Introduction
Stroke is one of the most common types of
cerebrovascular disease and has become a major neurological concern
worldwide due to its high morbidity, disability and mortality rates
(1). Ischemic stroke is the main
subtype of stroke, accounting for ~70% of the cases (2). Although reperfusion after cerebral
ischemia can rescue dying cells, it may also aggravate ischemic
cell injury. Therefore, the prevention and treatment of
ischemia-reperfusion (I/R) injury (IRI) may represent an important
strategy for the treatment of ischemic cerebrovascular disease.
Currently, thrombolysis is the only approved treatment for ischemic
stroke; however, the clinical effect remains unsatisfactory, as the
reperfusion of ischemic vessels after thrombolysis promotes
secondary injury. Previous studies that have attempted to target
the mechanisms underlying ischemic stroke have mainly focused on
the excitotoxicity of neurons, oxidative stress and inflammatory
injury (3-6).
Among these pathways, the activation of the immune-mediated
inflammatory response following a stroke has been widely
investigated, with promising results to date (7,8).
Therefore, the present study aimed to determine the
anti-inflammatory and antiapoptotic molecular targets of ischemic
stroke, which may provide further treatment options.
Long non-coding RNAs (lncRNAs) are a class of
non-coding RNAs of >200 nucleotides in length. lncRNAs can
regulate the expression of protein-coding genes at the
pre-transcriptional, mid-transcriptional and post-transcriptional
levels. lncRNAs have been a focus of research in recent years, and
numerous previous studies have reported that lncRNAs may be
implicated in the pathological process of IRI in several vital
organs, such as the heart, brain, liver, kidney and vascular
endothelial tissue (9). For
example, Li et al (10)
demonstrated that the expression levels of the lncRNA
metastasis-associated lung adenocarcinoma transcript 1 were
significantly upregulated in cerebral microvascular endothelial
cells of I/R model mice, which promoted autophagy and cell survival
by downregulating the expression levels of microRNA
(miRNA/miR)-26b. In addition, the knockdown of lncRNA AK139328
reduced myocardial IRI through inhibiting autophagy by targeting
miR-204-3p expression (11).
Testis-specific transcript Y-linked 15 (TTTY15), which is 5,263
base pairs in length, is located on chromosome Yq 11.21 of the
male-specific region of the Y chromosome. TTTY15 has only been
discovered as a new lncRNA in recent years. Although few studies
have reported the role of the TTTY15 gene family in different
diseases, the results to date appear to be promising. For example,
with regards to cancer diagnosis, the fusion of TTTY15 with
ubiquitin-specific peptidase 9 Y-linked was found to be able to
predict the biopsy results of lung cancer, hepatocellular carcinoma
and prostate cancer (12,13). In addition, a previous study
reported that the overexpression of TTTY15 significantly inhibited
the proliferation and metastasis of non-small cell lung cancer
cells (14). Huang et al
(15) also reported that TTTY15
could attenuate hypoxia-induced cardiomyocyte injury by targeting
miR-455-5p. Therefore, based on these aforementioned findings, it
was hypothesized that TTTY15 may exert a protective effect against
neuronal injury induced by I/R. miR-766-5p has been demonstrated to
be overexpressed in colorectal cancer and to promote the cancer
process of colorectal cancer. However, the role of miR-766-5p in
IRI has not been reported to date.
The present study employed an in vitro model
of neuronal IRI to determine the role and specific regulatory
mechanism of TTTY15 in I/R-induced neuronal injury.
Materials and methods
Cell culture and establishment of the
oxygen-glucose deprivation/reperfusion (OGD/R) model
PC12 neurons (rat adrenal pheochromocytoma cells)
were obtained from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences. The cells were cultured in DMEM
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and maintained at 37˚C in a humidified atmosphere with 5%
CO2. The proliferation of the cells was observed
regularly, and cells were passaged when required. Cells in the
logarithmic phase were collected for use in subsequent
experiments.
Treatment of PC12 cells with OGD/R was performed to
simulate cerebral IRI in vitro. The detailed treatment
protocol is described in a previously published study (16). Briefly, PC12 cells were cultured in
glucose-free DMEM without FBS at 37˚C in an incubator containing
95% N2 and 5% CO2 for 3 h. Subsequently, the
medium was replaced with glucose-containing DMEM supplemented with
10% FBS and reoxygenated for 24 h under normoxic conditions.
Following successful establishment of the cell model, the cells
were used for subsequent experiments.
Cell transfection
Short hairpin RNA (shRNA/sh) targeting TTTY15
(shRNA-TTTY15-1 and shRNA-TTTY15-2) and the corresponding negative
control (NC; shRNA-NC) were constructed by Shanghai GenePharma Co.,
Ltd. The TTTY15 overexpression plasmid (pcDNA3.1-TTTY15) and empty
vector (pcDNA-NC) were produced by OBiO Technology (Shanghai)
Corp., Ltd. miR-766-5p mimics and miR-NC were purchased from
Guangzhou RiboBio Co., Ltd. The aforementioned plasmids were
transfected into PC12 cells at a final concentration of 20 nM using
Lipofectamine® 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The transfection efficiency was verified using reverse
transcription-quantitative PCR (RT-qPCR) at 48 h
post-transfection.
RT-qPCR analysis
Briefly, total RNA was extracted from transfected
cells using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Total RNA was quantified using a NanoDrop
spectrophotometer (Thermo Fisher Scientific, Inc.) and
reverse-transcribed into cDNA using a SuperScript™ IV First-Strand
Synthesis system (Thermo Fisher Scientific, Inc.). The procedure
was performed according to the manufacturer's protocol. qPCR was
subsequently performed using a TaqMan assay (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
following thermocycling conditions were used for the qPCR: 35
cycles at 95˚C for 1 min, 55˚C for 30 sec and 72˚C for 30 sec. The
following primer sequences were used for the qPCR: TTTY15 forward,
CACCCAACCAGTCATCTGAGTA and reverse, 5'-GGTTGCAGTGGGCTATGACT-3';
miR-766-5p forward, 5'-TCGAGTACTTGAGATGGAGTTTT-3' and reverse,
5'-GGCCGCGTTGCAGTGAGCCGAG-3'; GAPDH forward,
5'-CTGGGCTACACTGAGCACC-3' and reverse, 5'-AAGTGGTCGTTGAGGGCAATG-3';
and U6 forward, 5'-CTCGCTTCGGCAGCACA-3' and reverse,
5'-AACGCTTCACGAATTTGCGT-3'. The expression levels were quantified
using the 2-ΔΔCq method (17) and normalized to either GAPDH or
U6.
