Introduction
Macular hole was first described by Knapp in 1869
and later by Noyes (1872) in patients with ocular trauma (1,2). A
macular hole may occur as a result of the development of macular
cystoid edema due to inflammation and retinal vascular disease
(diabetic retinopathy, vascular occlusions, hypertensive
retinopathy, myopia, macular pucker, retinal detachment, and less
frequent phototrauma) (3).
The internal limiting membrane (ILM), the basement
membrane of the Müller cells, is composed of collagen fibers,
glycosaminoglycans, laminin, and fibronectin and it serves as the
connection between the vitreous body and the retinal nerve fiber
layer. It is said to be almost 1.5 µm in the peripheral foveal
area, where it is the thickest (4).
The ILM helps the cellular proliferation of myofibroblasts,
fibrocytes, and retinal pigment epithelium (RPE) cells (5). The role of the ILM is fundamental in
the development, structure, and function of the retina, although it
can represent a pathologic component, especially in macular holes
(6).
Nowadays, surgical techniques such as ILM peeling
are widely used for macular hole, macular puckers, epiretinal
membranes, diabetic macular edema, retinal detachment, retinal vein
occlusions, vitreomacular traction, optic pit maculopathy, and
Terson syndrome (7). In a pilot
study performed in 1989, Kelly and Wendel performed vitrectomy and
posterior cortical removal to ease traction on the macula, shedding
light on ILM peeling as a possible therapy for the treatment of
full-thickness macular holes. Before this occurred, idiopathic
macular holes were considered to be untreatable (8). Not much longer after the pilot study,
in the 1990s, for the treatment of hemorrhagic macular cysts due to
Terson syndrome, Morris et al performed ILM peeling
(9).
Intraocular surgery uses non-therapeutic agents as
intraocular dyes for one or two steps when needed. The use of dyes
for these steps is optional, not mandatory, for surgical success.
These substances are efficient, and may even have a beneficial
effect on the vitreoretinal surgery. Staining these tissues using
vital dyes simplifies the surgical procedure. The first to perform
intravitreal injection of a dye on animals was Lobeck in
1932(10). Sodium fluorescein was
the first dye used on humans, in order to identify the transparent
vitreous during pars plana vitrectomy (PPV) by Abrams and
colleagues (11).
Intraocular dyes primarily used for chromovitrectomy
include triamcinolone acetonide (TA) for vitreous identification,
indocyanine green (ICG), Brilliant Blue for the identification of
the ILM and Trypan Blue (TrB) for epiretinal membrane (ERM)
identification (12). TA is a
synthetic insoluble corticosteroid. In ocular surgery, TA functions
like a dye to stain the vitreous, mainly because of crystal
deposition (13,14).
Due to the iodide component and affinity for RPE,
ICG introduced by Kadonoso et al is toxic and induces
chemical trauma (15).
TrB colors the cell membrane of dead tissues and has
a strong affinity for the ERM. Brilliant blue G (BBG) (16) is intense when staining ILM, is easy
to remove, and is unique among currently popular dyes. Recently, a
good safety profile has also been described for the use of lutein
and zeaxanthin-based dyes during ocular surgery.
There are two known techniques for staining the
vitreous cavity with vital dyes. The first one is the ‘dry
technique’, when the eye is full of air after removing the liquid.
The second one is the ‘wet method’, when injecting the dye with the
eye full of liquid, taking into account the fact that the dye
concentration should be lower because it is diluted in the vitreous
cavity fluid (17).
Due to the multifactorial etiology of macular holes
and in order to fulfill the inclusion and exclusion criteria, all
subjects enrolled in this study were tested for associated ocular
and general diseases. All patients enrolled in this study underwent
ophthalmological evaluation that included: Visual acuity,
intraocular pressure, anterior pole and posterior pole examination,
in order to establish the macular hole etiology and any surgery
contraindications. Also, a complete blood count was performed
during hospitalization (18-25).
The aim of this study was to present and investigate
the safety and efficacy of vital dyes for macular pathology after
PPV in large full-thickness macular holes (MHs). The design of the
study was constituted by comparative retrospective case series.
