Introduction
Ovarian cancer (OC) is one of the deadliest
gynecological malignancies, with high incidence and mortality rates
worldwide (1,2). There were ~239,000 new cases and
152,000 deaths worldwide annually with regard to this disease in
2017(3). Despite advancements in
treatment options, the 5-year survival rate of patients with OC
remains <35% globally due to distant metastasis and recurrence
(4,5). Thus, it is urgent to obtain an
improved understanding regarding the pathogenesis underlying OC to
identify and develop more effective therapeutic targets for the
treatment of this disease.
F-box and WD repeat domain containing 7 (FBXW7), a
member of the F-box protein family, is an evolutionarily conserved
F-box protein, containing two vital functional domains (F-box and
WD), which are indispensable for its function (6). FBXW7 is a critical tumor suppressor
gene, where mutations within this gene have been implicated in
different types of human cancer (7,8). For
instance, FBXW7 can target salt inducible kinase 2 for degradation,
leading to the disruption of target of rapamycin 2-AKT signaling to
inhibit pancreatic cancer cell proliferation and cell cycle
progression (9). It has also been
reported that FBXW7 suppresses oral squamous cell carcinoma
proliferation and invasion regulated by miR-27a through the
PI3K/AKT signaling pathway (10).
Increasing evidence has suggested that FBXW7 serves as a key
regulator in the proliferation, invasion, migration and apoptosis
of human cancer cells through the degradation of oncoproteins,
including c-Myc, in a proteasome-dependent manner (11,12).
Previous studies have demonstrated that FBXW7 is deleted or
methylated in epithelial OC, and its expression is negatively
associated with the malignant potential of ovarian tumors (13,14).
FBXW7 has been reported to act as a positive regulator of
angiogenesis in the endothelium of the growing vasculature
(15). Increasing evidence has
suggested that angiogenesis serves a crucial role in the invasion,
migration and metastasis of OC, with a positive association between
the rate and extent of angiogenesis and an unfavorable prognosis in
patients with OC (16,17). Vascular endothelial growth factor
(VEGF), a homodimeric glycoprotein, is the key mediator of
angiogenesis, which binds two important VEGF receptors, VEGFR1 and
VEGFR2 (18,19). However, the effects of FBXW7 on the
angiogenesis of OC, and whether FBXW7 functions by regulating VEGF
expression, remain unclear.
The present study aimed to investigate VEGF
expression following overexpression of FBXW7 in OC cells. In
addition, the role of FBXW7 on the invasion, migration and
angiogenesis of OC cells, and its potential regulatory effects on
VEGF expression were assessed.
Materials and methods
Cell culture
The normal human ovarian epithelial cell line
(IOSE-80) and several OC cell lines (ES-2, SKOV3, PA1 and OVCAR3)
were purchased from the American Type Culture Collection and
maintained in DMEM (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (HyClone; Cytiva) at 37˚C with 5%
CO2. Cells were treated with the β-catenin activator,
lithium chloride (LiCl; 20 mM; Sigma-Aldrich; Merck KGaA) for 3 h
at 37˚C.
Human umbilical vein endothelial cells (HUVECs) were
provided The Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences and cultured in a mixture containing RPMI-1640
medium (HyClone; Cytiva) and 10% FBS. The cells were incubated at
37˚C in a humidified atmosphere which was maintained at 5%
CO2.
Cell transfection
SKOV3 cells were seeded into 6-well plates at a
density of 2x105 cells/well and cultured at 37˚C until
they reached 80% confluence. pcDNA 3.1 containing FBXW7 (Ov-FBXW7)
or VEGF (pc-VEGF), and the corresponding empty vectors used as
negative controls (Ov-NC and pc-NC, respectively) were synthesized
by Shanghai GenePharma Co., Ltd. SKOV3 cells were transfected with
the respective plasmids (50 nM) using Lipofectamine®
2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) at 37˚C
for 24 h, according to the manufacturer's instructions. Cells were
collected and the transfection efficiency was assessed via reverse
transcription-quantitative (RT-q) PCR and western blot analyses 24
h post-transfection.
Invasion assay
Cell invasion was assessed using Transwell assay
(pore size, 8.0 µm; Corning Inc.) precoated with Matrigel (6.25
mg/l; BD Biosciences) overnight at 4˚C. A total of 2x105
transfected SKOV3 cells were resuspended in 200 µl serum-free DMEM
and plated in the upper chambers of Transwell plates, whilst 600 µl
DMEM supplemented with 10% FBS was used as the chemoattractant and
plated in the lower chambers. Following incubation for 24 h at
37˚C, the invasive cells were fixed with 4% formaldehyde for 30 min
at room temperature and subsequently stained with 0.1% crystal
violet for 30 min at room temperature. Stained cells were counted
in five randomly selected fields using an inverted light microscope
(Olympus Corporation; magnification, x100) and the results were
analyzed using ImageJ software (version 1.52r; National Institutes
of Health).
Wound healing assay
Cell migration was assessed using wound healing
assays. Briefly, SKOV3 cells were seeded into 6-well plates at a
density of 4x105 cells/well and cultured in DMEM
supplemented with 10% FBS until they reached 80% confluence. The
cell monolayers were scratched using sterile 200-µl pipette tips.
Cells were washed with PBS to elute the debris, and the medium was
replaced with serum-free DMEM. Following incubation for 24 h at
37˚C, the average distance of cells migrated into the wound surface
was observed under an inverted light microscope (Olympus
Corporation; magnification, x100). Quantitative analysis of the
wound healing area was performed using ImageJ software (version
1.52r; National Institutes of Health).
Tube formation assay
A total of 1.5x104 HUVECs were seeded
into 96-well plates precoated with Matrigel (10 mg/ml; BD
Biosciences) at 4˚C overnight and incubated for 6 h at 37˚C in a 5%
CO2 incubator with the supernatants from SKOV3 cells
transfected with Ov-FBXW7 or/and pc-VEGF. Tube formation was
observed under an inverted light microscope (Olympus Corporation).
The number of loops formed was counted in five randomly selected
fields and analyzed using ImageJ software (version 1.52r; National
Institutes of Health). A connection between two cells was counted
as one capillary tube formation.
