Introduction
Heart diseases cause more deaths than all types of
cancer combined (1). In effect,
cardiac diseases, such as chronic heart failure (CHF), are the
leading cause of hospitalization in numerous regions of the world
(2). The occurrence of CHF is
closely related to various other clinical diseases, such as
hypercholesterolemia, hypertension and diabetes mellitus (3). The goal of the treatment of heart
failure is not only to improve symptoms and quality of life, but
also to delay and prevent the development of cardiac remodeling and
reduce hospitalization and mortality of patients with heart failure
(4). Brain natriuretic peptide
(BNP) is a cardiac hormone with diuretic, natriuretic and
vasodilator properties. Measurement of plasma B-type natriuretic
peptide concentrations is increasingly used to aid the diagnosis,
assess prognosis and tailoring treatment in adults with congestive
heart failure (5). However, it may
also be significantly altered in other diseases, such as hepatitis
(6) and renal failure (7). Although the left ventricle ejection
fraction (LVEF) is frequently used to assess cardiac function in
patients with CHF (8), a certain
proportion of patients may have normal LVEFs. Therefore, novel
markers for the diagnosis and prognosis of CHF remain to be
explored and developed.
Long non-coding RNAs (lncRNAs) are a group of
non-protein coding RNAs that are >200 nucleotides in length.
Increasing evidence indicates that lncRNAs have a crucial role in
the pathophysiology of human diseases (9) and certain lncRNAs are also aberrantly
expressed in CHF. LncRNA nuclear-enriched abundant transcript 1
(NEAT1) is an lncRNA known to be closely related to myocardial
function. Interfering with NEAT1 levels protects myocardial cells
from hypoxia injury (10). NEAT1 is
able to competitively bind to microRNA (miR)-129-5p in myocardial
cells, thereby promoting apoptosis and inhibiting cell
proliferation (11). Studies have
indicated that miR-129-5p is able to improve cardiac function in
rats with CHF (12) and has a
biological function of inhibiting apoptosis of cardiomyocytes
(13). However, the expression
levels of the NEAT1/miR-129-5p axis in patients with CHF and its
clinical significance in the diagnosis and prognosis of CHF have
remained to be determined.
In the present study, the expression of lncRNA NEAT1
and miR-129-5p in the serum of patients with CHF was analyzed using
reverse transcription-quantitative (RT-q)PCR. Furthermore,
inter-indicator correlations were determined using Pearson
correlation coefficient analysis. Receiver operating characteristic
(ROC) curves were obtained to analyze the predictive ability of
NEAT1, miR-129-5p and BNP for the onset of CHF. In addition, the
prognostic value of the NEAT1/miR-129-5p axis was analyzed by
drawing Kaplan-Meier survival curves and performing Cox regression
analysis. The results suggested that patients with CHF have
increased NEAT1 and decreased miR-129-5p expression. The
NEAT1/miR-129-5p axis may provide novel non-invasive biomarkers for
CHF diagnosis and prognosis.
Patients and methods
Study population and sample
collection
A total of 70 patients with CHF and 62 age- and
sex-matched controls were collected from Weifang People's Hospital
(Weifang, China) between May 2016 and April 2018. The diagnosis of
CHF was made according to the criteria of the 2013 American College
of Cardiology Foundation/American Heart Association Guidelines for
the Management of Heart Failure and the 2016 European Society of
Cardiology (ESC) Guidelines for the diagnosis and treatment of
Acute and CHF (14,15). The control subjects were obtained
from a population of individuals referred for physical examination
at Weifang People's Hospital (Weifang, China) during a same time
period and were determined to not have any CHF. The patients with
CHF had an LVEF <40% and were clinically stable, with the New
York Heart Association (NYHA) stage ranging from II to IV based on
the 1928 and 1994 revised version of NYHA staging system (16). The exclusion criteria for both the
patients and controls were as follows: i) Presence of infection;
ii) cancer; iii) history of surgery within 1 year; iv) history of
cerebral vascular events within 6 months; v) heart assist devices;
or vi) liver or renal failure. To avoid fibrin interference in
plasma, serum was used as the experimental sample in this
experiment. Venous blood samples were collected from the
participants to ensure the accuracy of the analytical results,
serum separation was performed by centrifugation immediately after
blood sample collection to avoid hemolysis and the samples were
stored at -20˚C for further use. The experimental protocols were
approved by the Ethics Committee of Weifang People's Hospital
(Weifang, China) and written informed consent was provided by each
participant.
Therapy of patients and follow-up
survey
The 70 patients with CHF were treated with
conventional drugs, including diuretics, angiotensin-converting
enzyme inhibitors or angiotensin receptor blockers and β-blockers.
All patients underwent monthly telephone follow-up for a total of
24 months and survival information was collected and analyzed for
all patients in this study to record the deaths of patients with
CHF.
RNA extraction and RT-qPCR
Total RNA was extracted from fresh serum samples
using the GenElute Total RNA Purification Kit (Sigma-Aldrich; Merck
KGaA), and the concentration and quality of total RNA were analyzed
using a NanoDrop 2000 (Thermo Fisher Scientific, Inc.). RNA with an
optical density at 260 nm (OD260)/OD280 ratio close to 2.0 was used
for subsequent RT. RT was performed using the Applied Biosystems
High-Capacity complementary (c)DNA RT Kit (Thermo Fisher
Scientific, Inc.) with the following reaction conditions: 42˚C for
30 min, 85˚C for 5 sec, and the resulting cDNA was stored at -20˚C
for later use.