Patients and methods
Patients
Thirty eyes of 30 different patients with MHs were
utilized in the study. The cause of the visual acuity drop was an
idiopathic MH. We divided the patients into two groups, depending
on the dye used intraoperatively: 15 eyes colored with Brilliant
blue G (BBG group) and 15 eyes colored with lutein/zeaxanthin (L/Z
group).
The study was approved by the Ethics Committee
(number 403) of ‘Dr. Carol Davila’ Central Military Emergency
University Hospital Bucharest. Informed consent was signed by all
patients. Patients inclusion and exclusion criteria are presented
in Table I.
 | Table IPatient inclusion and exclusion
criteria. |
Table I
Patient inclusion and exclusion
criteria.
| Inclusion
criteria | Exclusion
criteria |
|---|
| Patients enrolled at
the Department of Ophthalmology, | Significant
epiretinal membrane |
| ‘Dr. Carol Davila’
Central Military University Emergency Hospital, Bucharest | Macular pathology
(neovascular age-related macular degeneration, foveal involving
geographic atrophy) |
| Large full-thickness
idiopathic MH (>400 microns) Minimum follow-up period of 6
months | Retinal pathology
(retinal laser, retinal vascular occlusion, diabetic
retinopathy) |
| VA >0.1 | Optic nerve pathology
(congenital anomalies, tumors, glaucoma) Previous intraocular
injection |
Surgery
Surgery was conducted under retrobulbar anesthesia,
with a 3-port 25-gauge vitrectomy, using the Alcon
CONSTELLATION® Vision System (Alcon Romania S.R.L.).
After core vitrectomy, intravitreal TA was injected to stain the
posterior cortical vitreous, and the ILM was stained with 0.25
mg/ml BBG solution or L/Z dyes. The inverted ILM flap technique was
applied using the inherent elastic properties of the retina. The
graft was held with Grieshaber™ end-grasping forceps (Alcon Romania
S.R.L.) to bring the edges of the macular hole closer. Fluid-gas
exchange was then performed. No intraoperative complications were
encountered. Postoperatively, the patient was postured supine for
one week, and the retina was attached with no postoperative
complications.
Assessment
We reviewed and collected data in regards to: IOP
(after 1 month), the Watzke-Allen test, visual acuity (VA) and
optical coherence tomography (OCT) parameters, six months after the
vitrectomy surgery with standard ILM peeling using BBG and L/Z
dyes. The VA measurements were performed using the Snellen method.
OCT changes were judged by a single retinal specialist. There are 2
types of macular hole closure based on OCT: Type 1 (closed without
retinal neurosensory foveal defect) and type 2 (closed with foveal
neurosensory retinal defect). The magnitude of the postoperative
visual improvement of type 1 closure was greater than that of type
2 closure. Allen test was used in the diagnosis of a macular hole
in the retina. Over the macula, a thin line of slit lamp light is
projected and the patient is asked to report on its appearance. A
line appearing broken may indicate a macular hole. A line appearing
deformed suggests epiretinal membrane. A line that seems to be thin
indicates macular edema or incipient macular hole (stage I,
II).
Statistical analysis
For the data systematization, we used the Excel
program of the Microsoft Office 365 suite. Graphical
representations and statistical analysis of the data were performed
using the ‘R’ program, 3.0.1 version.