RT-qPCR
Total RNA was extracted from SKOV3 cells using
TRIzol® reagent (Thermo Fisher Scientific, Inc.). Total
RNA was reverse transcribed into cDNA using the First Strand cDNA
synthesis kit (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. qPCR was subsequently performed with 2 µg
cDNA using the SYBR Premix Ex Taq (Takara Bio, Inc.) and ABI 7500
equipment (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
following thermocycling conditions were used: Initial denaturation
at 95˚C for 10 min; followed by 40 cycles of denaturation at 95˚C
for 15 sec and annealing at 60˚C for 1 min; and a final extension
of 10 min at 72˚C. The sequences of the gene-specific primers used
in the present study were as follows FBXW7 forward, 5'-CACAGG
CCTTCAAGAGTGGC-3' and reverse, 5'-TTGCATCATATG CTTCACTTGTGT-3';
VEGF forward, 5'-GGGCAGAATCAT CACGAAGT-3' and reverse,
5'-AAATGCTTTCTCCGCTC TGA-3'; CD31 forward, 5'-TGCAGTGGTTATCATCGGA
GTG-3' and reverse, 5'-CGTTGTTGGAGTTCAGAAGTG-3'; VEGFR1 forward,
5'-TGCCTCAGAAGAGCTGAAAAC-3' and reverse,
5'-CACAGACTCCCTGCTTTTGCT-3'; VEGFR2 forward,
5'-GCACATTGGTGGTGGCTGAC-3' and reverse, 5'-CTCTCCTTCGGCTGGCATCT-3'
and GAPDH forward, 5'-ACAACTTTGGTATCGTGGAAGG-3' and reverse,
5'-GCCATCACGCCACAGTTTC-3'. Relative expression levels were
calculated using the 2-ΔΔCq method and normalized to the
internal reference gene GAPDH (20).
Western blotting
Total protein was extracted from SKOV3 cells using
RIPA lysis buffer (Beyotime Institute of Biotechnology). Total
protein was quantified using the bicinchoninic acid kit (Beyotime
Institute of Biotechnology) and 4 µg protein/lane was separated by
10% SDS-PAGE. The separated proteins were subsequently transferred
onto polyvinylidene fluoride membranes (EMD Millipore) and blocked
with 5% skimmed milk for 1.5 h at room temperature. The membranes
were incubated with corresponding primary antibodies at 4˚C
overnight. Following the primary antibody incubation, membranes
were incubated with a goat anti-rabbit horseradish peroxidase
(HRP)-conjugated secondary antibody (1:3,000; cat. no. 7074S; Cell
Signaling Technology, Inc.) or horse anti-mouse HRP-conjugated
secondary antibody (1:3,000; cat. no. 7076S; Cell Signaling
Technology) for 1.5 h at room temperature. The immunoreactive
protein bands on the membranes were visualized using an enhanced
chemiluminescence assay (EMD Millipore). The relative intensity of
target bands were semi-quantified using ImageJ software (version
