Introduction
Breast cancer is the most common type of cancer
diagnosed in women and ranked as the fifth leading cause of death
(1). Cancer cells have an elevated
level of reactive oxygen species (ROS) compared with healthy cells,
and exhibit uncontrolled proliferative and high metabolic natures
(2). This elevated ROS level is
essential to maintain a cancer phenotype and makes cancer cells
more susceptible to oxidative stress. Consequently, cancer cells
resist this effect by increasing antioxidant levels, including that
in the thioredoxin/thioredoxin reductase (Trx/TrxR) system
(3).
The antioxidant function of Trx/TrxR system is not
only beneficial for healthy cells, but also for cancer cell
development as it has anti-apoptotic and angiogenesis functions
(4). In addition, it has been
reported that a high level of Trx/TrxR increases cancer resistance
to conventional treatment, such as chemotherapy and radiotherapy
(5-7).
Therefore, the last few years have witnessed an increasing interest
towards inhibiting the Trx/TrxR system by natural or synthetic
molecules, including the natural electrophilic inhibitor curcumin
(8).
Curcumin, a natural polyphenol extracted from
turmeric, has several therapeutic potentials due to its antioxidant
and anti-inflammatory activities. Various studies have reported its
tumor suppressive role via suppression of cellular proliferation,
induction of apoptosis and irreversible inhibition of the Trx/TrxR
system (9-11).
Furthermore, few reports have demonstrated the role of curcumin in
increasing tumor cell sensitivity to radiotherapy and cisplatin
in vitro using 2D monolayer culture (12,13).
Although the traditional 2D cell culture is an
established system for cell-based studies, it does not reflect the
real cellular behavior in vivo. Cells grown in monolayers
differ in their cellular and extracellular interactions, which
leads to altered morphologies, proliferative behaviors, genetic
expression levels, treatment responses, and impaired access to
metabolites and extracellular signals. As a result, the data can be
misleading regarding in vivo responses (14,15).
Using a 3D cell culture system overcomes the disadvantages of a 2D
system, as it represents a microenvironment similar to that in
vivo (16). Numerous
publications have focused on developing 3D models of the MCF-7 cell
line to gain a better understanding of breast cancer biology and
overcome the limitations associated with the traditional 2D models
(17,18).
Herein, the present study focused on studying the
effect of curcumin on the Trx/TrxR system in breast cancer cells
either alone or in combination with radio- or chemotherapy using 2
and 3D culture systems.
Materials and methods
Cell culture
The human breast adenocarcinoma MCF-7 cell line
(ATCC HTB-22™) was maintained in DMEM/F12 (Lonza Group,
Ltd.) supplemented with 10% fetal bovine serum (Sigma-Aldrich;
Merck KGaA), 100 IU/ml penicillin and 100 mg/ml streptomycin. Cells
were cultures in a humidified atmosphere at 37˚C with 5%
CO2.
For the 2D monolayer culture, cells continued to be
cultured in 6- and 96-well tissue culture plates as aforementioned.
Cells were examined under an inverted microscope after 48 h. MCF-7
cells formed a confluent adherent layer (70-80% confluence). After
completion of the treatment protocols listed below, MCF-7 monolayer
2D cells were harvested using 0.05% trypsin and 0.02% EDTA.
For the 3D spheroid cultures, 96-well plates were
pre-coated with 1.5% agarose and allowed to solidify for at least 2
h before loading the cells. MCF-7 cells were seeded in the
pre-coated wells at a density of 1x105 cells/well and
the plates were incubated in a humidified atmosphere with 5%
CO2 at 37˚C. The culture medium was changed every 2 days
until spheroids were formed. Spheroids were examined using a light
microscope (magnification, x40) and digital camera (Carl Zeiss
AG).
Treatments of MCF-7 cells
MCF-7 cells cultured in 2D monolayers and 3D
spheroids were treated with the following: i) Curcumin (Loba Chemie
Pvt. Ltd.) at concentrations of 10, 20, 30, 50 and 100 µM for 24
and 48 h; ii) 5-fluorouracil (5-FU)-Adriamycin-cyclophosphamide
(FAC)-based chemotherapy (22.5 µg/ml 5-fluorouracil, 0.579 µM
Adriamycin and 3 µg/ml cyclophosphamide) either alone or in
combination with various curcumin concentrations for 24, 48 and 72
h; or iii) radiation whereby cells were exposed to irradiation at
room temperature using a PRIMUSTM linear accelerator (Siemens AG)
at a dose of 200 MU/min for the time required to apply doses of 2,
4, 6 and 8 Gy. Radiation treatment was applied on untreated cells
and curcumin-treated cells. Control cells were treated with
DMSO.
Cell viability assay
The viability of MCF-7 cells was evaluated using an
MTT assay. After treatment, cells were incubated with 0.5 mg/ml of
MTT reagent for 3 h at 37˚C. After the MTT incubation, the
MTT/medium was removed, and the precipitated crystals were
dissolved in ethanol/DMSO (1:1) solution with agitation for 20 min.
The absorbance was measured at 540 nm.
Measurement of TrxR1
concentration
TrxR1 concentration was determined using a Human
TrxR1 ELISA kit (cat. no. In-Hu4061; Innova Biotech Co. Ltd.).
Following treatment, cells were washed with PBS, trypsinized, and
centrifuged at 24 x g for 5 min at 4˚C. The cells were lyzed by
repeated freezing/thawing cycles, and then the lysate was
centrifuged for 15 min at 18,600 x g at 4˚C. The supernatant was
collected for protein and activity measurements. Total protein
measurement was performing using the standard Lowry method
(19). For TrxR1 concentration
measurements, samples were processed according to the
manufacturer's protocol. The TrxR1 content is expressed as
TrxR1/protein (pg/mg).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The gene expression of Trx, TrxR1, Bcl2 and BAX was
determined using RT-qPCR. Total cellular RNA of monolayer cells and
spheroids were extracted using a QIAamp RNA Blood Mini kit (Qiagen,
Inc.) according to the manufacturer's instructions. Equal amounts
of RNA were reverse transcribed using a miScript II reverse
transcription kit (Qiagen, Inc.). The RNA samples were incubated
with reverse transcription mixture at 37˚C for 60 min followed by 5
min incubation at 95˚C. Subsequently, the RT-qPCR reaction was
performed on an Applied Biosystems Real-Time PCR system using
QuantiTecht SYBR Green RT-PCR Master mix (Qiagen, Inc.). The primer
sequences were as follows: Trx forward,
5'-CGAGTCTTGAAGCTCTGTTTGG-3' and reverse,
5'-TATCACCTGCAGCGTCCAAG-3'; TrxR1 forward,
5'-CTCAAATTCTTGCTTATCAGGAGGG-3' and reverse,
5'-GCGACATAGGATGCTCCAACA-3'; Bcl2 forward,
5'-ACAGGGTACGATAACCGGGA-3' and reverse, 5'-GCCCAGACTCACATCACCAA-3';
BAX forward, 5'-CCCTTTTGCTTCAGGGGATGAT-3' and reverse,
5'-GGCGTCCCAAAGTAGGAGAG 3'; GAPDH forward,
5'-CCACATCGCTCAGACACCAT-3' and reverse, 5' AGCCAAATTCGTTGTCATACTTCT
3'. The RT-qPCR cycling conditions were as follows: An initial
activation step of 15 min at 95˚C; 45 cycles of 15 sec at 94˚C, 30
sec at 59˚C and 30 sec at 70˚C. Results are presented as the
average fold change of target gene in test to control group using
the 2-ΔΔCq formula (20).