Analysis of the secretion of
inflammatory factors
The secretion of TNF-α (cat. no. ml002859-1), IL-1β
(cat. no. ml058228-1), IL-10 (cat. no. ml037888-1) and IL-18 (cat.
no. ml063131-1) into the cell culture supernatant was analyzed
using ELISA kits (Shanghai Enzyme-linked Biotechnology Co., Ltd.),
according to the manufacturers' protocols. Briefly, the treated
cells were harvested by centrifugation at 4˚C, 12,000 x g for 10
min. Then, the supernatant was collected and plated into ELISA
microplates to measure the absorbance of each well at a wavelength
of 450 nm using an automatic microplate reader (Syngene). Standard
curves were drawn, and the concentrations of the inflammatory
factors were expressed as pg/ml.
Flow cytometric analysis of
apoptosis
The levels of cell apoptosis were analyzed using an
Annexin V-FITC/propidium iodide (PI) apoptosis detection kit (BD
Biosciences), according to the manufacturer's protocol. Briefly,
the transfected cells were at a density of 1x105
cells/ml and incubated with 5 µl Annexin V-FITC and 5 µl PI in the
dark for 15 min. Following incubation, 20 µl Annexin V binding
solution was added to the cell suspension and incubated for 1 h.
Apoptotic cells were subsequently analyzed using a FACSCalibur flow
cytometer (BD Biosciences) and CellQuest 3.0.1 software (BD
Biosciences).
Dual luciferase reporter assay
StarBase v2.0 (http://starbase.sysu.edu.cn/index.php) was used to
predict whether miR-766-5p may be a direct target of TTTY15.
Subsequently, a dual luciferase reporter assay was performed to
validate the relationship between TTTY15 and miR-766-5p. The
wild-type (WT) 3'-untranslated region (UTR) of TTTY15 containing
the potential binding site for miR-766-5p was amplified and cloned
into a pGL3-Basic vector (Promega Corporation). A mutant (Mut)
3'-UTR sequence was also generated and cloned into the pGL3-Basic
vector. Then, PC12 cells were co-transfected via
Lipofectamine® 3000 (cat. no. L3000001; Thermo Fisher
Scientific, Inc.) with miR-NC or miR-766-5p mimics and TTTY15-WT or
TTTY15-Mut according to the manufacturer's protocol. The relative
luciferase activity was measured using a Dual Luciferase Reporter
assay kit (Promega Corporation).
Western blotting
The expression levels of the apoptotic proteins
Bcl-2, Bax, cleaved caspase-3, caspase-3, cleaved caspase-9 and
caspase-9 were analyzed using western blotting. Total protein was
extracted from cells using RIPA lysis buffer (Beyotime Institute of
Biotechnology). Total protein was quantified and 35 µg protein per
lane was separated via 12% SDS-PAGE. The separated proteins were
subsequently transferred onto PVDF membranes (Thermo Fisher
Scientific, Inc.) and blocked at room temperature with blocking
solution (Thermo Fisher Scientific, Inc.) for 1 h. The membranes
were then incubated with the following primary antibodies at 4˚C
overnight: Anti-Bcl-2 (1:1,000; cat. no. 15071; Cell Signaling
Technology, Inc.), anti-Bax (1:1,000; cat. no. 5023; Cell Signaling
Technology, Inc.), anti-cleaved caspase-3 (1:1,000; cat. no. 9661;
Cell Signaling Technology, Inc.), anti-caspase-3 (1:1,000; cat. no.
9662; Cell Signaling Technology, Inc.), anti-cleaved caspase-9
(1:1,000; cat. no. 20750; Cell Signaling Technology, Inc.) and
anti-caspase-9 (1:1,000; cat. no. 9502; Cell Signaling Technology,
Inc.). Following primary antibody incubation, the membranes were
incubated with HRP-conjugated secondary antibody (1:1,000; cat. no.
K4003; Dako; Agilent Technologies, Inc.) at room temperature for 2
h. Protein bands were visualized using an ECL reagent (cat. no.