1.52r; National Institutes of Health) and normalized by the
intensity of GAPDH. Anti-FBXW7 (cat. no. ab109617; 1:1,000)
antibody was provided by Abcam. Anti-VEGF (cat. no. 2463S;
1:1,000), anti-CD31 (cat. no. 3528S; 1:1,000), anti-VEGFR1 (cat.
no. 64094S; 1:1,000), VEGFR2 (cat. no. 9698S; 1:1,000),
anti-E-cadherin (E-cad; cat. no. 3195T; 1:1,000), anti-N-cadherin
(N-cad; cat. no. 13116T; 1:1,000), anti-Slug (cat. no. 9585T;
1:1,000) and anti-GAPDH (cat. no. 5174T; 1:1,000) antibodies were
all purchased from Cell Signaling Technology, Inc.
Statistical analysis
All experiments were repeated three times
independently. Statistical analysis was performed using GraphPad
Prism 8.0 (GraphPad Software, Inc.). Data are presented as the mean
± SD. An unpaired Student's t-test was used to compare differences
between two groups, while one-way ANOVA followed by Tukey's post
hoc test was used to compare differences among multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
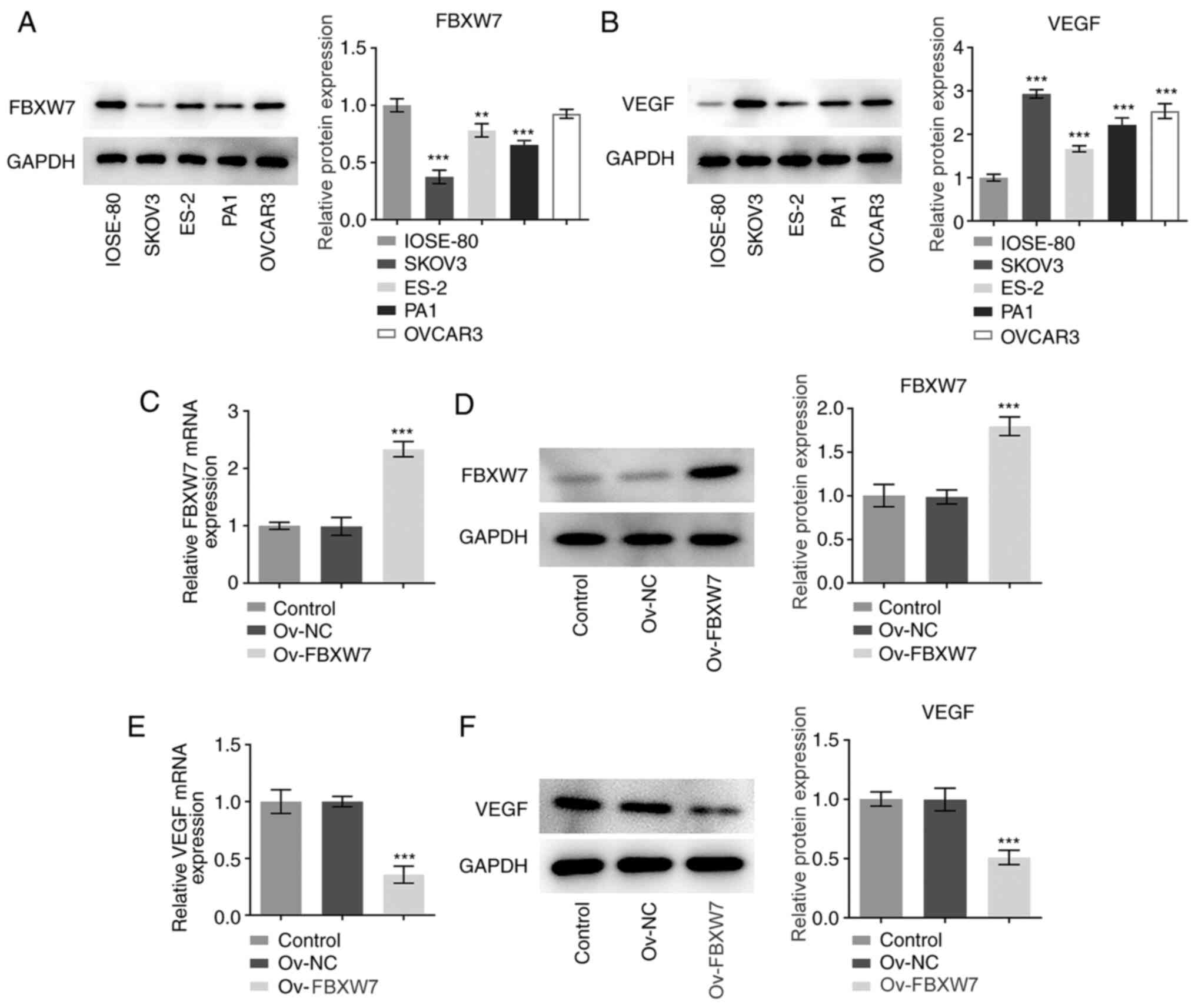
Overexpression of FBXW7 significantly
downregulates VEGF expression in OC cells
Firstly, the expression levels of FBXW7 and VEGF in
a normal human ovarian epithelial cell line (IOSE-80) and several
OC cell lines (ES-2, SKOV3, PA1 and OVCAR3) were detected via
western blot analysis. As shown in Fig.
1A and B, FBXW7 expression was
markedly downregulated, while VEGF expression was significantly
upregulated in OC cell lines compared with in IOSE-80 cells,
especially in SKOV3 cells, which were therefore used in subsequent
experiments. Subsequently, FBXW7 was overexpressed in SKOV3 cells.
As presented in Fig. 1C and
D, FBXW7 expression was
significantly upregulated at both the transcriptional and protein
levels following transfection with Ov-FBXW7 compared with those
after transfection with Ov-NC. Notably, overexpression of FBXW7
significantly decreased VEGF mRNA and protein expression compared
with the vector control group (Fig.
1E and F). Overall, these
results suggested that overexpression of FBXW7 inhibited VEGF
expression in OC cells.
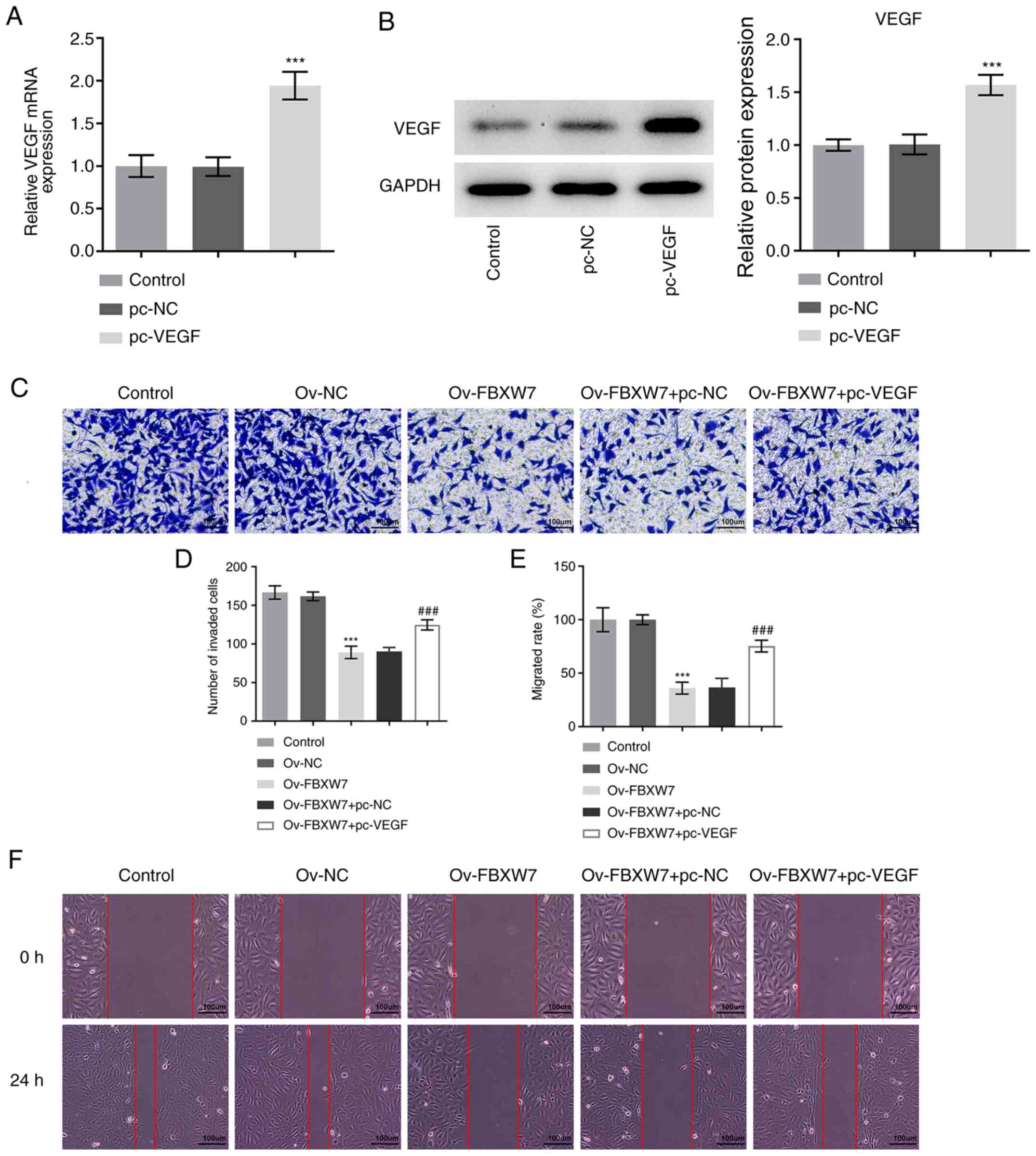
Overexpression of VEGF restores the
inhibitory effects of FBXW7 overexpression on the invasion,
migration and epithelial-to-mesenchymal transition (EMT) of OC
cells
To determine whether FBXW7 regulates VEGF expression
in OC, VEGF was overexpressed following transfection with a plasmid
containing VEGF. As presented in Fig.