Statistical analysis
Data were analyzed using SPSS software version 20.0.
(IBM Corp.). Normally distributed quantitative data are expressed
as the mean ± standard deviation. Student's t-tests were used for
comparing two variables or two groups and the F-test (ANOVA) was
used to compare >two groups with Tukey's post hoc test for
pairwise comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
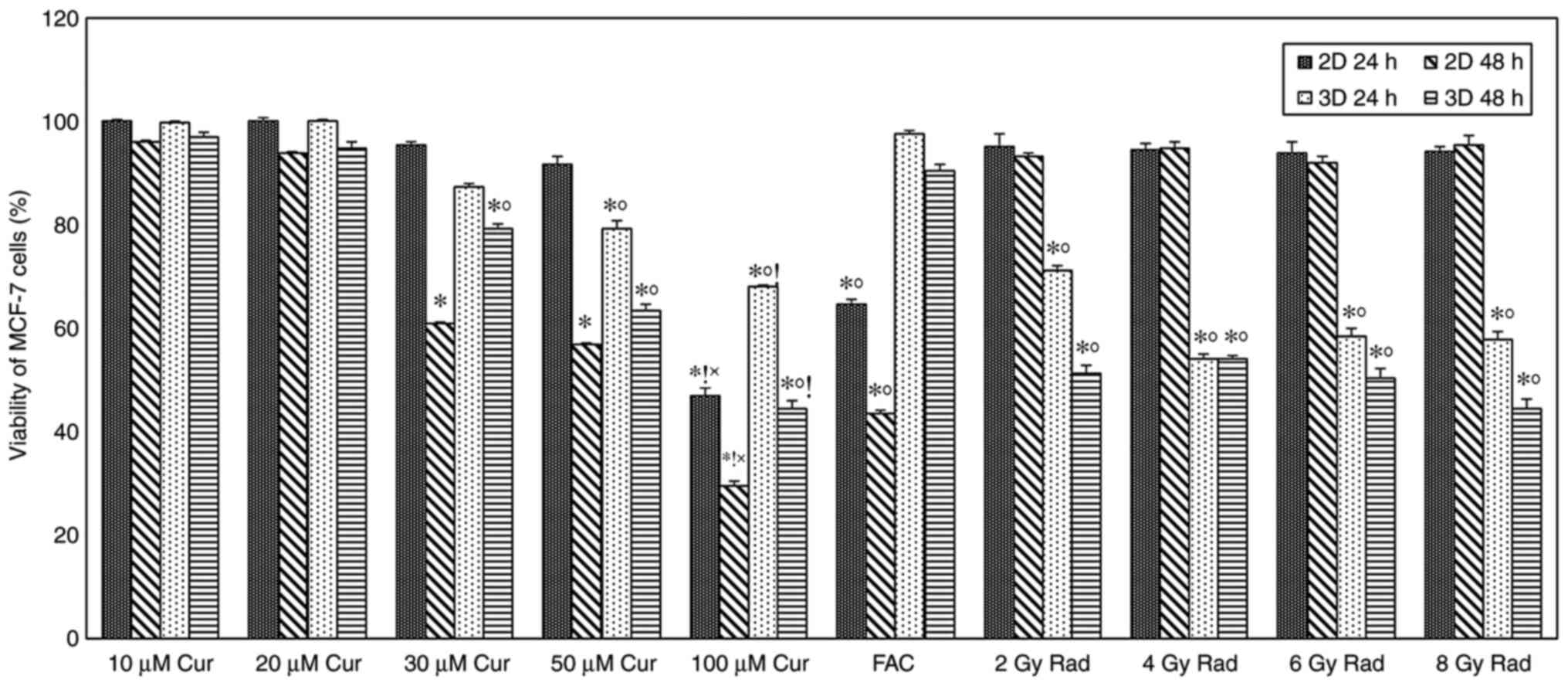
Cell viability
MCF-7 cells cultured as 2D monolayer and 3D
spheroids were treated with different curcumin concentrations (10,
20, 30, 50 and 100 µM), cell viability was determined after 24 and
48 h of treatment. As demonstrated in Fig. 1, time- and concentration-dependent
cytotoxic effects were observed in the 2 and 3D cultures. A
significant amount of cell death (P<0.001) was observed in both
cultures compared with the control at 30 µM concentration and
above, with a more pronounced effect after 48 h of treatment.
Furthermore, a significant difference (P<0.001) was observed
between the 2 and 3D cultures at 30 µM concentrations and above.
After 48 h treatment, results showed that 2D cultures were more
sensitive to the cytotoxic effect of curcumin compared with the 3D
cultures, cell viability percentages were 61, 57 and 30% for the 2D
monolayer, and 79, 63 and 45% for the 3D spheroids at 30, 50 and
100 µM concentrations, respectively (Fig. 1).
A time-dependent cytotoxic effect was observed in
cells treated with chemotherapy compared with the control group,
with significantly reduced viability in the 2D cultures compared
with that in the 3D cultures (P<0.001). Cell viability
percentages were 65 and 44% for the 2D monolayer, and 98 and 91%
for the 3D spheroids after 24 and 48 of treatment, respectively
(Fig. 1). Exposing cells to
different doses of ionizing radiation showed a slight amount of
cell death in the 2D monolayer, while in the 3D spheroid group,
cell death was significantly increased compared with the control
(P<0.001 for all doses; Fig.
1).
Comparing the effect of curcumin treatment on cell
viability with standard treatments did not show any significant
difference at low doses; however, the viability of 2D cells treated
with 100 µM curcumin was significantly lower compared with those
treated with FAC and radiation. In the 3D model, 100 µM
curcumin-treated cells had significantly lower viability compared
with FAC, but not with radiation (Fig.
1).