6883; Cell Signaling Technology, Inc.) and densitometric analysis
was performed using ImageJ software (version 1.4.3; National
Institutes of Health). GAPDH was used as the internal reference
protein.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism, version 6.0 (GraphPad Software, Inc.). Data are presented as
the mean ± SD of three independent experiments. Unpaired Student's
t-tests or a one-way ANOVA followed by Tukey's post hoc test were
performed to determine the statistical significance of the
differences between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
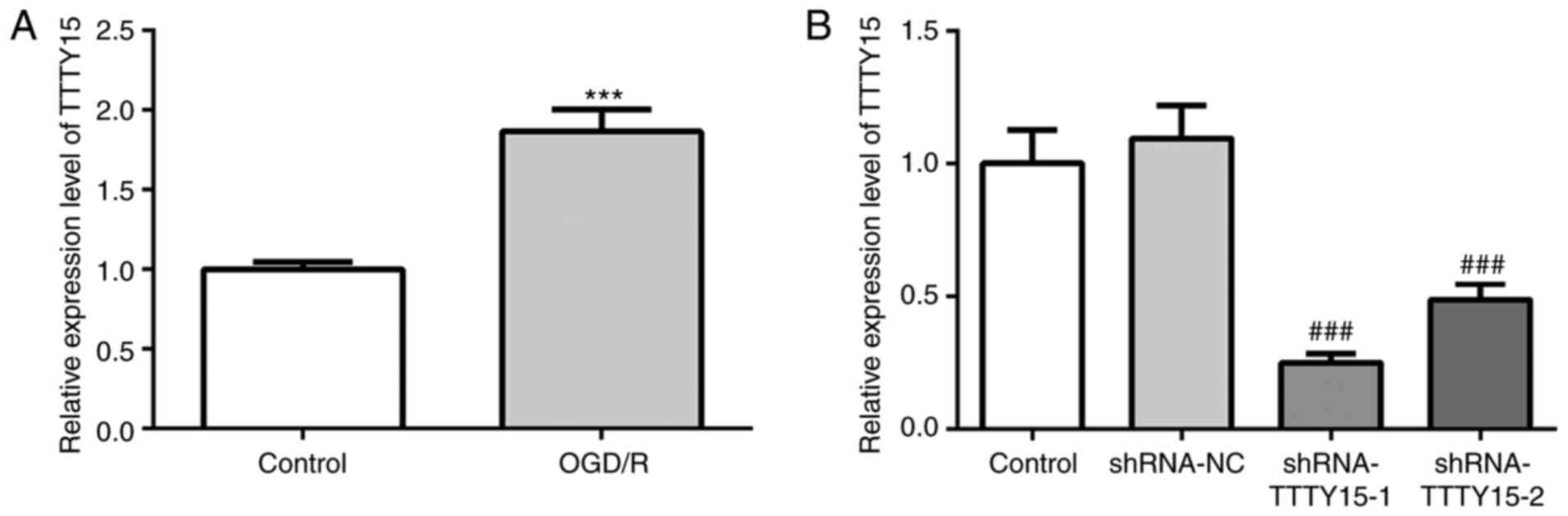
lncRNA TTTY15 expression is
upregulated in PC12 cells following OGD/R
First, the expression levels of TTTY15 in PC12 cells
were analyzed before and after OGD/R treatment. As shown in
Fig. 1A, TTTY15 expression was
upregulated in OGD/R-treated PC12 cells compared with the control
group, indicating that TTTY15 may be involved in OGD/R-induced
neuronal cell injury. Subsequently, cells were transfected with
shRNA-TTTY15 plasmids to knock down the expression of TTTY15. As
shown in Fig. 1B, the expression
levels of TTTY15 in the shRNA-TTTY15-1 group were downregulated to
the greatest extent. Thus, shRNA-TTTY15-1 was selected for use in
further experiments.
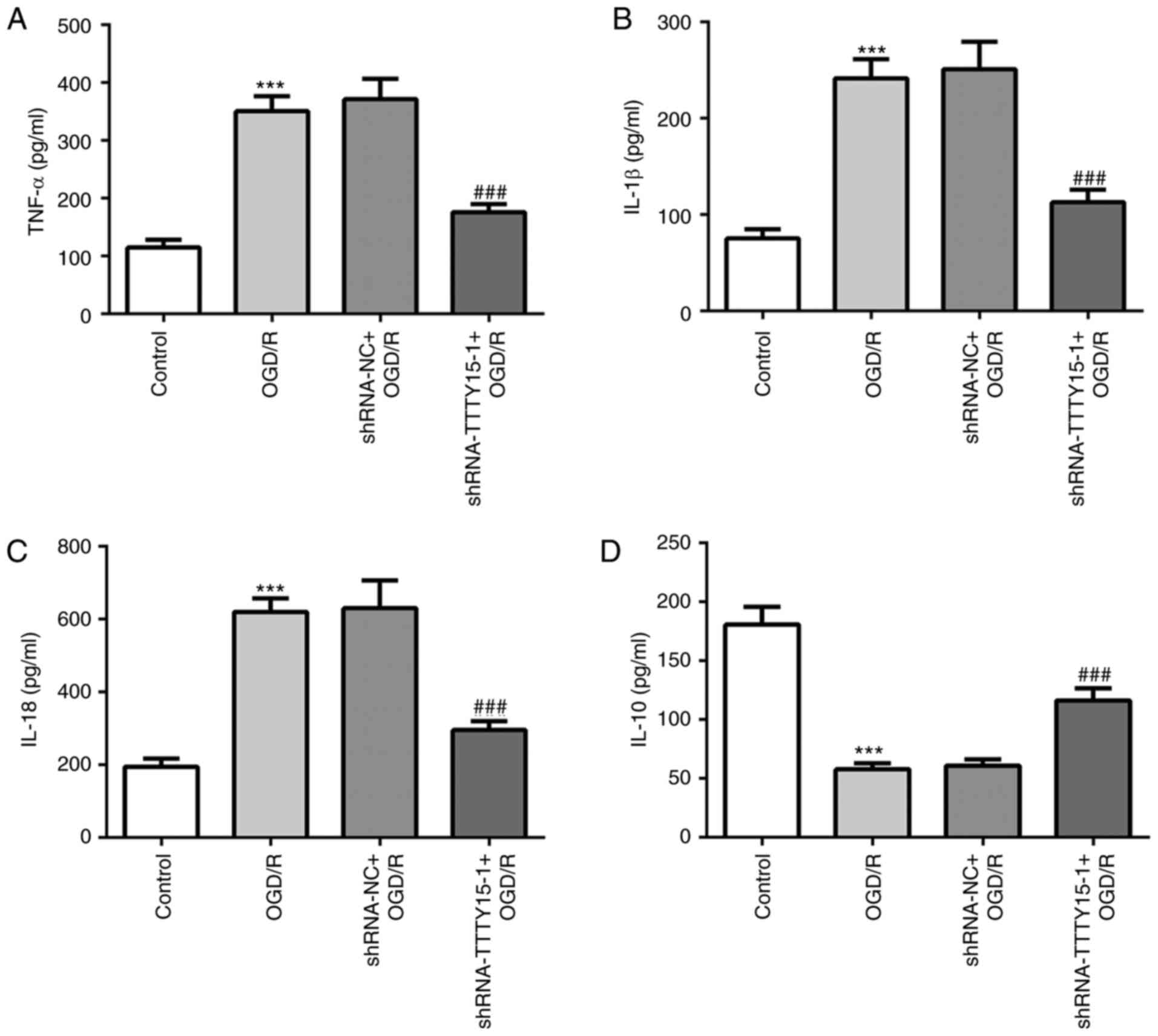
Knockdown of TTTY15 relieves the
inflammatory response in OGD/R-induced PC12 cell injury
The secretory levels of inflammatory cytokines in
PC12 cells following OGD/R were determined using ELISA. The results
revealed that the levels of inflammation in PC12 cells treated with
OGD/R were significantly increased compared with the control group.