2A and B, VEGF mRNA and protein
expression was significantly increased in the pc-VEGF group
compared with in the pc-NC group.
Transwell and wound healing assays were performed to
assess the invasive and migratory abilities of SKOV3 cells,
respectively. As presented in Fig.
2C-F, overexpression of FBXW7 significantly inhibited the
invasive and migratory abilities of SKOV3 cells compared with the
Ov-NC group. Conversely, co-transfection with VEGF and FBXW7
overexpression plasmids significantly promoted the invasive and
migratory abilities of SKOV3 cells compared with cells transfected
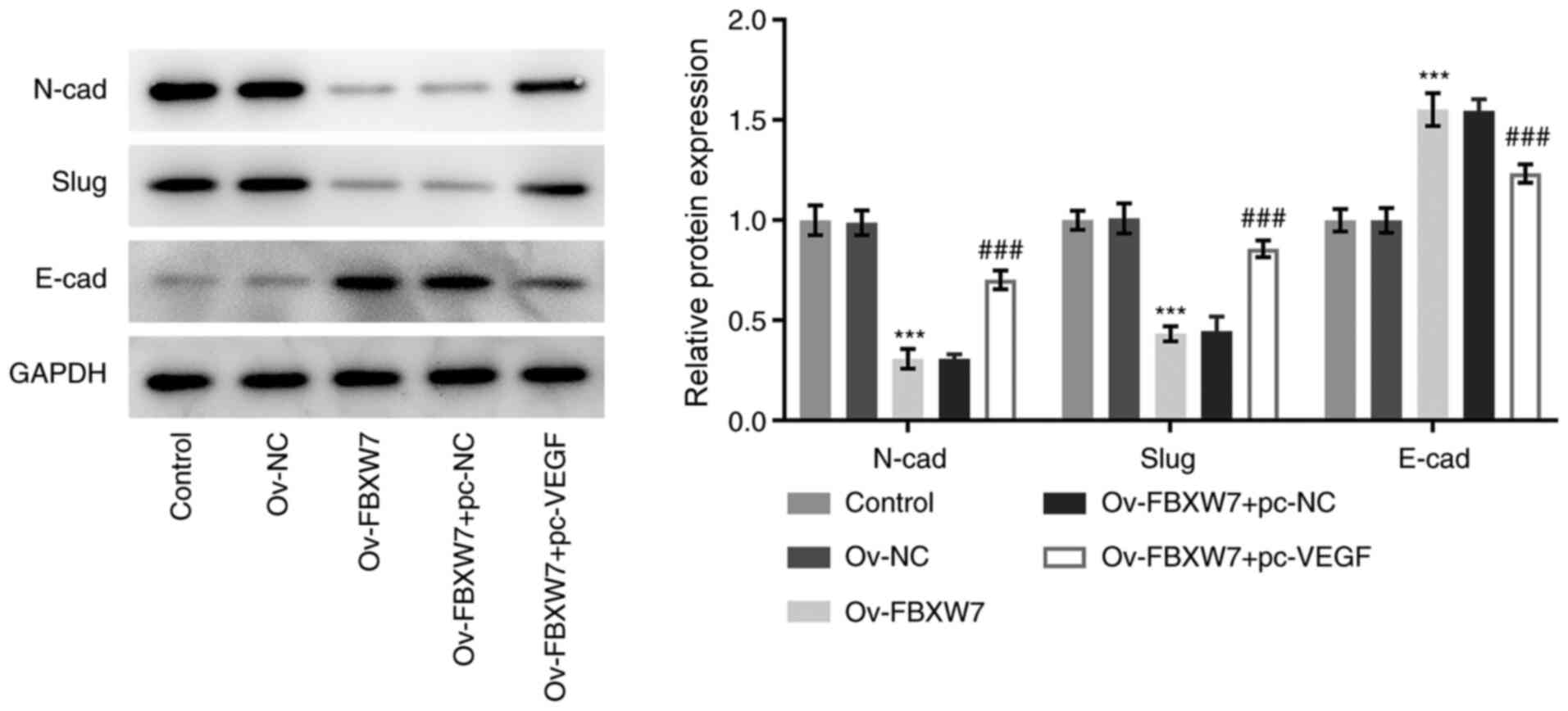
with the FBXW7 overexpression plasmid alone (Fig. 2C-F). Additionally, overexpression of
FBXW7 significantly decreased the expression levels of N-cadherin
and slug, while significantly increasing E-cadherin expression, and
these effects were reversed following co-transfection with the VEGF
overexpression plasmid (Fig. 3).
Collectively, these results indicated that overexpression of FBXW7
inhibited the invasion, migration and EMT process of OC cells by
suppressing VEGF expression.