Results
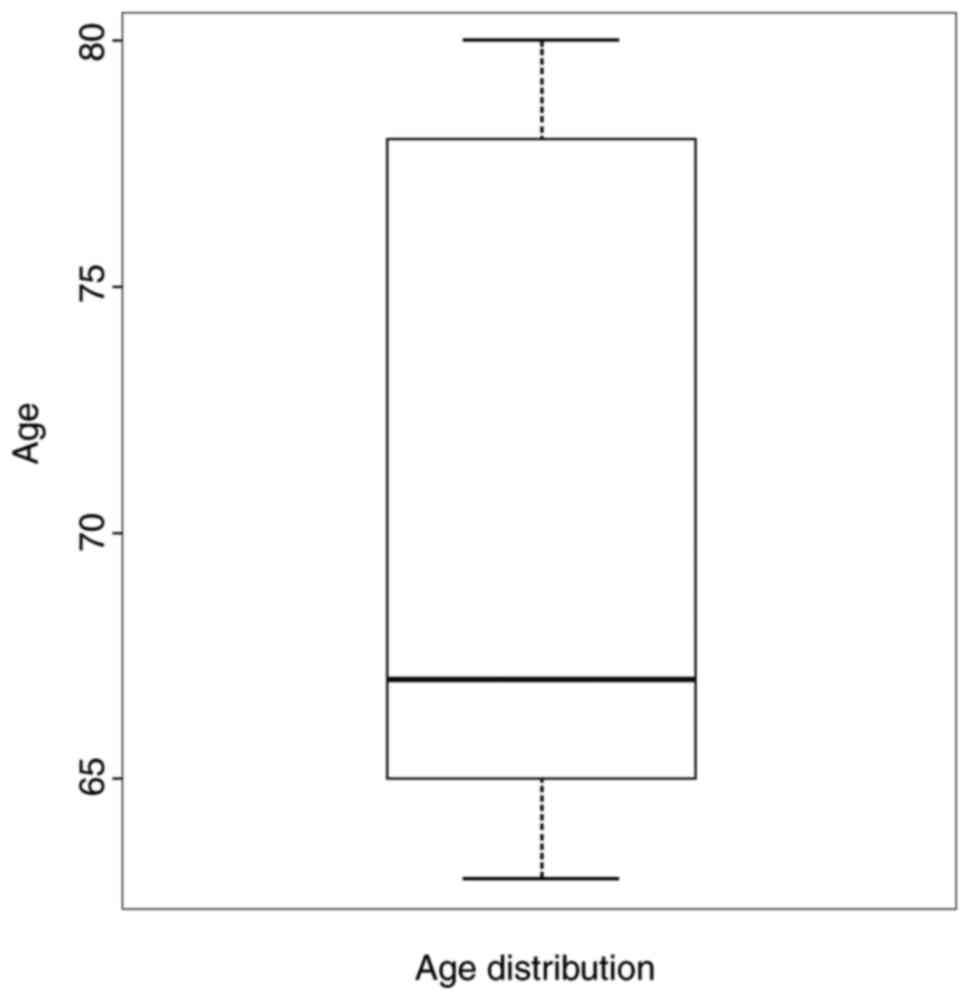
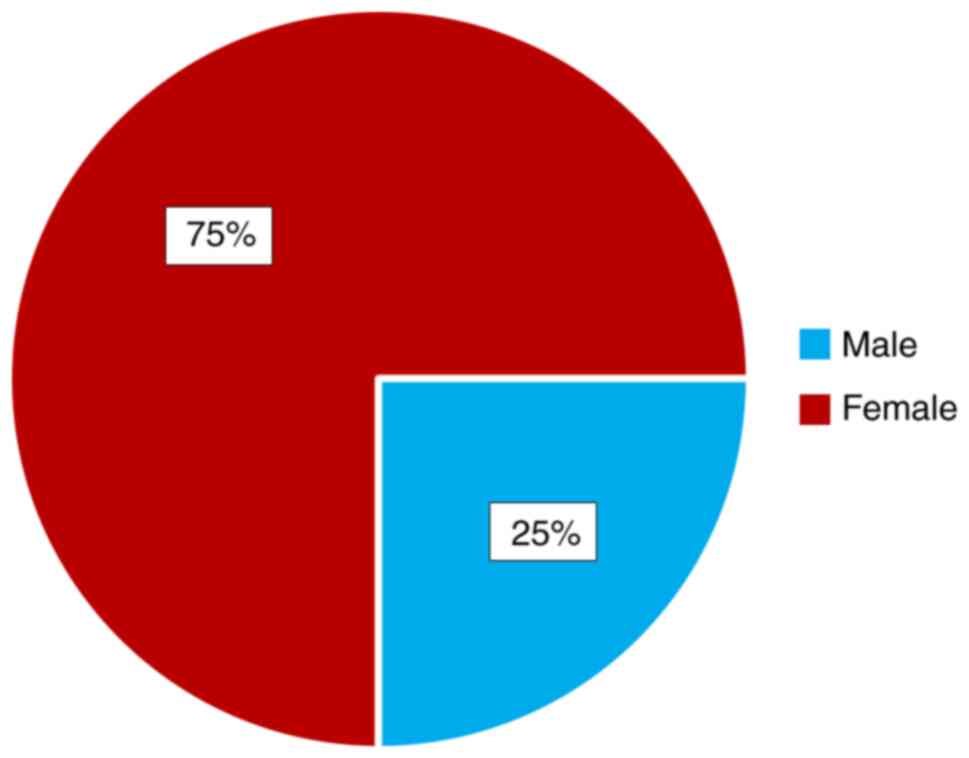
The distribution of the patients by age group and
sex are shown (Figs. 1 and 2). We observed that the most represented
age group was from 65 to 70 years. Gender impact is to be
considered, as females are more likely to develop a MH.