The secretory levels of the proinflammatory factors TNF-α, IL-1β
and IL-18 were decreased in PC12 cells following OGD/R compared
with the shRNA-NC + OGD/R group (Fig.
2A-C). Conversely, the secretory levels of the
anti-inflammatory factor IL-10 were increased in PC12 cells
following OGD/R compared with the shRNA-NC + OGD/R group (Fig. 2D). These results suggested that
TTTY15 knockdown may reduce the inflammatory response in
OGD/R-induced PC12 cell injury.
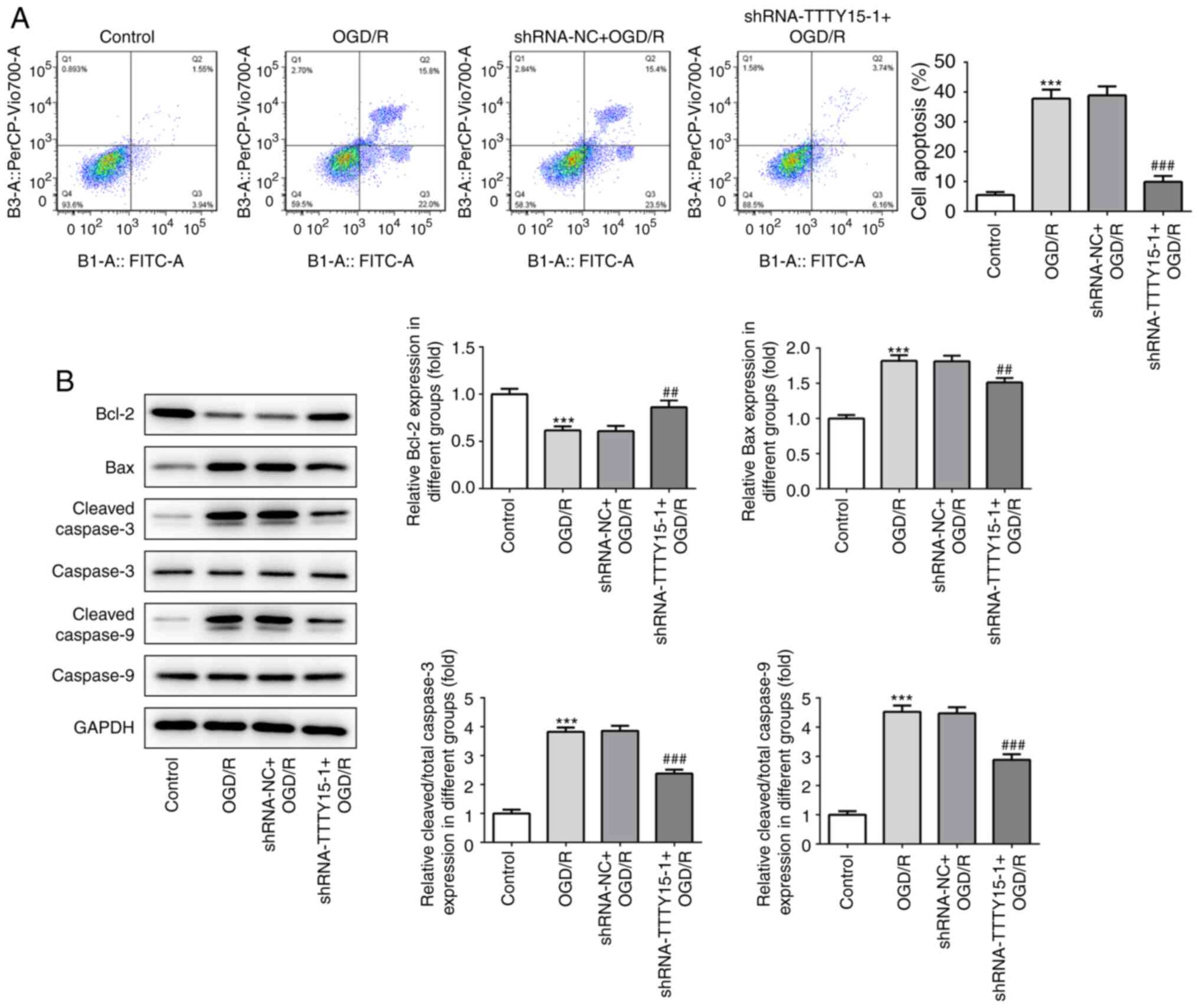
Knockdown of TTTY15 inhibits the
apoptosis of PC12 cells following OGD/R
Subsequently, the effects of TTTY15 on the apoptosis
of PC12 cells following OGD/R were analyzed using flow cytometry
and western blotting. The results from the flow cytometric analysis
revealed that TTTY15 silencing significantly decreased the
apoptotic rate in PC12 cells following OGD/R (Fig. 3A). The western blotting results
demonstrated that the expression levels of the proapoptotic
proteins Bax, caspase-3 and caspase-9 were downregulated, while the
expression levels of the anti-apoptotic protein Bcl-2 were
upregulated following TTTY15 gene silencing (Fig. 3B). Taken together, these findings
suggested that the knockdown of TTTY15 in PC12 cells may exert a
neuroprotective effect in PC12 cells following OGD/R.