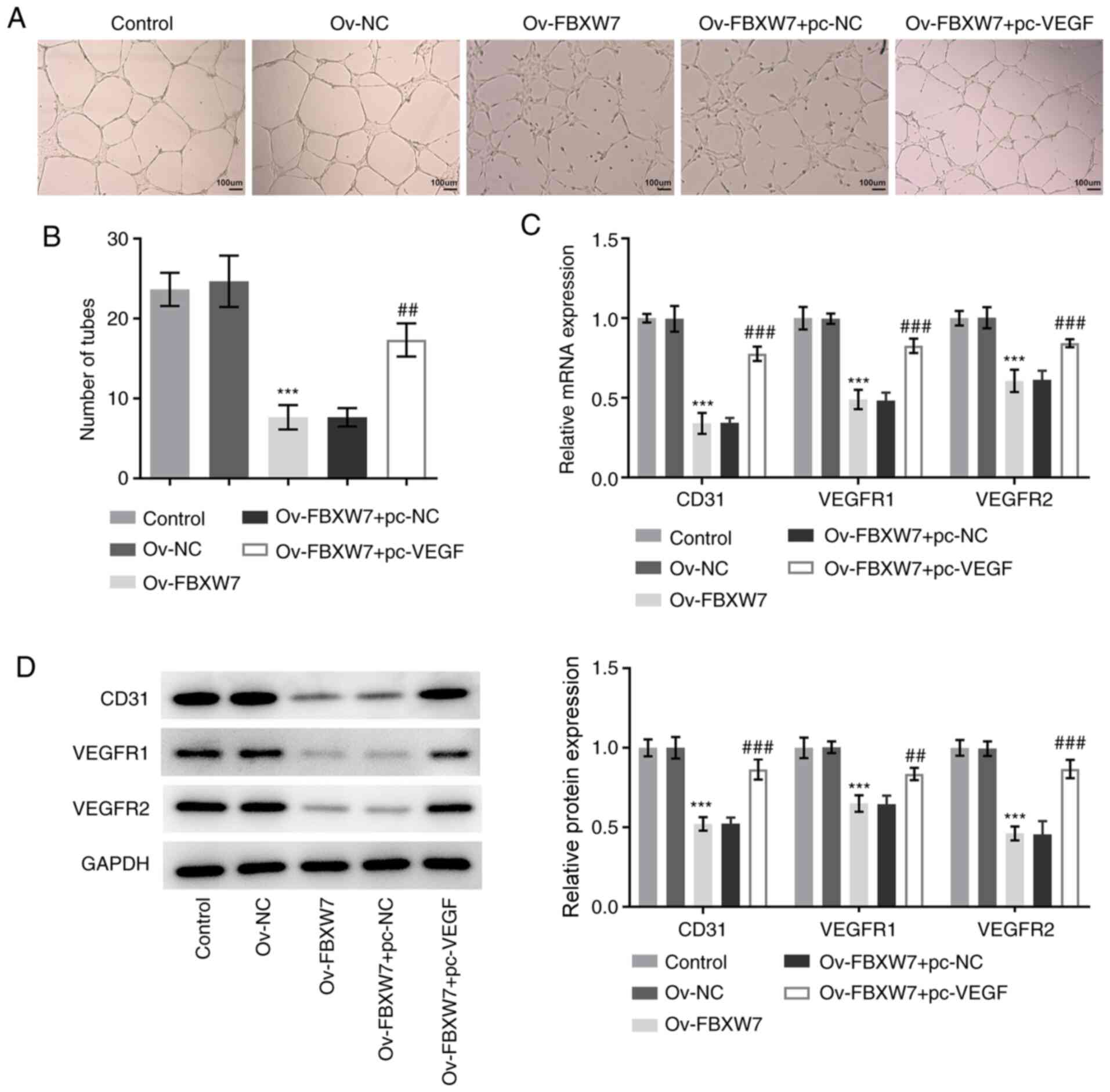
Overexpression of VEGF partially
counteracts the impact of FBXW7 overexpression on the angiogenesis
of OC cells
To investigate the effect of FBXW7 on the
angiogenesis of OC cells, a tube formation assay was performed. As
presented in Fig. 4A and B, there was no significant difference in
the number of tubes formed between the control and the Ov-NC
groups. However, a significantly decreased number of tubes was
observed in the Ov-FBXW7 group compared with in the Ov-NC group
(Fig. 4A and B). Notably, overexpression of VEGF
enhanced the number of tubes formed compared with the
Ov-FBXW7+pc-NC group (Fig. 4A and
B). RT-qPCR and western blot
analyses were performed to detect the expression levels of proteins
associated with angiogenesis. The results demonstrated that
overexpression of FBXW7 downregulated the expression levels of
CD31, VEGFR1 and VEGFR, whereas co-transfection with FBXW7 and VEGF
plasmids significantly increased their expression levels compared
with the Ov-FBXW7+pc-NC group (Fig.
4C and D), which is consistent
with the results of the tube formation assay. Overall, these
results suggested that overexpression of FBXW7 suppressed the
angiogenesis of OC cells by suppressing VEGF expression.