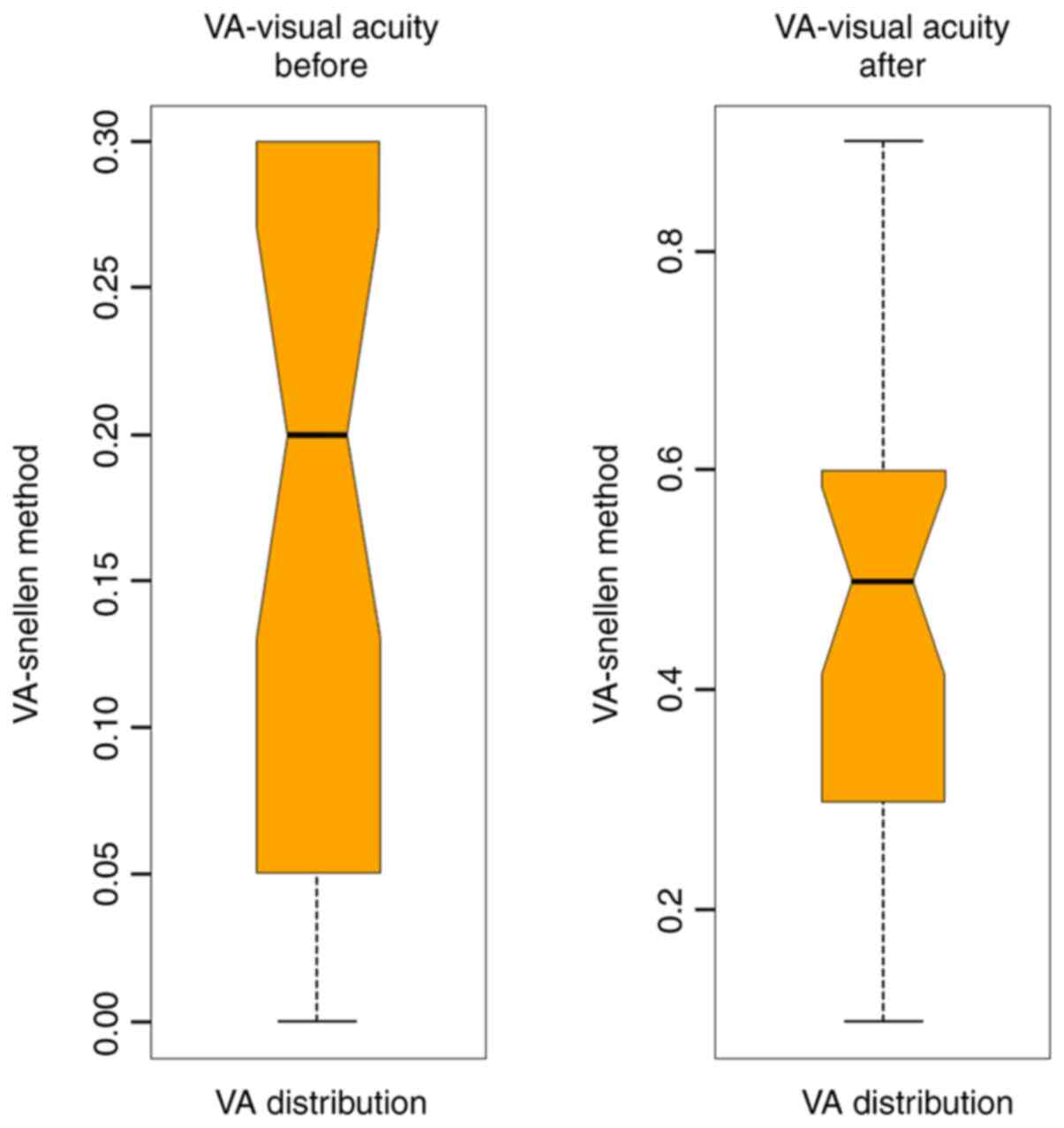
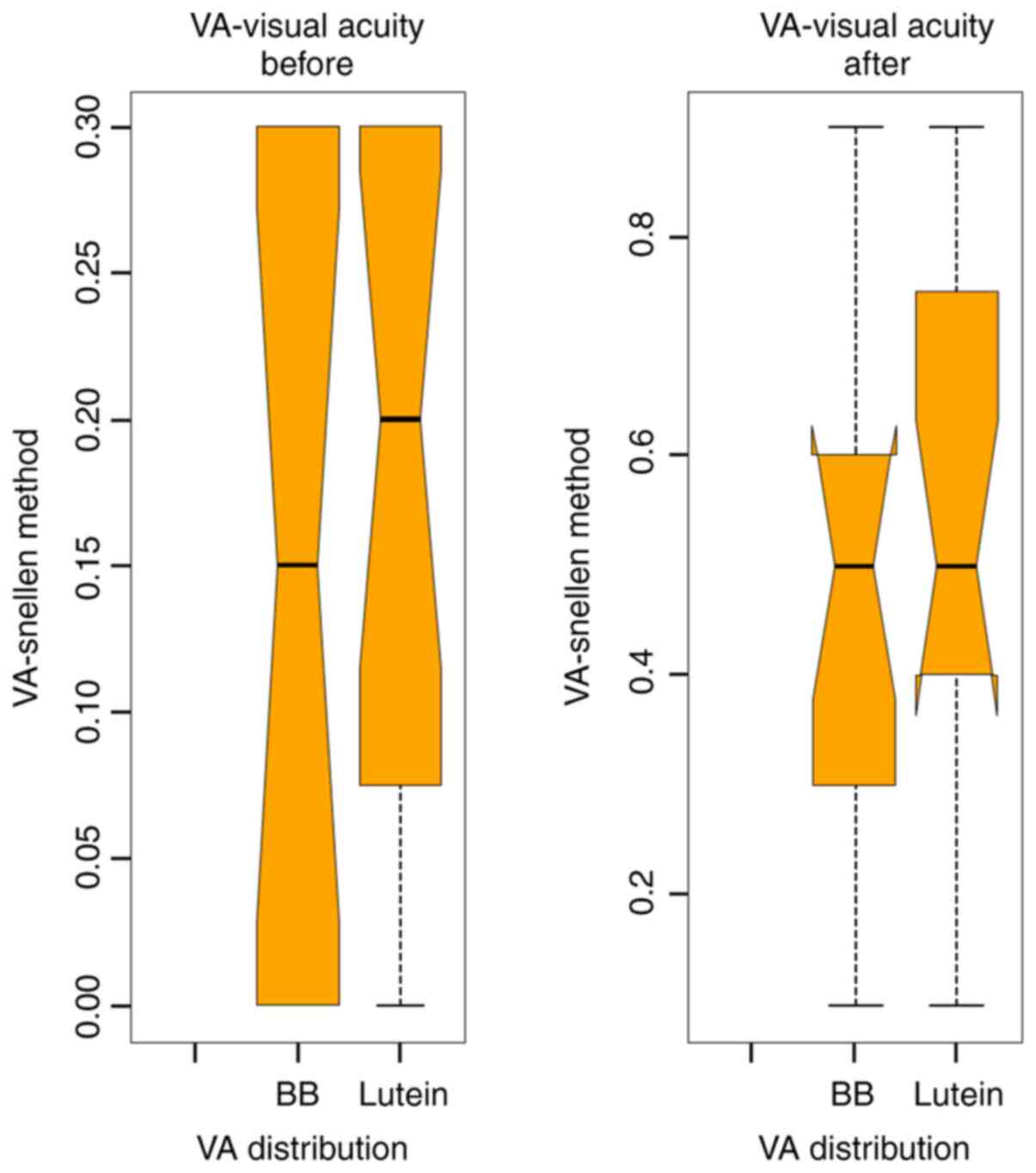
Average VA before surgery for the BBG group was 0.18
and for the L/Z group was 0.15. After surgery, the mean VA for the
BBG group was 0.45 and for the L/Z group was 0.42 (Figs. 3 and 4).