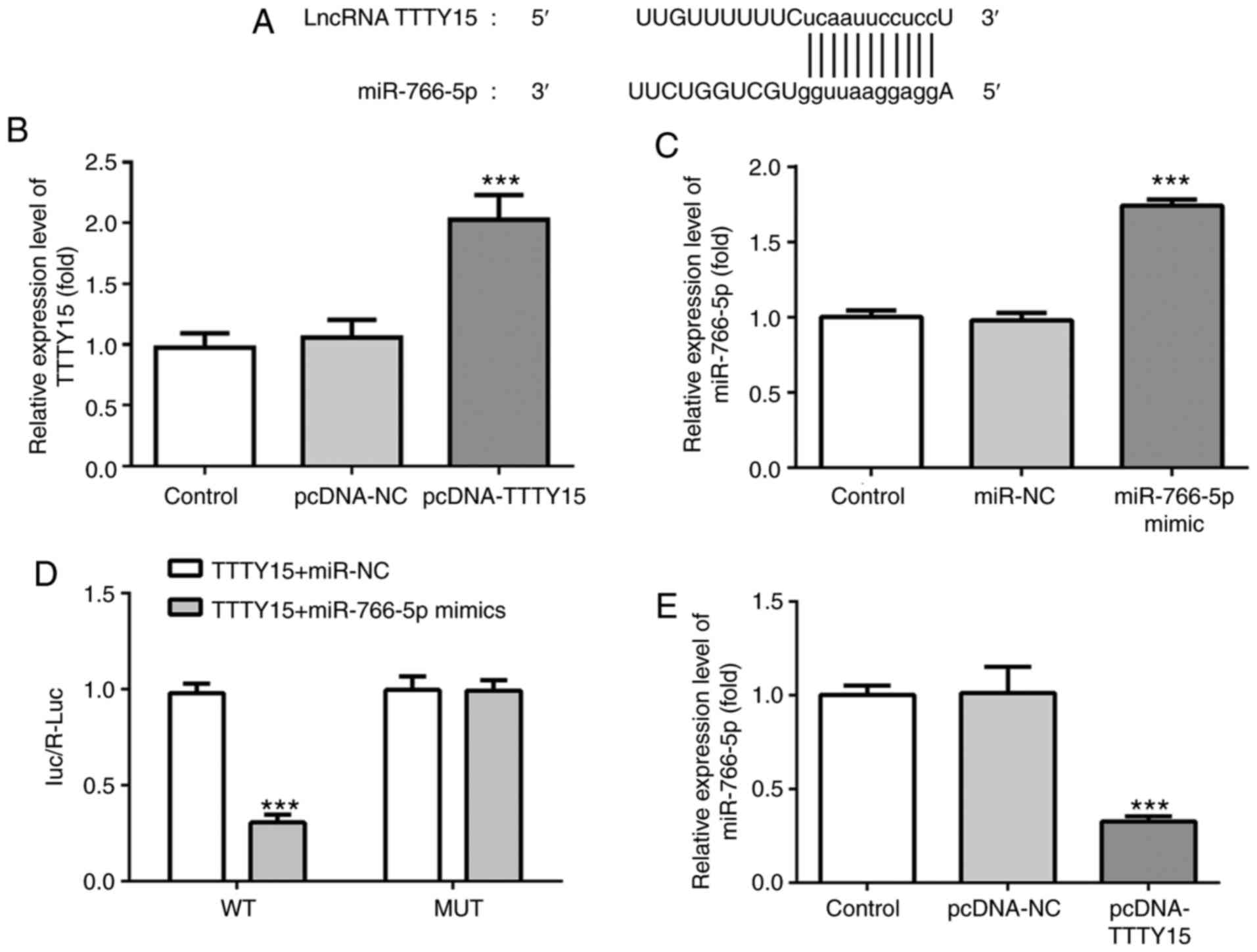
TTTY15 negatively regulates miR-766-5p
expression
To investigate the mechanism of action of TTTY15 in
OGD/R-induced injury, the potential target of TTTY15 was predicted
using StarBase. Through screening, miR-766-5p was predicted to be
the direct target of TTTY15 (Fig.
4A). Subsequently, a TTTY15 overexpression plasmid
(pcDNA-TTTY15) and miR-766-5p mimic were constructed and
successfully transfected into PC12 cells (Fig. 4B and C). The results of the dual luciferase
reporter assay revealed that the relative luciferase activity of
PC12 cells co-transfected with the TTTY15-WT 3'-UTR and miR-766-5p
mimics was significantly decreased compared with the cells
co-transfected with the TTTY15-WT 3'-UTR and miR-NC. Notably, the
relative luciferase activity of the PC12 cells transfected with
TTTY15-Mut reporter was not altered (Fig. 4D). In addition, the overexpression
of TTTY15 significantly downregulated the expression levels of
miR-766-5p in PC12 cells treated with OGD/R (Fig. 5E). Taken together, these data
suggested that TTTY15 may negatively regulate miR-766-5p.
TTTY15 promotes OGD/R-induced
inflammation and apoptosis by downregulating miR-766-5p
expression
To further determine the effects of TTTY15 on
inflammation and PC12 cell apoptosis following OGD/R injury, the
TTTY15 overexpression plasmid and miR-766-5p mimic were
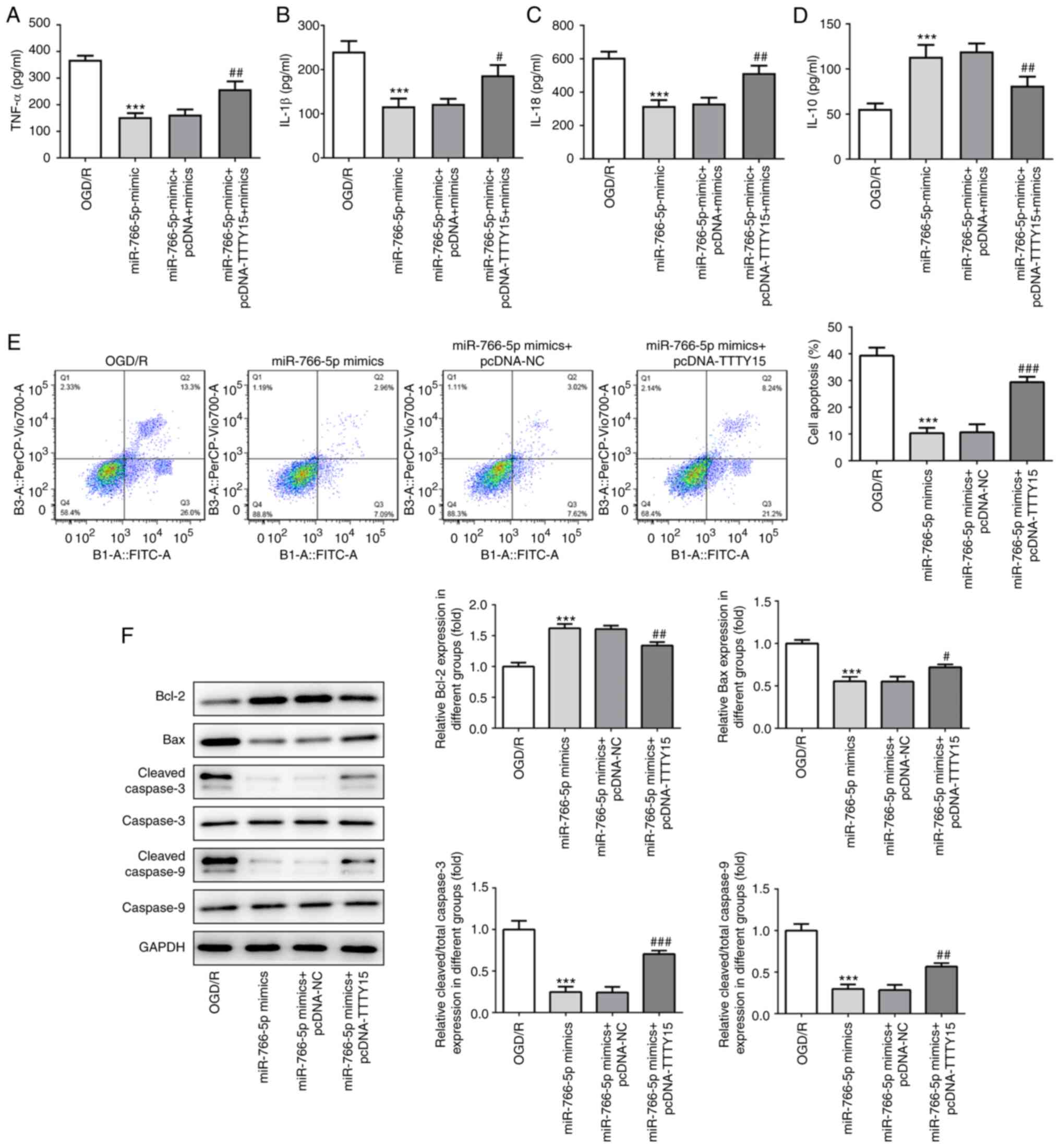
co-transfected into PC12 cells following OGD/R. The results of the
ELISA demonstrated that miR-766-5p overexpression inhibited
OGD/R-induced PC12 cell injury by decreasing the levels of the
proinflammatory factors TNF-α, IL-1β and IL-18, and increasing the
levels of the anti-inflammatory factor IL-10. Subsequent
transfection with the TTTY15 overexpression plasmid attenuated the
miR-766-5p overexpression-induced changes (Fig. 5A-D). In addition, miR-766-5p
overexpression significantly reduced OGD/R-induced apoptosis, which
could be alleviated through the overexpression of TTTY15 (Fig. 5E). The results of the western
blotting experiments revealed that the overexpression of miR-766-5p
significantly upregulated the expression of the anti-apoptotic
protein Bcl-2 and downregulated the expression of the proapoptotic
proteins Bax, caspase-3 and caspase-9. Similar to the previous
results, the overexpression of TTTY15 attenuated the miR-766-5p
overexpression-induced changes in the expression levels of the
aforementioned proteins (Fig. 5F).
Taken together, these results suggested that TTTY15 may promote
OGD/R-induced inflammation and apoptosis by downregulating
miR-766-5p expression.
Discussion
I/R is a common pathological process that is
involved in numerous pathological conditions, such as myocardial
infarction, acute ischemic stroke, acute renal injury, trauma and
circulatory failure, among which ischemic and hypoxic
cerebrovascular diseases, including cerebral stroke, account for
the highest morbidity and mortality rates worldwide (18). Blood reperfusion is the preferred
