. |
Table I
Clinical features of children with GHD
and ISS.
| Variable | GHD | ISS | P-value |
|---|
| Age, years | 9.07±2.59 | 8.61±2.76 | 0.101 |
| Sex | | | 0.246 |
|
Male | 138 | 134 | |
|
Female | 52 | 38 | |
| ALT, IU | 16.80±12.17 | 15.30±8.77 | 0.177 |
| Ca, mmol/l | 1.60±0.08 | 1.61±0.08 | 0.85 |
| IGF-1, ng/ml | 186.33±81.94 | 206.94±106.35 | 0.041 |
| IGFBP-3, µg/ml | 3.89±0.84 | 4.11±0.94 | 0.018 |
Feature selection and performance in
the predictive model
Two clinical features, IGF-1 and IGFBP-3, were
chosen to establish the clinical model since the P-values of these
two clinical features were statistically different between children
with ISS and GHD (P<0.05; Table
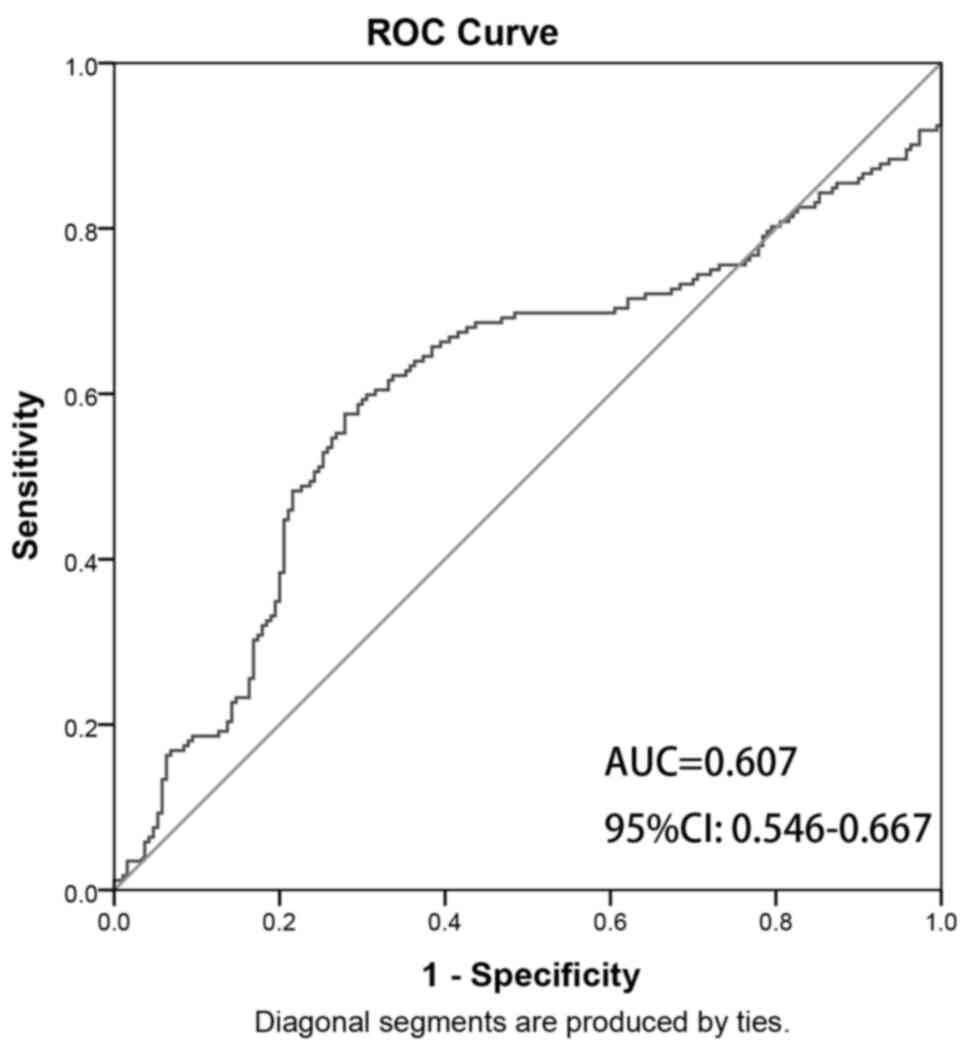
I). The ROC curve was as shown in Fig. 4. The area under the curve (AUC)
value of the clinical model in differentiating ISS from GHD was
0.607 (95% CI, 0.546-0.667), with an accuracy of 0.645, a
sensitivity of 0.576, a specificity of 0.721, a PPV of 0.279 and an
NPV of 0.424.