The expression levels of NEAT1 and miR-129-5p were
measured by qPCR, which was performed using a SYBR green I Master
Mix kit (Invitrogen; Thermo Fisher Scientific, Inc.) on a 7500
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. U6 was used as an
endogenous control for miR-129-5p and GAPDH was used as an
endogenous control for NEAT1. The following thermocycling
conditions were used for the qPCR: Initial denaturation at 95˚C for
10 min; followed by 40 cycles of 95˚C for 20 sec, 60˚C for 15 sec
and 72˚C for 20 sec. The following primer sequences were used for
the qPCR: miR-129-5p forward: 5'-GCCGAGCTTTTTGCGGTCTGGG-3' and
reverse, 5'-CTCAACTGGTGTCGTGGA-3'; NEAT1 forward,
5'-CTTCCTCCCTTTAACTTATCCATTCAC-3' and reverse,
5'-CTCTTCCTCCACCATTACCAACAATAC-3'; U6 forward,
5'-GCTTCGGCAGCACATATACTAAAAT-3' and reverse,
5'-CGCTTCACGAATTTGCGTGTCAT-3' and GAPDH forward,
5'-TGCACCACCAACTGCTTAGC-3' and reverse:
5'-GGCATGCACTGTGGTCATGAG-3'; The final expression value was
calculated using the 2-ΔΔCq method (17).
Statistical analysis
Values are expressed as the mean ± standard
deviation and analyzed using SPSS 21.0 (IBM Corp.) and GraphPad 7.0
(GraphPad Software, Inc.). Each experiment was performed for at
least three times. Differences between groups were analyzed with an
unpaired Student's t-test or one-way ANOVA followed by Tukey's
multiple-comparisons test. Pearson correlation analysis was used to
determine the correlation between various indicators. ROC curves
for the predictive value of the expression values of BNP, NEAT1 and
miR-129-5p regarding CHF were plotted using SPSS software. The area
under the ROC curve (AUC) was calculated and the sensitivity and
specificity were obtained at the optimal cutoff value. To evaluate
the synthetic role of BNP, NEAT1 and miR-129-5p to distinguish CHF
patients, logistic analysis was used to calculate the probability
values of the combination of the parameters. Survival analysis was
performed using the Kaplan-Meier method and a log-rank test was
used to determine statistically significant differences between
curves for high and low expression. The prognostic value of NEAT1
and miR-129-5p was evaluated by Cox logistic regression analysis,
in which the clinical data, NEAT1 and miR-129-5p were included to
evaluate their relationship with the survival of patients.
Results
Baseline characteristics and clinical
parameters of the participants
In the present study, 70 patients with CHF and 62
matched controls were enrolled. The CHF patients included 37 males
and 33 females with an average age of 67.24±1.60 years and the
control included 34 males and 28 females with an average age of
66.71±1.65 years. Their basic information is listed in Table I. The results suggested that there