treatment option for these conditions; however, the accompanying
IRI poses a major concern to clinicians and researchers. Therefore,
reducing IRI in the clinical setting is a difficult problem and
should be urgently addressed. An increasing number of studies have
reported that the inflammatory response plays an important role in
the process of cerebral IRI (19,20).
Numerous different proinflammatory cytokines are produced and
released during the process of inflammation, including TNF-α, IL-1β
and IL-18. These proinflammatory cytokines have been discovered to
activate neurons and vascular endothelial cells, lead to the death
of neurons and glial cells, and aggravate the infiltration of
immune cells during cerebral ischemia, thus promoting the
inflammatory response (21,22). In the present study, the expression
levels of TTTY15 were found to be upregulated in PC12 cells
following OGD/R, whereas TTTY15 gene knockdown significantly
inhibited the inflammatory response and OGD/R-induced apoptosis of
PC12 cells by upregulating miR-766-5p expression.
lncRNAs are a class of non-coding RNA of >200
nucleotides in length that lack an open reading frame (23). In recent years, accumulating studies
have reported that lncRNAs serve as key regulators in epigenetics
and various types of disease, including cancer, nervous system
diseases, cardiovascular diseases and mental disorders (24,25).
Ischemic stroke is mediated by a variety of mechanisms, including
oxidative stress, inflammation, vascular dysfunction, apoptosis and
autophagy (26). An increasing
number of studies have revealed that lncRNAs play an important role
in the pathophysiological mechanisms implicated in ischemic stroke.
For example, the analysis of lncRNAs in the cerebral cortex of rats
revealed that the expression levels of chromatin-modifying
protein-related lncRNAs after a stroke may regulate the epigenetics
following ischemia (27). In
addition, Deng et al (28)
found that the expression levels of a large number of lncRNAs were
significantly upregulated in patients with ischemic stroke compared
with healthy individuals (29).
These results revealed the potential of targeting lncRNAs in
ischemic injury.
TTTY15 is a newly discovered lncRNA and, to the best
of our knowledge, few studies to date have investigated the effect
of TTTY15 in IRI. In addition to its reported important role in
cancer diagnosis, a previous study demonstrated that the silencing
of TTTY15 could protect cardiomyocytes against IRI by targeting
miR-455-5p (15). In addition,
TTTY15 was discovered to protect vascular endothelial cells from
IRI by targeting miR-186-5p (30).
These results confirmed the protective role of TTTY15 in IRI.
However, to the best of our knowledge, the role of TTTY15 in nerve
cell injury induced by OGD/R remains unknown. Therefore, to
understand the effect of TTTY15 on I/R-induced nerve cells, the
present study aimed to determine the biological function and
underlying mechanisms of TTTY15 in OGD/R-induced PC12 cell injury.