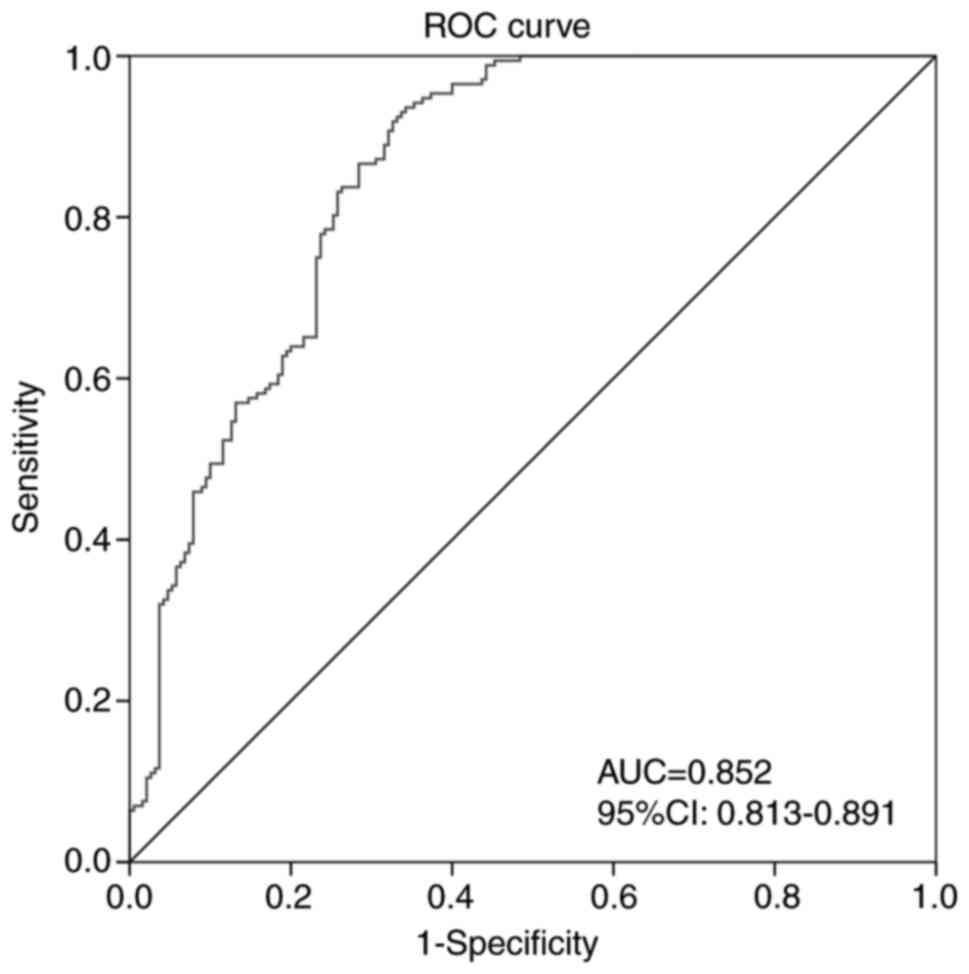
Three MRI texture features: Fractal dimension,
normalized entropy and fourth-order moment (kurtosis), were chosen
to establish the texture model since the P-values of the three
features were statistically different (P<0.05; Table II). The ROC curve was shown in
Fig. 5. The AUC value of the MRI
texture model in differentiating ISS from GHD was 0.852 (95% CI,
0.813-0.891), with an accuracy of 0.804, a sensitivity of 0.936, a
specificity of 0.658, a PPV of 0.751 and an NPV of 0.903. Fractal
dimension mainly describes the most important fractal parameters,
and the similarity of textures or structures obtained by random
processes. Normalized entropy is the value of entropy after
normalization. Entropy value expresses the asymmetry of image
histogram distribution. The higher the entropy value is, the more
asymmetrical the histogram (lesion) distribution is; the smaller
the value is, the more symmetrical the histogram distribution is.