were no differences in age, sex, body mass index, smoking history,
drinking history, total cholesterol, triglycerides, low-density
lipoprotein cholesterol, high-density lipoprotein cholesterol or
uric acid between the two groups. In terms of complications, there
were no differences in hypertension and diabetes between the two
groups. The CHF group had significantly higher levels of BNP and
significantly lower levels of LVEF compared to the controls (both
P<0.001).
 | Table IBaseline characteristics and clinical
parameters of the participants. |
Table I
Baseline characteristics and clinical
parameters of the participants.
| Feature | Controls (n=62) | CHF (n=70) | P-value |
|---|
| Age (years) | 66.71±1.65 | 67.24±1.60 | 0.061 |
| Sex
(male/female) | 34/28 | 37/33 | 0.820 |
| BMI
(kg/m2) | 25.04±0.377 | 24.95±0.360 | 0.171 |
| Smoking history
(never/ever) | 43/19 | 44/26 | 0.432 |
| Drinking history
(never/ever) | 40/22 | 40/30 | 0.387 |
| TC (nM) | 4.65±0.16 | 4.63±0.19 | 0.779 |
| TG (nM) | 1.37±0.65 | 1.44±0.63 | 0.476 |
| LDL-C (nM) | 2.97±0.13 | 3.00±0.14 | 0.153 |
| HDL-C (nM) | 1.19±0.31 | 1.16±0.03 | 0.542 |
| UA (µM) | 353.76±15.15 | 356.31±13.69 | 0.312 |
| BNP (ng/l) | 67.24±20.66 |
1,519.83±853.73 | <0.001 |
| LVEF (%) | 59.89±0.63 | 30.17±3.44 | <0.001 |
| Complication
(no/yes) | | | |
|
Hypertension | 26/36 | 22/48 | 0.210 |
|
Diabetes | 30/32 | 24/46 | 0.100 |
|
COPD | - | 58/12 | - |
|
Anemia | - | 62/8 | - |
|
Fluid and
electrolyte imbalance | - | 39/31 | - |
| NYHA stage | | | |
|
II | - | 36 | - |
|
III | - | 19 | - |
|
IV | - | 15 | - |
Expression of NEAT1 and miR-129-5p in
patients with CHF
To further understand the role of NEAT1 and
miR-129-5p in CHF, their serum levels in patients with CHF were
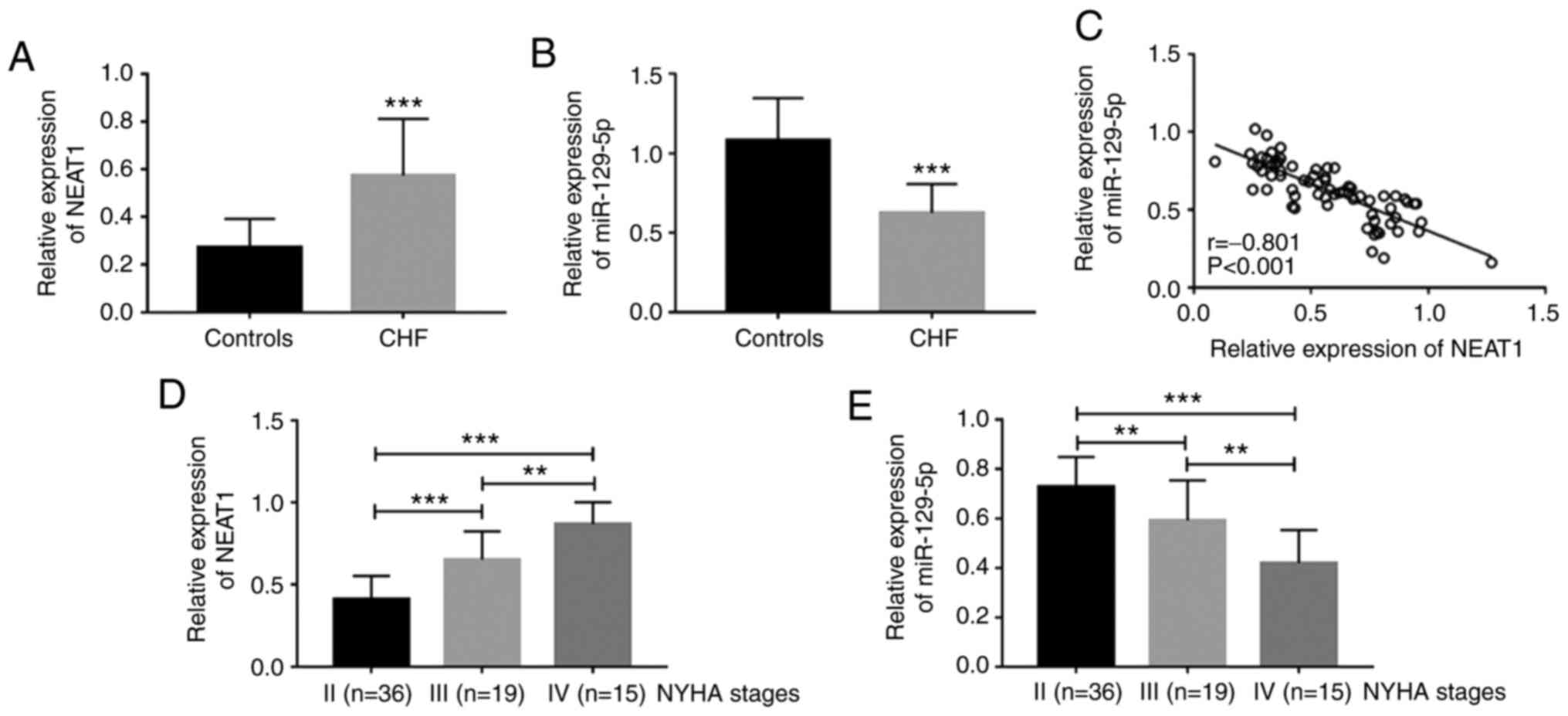
quantified by RT-qPCR. It was observed that compared to the healthy
control, patients with CHF had upregulated serum NEAT1 (P<0.001;
Fig. 1A) and downregulated serum
miR-129-5p (P<0.001; Fig. 1B),
and serum NEAT1 and miR-129-5p levels were significantly negatively
correlated in patients with CHF (r=-0.801, P<0.001; Fig. 1C). In addition, the expression level
of NEAT1 was upregulated with the increase of the NYHA stage (all
P<0.01; Fig. 1D), while the
expression level of miR-129-5p decreased with the increase of the
NYHA stage (all P<0.01; Fig.
1E).
Correlation of the NEAT1/miR-129-5p
axis with BNP and LVEF in patients with CHF
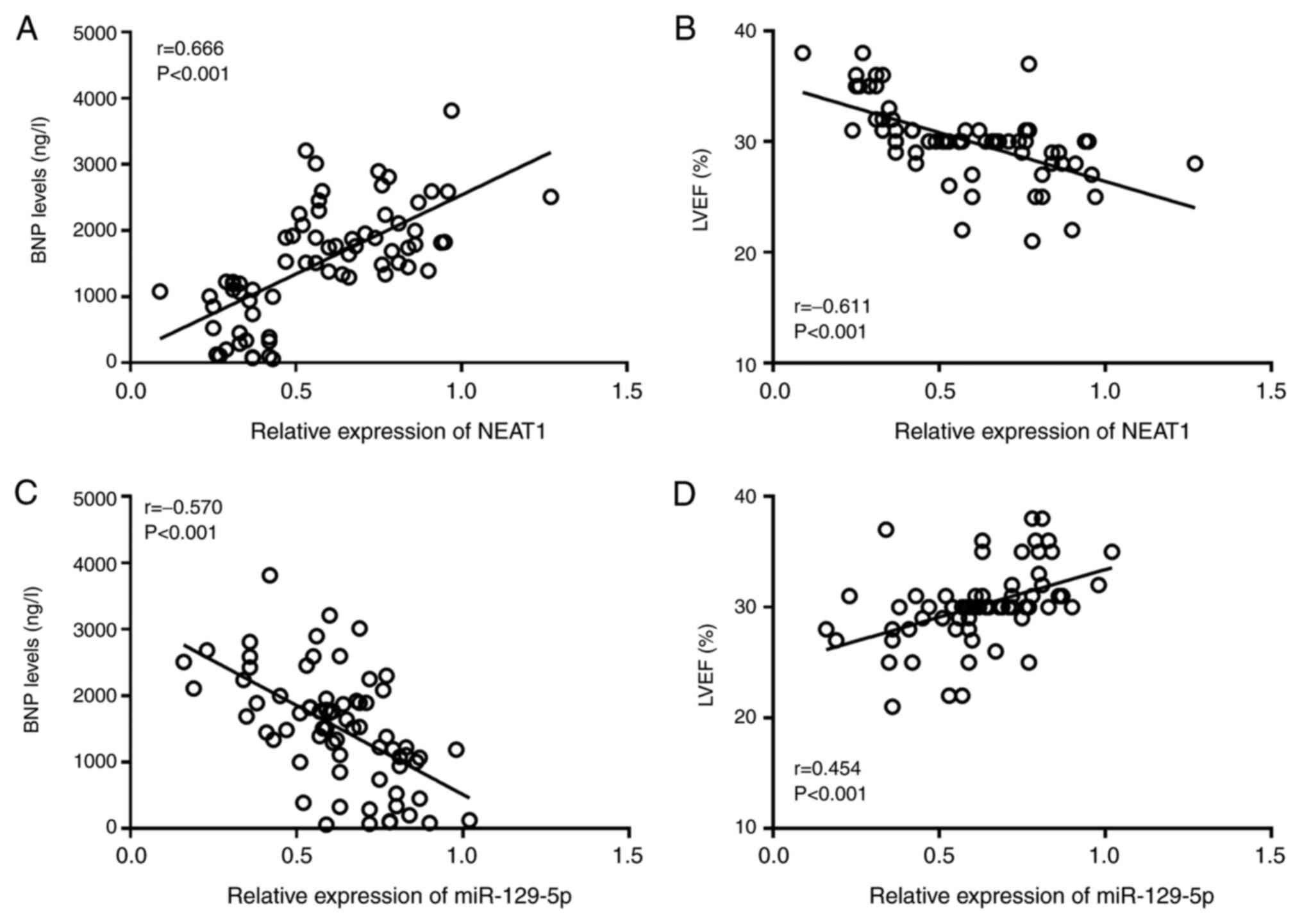
The results of the Pearson correlation analysis
indicated that the expression level of NEAT1 was positively
correlated with BNP (r=0.666, P<0.001; Fig. 2A) and negatively correlated with
LVEF (r=-0.611, P<0.001; Fig.