The results revealed that TTTY15 expression levels were upregulated
in PC12 cells following OGD/R, and TTTY15 silencing significantly
inhibited the inflammatory reaction and apoptosis of PC12 cells
treated with OGD/R. These results suggested the potential
protective role of TTTY15 against OGD/R-induced neuronal
damage.
miRNAs are small non-coding RNA molecules of 18-25
nucleotides in length that can regulate gene expression by
targeting specific mRNAs. miRNAs have been found to exert a wide
range of biological effects on nerve cell growth, apoptosis and fat
metabolism (31,32). Previous studies have reported
differences in the expression levels of miRNAs in the peripheral
blood of patients with stroke compared with healthy individuals
(33,34). In addition, an increasing number of
studies have demonstrated that certain miRNAs may exert important
cerebroprotective effects against IRI (35,36).
miR-766-5p has been reported to exert anti-inflammatory effects on
human rheumatoid arthritis fibroblast-like synoviocyte MH7A cells
(37). However, it remains unknown
whether miR-766-5p, which was predicted as a potential target of
TTTY15 through bioinformatics analysis, is involved in IR-induced
nerve injury. In the present study, miR-766-5p was predicted and
verified to be a target of TTTY15 using bioinformatics software and
a dual luciferase reporter assay. Furthermore, TTTY15
overexpression significantly alleviated the inhibitory effect of
miR-766-5p overexpression on the OGD/R-induced inflammation and
apoptosis of PC12 cells. These data suggested that the effects of
TTTY15 on OGD/R-induced injury in PC12 cells may be mediated by
miR-766-5p.
Of note, the incidence of sex-unspecific tumors in
men is significantly higher compared with in women; however, the
reason for this remains unclear (33). While the Y chromosome is a
male-specific chromosome with a unique evolutionary process, the
function of the genes located on the Y chromosome are not
sufficient to fully explain the high incidence of cancers in men
(38). A previous study reported
that the inflammatory response in men may be enhanced by
testosterone, which subsequently increases the susceptibility of
renal tissue to IRI; however, the specific differences between men
and women should be further studied (39). At present, there are few studies
reporting the relationship between the Y chromosome and I/R, and
whether the results of the present study may be applicable to
female mice remains to be examined. Thus, the effects of TTTY15 on
OGD/R-induced nerve injury should be further verified in
vivo. Future experiments should focus on the functions of
TTTY15 in female subjects, which may help to elucidate the role of
TTTY15 as a potential target for the treatment of neuronal IRI in
both sexes.
In conclusion, the findings of the present study
revealed that the expression levels of TTTY15 were upregulated in
PC12 cells following OGD/R. The knockdown of TTTY15 improved the
OGD/R-induced injury of PC12 cells by upregulating miR-766-5p
expression. These results suggested that TTTY15 may be considered
as a potential target for the treatment of I/R.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
CH and SC conceived and designed the study;
conducted the experiments; analyzed the data and wrote the paper.
Both authors have read and consent to the publication of the final
version of the manuscript, and confirm the authenticity of the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
GBD 2015 Mortality and Causes of Death
Collaborators. Global, regional, and national life expectancy,
all-cause mortality, and cause-specific mortality for 249 causes of
death, 1980-2015: A systematic analysis for the Global Burden of
Disease Study 2015. Lancet. 388:1459–1544. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Piao JM, Wu W, Yang ZX, Li YZ, Luo Q and
Yu JL: MicroRNA-381 favors repair of nerve injury through
regulation of the SDF-1/CXCR4 signaling pathway via LRRC4 in acute
cerebral ischemia after cerebral lymphatic blockage. Cell Physiol
Biochem. 46:890–906. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chamorro Á, Dirnagl U, Urra X and Planas
AM: Neuroprotection in acute stroke: Targeting excitotoxicity,
oxidative and nitrosative stress, and inflammation. Lancet Neurol.
15:869–881. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hill MD, Martin RH, Mikulis D, Wong JH,
Silver FL, Terbrugge KG, Milot G, Clark WM, Macdonald RL, Kelly ME,
et al: Safety and efficacy of NA-1 in patients with iatrogenic
stroke after endovascular aneurysm repair (ENACT): A phase 2,
randomised, double-blind, placebo-controlled trial. Lancet Neurol.
11:942–950. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Feng S, Yang Q, Liu M, Li W, Yuan W, Zhang
S, Wu B and Li J: Edaravone for acute ischaemic stroke. Cochrane
Database Syst Rev. (CD007230)2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Di Menna L, Molinaro G, Di Nuzzo L, Riozzi
B, Zappulla C, Pozzilli C, Turrini R, Caraci F, Copani A, Battaglia
G, et al: Fingolimod protects cultured cortical neurons against
excitotoxic death. Pharmacol Res. 67:1–9. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Iadecola C and Anrather J: The immunology
of stroke: From mechanisms to translation. Nat Med. 17:796–808.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Moskowitz MA, Lo EH and Iadecola C: The
science of stroke: Mechanisms in search of treatments. Neuron.
67:181–198. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yoon JH, Abdelmohsen K and Gorospe M:
Functional interactions among microRNAs and long noncoding RNAs.