The fourth-order moment (kurtosis) feature describes the shape of
the flatness or peak of the probability density function. The peak
value of the peak distribution is greater than that of the plane
distribution.
 | Table IIOne-way ANOVA analysis for
differentiating GHD from ISS. |
Table II
One-way ANOVA analysis for
differentiating GHD from ISS.
| Variable | GHD | ISS | P-value |
|---|
| Fractal
dimension | 2.970±0.278 | 3.324±0.328 | <0.001 |
| Normalized
entropy | 0.058±0.015 | 0.066±0.023 | <0.001 |
| Fourth-order
moment | 0.018±0.023 | 0.070±0.193 | 0.001 |
Formula results and
interpretation
The clinical model prediction probability of ISS can
be calculated using the following formula: Ln[P/(1-P)]=-1.194+0.001
x IGF-1+0.213 x IGFBP3. The texture model prediction probability of
ISS can be calculated using the following formula:
Ln[P/(1-P)]=-11.352+8.853 x fractal dimension -5.189 x normalized
entropy+3.595 x fourth-order moment (kurtosis). Firstly, the
P-value was selected, then the features for which the P-value was
<0.05 were selected. Secondly, binary logistic regression
analysis was used to calculate the coefficient of each feature.
Finally, the formula Ln[P/(1-P)]=α+β1X1+β2X2+βnXn was used; α
represents the constant, βn represents the B value and Xn
represents each feature.
The pituitary gland image was inputted into Matlab
software for one new child with short stature for whom it was not
known whether they had GHD or ISS. The fractal dimension,
normalized entropy and kurtosis of the image were extracted. The
IGF-1 and IGFBP3 of the child were inputted into the clinical model
formula, and the fractal dimension, normalized entropy and kurtosis
of the child were inputted into the texture model formula; the
P-value was obtained through the aforementioned calculation. When
the P-value was >0.5, the child was considered more likely to
have ISS, and when the P-value was <0.5, the child was
considered more likely to have GHD. SPSS software defined the
threshold as 0.5.
Consistency test
The intraobserver and interobserver ICCs among the
57 MRI texture features ranged between 0.809 and 0.967 (data not
shown). These findings indicated that the intra- and interobserver
consistency was reliable.
Discussion
To the best of our knowledge, the present study is
the first to use predictive models to distinguish GHD from ISS in
children. This is particularly noteworthy, because the present
study did not assess children with GHD and ISS according to
pituitary volume or height, as numerous previous studies have done
(1,6-8).
In the present study, a predictive model was established that used
MRI texture features to distinguish between children with GHD and
those with ISS. This MRI texture prediction model was superior to
the clinical prediction model for distinguishing between children
with GHD and ISS. The ROC analysis indicated that the predictive
AUC values had good predictive capacity. In the present study, the
children with ISS had higher IGF-1 and IGFBP-3 levels than the
children with GHD, and the difference was statistically
significant. This result was concordant with previous studies
(9,10). IGF-1 is mainly synthesized and
secreted by the liver, and its expression varies under the
influence of various factors, including GH. GH has a direct effect
on target cells, stimulating the synthesis and secretion of IGF-1,
and inhibiting the secretion of GH through negative feedback
(11,12).
Fractal analysis is a type of mathematical
structure, which can be used to evaluate and quantitatively analyze
the texture or heterogeneity of tumors; therefore, it has great
application value in brain tumors (13-16).
Entropy refers to the uniformity of pixel value distribution in the
image histogram. The higher the entropy is, the more evenly the
image pixel value is distributed. Kurtosis refers to the
approximate state when the pixel value of the image is close to the
mean value. The smaller the peak, the more concentrated it is.