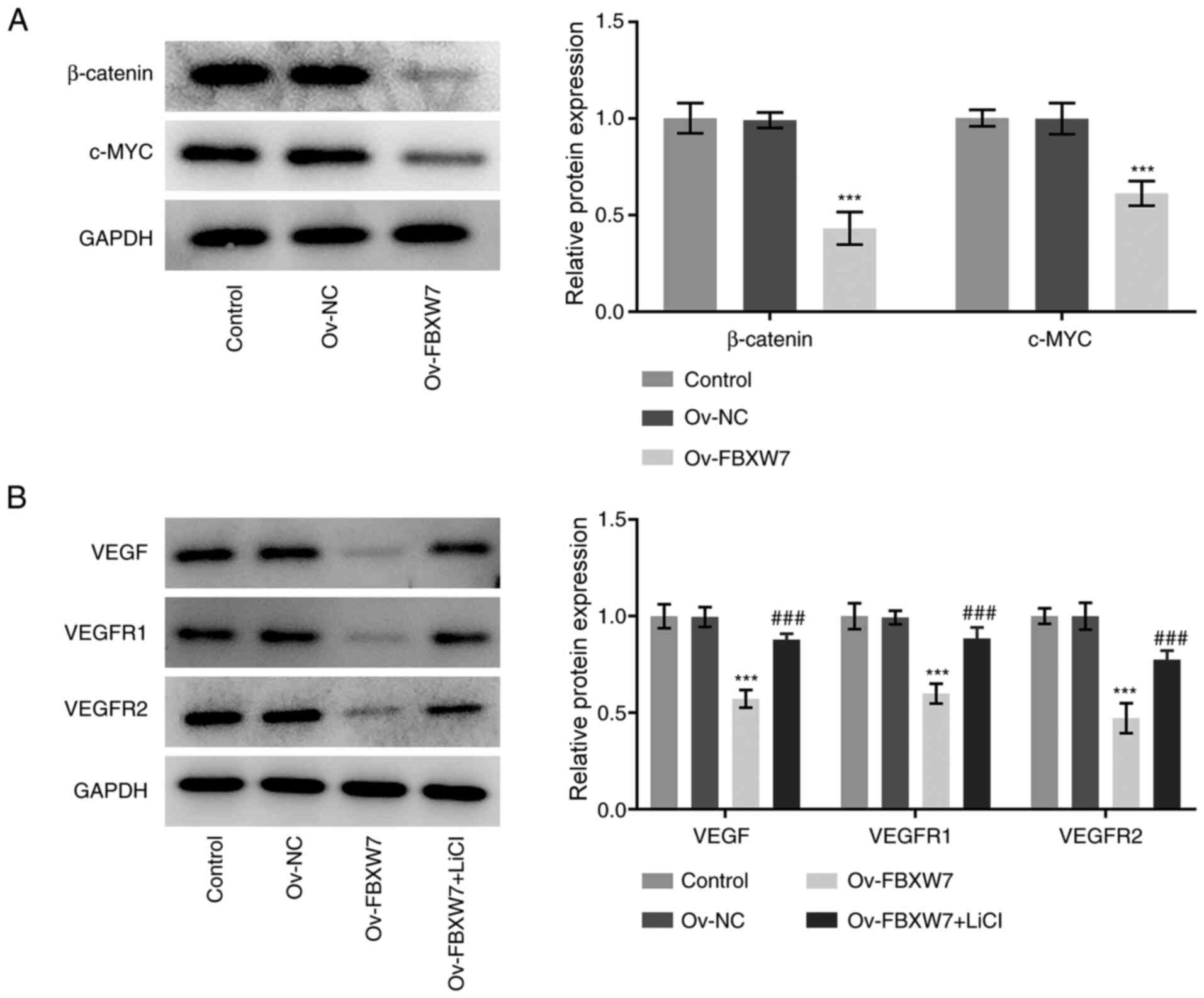
FBXW7 inhibits VEGF expression through
inactivation of β-catenin signaling
To further elucidate the potential molecular
mechanism by which FBXW7 mediates its antitumor effects in OC,
western blot analysis was performed to detect the expression levels
of key proteins in β-catenin signaling. As presented in Fig. 5A, overexpression of FBXW7
significantly decreased the expression levels of β-catenin and
c-Myc compared with the empty vector group. Furthermore, LiCl, an
agonist of β-catenin signaling, was used to treat SKOV3 cells
transfected with the FBXW7 plasmid, and the expression levels of
angiogenesis-associated proteins were determined. As presented in
Fig. 5B, treatment with LiCl
significantly abrogated the inhibitory effects of FBXW7
overexpression on the expression levels of VEGF, VEGFR1 and VEGFR2.
Collectively, these results suggested that FBXW7 may inhibit VEGF
expression through inactivation of β-catenin signaling in SKOV3
cells.
Discussion
OC is one of the most common and lethal types of
cancer in women, and has been a significant public health burden
worldwide (21). Thus, it is of
great importance to further understand the molecular mechanisms of
OC tumorigenesis and progression to identify and develop effective
therapeutic strategies. The results of the present study
demonstrated that FBXW7 efficiently inhibited SKOV3 cell invasion
and migration, as well as tube formation of HUVECs.
Mechanistically, FBXW7 suppressed VEGF expression by inactivating
β-catenin signaling.
Invasion and migration are two hallmarks of the
malignant biological behavior of OC, and interdiction of these
progresses is a critical factor to improve biomedical treatment
worldwide (22,23). EMT, a process in which stationary
epithelial cells attain a highly active mesenchymal phenotype, is
an essential and important step in tumor cell invasion, migration
and relocalization (24-26).
Downregulated expression levels of E-cadherin (a crucial epithelial
marker) in epithelial cells, along with upregulated expression
levels of mesenchymal proteins, including N-cadherin and slug, are
common hallmarks of EMT (27,28).
Angiogenesis refers to the generation of new blood vessels by the
sprouting of endothelial cells from preexisting ones (29). It has been reported that tumors
depend on the constant growth of new blood vessels, whereby
interruption of the blood supply may eliminate the cancer (30). Increasing evidence has suggested
that angiogenesis is essential for cancer development by
participating in the growth, invasion, migration and metastasis of
cancer (31,32). FBXW7 is a vital tumor suppressor
gene, and mutations in this gene have been implicated in different
types of human cancer. For example, upregulated FBXW7 expression
inhibits tumor invasion, migration, EMT and angiogenesis, including
oral squamous cell carcinoma, breast cancer and non-small-cell lung
cancer (10,33-35).
Notably, FBXW7 has been reported to act as a potent positive
regulator of angiogenesis in the endothelium of the growing
vasculature (15). FBXW7 expression
is downregulated in OC tissues, and low FBXW7 expression is
negatively associated with the malignant potential of OC (14). Increasing evidence has suggested
that FBXW7-knockdown can promote OC cell invasion and migration
(36). The results of the present
study demonstrated that overexpression of FBXW7 inhibited the
invasion, migration, EMT and angiogenesis of OC cells. To the best
of our knowledge, the present study was the first to demonstrate
the inhibitory effect of FBXW7 on the angiogenesis of OC.
VEGF, a homodimeric glycoprotein, is one of the most
potent and specific angiogenic factors of tumor-induced
angiogenesis and binds to VEGFR1 and VEGFR2 (18,19).
Elevated VEGF expression has been observed in several OC cell lines
and OC biopsies of different histological grades (37). A previous study has demonstrated
that FBXW7 can block the effect of microRNA-182 on VEGF induction
and angiogenesis in breast cancer cells (38). However, whether FBXW7 regulates VEGF
expression in the progression of OC remains unclear.