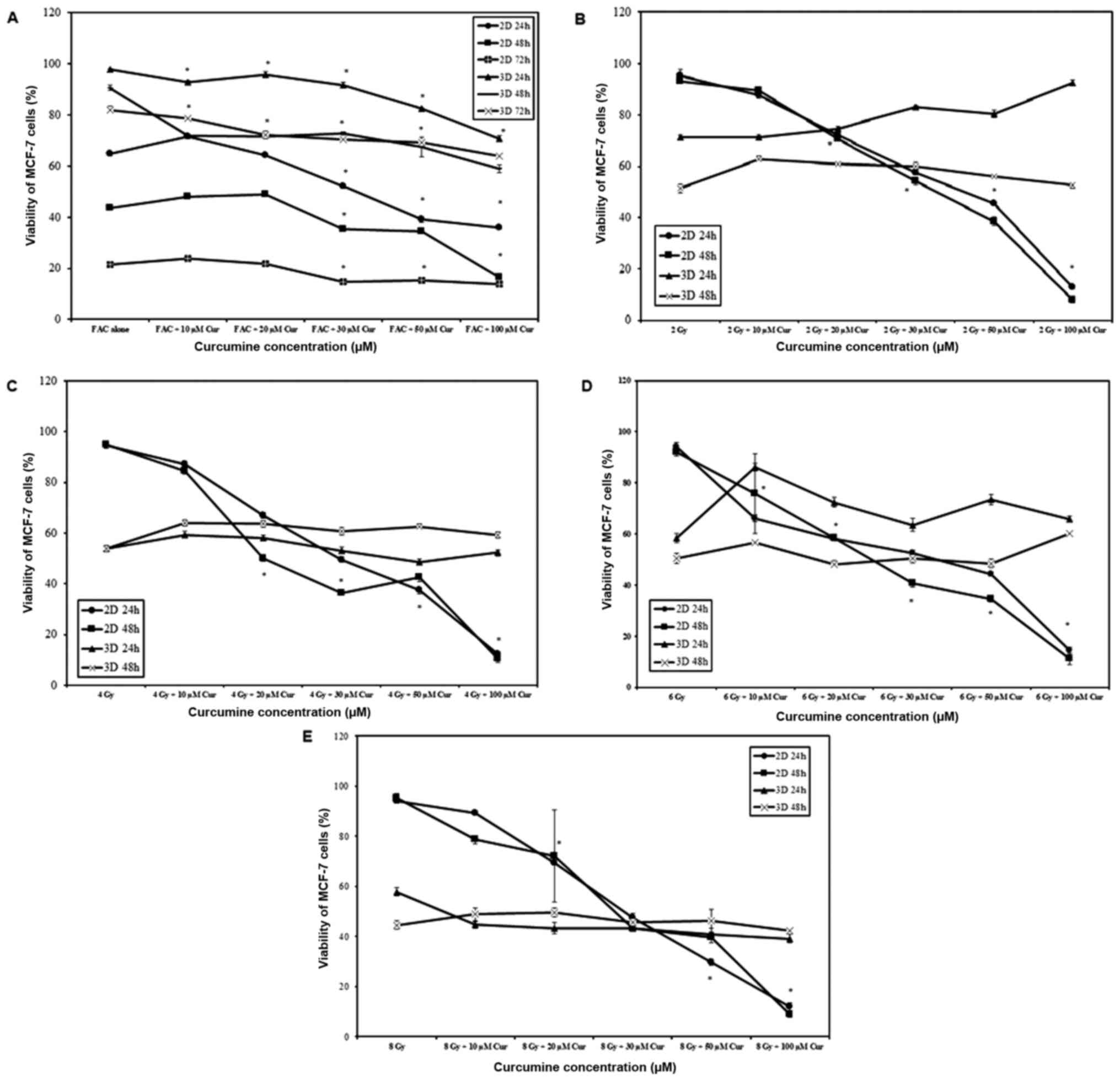
When chemotherapy was combined with curcumin, cell
viability was significantly reduced with increasing curcumin
concentration and treatment duration in 2 and 3D cultures
(P<0.001 for all comparisons). A significantly higher rate of
cell death was observed in 2D cultures compared with 3D cultures at
all-time intervals (P<0.001; Fig.
2A). Increasing curcumin concentration resulted in increased
cell death (P<0.001) in 2D monolayers, which was a behavior
independent on the irradiation dose (Fig. 2B-E). However, in 3D spheroids the
effect on cell viability was minimal and not proportional to
irradiation dose (Fig. 2B-E).
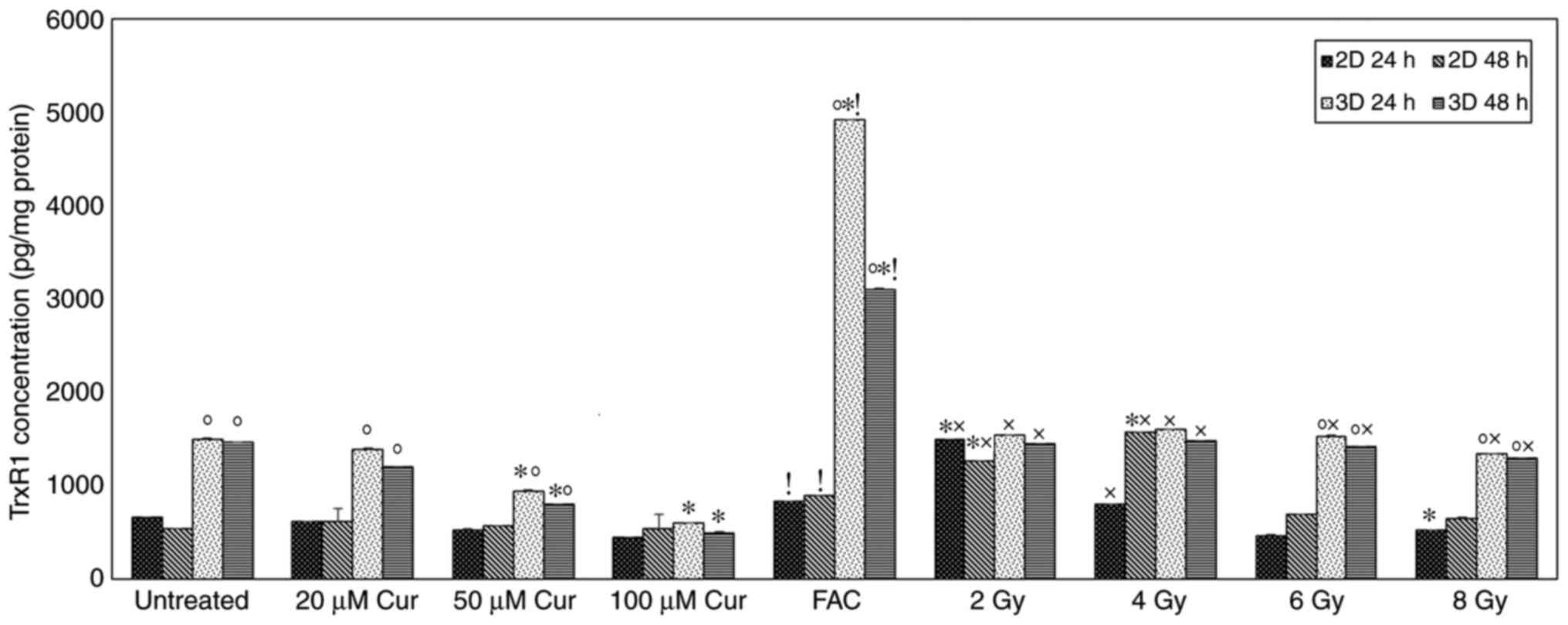
TrxR1 concentration
The level of TrxR1 in 2 and 3D MCF-7 cell cultures
treated with or without curcumin is shown in Fig. 3. The Trxs R1 level in 3D spheroids
was significantly higher compared with that in 2D monolayers in
untreated, curcumin (20 and 50 µM), FAC and radiation (6 and 8 Gy)
treated groups at both time intervals (P<0.001). After 24 h of
treatment, 3D cultures showed a significant decrease in the TrxR1
level compared with that in the untreated cells at the 50 and 100
µM curcumin dose (P<0.001). At a concentration of 100 µM
curcumin, 1.5- and 2.5-fold reductions in TrxR1 levels were
detected in the 2 and 3D cultures, respectively. After 48 h of
treatment, no significant change in the TrxR1 level was observed in
the 2D cultures. By contrast, a significant decrease was detected
in 3D cultures with increasing curcumin dose. Chemotherapy led to a
significant elevation in TrxR1 level in compared with the untreated
2 and 3D cultures at both time intervals (P=0.001). Radiation
treatment at lower doses (2 and 4 Gy) caused a significant
elevation in TrxR1 levels in 2 and 3D cultures compared with the
untreated cells after 48 h. This effect decreased with increasing
radiation dose, whereby the TrxR1 level was significantly reduced
compared with the untreated cells at 8 Gy in 2D culture after 24 h
(Fig. 3). The TrxR1 level in cells
treated with 100 µM of curcumin was significantly lower compared
with those treated with FAC in both 2 and 3D cultures at both time
intervals (P<0.001; Fig. 3). In
the radiotherapy treated groups, 100 µM curcumin-treated 2D and
showed a significantly lower TrxR1 level compared to those treated
with lower doses of radiation (2 and 4 Gy) at both time intervals
(Fig. 3). However, at higher
radiation doses (6 and 8 Gy), only 3D cultures showed a significant
increase in TrxR1 levels compared to 100 µM curcumin treated group
(P<0.001; Fig. 3).