Semin Cell Dev Biol. 34:9–14. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li Z, Li J and Tang N: Long noncoding RNA
Malat1 is a potent autophagy inducer protecting brain microvascular
endothelial cells against oxygen-glucose
deprivation/reoxygenation-induced injury by sponging miR-26b and
upregulating ULK2 expression. Neuroscience. 354:1–10.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yu SY, Dong B, Fang ZF, Hu XQ, Tang L and
Zhou SH: Knockdown of lnc RNA AK 139328 alleviates myocardial
ischaemia/reperfusion injury in diabetic mice via modulating
miR-204-3p and inhibiting autophagy. J Cell Mol Med. 22:4886–4898.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhu Y, Ren S, Jing T, Cai X, Liu Y, Wang
F, Zhang W, Shi X, Chen R, Shen J, et al: Clinical utility of a
novel urine-based gene fusion TTTY15-USP9Y in predicting prostate
biopsy outcome. Urol Oncol. 33:384.e9–e20. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ren S, Peng Z, Mao JH, Yu Y, Yin C, Gao X,
Cui Z, Zhang J, Yi K, Xu W, et al: RNA-seq analysis of prostate
cancer in the Chinese population identifies recurrent gene fusions,
cancer-associated long noncoding RNAs and aberrant alternative
splicings. Cell Res. 22:806–821. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lai IL, Chang YS, Chan WL, Lee YT, Yen JC,
Yang CA, Hung SY and Chang JG: Male-specific long noncoding RNA
TTTY15 inhibits non-small cell lung cancer proliferation and
metastasis via TBX4. Int J Mol Sci. 20(3473)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Huang S, Tao W, Guo Z, Cao J and Huang X:
Suppression of long noncoding RNA TTTY15 attenuates hypoxia-induced
cardiomyocytes injury by targeting miR-455-5p. Gene. 701:1–8.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhong Y, Yu C and Qin W: LncRNA SNHG14
promotes inflammatory response induced by cerebral
ischemia/reperfusion injury through regulating miR-136-5p/ROCK1.
Cancer Gene Ther. 26:234–247. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mavridis K, Stravodimos K and Scorilas A:
Downregulation and prognostic performance of microRNA 224
expression in prostate cancer. Clin Chem. 59:261–269.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–1401.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Carbone F, Bonaventura A and Montecucco F:
Neutrophil-related oxidants drive heart and brain remodeling after
ischemia/reperfusion injury. Front Physiol. 10(1587)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhuo Y and Zhuo J: Tranilast treatment
attenuates cerebral ischemia-reperfusion injury in rats through the
inhibition of inflammatory responses mediated by NF-κB and PPARs.
Clin Transl Sci. 12:196–202. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Allan SM and Rothwell NJ: Cytokines and
acute neurodegeneration. Nat Rev Neurosci. 2:734–744.
2001.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Chamorro A and Hallenbeck J: The harms and
benefits of inflammatory and immune responses in vascular disease.
Stroke. 37:291–293. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Carninci P, Kasukawa T, Katayama S, Gough
J, Frith MC, Maeda N, Oyama R, Ravasi T, Lenhard B, Wells C, et al:
The transcriptional landscape of the mammalian genome. Science.
309:1559–1563. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Leung A and Natarajan R: Non-coding RNAs
in vascular disease. Curr Opin Cardiol. 29:199–206. 2014.
|
|
25
|
Li J, Xuan Z and Liu C: Long non-coding
RNAs and complex human diseases. Int J Mol Sci. 14:18790–18808.
2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kalogeris T, Baines CP, Krenz M and
Korthuis RJ: Cell biology of ischemia/reperfusion injury. Int Rev
Cell Mol Biol. 298:229–317. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dharap A, Pokrzywa C and Vemuganti R:
Increased binding of stroke-induced long non-coding RNAs to the
transcriptional corepressors Sin3A and coREST. ASN Neuro.
5:283–289. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Deng QW, Li S, Wang H, Sun HL, Zuo L, Gu
ZT, Lu G, Sun CZ, Zhang HQ and Yan FL: Differential long noncoding
RNA expressions in peripheral blood mononuclear cells for detection
of acute ischemic stroke. Clin Sci (Lond). 132:1597–1614.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zheng J, Zhuo YY, Zhang C, Tang GY, Gu XY
and Wang F: LncRNA TTTY15 regulates hypoxia-induced vascular
endothelial cell injury via targeting miR-186-5p in cardiovascular
disease. Eur Rev Med Pharmacol Sci. 24:3293–3301. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chang HL, Wang HC, Chunag YT, Chou CW, Lin
IL, Lai CS, Chang LL and Cheng KI: miRNA expression change in
dorsal root ganglia after peripheral nerve injury. J Mol Neurosci.
61:169–177. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lefai E, Blanc S, Momken I, Antoun E,
Chery I, Zahariev A, Gabert L, Bergouignan A and Simon C: Exercise
training improves fat metabolism independent of total energy
expenditure in sedentary overweight men, but does not restore lean
metabolic phenotype. Int J Obes (Lond). 41:1728–1736.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Stary CM, Xu L, Sun X, Ouyang YB, White
RE, Leong J, Li J, Xiong X and Giffard RG: MicroRNA-200c
contributes to injury from transient focal cerebral ischemia by
targeting Reelin. Stroke. 46:551–556. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xue Y, Yin P, Li G and Zhong D:
Genome-wide integration study of circulating miRNAs and peripheral
whole-blood mRNAs of male acute ischemic stroke patients.
Neuroscience. 380:27–37. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu G, Cao C and Zhu M: Peripheral blood
miR-451 may serve as a biomarker of ischemic stroke. Clin Lab.
65:2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Liu P, Zhao H, Wang R, Wang P, Tao Z, Gao
L, Yan F, Liu X, Yu S, Ji X and Luo Y: MicroRNA-424 protects
against focal cerebral ischemia and reperfusion injury in mice by
suppressing oxidative stress. Stroke. 46:513–519. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hayakawa K, Kawasaki M, Hirai T, Yoshida
Y, Tsushima H, Fujishiro M, Ikeda K, Morimoto S, Takamori K and
Sekigawa I: MicroRNA-766-3p contributes to anti-inflammatory
responses through the indirect inhibition of NF-κB signaling. Int J
Mol Sci. 20(809)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Forsberg LA, Rasi C, Malmqvist N, Davies
H, Pasupulati S, Pakalapati G, Sandgren J, Diaz de Ståhl T,
Zaghlool A, Giedraitis V, et al: Mosaic loss of chromosome Y in
peripheral blood is associated with shorter survival and higher
risk of cancer. Nat Genet. 46:624–628. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
38
|
Prensner JR and Feng FY: ‘Lincing’ the Y
chromosome to prostate cancer: TTTY15 takes center stage. Eur Urol.
Sep. 76:327–328. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kang KP, Lee JE, Lee AS, Jung YJ, Kim D,
Lee S, Hwang HP, Kim W and Park SK: Effect of gender differences on
the regulation of renal ischemia-reperfusion-induced inflammation
in mice. Mol Med Rep. 9:2061–2068. 2014.PubMed/NCBI View Article : Google Scholar
|