2B) in patients with CHF. The expression level of miR-129-5p
was negatively correlated with the BNP level (r=-0.570, P<0.001;
Fig. 2C) and positively correlated
with the LVEF (r=0.454, P<0.001; Fig. 2D).
Diagnostic performance of the
NEAT1/miR-129-5p axis in patients with CHF
As a traditional diagnostic marker for CHF, the
BNP-based ROC curve was first drawn and the diagnostic value of
NEAT1 and miR-129-5p was further evaluated, which indicated that
both NEAT1 and miR-129-5p had a certain diagnostic value (Fig. 3). ROC analysis revealed that the AUC
of BNP was 0.982. The AUC of the ROC curve based on serum NEAT1 was
0.868 with a sensitivity and specificity of 62.9 and 96.8%,
respectively, at a cutoff value of 0.465. miR-129-5p had a good
diagnostic value with an AUC of 0.921, and the sensitivity was
95.7% and the specificity was 77.4% at a cutoff value of 1.130. Of
note, the combination of NEAT1 and miR-129-5p had high diagnostic
accuracy (AUC=0.970) and the combination of NEAT1, miR-129-5p and
BNP had the best diagnostic value (AUC=0.998), indicating that this
combination resulted in a significant increase in the diagnostic
power compared with BNP alone (Table
II).
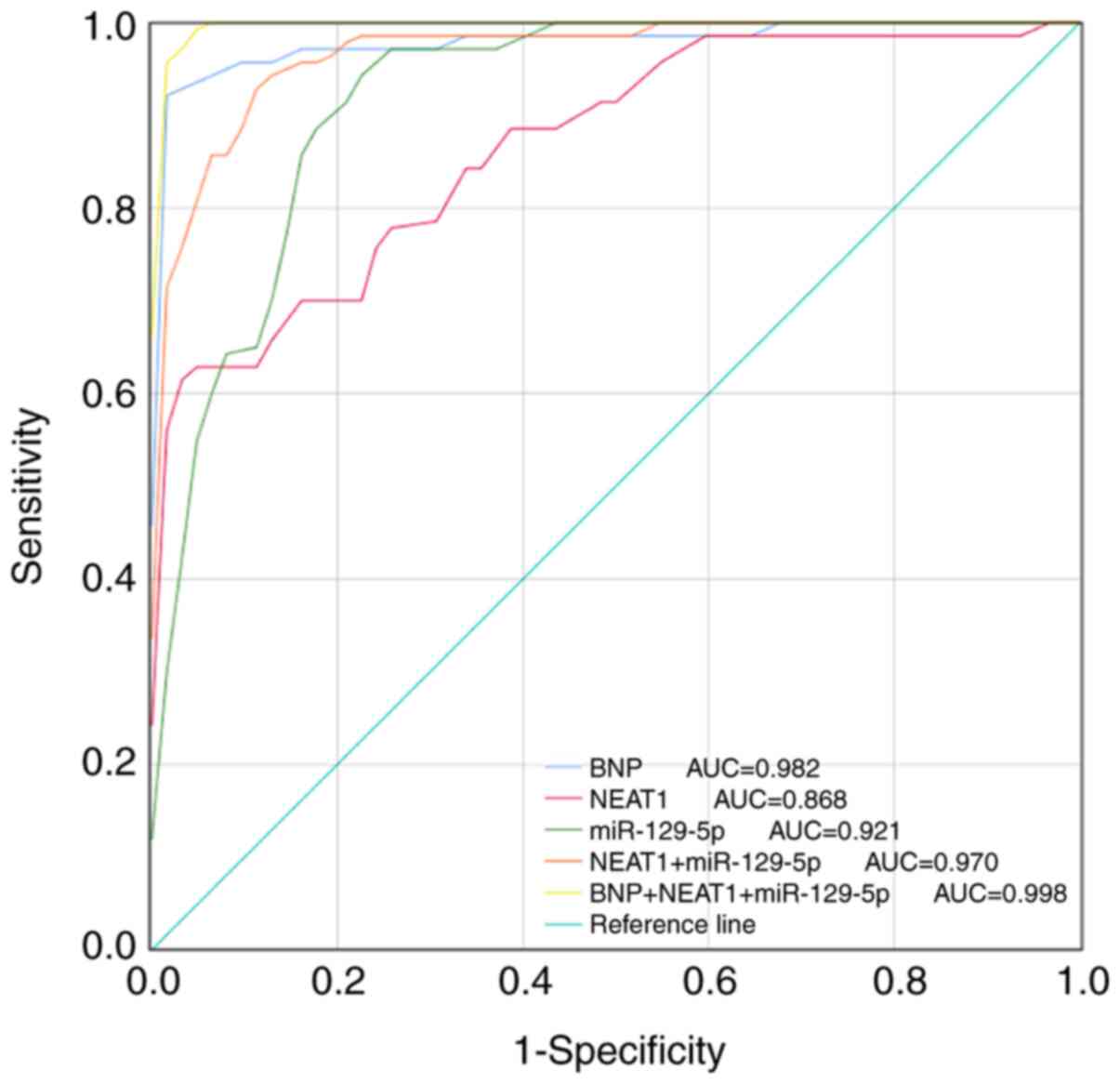 | Figure 3ROC analysis of the diagnostic
performance of the NEAT1/miR-129-5p axis for CHF. The AUC of BNP,
NEAT1, miR-129-5p, NEAT1+miR-129-5p and BNP+NEAT1+miR-129-5p to
diagnose CHF were 0.982, 0.868, 0.921, 0.970 and 0.998,
respectively. CHF, chronic heart failure; miR, microRNA; NEAT1,
nuclear-enriched abundant transcript 1; BNP, brain natriuretic
peptide; AUC, area under the ROC curve; ROC, receiver operating
characteristic. |
 | Table IIROC curve analysis results for
patients with chronic heart failure. |
Table II
ROC curve analysis results for
patients with chronic heart failure.