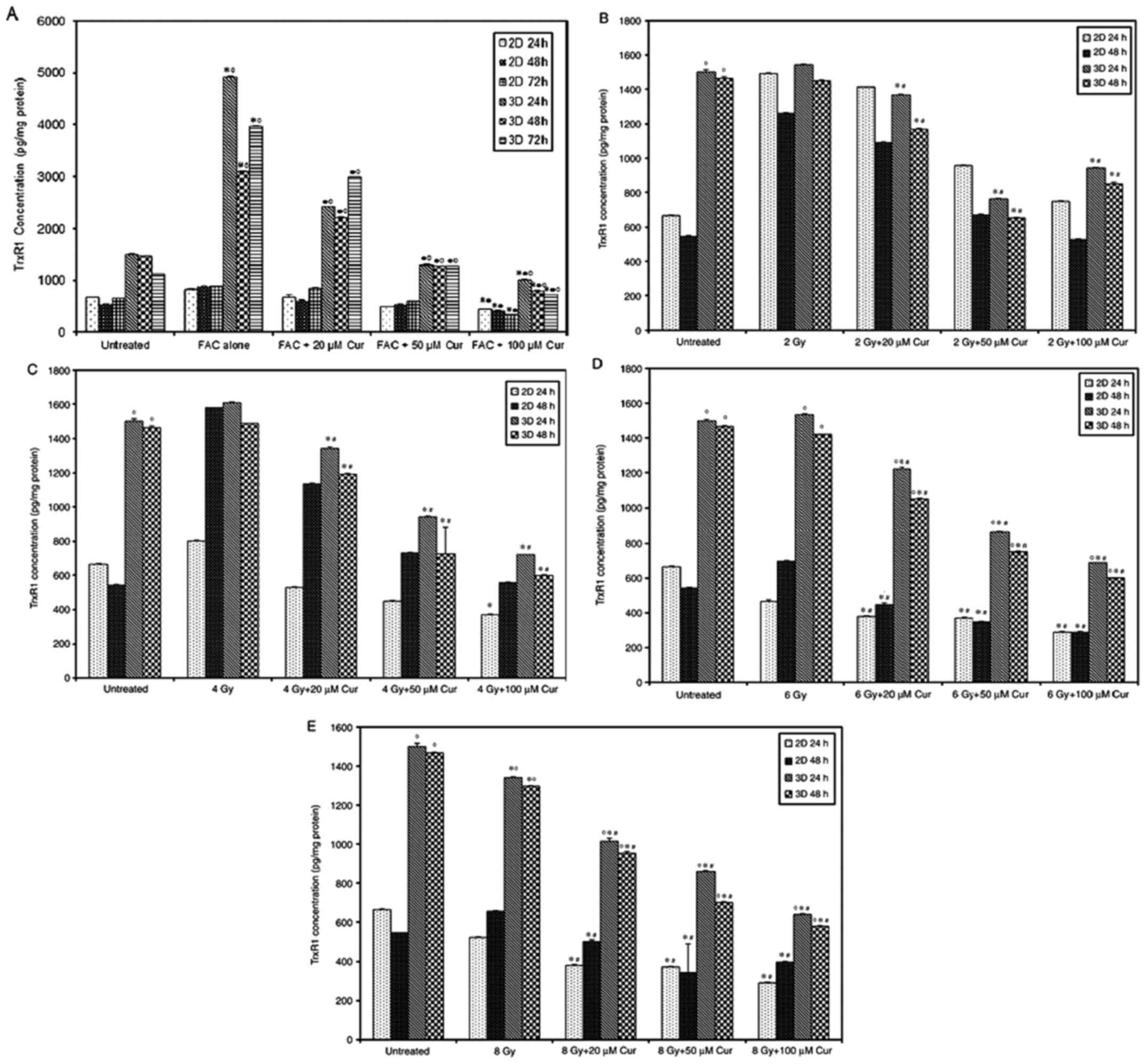
The combined treatment of chemotherapy and 100 µM
curcumin led to a significant reduction in TrxR1 level compared
with that in the untreated group at all treatment intervals and
both culture settings (P<0.001, Fig.
4A). Compared with that after chemotherapy, the combined
treatment also led to a significant reduction of TrxR1 levels in 3D
cultures in a dose-dependent manner at all time intervals. However,
2D culture only showed a significant reduction at FAC + 100 µM Cur
concentration (P<0.001, Fig.
4A). TrxR1 levels were significantly higher in 3D spheroids
compared with that in 2D monolayers at all treatment conditions and
time intervals (P<0.001, Fig.
4A). The combination of radiation and curcumin also led to a
significant reduction in TrxR1 levels in a dose-dependent manner in
all 3D culture settings compared to both control and groups treated
with radiation alone (P<0.001; Fig.
4B-E). However, the 2D cultures didn't show a similar response
unless the radiation doses was increased up to 6 and 8 Gy
(P<0.001; Fig. 4D and E). The 3D spheroids demonstrated
significantly higher TrxR1 levels compared with that in the 2D
monolayer group (P<0.001) and the difference was more prominent
with higher radiation doses (Fig.
4B-E).