For the entire study lot, the mean VA after surgery
increased by 30%. The VA improvement has a positive impact on the
patient quality of life. By comparing the two groups, VA
improvement was relatively similar.
This improvement takes into account numerous factors
that involve conditions related to the surgery and other associated
conditions, such as preoperative lens opacification, degree of
post-op opacification, MH etiology, and patient compliance.
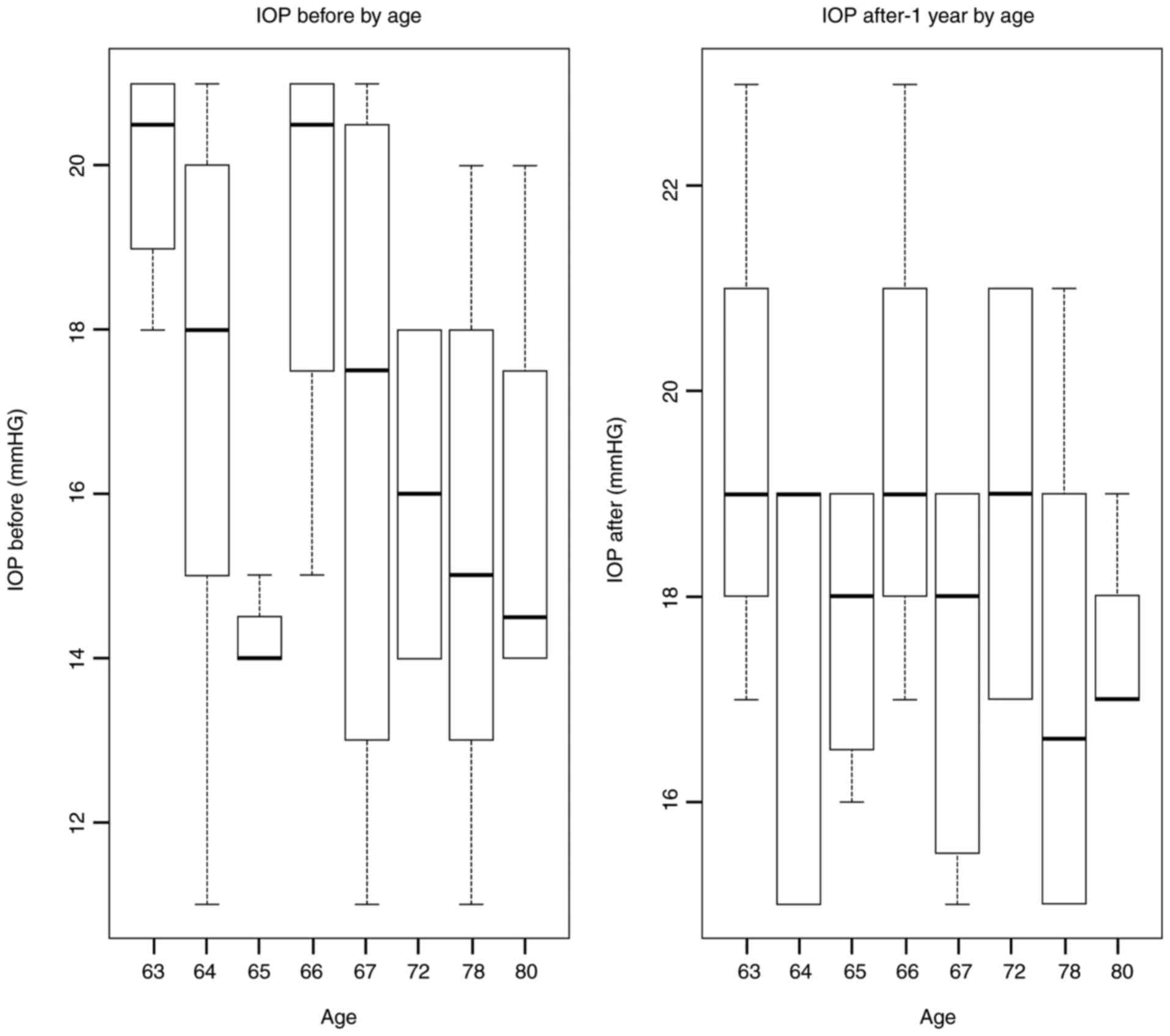
The average IOP before surgery was 16 mmHg; after
surgery (1 month) it was 18.5 mmHg. This asymmetry comes from the
fact that TA was used to highlight the vitreous gel. TA persists in
the eyes for about 9-12 months. Increases in IOP being recorded
mainly in the first days-4 weeks. Later, due to the dilution of TA,
IOP increases are less frequent.