| Variable | AUC | Cutoff value | Sensitivity
(%) | Specificity
(%) |
|---|
| BNP | 0.982 | 127.455 | 92.9 | 98.4 |
| NEAT1 | 0.868 | 0.465 | 62.9 | 96.8 |
| miR-129-5p | 0.921 | 1.130 | 95.7 | 77.4 |
|
NEAT1+miR-129-5p | 0.970 | - | 94.3 | 88.7 |
|
BNP+NEAT1+miR-129-5p | 0.998 | - | 98.6 | 96.8 |
Prognostic value of NEAT1/miR-129-5p
in predicting survival rates of patients with CHF
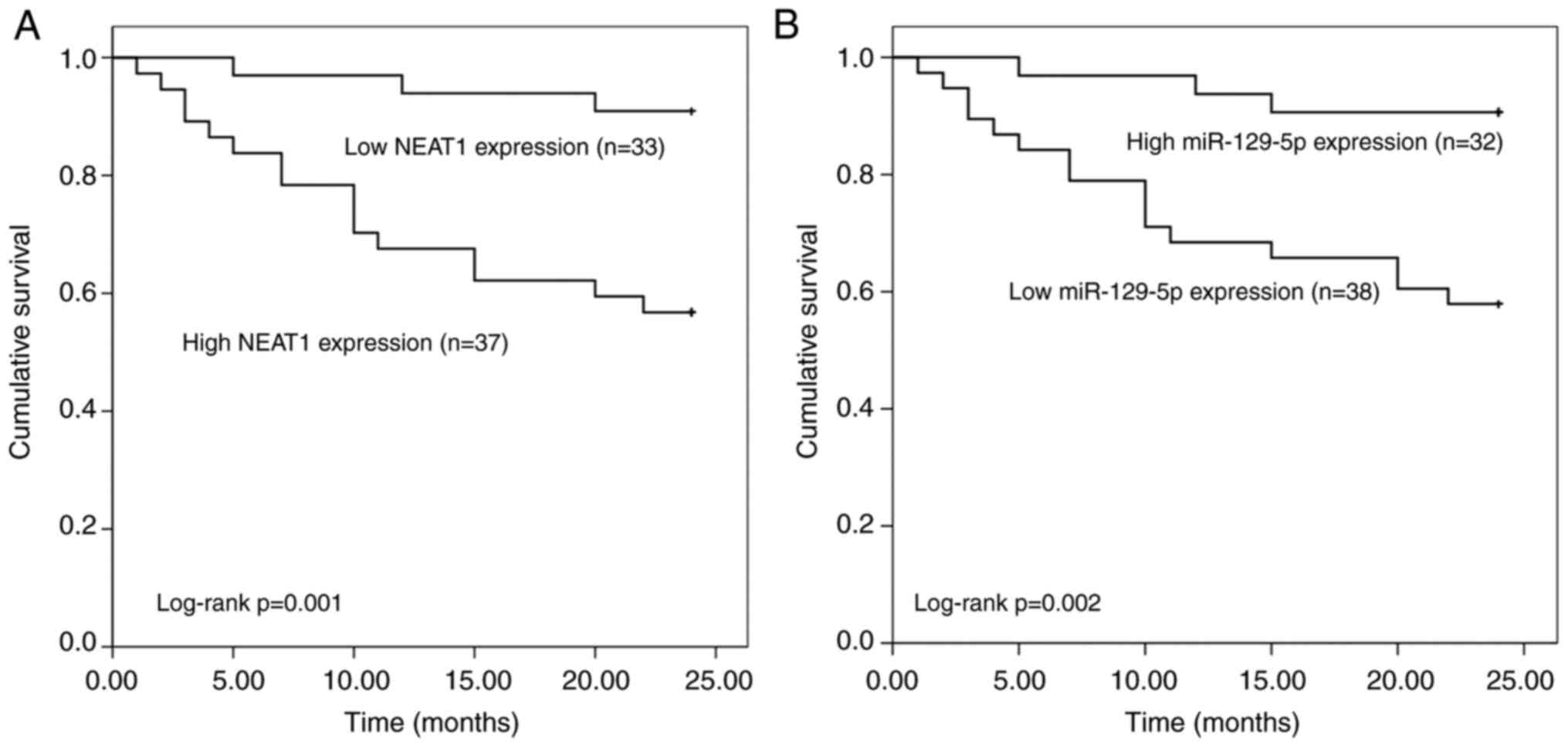
The association of NEAT1 and miR-129-5p expression
with the overall survival of patients was estimated by plotting the
Kaplan-Meier survival curves (Fig.
4). The Kaplan-Meier curves indicated that patients with low
NEAT1 expression levels had better overall survival than those with
high NEAT1 expression levels (log-rank P=0.001) and patients with
low miR-129-5p expression levels had lower overall survival than
those with high miR-129-5p expression levels (log-rank P=0.002).
Furthermore, the multivariate Cox analysis demonstrated that BNP
[hazard ratio (HR)=3.998, 95% CI=1.752-6.874, P=0.022], NYHA stage
(HR=3.597, 95% CI=1.884-6.496, P=0.016), NEAT1 (HR=3.197, 95%
CI=1.702-5.879, P=0.006) and miR-129-5p (HR=3.549, 95%
CI=1.975-6.365, P=0.012) were independent prognostic factors for
the survival of patients with CHF (Table III).
 | Table IIIMultivariate Cox regression analysis
for patients with chronic heart failure. |
Table III
Multivariate Cox regression analysis
for patients with chronic heart failure.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (≥67 years vs.