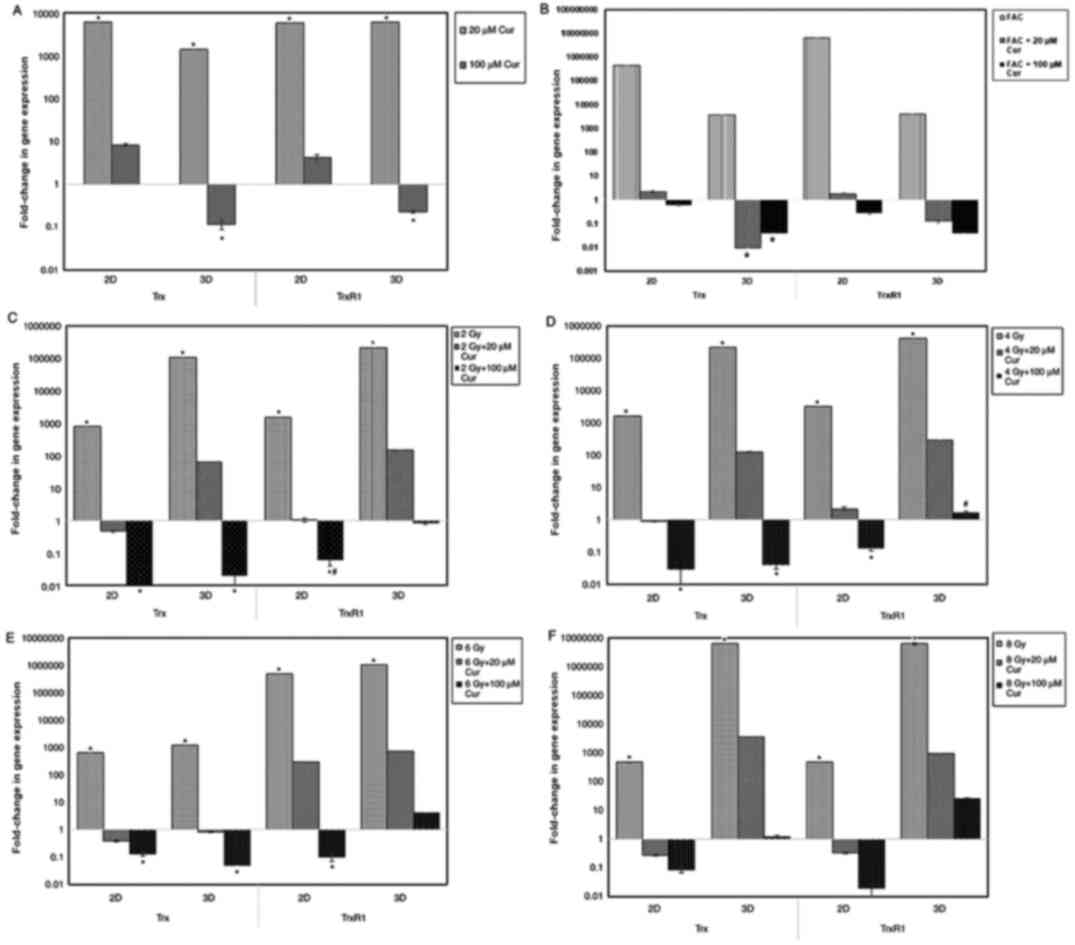
Trx and TrxR1 gene expression
The results of the Trx and TrxR1 gene expression
assays in 2 and 3D cultures are presented in Fig. 5 as a fold-change of the control. In
2 and 3D cultures, treatment with 20 µM curcumin led to a
significant increase (P<0.001) in Trx and TrxR1 gene expression
in compared with the control (Fig.
5A). At 100 µM concentration, the expression of Trx and TrxR1
in 2D cultures was slightly increased, while in 3D cultures, it was
significantly lower compared with the control.
In all culture settings, treatment with chemotherapy
alone significantly increased Trx and TrxR1 expression compared
with those in the control group (Fig.
5B). Furthermore, combination treatment with curcumin resulted
in a significant reduction in Trx and TrxR1 expression in compared
with control, particularly with higher curcumin doses (100 µM).
With radiation treatment, Trx and TrxR1 expression
in all culture settings were significantly increased in compared
with the control (P<0.001). Combining radiotherapy with curcumin
led to a significant reduction in Trx and TrxR1 expression in both
culture settings (P<0.001). However, the reduction in 2D was
more prominent compared with that in 3D cultures (P<0.001;
Fig. 5).
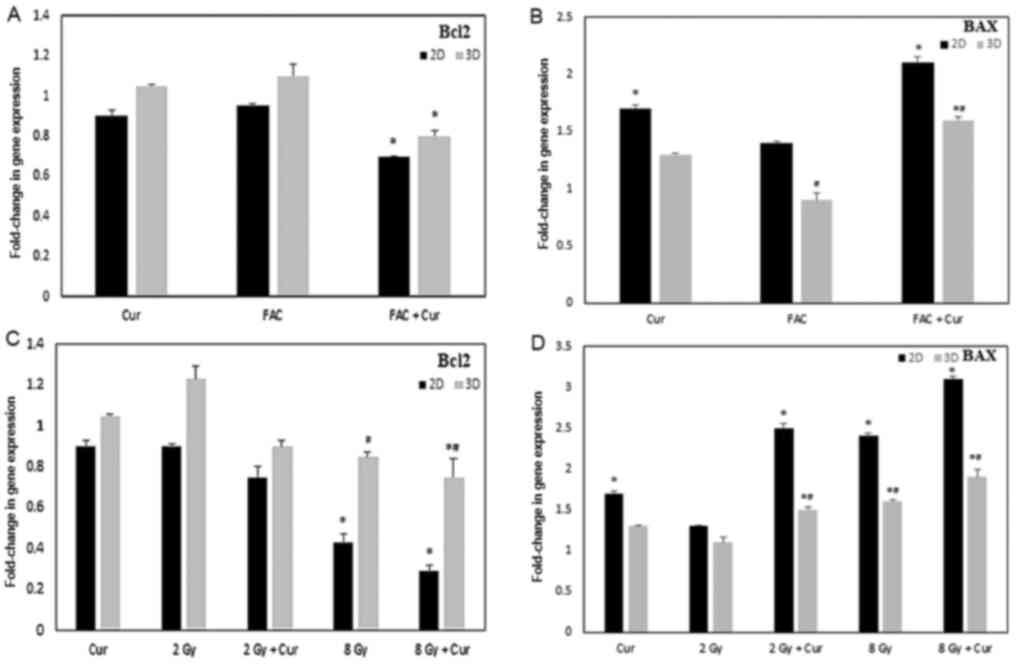
Bcl2 and BAX gene expression
The results of the Bcl2 and BAX gene expression
assays in 2 and 3D cultures are presented in Fig. 6 as a fold-change of the control. In
2 and 3D cultures, treatment with 100 µM curcumin combined either
with FAC or 8 Gy radiation led to a significant downregulation of
Bcl2 genetic expression compared to the cells treated with 100 µM
curcumin alone (P<0.001). However, the reduction in Bcl2
expression was significantly lower in the 2D cultures compared with
the 3D cultures.