Based on this, the present study aimed to assess whether the
features of the normal pituitary gland were related to its hormone
secretion level. In the present study, the accuracy of using the
three features to distinguish GHD from ISS was 80.4%. MRI texture
analysis is a quantitative tool (17); through MRI texture analysis, more
detailed and quantitative data of the ROI can be obtained compared
with that obtained from the naked eye. Texture analysis is applied
to the pituitary gland for several reasons. Pituitary and
surrounding slope structures make the pituitary gland an ideal
object to evaluate. There is a magnetic resonance signal difference
between the pituitary gland and the slope, so it is helpful for the
physician to sketch the pituitary gland. There is a difference in
the pituitary gland which is hard to distinguish between GHD and
ISS with the naked eye. The present study assessed children with
short stature and normal pituitary glands; therefore, GHD and ISS
could not be distinguished with the naked eye. Therefore, texture
analysis was used to identify GHD and ISS. Another study, similar
to the present study, has previously been published (18). This previous study reported that the
visual image texture of EGFR mutations in patients with lung cancer
could not be distinguished by the naked eye alone (18). Similarly, the present study was
unable to distinguish the visual images between children with GHD
and ISS by the naked eye.
Children with short stature are often diagnosed by
pediatric endocrinologists as having GHD or ISS. Cohen et al
(19) reported that ISS may
represent a partial GH-insensitive state that manifests during
treatment with higher doses of GH. To distinguish between the two
conditions, endocrinologists rely on the GH stimulation test;
however, this test requires frequent blood sampling of children.
More likely, GH production is continuous, and although some
patients classified as ISS can show normal GH production in GH
stimulation trials, they may not be able to consistently produce
enough GH to support optimal linear growth under physiological
conditions. Therefore, it is particularly important to distinguish
GHD from ISS.
The present study has some limitations. Firstly,
this is a retrospective study, and the number of children with
short stature was small. In future studies, we aim to include more
children with short stature. Secondly, this study only selected the
maximum level of the sagittal position of the T1WI adenohypophysis,
which may result in the loss of part of the MRI information.
Finally, this procedure is not suitable for children who could not
undergo the GH stimulation test; this situation could not be
overcome. Because there were a series of problems in the pituitary
gland of the child (such as pituitary stalk interruption syndrome
and pituitary adenoma), which were mentioned in the inclusion and
exclusion criteria, and the GH stimulation test could not be
performed, it was impossible to distinguish whether the child had
GHD or ISS.
In conclusion, the present study indicated that a
predictive model using MRI texture features may have potential for
differentiating between GHD and ISS in children.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MC collected clinical data on short stature, MRI and
outlined the ROI, and was a major contributor in writing the
manuscript. SQ used the software to extract the texture features of
the ROI. RL made substantial contributions to design and conception
of the study. HS outlined ROI of the pituitary gland. LC collected
the clinical and imaging data. ZH made substantial contributions to
conception and design of the study, and the acquisition, analysis
and interpretation of data. MC and SQ confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This retrospective analysis was approved by the
ethical review board of our hospital, and the need for informed
consent was waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pedicelli S, Peschiaroli E, Violi E and
Cianfarani S: Controversies in the definition and treatment of
idiopathic short stature (ISS). J Clin Res Pediatr Endocrinol.
1:105–115. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wilson TA, Rose SR, Cohen P, Rogol AD,
Backeljauw P, Brown R, Hardin DS, Kemp SF, Lawson M, Radovick S, et
al: Update of guidelines for the use of growth hormone in children:
The lawson wilkins pediatric endocrinology society drug and
therapeutics committee. J Pediatr. 143:415–421. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Albrecht A, Penger T, Marx M, Hirsch K and
Dörr HG: Short-term adverse effects of testosterone used for
priming in prepubertal boys before growth hormone stimulation test.
J Pediatr Endocrinol Metab. 31:21–24. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Grimberg A, DiVall S, Polychronakos C,
Allen DB, Cohen LE, Quintos JB, Rossi WC, Feudtner C and Murad MH:
Drug and Therapeutics Committee and Ethics Committee of the
Pediatric Endocrine Society. Guidelines for growth hormone and
insulin-like growth factor-i treatment in children and adolescents:
Growth hormone deficiency, idiopathic short stature, and primary
insulin-like growth factor-i deficiency. Horm Res Paediatr.