The results of the present study demonstrated that
overexpression of FBXW7 suppressed VEGF expression, while
overexpression of VEGF partially counteracted the inhibitory
effects of FBXW7 overexpression on the invasion, migration, EMT and
angiogenesis of OC cells. To further elucidate the potential
molecular mechanism by which FBXW7 mediates its antitumor effects
in OC, the expression levels of key proteins in β-catenin signaling
were analyzed. Increasing evidence suggests that inhibition of the
β-catenin signaling pathway restrains the proliferation, invasion,
migration and angiogenesis of different types of cancer, including
retinoblastoma, gastric cancer and OC (39-41).
Previous studies have demonstrated that β-catenin signaling helps
VEGF regulate angiogenesis, and that FBXW7 promotes the degradation
of β-catenin (42,43). The results of the present study
demonstrated that overexpression of FBXW7 inhibited the expression
levels of VEGF, β-catenin and c-Myc. Notably, treatment with LiCl,
an agonist of β-catenin signaling, increased the expression levels
of VEGF, VEGFR1 and VEGFR2. Overall, the current results suggested
that FBXW7 may inhibit VEGF expression through inactivation of
β-catenin signaling in SKOV3 cells.
In conclusion, the results of the present study
demonstrated that FBXW7 inhibited the invasion, migration and
angiogenesis of OC cells. To the best of our knowledge, the present
study was the first to provide insight into the anti-angiogenesis
effects of FBXW7 on OC, and to suggest a promising therapeutic
potential of FBXW7 in the targeted treatment of this disease.
However, the use of a single OC cell line to analyze FBXW7
expression and its potential mechanisms in OC is a limitation of
the present study. Therefore, future studies should confirm the
results of the present study using multiple OC cell lines and in
vivo models.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and Technology
Planning Project of Huzhou City, Zhejiang Province (grant no.
2019GY01).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ and YP searched the literature, designed the
experiments and performed the experiments. YP and JS analyzed and
interpreted the data. LZ wrote the manuscript. JS revised the
manuscript. LZ and JS can authenticate the raw data in the present
study. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ebell MH, Culp MB and Radke TJ: A
systematic review of symptoms for the diagnosis of ovarian cancer.
Am J Prev Med. 50:384–394. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Reid BM, Permuth JB and Sellers TA:
Epidemiology of ovarian cancer: A review. Cancer Biol Med. 14:9–32.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pei H, Yang Y, Cui L, Yang J, Li X, Yang Y
and Duan H: Bisdemethoxycurcumin inhibits ovarian cancer via
reducing oxidative stress mediated MMPs expressions. Sci Rep.
6(28773)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rodriguez-Garcia A, Sharma P, Poussin M,
Boesteanu AC, Minutolo NG, Gitto SB, Omran DK, Robinson MK, Adams
GP, Simpkins F, et al: CAR T Cells Targeting MISIIR for the
Treatment of Ovarian Cancer and Other Gynecologic Malignancies. Mol
Ther. 28:548–560. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xiao G, Zhang B, Meng J, Wang J, Xu C,
Tang SC, Li X, Zhang J, Liang R, Ren H, et al: miR-367 stimulates
Wnt cascade activation through degrading FBXW7 in NSCLC stem cells.
Cell Cycle. 16:2374–2385. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yeh CH, Bellon M and Nicot C: FBXW7: A
critical tumor suppressor of human cancers. Mol Cancer.
17(115)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhan P, Wang Y, Zhao S, Liu C, Wang Y, Wen
M, Mao JH, Wei G and Zhang P: FBXW7 negatively regulates ENO1
expression and function in colorectal cancer. Lab Invest.
95:995–1004. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang MX, Wang H and Sun GP:
Tumor-suppressor Fbxw7 targets SIK2 for degradation to interfere
with TORC2-AKT signaling in pancreatic cancer. Cell Biol Int.
44:1900–1910. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li C, Lin XF, Wang JN and Ren XS: FBXW7
inhibited cell proliferation and invasion regulated by miR-27a
through PI3K/AKT signaling pathway and epithelial-to-mesenchymal
transition in oral squamous cell carcinoma. Eur Rev Med Pharmacol
Sci. 24:3701–3709. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mao JH, Perez-Losada J, Wu D, Delrosario
R, Tsunematsu R, Nakayama KI, Brown K, Bryson S and Balmain A:
Fbxw7/Cdc4 is a p53-dependent, haploinsufficient tumour suppressor
gene. Nature. 432:775–779. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhao J, Wang Y, Mu C, Xu Y and Sang J:
MAGEA1 interacts with FBXW7 and regulates ubiquitin ligase-mediated
turnover of NICD1 in breast and ovarian cancer cells. Oncogene.
36:5023–5034. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
De La Chesnaye E, Méndez JP, López-Romero
R, De Los Angeles Romero-Tlalolini M, Vergara MD, Salcedo M and
Ojeda SR: FBXW12, a novel F box protein-encoding gene, is deleted
or methylated in some cases of epithelial ovarian cancer. Int J
Clin Exp Pathol. 8:10192–10203. 2015.PubMed/NCBI
|
|
14
|
Kitade S, Onoyama I, Kobayashi H, Yagi H,
Yoshida S, Kato M, Tsunematsu R, Asanoma K, Sonoda K, Wake N, et
al: FBXW7 is involved in the acquisition of the malignant phenotype
in epithelial ovarian tumors. Cancer Sci. 107:1399–1405.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Izumi N, Helker C, Ehling M, Behrens A,
Herzog W and Adams RH: Fbxw7 controls angiogenesis by regulating
endothelial Notch activity. PLoS One. 7(e41116)2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rivera LB and Bergers G: CANCER. Tumor
angiogenesis, from foe to friend. Science. 349:694–695.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ye W, Ni Z, Yicheng S, Pan H, Huang Y,
Xiong Y and Liu T: Anisomycin inhibits angiogenesis in ovarian
cancer by attenuating the molecular sponge effect of the lncRNA
Meg3/miR 421/PDGFRA axis. Int J Oncol. 55:1296–1312.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Carmeliet P: VEGF as a key mediator of
angiogenesis in cancer. Oncology. 69 (Suppl 3):4–10.