Cells treated with curcumin showed significant
upregulation of BAX in 2D cultures either alone or in combination
with either FAC or 2-8 Gy radiation (P<0.001). Regarding the 3D
cultures, the levels of BAX was significantly lower compared with
the 2D cultures (P<0.001); however, treatment with curcumin +
FAC or 8 Gy radiation lead to a significant upregulation in BAX
gene levels.
Discussion
Targeting the Trx/TrxR system inhibition is a
promising approach for cancer treatment. Recently, many natural and
synthetic compounds were developed to be used either alone or as
adjuvants to existing cancer therapies, such as curcumin (8). In the current study, the use of
curcumin as an anticancer/sensitizer agent was evaluated, through
studying its effect on breast cancer (MCF-7) cell viability, TrxR1
activity and Trx/TrxR1 gene expression. MCF-7 2 and 3D cell culture
systems were used, and cells were treated with different
concentrations of curcumin alone or in combination with
conventional treatment regimens, including chemo- and radiotherapy
for different periods.
Curcumin significantly decreased MCF-7 cells
viability in dose- and time-dependent manners in 2 and 3D systems.
However, cell viability in 2D cultures was significantly lower
compared to 3D cultures; most probably due to cell clustering
effect that may prevent curcumin penetration to inner cells in
spheroids. The results of the present study are in agreement with
other studies in which a marked decrease in cell proliferation was
associated with curcumin treatment in different types of cancer,
including bladder (21), prostate
(22), liver (23), and breast cancer (10). Curcumin function has been reported
to be through p53-associated, caspase-dependent and mitochondrial
mechanisms (24). Recently,
Vallianou et al (25)
proposed that curcumin can induce apoptosis through the induction
of severe endoplasmic reticulum stress. In apoptosis-resistant cell
lines, curcumin potentially activates cell death mechanisms, such
as mitotic catastrophe, which is characterized by aberrant mitosis,
multinucleated cells and giant cells (26).
The efficiency of chemotherapeutics is compromised
by several metabolic and epigenetic alterations, and a constant
shift in the tumor microenvironment leading to drug resistance
(27). Although these results
indicated that chemotherapy alone exhibited a substantial effect on
MCF-7 cells in 2D cultures, the 3D culture showed a different
response pattern to chemotherapy as no significant reductions in
cell viability were observed after 24 or 48 h, and cell viability
reached 82% after 72 h. The 2D cultures demonstrate maximum
exposure to drugs and external stimuli in addition to limited
cell-cell interaction, unlike 3D cultures that are multilayered
with a hypoxic core, and show different exposure patterns to
oxygen, nutrients and drugs. Hypoxic conditions in 3D spheroids
resemble those observed in vivo in solid tumors, therefore,
certain cytotoxic agents, such as doxorubicin, 5-FU or cisplatin,
which work with oxygen, are less effective in 3D models (28,29).
In the presence of curcumin with chemotherapy, the
same pattern of effect between 2 and 3D cultures was noted in the
present study. A significant reduction in cell viability and
pro-apoptotic gene expression was observed in 2D cultures compared
with in 3D cultures. In agreement with the results of the current
study, Vinod et al (30)
reported that curcumin works as a chemosensitizer for chemotherapy
by silencing thymidylate synthase enzyme in breast cancer cells.
Furthermore, Serasanambati et al (31), observed that 20 µM of curcumin
enhanced the antitumor effects of chemotherapy on MCF-7 breast
cancer cells by decreasing cell proliferation (31). Additionally, Chen et al
(32) reported that chemotherapy
combined with curcumin showed modestly improved efficacy.
Regardless of the wide application of radiotherapy
for breast cancer treatment, little is known about the response of
3D spheroids to radiation exposure. When exploring the effect of
radiation on MCF-7 cell viability, 2D cultures showed little
radiosensitivity in the present study. On the other hand, 3D
cultures showed a significant reduction in cell viability in a
time-dependent manner. By increasing radiation dose, the viability
of 2D cultures did not show any significant change, whilst the
viability of cells cultured in the 3D system showed a mild
decrease. This is contradictory to other published reports that
stated that cells cultured in a 3D system exhibit higher
radioresistance levels compared with cells cultured in a 2D system
(33,34) These observations may be associated
to the reduced thickness of monolayer cultured cells, which allows
for little energy deposition within the cells. Meanwhile, the
increased thickness of spheroids in 3D cultures may allow for
higher energy deposition and subsequently an increased effect. In
addition, cell-cell interactions may promote the response to
radiotherapy through a bystander effect. El-Ashmawy et al
(35) reported that irradiation of
human lung epithelial 3D cell cultures showed reduced frequency of
progression toward malignant phenotypes compared with 2D monolayers
regardless of the comparable number of colonies (35).
Treatment of 2D monolayers with curcumin in
combination with irradiation resulted in immediate
radiosensitization in a time- and dose-dependent manner, which was
the opposite to that observed on the 3D spheroids in which the
effect was minimal. In line with the current results, the
radiosensitizing effect of curcumin on 2D-radioresistant MCF-7
cells has been reported (36).
Similar results were also reported in other types of cancer,
including prostate cancer, musculoskeletal cancer (37), non-Hodgkin's lymphoma (38) and aggressive lymphomas (39). The discrepancy in culture viability
may be explained by the downregulation of Bcl2 and upregulation of
BAX, which leads to a shift in the Bcl2/BAX ratio towards a
pro-apoptotic signal in curcumin-treated cells. A previous study
indicated that curcumin may counteract the radiation-induced
anti-apoptotic signals, including Bcl2 elevation (40). Further investigations including
Ki67/caspase-3 immunohistochemistry staining or flow cytometry
would have been beneficial to back up these findings.
The effect of curcumin on cell viability was
suspected to occur through Trx/TrxR1 modulation. TrxR1 is an
oxidoreductase enzyme that serves a key role in maintaining
redox-regulated cellular functions, including transcription, DNA
damage recognition and repair, proliferation, and apoptosis
(41). Cytosolic TrxR1 expression
is often upregulated in human cancer, where it is associated with
aggressive tumor growth and poor prognosis (42). The activity and ability of TrxR1 to
overcome oxidative stress induced in tumor cells via different
treatment modalities depends on numerous factors, most importantly
the availability of Trx (43).
The results of the current study demonstrated that
TrxR1 concentrations in 3D cultures were significantly higher
(approximately three times) compared with that in cells cultured in
a 2D system; however, the levels significantly decreased upon
curcumin treatment in a dose-dependent manner. At the
transcriptional level, low curcumin concentration (20 µM) resulted
in a large induction of TrxR1 and Trx genes, in 2 and 3D settings.
Furthermore, high curcumin concentration (100 µM) resulted in a
mild induction of both genes in 2D cultures and significantly
increased expression inhibition of both genes in 3D cultures. These
results support the hypothesis of curcumin exhibiting dual
functions. It has been postulated that curcumin may serve a
protective role against oxidative stress at low concentrations and
the opposite at high concentrations (44).