<67 years) | 1.311 | 0.702-2.213 | 0.485 | 1.281 | 0.672-2.120 | 0.551 |
| Sex (male vs.
female) | 1.248 | 0.589-2.096 | 0.669 | 1.201 | 0.531-1.705 | 0.732 |
| BMI (≥24
kg/m2 vs. <24 kg/m2) | 1.587 | 0.781-2.406 | 0.326 | 1.658 | 0.730-2.888 | 0.256 |
| Smoking (ever vs.
never) | 1.401 | 0.728-1.757 | 0.384 | 1.389 | 0.657-2.218 | 0.408 |
| Drinking (ever vs.
never) | 1.399 | 0.697-2.188 | 0.455 | 1.497 | 0.687-2.240 | 0.469 |
| TC (high vs.
low) | 1.502 | 0.759-2.337 | 0.412 | 1.584 | 0.726-2.553 | 0.352 |
| TG (high vs.
low) | 1.608 | 0.714-2.587 | 0.287 | 1.822 | 0.796-2.854 | 0.246 |
| LDL-C (high vs.
low) | 1.912 | 0.951-3.841 | 0.062 | 2.077 | 0.906-3.895 | 0.069 |
| HDL-C (low vs.
high) | 1.857 | 0.912-2.816 | 0.118 | 1.912 | 0.856-3.511 | 0.130 |
| UA (high vs.
low) | 2.022 | 0.979-4.005 | 0.059 | 1.923 | 0.859-3.687 | 0.072 |
| BNP (high vs.
low) | 4.106 | 1.855-6.304 | 0.014 | 3.998 | 1.725-6.874 | 0.022 |
| LVEF (low vs.
high) | 2.027 | 1.612-2.419 | 0.038 | 2.114 | 0.982-3.763 | 0.059 |
| Complication (yes
vs. no) | 2.409 | 0.989-3.205 | 0.055 | 2.541 | 0.984-4.167 | 0.064 |
| NYHA stage (high
vs. low) | 3.995 | 1.974-6.207 | 0.008 | 3.597 | 1.884-6.496 | 0.016 |
| NEAT1 (high vs.
low) | 3.228 | 1.846-4.789 | <0.001 | 3.197 | 1.702-5.879 | 0.006 |
| miR-129-5p (low vs.
high) | 3.666 | 2.017-5.294 | 0.002 | 3.549 | 1.975-6.365 | 0.012 |
Discussion
Numerous studies have indicated that NEAT1
overexpression may improve diseases by downregulating miR-129-5p,
including epilepsy (18) and
alcoholic steatohepatitis (19). In
the present study, the clinical value of NEAT1 and miR-129-5p in
patients with CHF was investigated. It was confirmed that NEAT1
expression was upregulated and miR-129-5p expression was
downregulated in CHF, and they may serve as two non-invasive
diagnostic and prognostic biomarkers for CHF.
CHF is the end stage of multiple heart diseases. The
diagnosis and prognostication of patients with CHF remain a
challenge (4). With efforts to
improve the treatment and prevention of CHF, the mortality rate of
sudden death among patients with CHF have markedly decreased over
the past decades (20). However,
the overall mortality of these patients remains high (21). In recent years, BNP in the
diagnosis, treatment and prognosis of cardiovascular diseases has
become a research focus (22). It
was indicated that in the clinic, certain cases presented with
symptoms of CHF with normal BNP values. There are also cases with
heterophile antibody interference on immunodetection, resulting in
false elevation of BNP. These issues all influence the diagnostic
yield of BNP (23). Therefore, it
is important to identify patients with CHF with a high risk of
death, improve their diagnosis and prognostic efficacy and increase
the survival of those patients. Certain lncRNAs are abnormally
expressed during the development of diseases and lncRNAs may thus
become novel biomarkers for prognosis and diagnosis (24). For instance, Song et al
(25) provided an innovative lncRNA
expression signature that may be a useful biomarker for the
prognosis of patients with gastric cancer based on bioinformatics
analysis. Furthermore, lncRNA-D16366 was identified to be decreased
in hepatocellular carcinoma and may be an independent diagnostic
and prognostic indicator for this disease (26). In cervical cancer, upregulated
expression of lncRNA focally amplified lncRNA on chromosome 1 may
serve as a noninvasive diagnostic and prognostic biomarker
(27). However, studies on the
clinical significance of lncRNAs in CHF are currently limited.
LncRNA NEAT1 is transcribed from multiple endocrine
neoplasia sites and is involved in cancer progression (28). Aberrant overexpression of the long
non-coding RNA NEAT1 has been demonstrated in different types of
disease and its abnormal expression levels were associated with
diagnosis and prognosis (29). For
instance, in colorectal cancer, high expression of NEAT1 may serve
as a novel biomarker for diagnosis and prognostication of patients
(30). LncRNA NEAT1 may also be
used as a diagnostic biomarker for ovarian cancer (31). Huang et al (32) indicated that lncRNA NEAT1 correlates
with increased unfavorable prognosis in patients with sepsis.
However, the effect of NEAT1 on the CHF process has remained
elusive. Previous studies have indicated that lncRNA NEAT1 is able
to competitively bind to miR-129-5p in cardiomyocytes, thereby
promoting apoptosis and inhibiting cell proliferation (11). In the present study, the RT-qPCR
results revealed that NEAT1 was significantly upregulated, while
miR-129-5p was downregulated in the serum of patients with CHF when
compared to normal controls. Pearson correlation coefficient
analysis indicated a significant negative correlation between NEAT1
and miR-129-5p expression levels in patients with CHF. Furthermore,
with the increase of the NYHA stage, the expression level of
miR-129-5p was downregulated and the expression level of NEAT1 was
upregulated. The results all suggested that NEAT1 and miR-129-5p
may have a role in CHF. This may provide an approach to improve the
treatment of CHF, namely by screening out patients at the early
stage of NYHA based on the expression levels of NEAT1 and
miR-129-5p.