2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sadremomtaz A, Mansouri K, Alemzadeh G,
Safa M, Rastaghi AE and Asghari SM: Dual blockade of VEGFR1 and
VEGFR2 by a novel peptide abrogates VEGF-driven angiogenesis, tumor
growth, and metastasis through PI3K/AKT and MAPK/ERK1/2 pathway.
Biochim Biophys Acta Gen Subj. 1862:2688–2700. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ai B, Bie Z, Zhang S and Li A: Paclitaxel
targets VEGF-mediated angiogenesis in ovarian cancer treatment. Am
J Cancer Res. 6:1624–1635. 2016.PubMed/NCBI
|
|
22
|
Xu J, Zhang P, Sun H and Liu Y:
LINC01094/miR-577 axis regulates the progression of ovarian cancer.
J Ovarian Res. 13(122)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kappes L, Amer RL, Sommerlatte S, Bashir
G, Plattfaut C, Gieseler F, Gemoll T, Busch H, Altahrawi A,
Al-Sbiei A, et al: Ambrisentan, an endothelin receptor type
A-selective antagonist, inhibits cancer cell migration, invasion,
and metastasis. Sci Rep. 10(15931)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ombrato L and Malanchi I: The EMT
universe: Space between cancer cell dissemination and metastasis
initiation. Crit Rev Oncog. 19:349–361. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ji L, Li X, Zhou Z, Zheng Z, Jin L and
Jiang F: LINC01413/hnRNP-K/ZEB1 axis accelerates cell proliferation
and EMT in colorectal cancer via inducing YAP1/TAZ1 translocation.
Mol Ther Nucleic Acids. 19:546–561. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Peng Y, Li Y, Li Y, Wu A, Fan L, Huang W,
Fu C, Deng Z, Wang K, Zhang Y, et al: HOXC10 promotes tumour
metastasis by regulating the EMT-related gene Slug in ovarian
cancer. Aging (Albany NY). 12:19375–19398. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454.
2002.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Xin L, Zhao R, Lei J, Song J, Yu L, Gao R,
Ha C, Ren Y, Liu X, Liu Y, et al: SND1 acts upstream of SLUG to
regulate the epithelial-mesenchymal transition (EMT) in SKOV3
cells. FASEB J. 33:3795–3806. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Folkman J: Angiogenesis. Annu Rev Med.
57:1–18. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zheng Y, Chen H, Zhao Y, Zhang X, Liu J,
Pan Y, Bai J and Zhang H: Knockdown of FBXO22 inhibits melanoma
cell migration, invasion and angiogenesis via the HIF-1α/VEGF
pathway. Invest New Drugs. 38:20–28. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen L, Lin G, Chen K, Liang R, Wan F,
Zhang C, Tian G and Zhu X: VEGF promotes migration and invasion by
regulating EMT and MMPs in nasopharyngeal carcinoma. J Cancer.
11:7291–7301. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wu XP, Chen H, Wu MT, Peng SG and Zhang L:
Downregulation of miR-182-5p inhibits the proliferation and
invasion of triple-negative breast cancer cells through regulating
TLR4/NF-kappa B pathway activity by targeting FBXW7. Ann Transl
Med. 8(13)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chiang CH, Chu PY, Hou MF and Hung WC:
MiR-182 promotes proliferation and invasion and elevates the HIF-1
alpha-VEGF-A axis in breast cancer cells by targeting FBXW7. Am J
Cancer Res. 6:1785–1798. 2016.PubMed/NCBI
|
|
35
|
Xiao G, Li Y, Wang M, Li X, Qin S, Sun X,
Liang R, Zhang B, Du N, Xu C, et al: FBXW7 suppresses
epithelial-mesenchymal transition and chemo-resistance of
non-small-cell lung cancer cells by targeting snai1 for
ubiquitin-dependent degradation. Cell Prolif.
51(e12473)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Guo Y, Zhang Z, Wang Z, Liu G, Liu Y and
Wang H: Astragalus polysaccharides inhibit ovarian cancer cell
growth via microRNA-27a/FBXW7 signaling pathway. Biosci Rep: Mar
17, 2020 (Epub ahead of print). doi: 10.1042/BSR20193396.
|
|
37
|
Inan S, Vatansever S, Celik-Ozenci C,
Sanci M, Dicle N and Demir R: Immunolocalizations of VEGF, its
receptors flt-1, KDR and TGF-beta's in epithelial ovarian tumors.
Histol Histopathol. 21:1055–1064. 2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chiang CH, Chu PY, Hou MF and Hung WC:
MiR-182 promotes proliferation and invasion and elevates the
HIF-1α-VEGF-A axis in breast cancer cells by targeting FBXW7. Am J
Cancer Res. 6:1785–1798. 2016.PubMed/NCBI
|
|
39
|
Liao YJ, Yin XL, Deng Y and Peng XW: PRC1
gene silencing inhibits proliferation, invasion, and angiogenesis
of retinoblastoma cells through the inhibition of the Wnt/β-catenin
signaling pathway. J Cell Biochem. 120:16840–16852. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang F, Zhu W, Yang R, Xie W and Wang D:
LncRNA ZEB2-AS1 contributes to the tumorigenesis of gastric cancer
via activating the Wnt/β-catenin pathway. Mol Cell Biochem.
456:73–83. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Dai J, Wei R, Zhang P and Kong B:
Overexpression of microRNA-195-5p reduces cisplatin resistance and
angiogenesis in ovarian cancer by inhibiting the PSAT1-dependent
GSK3β/β-catenin signaling pathway. J Transl Med.
17(190)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Shi L, Yang F, Luo F, Liu Y, Zhang F, Zou
M and Liu Q: Evodiamine exerts anti-tumor effects against
hepatocellular carcinoma through inhibiting β-catenin-mediated
angiogenesis. Tumour Biol. 37:12791–12803. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Jiang JX, Sun CY, Tian S, Yu C, Chen MY
and Zhang H: Tumor suppressor Fbxw7 antagonizes WNT signaling by
targeting β-catenin for degradation in pancreatic cancer. Tumour
Biol. 37:13893–13902. 2016.PubMed/NCBI View Article : Google Scholar
|