The different expression patterns of TrxR1 between 2
and 3D cultures have been reported previously in a colon cancer
cell line. Lechner et al (45) reported that >70% of HT-29 cells
grown in a monolayer were positive for TrxR, while cells grown as
spheroids or as tumors in mice, were negative for TrxR1.
Nevertheless, to the best of our knowledge, the discrepancy in the
effects of curcumin on TrxR1 concentration in 2D vs. 3D cultured
cells were reported here for the first time. This observation
reflects that cell-cell interactions, which are present in 3D
cultured and are missing in 2D cultures, may impact the cellular
response to therapeutic agents, such as curcumin.
It has been observed that cells overexpressing TrxR1
exhibit higher resistance to certain anticancer agents (46). The present work highlighted that
cellular susceptibility to FAC is remarkably increased in
curcumin-treated cells and hence enhanced cancer apoptosis. One
possible explanation to rationalize this consequence may be the
reduction of TrxR1 via curcumin, which stimulates increasing ROS
formation and consequently increases the cancer cell death rate
(47). Similar results have also
been reported for 5-FU, whereby curcumin was found to increase the
sensitization of HCT116R cells to 5-FU-based chemotherapy (48). Although it is not clear whether the
mechanism of sensitization involves TrxR1 or not (48). In agreement with the results of the
current study, curcumin has been demonstrated to induce
time-dependent ROS accumulation through suppression of TrxR1
activity and increase the level of the oxidized Trx form in cancer
cells (49).
Upon exposure to radiotherapy, TrxR1 levels were
elevated in 2 and 3D cultures following exposure to low doses of
radiation; however, at higher radiation doses its levels were
diminished in this study. The initial elevation in TrxR1
concentration is probably associated with high ROS generation by
radiation in cell cytoplasm, which elicits an induced elevation of
TrxR1 as an antioxidant defense mechanism. However, the subsequent
reduction in TrxR1 concentration with increased radiation dose may
be associated with increased apoptosis and/or impaired cellular
responses resulting from radiation damage (14).
The present study possesses certain limitations.
These limitations include the lack of western blotting technique to
confirm the effect of curcumin on the translational level of TrxR1
and the lack of use of specific TrxR1 inhibitor for comparative
studies. Morphological investigations should be analyzed to fully
characterize the mechanisms underlying the potential involvement of
the Trx/TrxR1 system on the effects of curcumin on 2 and 3D MCF-7
cells.
In conclusion, the present findings showed that the
cell culture setting has a significant effect on the cellular
behavior and expression patterns, where the 3D model was shown to
be a promising tool to represent tumors. The modulation of Trx/TrxR
system expression suggests that it may be associated with increased
cell death upon treatment with curcumin. These results also suggest
that curcumin may have a potential role as a radiosensitizing
and/or a chemosensitizing agent, providing a novel way to overcome
cancer drug resistance.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SEEF: Sharing in study design and methodology,
primary and additional laboratory analyses. MAGM: Sharing in
conceptualization and manuscript revision and can authenticate the
raw data. NAAEM: Determination of standard treatments' regimens,
dose calculations and manuscript revision. ERZ: sharing in
conceptualization, data analysis and interpretation and can
authenticate the raw data. SAK: Co-participation in cell culture
procedures, enzymatic protein quantification and molecular biology
investigations. LMAA: Study design and methodology, primary and
additional laboratory investigations. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hornsveld M and Dansen TB: The hallmarks
of cancer from a redox perspective. Antioxid Redox Signal.
25:300–325. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lu J and Holmgren A: Thioredoxin system in
cell death progression. Antioxid Redox Signal. 17:1738–1747.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang J, Li X, Han X, Liu R and Fang J:
Targeting the thioredoxin system for cancer therapy. Trends
Pharmacol Sci. 38:794–808. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Haas B, Schütte L, Wos-Maganga M,
Weickhardt S, Timmer M and Eckstein N: Thioredoxin confers
intrinsic resistance to cytostatic drugs in human glioma cells. Int
J Mol Sci. 19(2874)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Selenius M, Hedman M, Brodin D, Gandin V,
Rigobello MP, Flygare J, Marzano C, Bindoli A, Brodin O, Björnstedt
M and Fernandes AP: Effects of redox modulation by inhibition of
thioredoxin reductase on radiosensitivity and gene expression. J
Cell Mol Med. 16:1593–1605. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kim SJ, Miyoshi Y, Taguchi T, Tamaki Y,
Nakamura H, Yodoi J, Kato K and Noguchi S: High thioredoxin
expression is associated with resistance to docetaxel in primary
breast cancer. Clin Cancer Res. 11:8425–8430. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fang J, Lu J and Holmgren A: Thioredoxin
reductase is irreversibly modified by curcumin: A novel molecular
mechanism for its anticancer activity. J Biol Chem.
280:25284–25290. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Panda AK, Chakraborty D, Sarkar I, Khan T
and Sa G: New insights into therapeutic activity and anticancer
properties of curcumin. J Exp Pharmacol. 9:31–45. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu J, Pan Y, Chen O, Luan Y, Xue X, Zhao
J, Liu L and Jia HY: Curcumin inhibits MCF-7 cells by modulating
the NF-κB signaling pathway. Oncol Lett. 14:5581–5584.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hewlings SJ and Kalman DS: Curcumin: A
review of its' effects on human health. Foods. 6(92)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zou J, Zhu L, Jiang X, Wang Y, Wang Y,
Wang X and Chen B: Curcumin increases breast cancer cell
sensitivity to cisplatin by decreasing FEN1 expression. Oncotarget.
9:11268–11278. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Javvadi P, Hertan L, Kosoff R, Datta T,
Kolev J, Mick R, Tuttle SW and Koumenis C: Thioredoxin reductase-1
mediates curcumin-induced radiosensitization of squamous carcinoma
cells. Cancer Res. 70:1941–1950. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Duval K, Grover H, Han LH, Mou Y, Pegoraro
AF, Fredberg J and Chen Z: Modeling physiological events in 2D vs.
3D cell culture. Physiology (Bethesda). 32:266–277. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kapałczyńska M, Kolenda T, Przybyła W,
Zajączkowska M, Teresiak A, Filas V, Ibbs M, Bliźniak R, Łuczewski
Ł and Lamperska K: 2D and 3D cell cultures-a comparison of
different types of cancer cell cultures. Arch Med Sci. 4:910–919.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Knight E and Przyborski S: Advances in 3D
cell culture technologies enabling tissue-like structures to be
created in vitro. J Anat. 227:746–756. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vantangoli MM, Madnick SJ, Huse SM, Weston
P and Boekelheide K: MCF-7 human breast cancer cells form
differentiated microtissues in scaffold-free hydrogels. PLoS One.
10(e0135426)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Imamura Y, Mukohara T, Shimono Y,
Funakoshi Y, Chayahara N, Toyoda M, Kiyota N, Takao S, Kono S,
Nakatsura T and Minami H: Comparison of 2D- and 3D-culture models
as drug-testing platforms in breast cancer. Oncol Rep.