The NEAT1/miR-129-5p axis has an important role in
the occurrence and development of numerous diseases. For instance,
lncRNA NEAT1 suppression was able to inhibit papillary thyroid
cancer progression by upregulating miR-129-5p (33). In breast tumorigenesis,
dysregulation of the BRCA1/NEAT1/miR-129-5p/WNT4 signaling axis has
a promotive role (34). Fu et
al (35) indicated that NEAT1
expression was aberrantly increased in hepatoblastoma and that it
may promote the metastasis of hepatoblastoma cells by inhibiting
miR-129-5p. In addition, certain studies investigated the potential
mechanisms of NEAT1 and miR-129-5p. For instance, NEAT1 was
indicated to regulate the expression of inflammatory chemokines and
cytokines to affect the MAPK pathway in systemic lupus
erythematosus (36). Zhang et
al (13) determined that
miR-129-5p was able to partially inhibit hydrogen peroxide-induced
cell autophagy and apoptosis by downregulating autophagy related 14
expression levels through activation of the PI3K/AKT/mTOR pathway.
miR-129-5p was able to regulate hydrogen peroxide-induced injury of
cardiomyocytes by mediating NEAT1. However, the clinical
application of NEAT1 and miR-129-5p remains to be established
(11). Based on the above, it is
indicated that the NEAT1/miR-129-5p axis has an important role in
CHF and may be of clinical value. In the present study, correlation
analyses suggested that both NEAT1 and miR-129-5p had linear
correlations with diagnostic markers (BNP, LVEF) of CHF. Thus, the
NEAT1/miR-129-5p axis may serve as a novel CHF marker, and it is
suggested that a high level of NEAT1 and low level of miR-129-5p
are closely associated with poor prognosis of patients with CHF.
The present study determined that the diagnostic power of NEAT1 and
miR-129-5p for CHF was high; the good diagnostic value of NEAT1 was
demonstrated by an ROC curve with an AUC of 0.868 and miR-129-5p
also has a high diagnostic value with an AUC of 0.921. Of note,
compared with BNP alone, the combination of NEAT1, miR-129-5p and
BNP had the best diagnostic value and significantly improved the
diagnostic accuracy. Thus, NEAT1 and miR-129-5p may be potential
diagnostic biomarkers for CHF and may be used to enhance the
diagnostic accuracy of BNP in CHF. The Kaplan-Meier survival curves
indicated that patients with high NEAT1 or low miR-129-5p
expression had poor overall survival. Multivariate Cox analysis
revealed that NEAT1 and miR-129-5p were independent prognostic
factors for survival in patients with CHF. These results
demonstrated that NEAT1 and miR-129-5p may be used as ideal
biomarkers for the diagnosis and prognosis of CHF.
In conclusion, the present study was the first to
demonstrate the deregulation of serum NEAT1 and miR-129 and their
clinical significance in patients with CHF. The results of the ROC
analysis indicated that NEAT1 and miR-129-5p had high diagnostic
accuracy and considerable potential to improve the diagnostic
accuracy of BNP in CHF. Higher NEAT1 and lower miR-129-5p were
predictive of poor prognosis for patients with CHF. Therefore, the
NEAT1/miR-129-5p axis may provide noninvasive diagnostic and
prognostic biomarkers for CHF. However, the present study has
certain limitations, such as the limited sample size and the
follow-up time to assess prognosis may have been relatively short.
In addition, the underlying mechanisms of the implication of the
NEAT1/miR-129-5p axis in CHF were not fully explored in the present
study and in a future study by our group, the roles of the
NEAT1/miR-129-5p axis in relation to CHF will be further
investigated in cardiomyocytes using a well-established
experimental protocol.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ and XL designed the present study and were
responsible for writing and revising the manuscript. NZ and WJ
collected the clinical samples and data. HZ and XL analyzed the
data and confirmed the authenticity of the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from each
patient and the experimental procedures were approved by the Ethics
Committee of Weifang People's Hospital (Weifang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Weir HK, Anderson RN, Coleman King SM,
Soman A, Thompson TD, Hong Y, Moller B and Leadbetter S: Heart
disease and cancer deaths-trends and projections in the united
states, 1969-2020. Prev Chronic Dis. 13(E157)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Writing Group Members. Mozaffarian D,
Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de
Ferranti S, Després JP, et al: Heart disease and stroke
statistics-2016 update: A report from the american heart
association. Circulation. 133:e38–e360. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bertinchant JP: Brain natriuretic peptide
(BNP) and N-terminal-pro BNP in chronic haemodialysed renal
failure. Arch Mal Coeur Vaiss. 97:881–888. 2004.PubMed/NCBI(In French).
|
|
4
|
Skrzypek A, Mostowik M, Szeliga M,
Wilczynska-Golonka M, Debicka-Dabrowska D and Nessler J: Chronic
heart failure in the elderly: Still a current medical problem.
Folia Med Cracov. 58:47–56. 2018.PubMed/NCBI
|
|
5
|
Carella DM: Brain natriuretic peptide:
It's not about the brain or just another smart polypeptide-It's
about the heart. Neonatal Netw. 34:355–359. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Antonelli A, Ferri C, Ferrari SM, Colaci
M, Sebastiani M, Zignego AL, Ghiri E, Goglia F and Fallahi P: High
levels of circulating N-terminal pro-brain natriuretic peptide in
patients with hepatitis C. J Viral Hepat. 17:851–853.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wettersten N, Horiuchi Y, van Veldhuisen
DJ, Mueller C, Filippatos G, Nowak R, Hogan C, Kontos MC, Cannon
CM, Müeller GA, et al: B-type natriuretic peptide trend predicts
clinical significance of worsening renal function in acute heart
failure. Eur J Heart Fail. 21:1553–1560. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jurado-Roman A, Agudo-Quilez P,
Rubio-Alonso B, Molina J, Díaz B, García-Tejada J, Martín R and
Tello R: Superiority of wall motion score index over left ventricle
ejection fraction in predicting cardiovascular events after an
acute myocardial infarction. Eur Heart J Acute Cardiovasc Care.