86:361–397. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Maghnie M, Lindberg A, Koltowska-Häggström
M and Ranke MB: Magnetic resonance imaging of CNS in 15,043
children with GH deficiency in KIGS (Pfizer International Growth
Database). Eur J Endocrinol. 168:211–217. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Han X, Xiu J, Huang Z, Zhang J, Zhang Z,
Dong Y, Yuan X and Liu Q: Three-dimensional magnetic resonance
volumetry of the pituitary gland is effective in detecting short
stature in children. Exp Ther Med. 8:551–556. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kessler M, Tenner M, Frey M and Noto R:
Pituitary volume in children with growth hormone deficiency,
idiopathic short stature and controls. J Pediatr Endocrinol Metab.
29:1195–1200. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fink AM, Vidmar S, Kumbla S, Pedreira CC,
Kanumakala S, Williams C, Carlin JB and Cameron FJ: Age-related
pituitary volumes in prepubertal children with normal endocrine
function: Volumetric magnetic resonance data. J Clin Endocrinol
Metab. 90:3274–3278. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ren S, Nie Y and Wang A: Effects of
recombinant human growth hormone in the treatment of dwarfism and
relationship between IGF-1, IGFBP-3 and thyroid hormone. Exp Ther
Med. 12:3579–3582. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang Y, Zhang H, Cao M, Kong L and Ge X:
Analysis of the value and correlation of IGF-1 with GH and IGFBP-3
in the diagnosis of dwarfism. Exp Ther Med. 17:3689–3693.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Clayton PE, Cuneo RC, Juul A, Monson JP,
Shalet SM and Tauber M: European Society of Paediatric
Endocrinology. Consensus statement on the management of the
GH-treated adolescent in the transition to adult care. Eur J
Endocrinol. 152:165–170. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Takeshita H, Fujihara J, Soejima M, Koda
Y, Kimura-Kataoka K, Ono R, Yuasa I, Iida R, Ueki M, Nagao M and
Yasuda T: Confirmation that SNPs in the high mobility group-A2 gene
(HMGA2) are associated with adult height in the Japanese
population; Wide-ranging population survey of height-related SNPs
in HMGA2. Electrophoresis. 32:1844–1851. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu S, Fan X, Zhang C, Wang Z, Li S, Wang
Y, Qiu X and Jiang T: MR imaging based fractal analysis for
differentiating primary CNS lymphoma and glioblastoma. Eur Radiol.
29:1348–1354. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Czyz M, Radwan H, Li JY, Filippi CG,
Tykocki T and Schulder M: Fractal analysis may improve the
preoperative identification of atypical meningiomas. Neurosurgery.
80:300–308. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Marusina MY, Mochalina AP, Frolova EP,
Satikov VI, Barchuk AA, Kuznetcov VI, Gaidukov VS and Tarakanov SA:
MRI image processing based on fractal analysis. Asian Pac J Cancer
Prev. 18:51–55. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Smitha KA, Gupta AK and Jayasree RS:
Fractal analysis: Fractal dimension and lacunarity from MR images
for differentiating the grades of glioma. Phys Med Biol.
60:6937–6947. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Herlidou-Même S, Constans JM, Carsin B,
Olivie D, Eliat PA, Nadal-Desbarats L, Gondry C, Le Rumeur E,
Idy-Peretti I and de Certaines JD: MRI texture analysis on texture
test objects, normal brain and intracranial tumors. Magn Reson
Imaging. 21:989–993. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jia TY, Xiong JF, Li XY, Yu W, Xu ZY, Cai
XW, Ma JC, Ren YC, Larsson R, Zhang J, et al: Identifying EGFR
mutations in lung adenocarcinoma by noninvasive imaging using
radiomics features and random forest modeling. Eur Radiol.
29:4742–4750. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cohen P, Germak J, Rogol AD, Weng W,
Kappelgaard AM and Rosenfeld RG: American Norditropin Study Group.
Variable degree of growth hormone (GH) and insulin-like growth
factor (IGF) sensitivity in children with idiopathic short stature
compared with GH-deficient patients: Evidence from an IGF-based
dosing study of short children. J Clin Endocrinol Metab.
95:2089–2098. 2010.PubMed/NCBI View Article : Google Scholar
|