33:1837–1843. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shi J, Zhang X, Shi T and Li H: Antitumor
effects of curcumin in human bladder cancer in vitro. Oncol
Lett. 14:1157–1161. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Teiten MH, Gaascht F, Cronauer M, Henry E,
Dicato M and Diederich M: Anti-proliferative potential of curcumin
in androgen-dependent prostate cancer cells occurs through
modulation of the wingless signaling pathway. Int J Oncol.
38:603–611. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang J, Wang C and Bu G: Curcumin inhibits
the growth of liver cancer stem cells through the
phosphatidylinositol 3-kinase/protein kinase B/mammalian target of
rapamycin signaling pathway. Exp Ther Med. 15:3650–3658.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Perrone D, Ardito F, Giannatempo G,
Dioguardi M, Troiano G, Lo Russo L, DE Lillo A, Laino L and Lo
Muzio L: Biological and therapeutic activities, and anticancer
properties of curcumin. Exp Ther Med. 10:1615–1623. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vallianou NG, Evangelopoulos A, Schizas N
and Kazazis C: Potential anticancer properties and mechanisms of
action of curcumin. Anticancer Res. 35:645–651. 2015.PubMed/NCBI
|
|
26
|
Salvioli S, Sikora E, Cooper EL and
Franceschi C: Curcumin in cell death processes: A challenge for CAM
of age-related pathologies. Evid Based Complement Alternat Med.
4:181–190. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Das T, Sa G, Saha B and Das K: Multifocal
signal modulation therapy of cancer: Ancient weapon, modern
targets. Mol Cell Biochem. 336:85–95. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hoarau-Véchot J, Rafii A, Touboul C and
Pasquier J: Halfway between 2D and animal models: Are 3D cultures
the ideal tool to study cancer-microenvironment interactions? Int J
Mol Sci. 19(181)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lv D, Hu Z, Lu L, Lu H and Xu X: Three
dimensional cell culture: A powerful tool in tumor research and
drug discovery. Oncol Lett. 14:6999–7010. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Vinod BS, Antony J, Nair HH,
Puliyappadamba VT, Saikia M, Narayanan SS, Bevin A and Anto RJ:
Mechanistic evaluation of the signaling events regulating
curcumin-mediated chemosensitization of breast cancer cells to
5-fluorouracil. Cell Death Dis. 4(e505)2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Serasanambati M, Chilakapati SR, Manikonda
PK, Kanala JR and Chilakapati DR: Anticancer effects of brucine and
gemcitabine combination in MCF-7 human breast cancer cells. Nat
Prod Res. 29:484–490. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen S, Liang Q, Xie S, Liu E, Yu Z, Sun
L, Shin MC, Lee SJ, He H and Yang VC: Curcumin based combination
therapy for anti-breast cancer: From in vitro drug screening to in
vivo efficacy evaluation. Front Chem Sci Eng. 10:383–388. 2016.
|
|
33
|
Hehlgans S, Eke I, Storch K, Haase M,
Baretton GB and Cordes N: Caveolin-1 mediated radioresistance of 3D
grown pancreatic cancer cells. Radiother Oncol. 92:362–370.
2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Storch K, Eke I, Borgmann K, Krause M,
Richter C, Becker K, Schrock E and Cordes N: Three-dimensional cell
growth confers radioresistance by chromatin density modification.
Cancer Res. 70:3925–3934. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
El-Ashmawy M, Coquelin M, Luitel K, Batten
K and Shay JW: Organotypic culture in three dimensions prevents
radiation-induced transformation in human lung epithelial cells.
Sci Rep. 6(31669)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Girdhani S, Ahmed MM and Mishra KP:
Enhancement of gamma radiation-induced cytotoxicity of breast
cancer cells by curcumin. Mol Cell Pharmacol. 1:208–217. 2009.
|
|
37
|
Peddada KV, Peddada KV, Shukla SK, Mishra
A and Verma V: Role of curcumin in common musculoskeletal
disorders: A review of current laboratory, translational, and
clinical data. Orthop Surg. 7:222–231. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Qiao Q, Jiang Y and Li G: Curcumin
enhances the response of non-Hodgkin's lymphoma cells to ionizing
radiation through further induction of cell cycle arrest at the
G2/M phase and inhibition of mTOR phosphorylation. Oncol Rep.
29:380–386. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Qiao Q, Jiang Y and Li G: Inhibition of
the PI3K/AKT-NF-κB pathway with curcumin enhanced radiation-induced
apoptosis in human Burkitt's lymphoma. J Pharmacol Sci.
121:247–256. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lia G, Wanga Z, Chonga T, Yangb J, Lia H
and Chena H: Curcumin enhances the radiosensitivity of renal cancer
cells by suppressing NF-κB signaling pathway. Biomed Pharmacother.
94:974–981. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Koháryová M and Kollárová M: Thioredoxin
system-a novel therapeutic target. Gen Physiol Biophys. 34:221–233.
2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lu J and Holmgren A: The thioredoxin
antioxidant system. Free Radic Biol Med. 66:75–87. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Stafford WC, Peng X, Olofsson MH, Zhang X,
Luci DK, Lu L, Cheng Q, Trésaugues L, Dexheimer TS, Coussens NP, et
al: Irreversible inhibition of cytosolic thioredoxin reductase 1 as
a mechanistic basis for anticancer therapy. Sci Transl Med.
10(eaaf7444)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Daverey A and Agrawal SK: Curcumin
alleviates oxidative stress and mitochondrial dysfunction in
astrocytes. Neuroscience. 333:92–103. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lechner S, Müller-Ladner U, Neumann E,
Spöttl T, Schlottmann K, Rüschoff J, Schölmerich J and Kullmann F:
Thioredoxin reductase 1 expression in colon cancer: Discrepancy
between in vitro and in vivo findings. Lab Invest. 83:1321–1331.
2003.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Koedrith P and Seo YR: Induction of
doxorubicin-mediated apoptosis via thioredoxin reductase 1RNAi in
human colon cancer cells. Mol Cell Toxicol. 7:112–119. 2011.
|
|
47
|
Eriksson SE, Prast-Nielsen S, Flaberg E,
Szekely L and Arnér ES: High levels of thioredoxin reductase 1
modulate drug-specific cytotoxic efficacy. Free Radic Bio Med.
47:1661–1671. 2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Shakibaei M, Kraehe P, Popper B, Shayan P,
Goel A and Buhrmann C: Curcumin potentiates antitumor activity of
5-fluorouracil in a 3D alginate tumor microenvironment of
colorectal cancer. BMC Cancer. 15(205)2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Shao FY, Du ZY, Ma DL, Chen WB, Fu WY,
Ruan BB, Rui W, Zhang JX, Wang S, Wong NS, et al: B5, a thioredoxin
reductase inhibitor, induces apoptosis in human cervical cancer
cells by suppressing the thioredoxin system, disrupting
mitochondrion-dependent pathways and triggering autophagy.
Oncotarget. 6:30939–30956. 2015.PubMed/NCBI View Article : Google Scholar
|