8:78–85. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li J, Li Z, Zheng W, Li X, Wang Z, Cui Y
and Jiang X: LncRNA-ATB: An indispensable cancer-related long
noncoding RNA. Cell Prolif. 50(e12381)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gidlöf O, Bader K, Celik S, Grossi M,
Nakagawa S, Hirose T, Metzler B, Olde B and Erlinge D: Inhibition
of the long non-coding RNA NEAT1 protects cardiomyocytes from
hypoxia in vitro via decreased pri-miRNA processing. Cell Death
Dis. 11(677)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wei Q, Zhou HY, Shi XD, Cao HY and Qin L:
Long noncoding RNA NEAT1 promotes myocardiocyte apoptosis and
suppresses proliferation through regulation of miR-129-5p. J
Cardiovasc Pharmacol. 74:535–541. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xiao N, Zhang J, Chen C, Wan Y, Wang N and
Yang J: miR-129-5p improves cardiac function in rats with chronic
heart failure through targeting HMGB1. Mamm Genome. 30:276–288.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang H, Zhang X and Zhang J: MiR-129-5p
inhibits autophagy and apoptosis of H9c2 cells induced by hydrogen
peroxide via the PI3K/AKT/mTOR signaling pathway by targeting
ATG14. Biochem Biophys Res Commun. 506:272–277. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yancy CW, Jessup M, Bozkurt B, Butler J,
Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi
JL, et al: 2013 ACCF/AHA guideline for the management of heart
failure: A report of the American College of Cardiology
Foundation/American Heart Association Task Force on Practice
Guidelines. J Am Coll Cardiol. 62:e147–e239. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ponikowski P, Voors AA, Anker SD, Bueno H,
Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP,
Jankowska EA, et al: 2016 ESC Guidelines for the diagnosis and
treatment of acute and chronic heart failure. Rev Esp Cardiol (Engl
Ed). 69(1167)2016.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Caraballo C, Desai NR, Mulder H, Alhanti
B, Wilson FP, Fiuzat M, Felker GM, Piña IL, O'Connor CM, Lindenfeld
J, et al: Clinical implications of the New York Heart association
classification. J Am Heart Assoc. 8(e014240)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wan Y and Yang ZQ: LncRNA NEAT1 affects
inflammatory response by targeting miR-129-5p and regulating Notch
signaling pathway in epilepsy. Cell Cycle. 19:419–431.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ye J, Lin Y, Yu Y and Sun D: LncRNA
NEAT1/microRNA-1 29-5p/SOCS2 axis regulates liver fibrosis in
alcoholic steatohepatitis. J Transl Med. 18(445)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
McCarthy CP, McCarthy KJ and McEvoy JW:
Declining risk of sudden death in heart failure. N Engl J Med.
377(1793)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ponikowski P, Anker SD, AlHabib KF, Cowie
MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, et al:
Heart failure: Preventing disease and death worldwide. ESC Heart
Fail. 1:4–25. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Farnsworth CW, Bailey AL, Jaffe AS and
Scott MG: Diagnostic concordance between NT-proBNP and BNP for
suspected heart failure. Clin Biochem. 59:50–55. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Collin-Chavagnac D, Manchon M, Traulle C
and Bernon H: False-positive BNP results in a 78-year-old man
caused by monoclonal IgM-kappa: A case report. Clin Chim Acta.
384(179)2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ferre F, Colantoni A and Helmer-Citterich
M: Revealing protein-lncRNA interaction. Brief Bioinform.
17:106–116. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Song P, Jiang B, Liu Z, Ding J, Liu S and
Guan W: A three-lncRNA expression signature associated with the
prognosis of gastric cancer patients. Cancer Med. 6:1154–1164.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chao Y and Zhou D: lncRNA-D16366 Is a
potential biomarker for diagnosis and prognosis of hepatocellular
carcinoma. Med Sci Monit. 25:6581–6586. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Naizhaer G, Kuerban A, Meilipa Kuerban R
and Zhou P: Up-regulation of lncRNA FALEC indicates prognosis and
diagnosis values in cervical cancer. Pathol Res Pract.
215(152495)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li Z, Wei D, Yang C, Sun H, Lu T and Kuang
D: Overexpression of long noncoding RNA, NEAT1 promotes cell
proliferation, invasion and migration in endometrial endometrioid
adenocarcinoma. Biomed Pharmacother. 84:244–251. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yu X, Li Z, Zheng H, Chan MT and Wu WK:
NEAT1: A novel cancer-related long non-coding RNA. Cell Prolif.
50(e12329)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wu Y, Yang L, Zhao J, Li C, Nie J, Liu F,
Zhuo C, Zheng Y, Li B, Wang Z and Xu Y: Nuclear-enriched abundant
transcript 1 as a diagnostic and prognostic biomarker in colorectal
cancer. Mol Cancer. 14(191)2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pils D, Tong D, Hager G, Obermayr E, Aust
S, Heinze G, Kohl M, Schuster E, Wolf A, Sehouli J, et al: A
combined blood based gene expression and plasma protein abundance
signature for diagnosis of epithelial ovarian cancer-a study of the
OVCAD consortium. BMC Cancer. 13(178)2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Huang Q, Huang C, Luo Y, He F and Zhang R:
Circulating lncRNA NEAT1 correlates with increased risk, elevated
severity and unfavorable prognosis in sepsis patients. Am J Emerg
Med. 36:1659–1663. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang H, Cai Y, Zheng L, Zhang Z, Lin X
and Jiang N: Long noncoding RNA NEAT1 regulate papillary thyroid
cancer progression by modulating miR-129-5p/KLK7 expression. J Cell
Physiol. 233:6638–6648. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lo PK, Zhang Y, Wolfson B, Gernapudi R,
Yao Y, Duru N and Zhou Q: Dysregulation of the BRCA1/long
non-coding RNA NEAT1 signaling axis contributes to breast
tumorigenesis. Oncotarget. 7:65067–65089. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fu MC, Yuan LQ, Zhang T, Yan XM, Zhou Y,
Xia HL, Wu Y, Xu LX, Cao X and Wang J: Nuclear paraspeckle assembly
transcript 1 promotes the metastasis and epithelial-mesenchymal
transition of hepatoblastoma cells by inhibiting miR-129-5p. Oncol
Lett. 14:5773–5778. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang F, Wu L, Qian J, Qu B, Xia S, La T,
Wu Y, Ma J, Zeng J, Guo Q, et al: Identification of the long
noncoding RNA NEAT1 as a novel inflammatory regulator acting
through MAPK pathway in human lupus. J Autoimmun. 75:96–104.
2016.PubMed/NCBI View Article : Google Scholar
|