Patients did not follow glaucomatous treatment
before and after surgery as long as this pathology fell into the
exclusion criteria (Fig. 5).
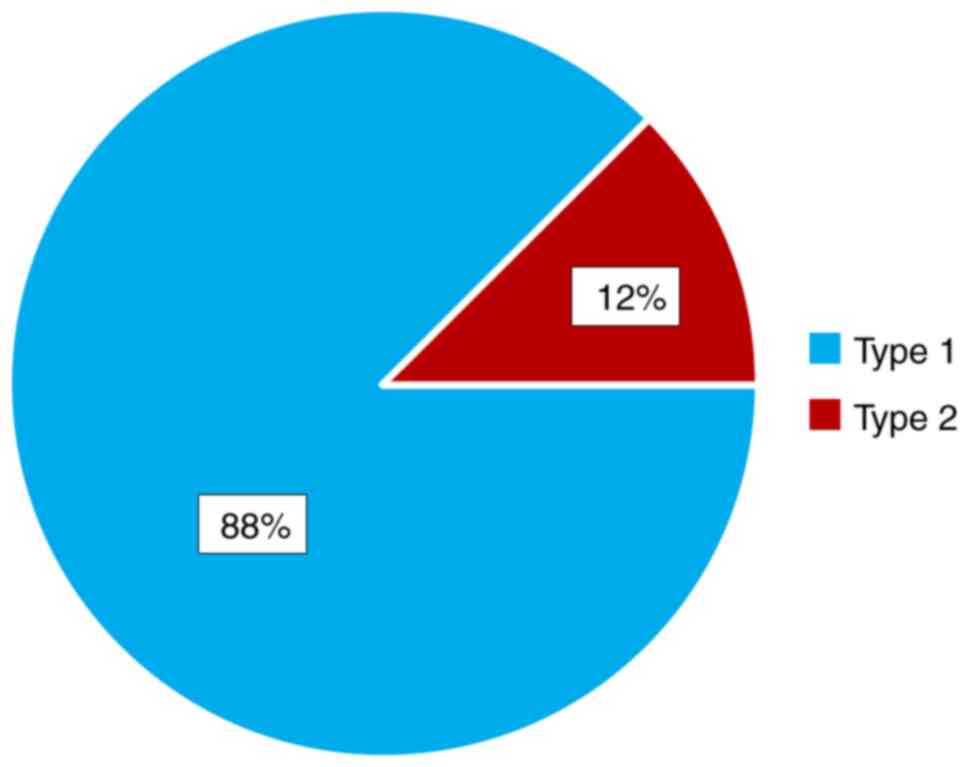
We evaluated the MH closure rate and we noted that
it was more than 85% type 1 in both studied groups, without
statistical differences between them (Fig. 6).
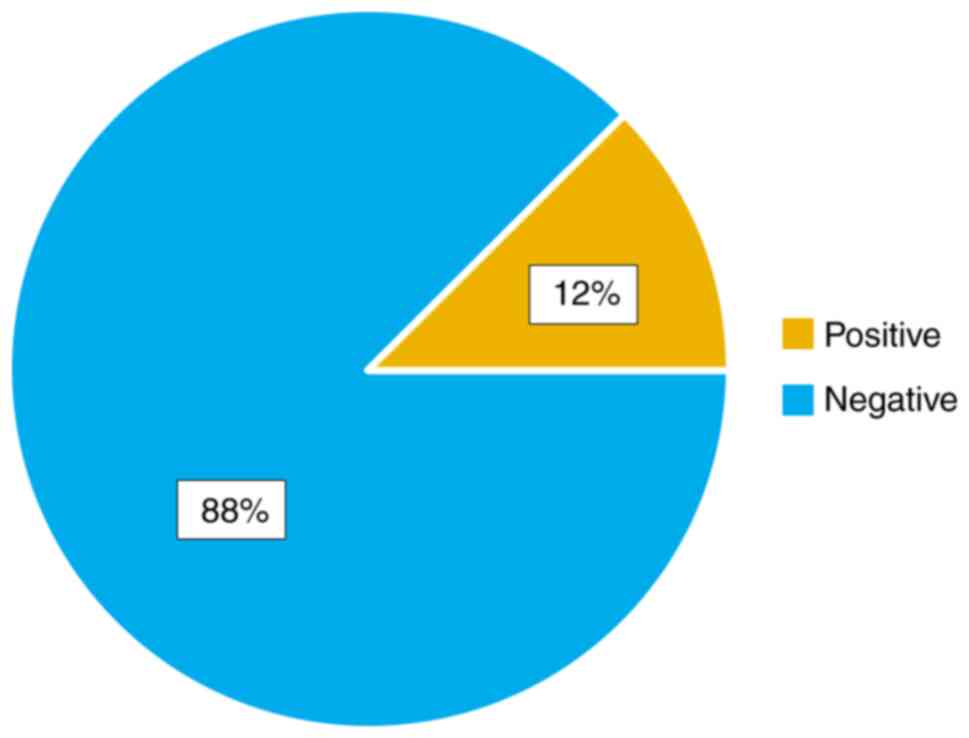
In our case series, we observed that VA improvement
occurred in the first month after pars plana vitrectomy.
The typical manifestation that consisted of
metamorphopsia, central scotoma was remedied postoperatively. The
Watzke-Allen test was negative in a high percentage after surgery
(Fig. 7).
BB, lutein and zeaxanthin dyes color the target
tissue offering safety issue and prospects.
Discussion
In 2012, 102 eyes were analyzed after lutein dye
solution alone or combined with Brilliant blue (BB) or TrB was
injected directly over the different intraocular structures and
improved the ability to initiate peeling in cadaveric eyes, with no
clinical or histologic signs of toxicity (26). In addition, in 2012, 60 cadaveric
eyes were stained along with lutein and zeaxanthin dye or in
combination with different Brilliant Blue concentrations and
efficiently stained vitreous and internal limiting membrane, with
no dye solutions in the eyes after the membrane removal (27).
Badaro et al reported that a combination of
soluble lutein/zeaxanthin (LZ) 1% and Brilliant blue (BB) 0.025%,
facilitated surgical steps and showed no signs of toxicity at 1
month of follow-up in 18 eyes treated surgically for a macular hole
(MH) or epiretinal membranes (ERM) (28).
Casaroli-Marano et al demonstrated that
L/Z-based dye solutions, either alone or in association with BB or
TrB did not significantly alter mitochondrial activity in the cell
lines tested; in addition, no structural alterations were observed
in the neurosensory retina, retinal pigment epithelium (RPE), or
choriocapillaris-choroidal complex (29).
In 2014, Maia et al evaluated 12 eyes that
underwent surgery using lutein-based dye. They histologically
examined the peeled membranes and claimed successful intraoperative
identification (30).
A crystalline lutein-based dye called Vitreodyne™
proved to be superior to the existing alternative dyes after
staining 18 patients with a diagnosis of ERM and MHs (31).
In the present study, we used BB dye for the first
group and L/Z dyes for the second group, and our results confirmed
that both dyes are useful intraocular tools in order to obtain good
surgical results.
The study had its limitations, because of the
reduced number of study eyes and because of the lack of
electrophysiology studies.
In conclusion, optical coherence tomography (OCT),
the gold standard diagnostic tool for retinal diseases, is
extremely useful for preoperative evaluation of MHs and
postoperative surgical results. MH size is typically predictive of
postoperative outcomes.
Identifying the internal limiting membrane (ILM) is
a challenging step in surgery, since the ILM is a barely visible
membrane; identifying and removal of the ILM is difficult even for
experienced retinal surgeons. Staining can reduce surgical trauma
to the retina during ILM removal, thus ILM staining with vital dyes
is essential for increased visibility of the ILM. Coating materials
only cover the membrane surface and do not stain the ILM.
Our results indicate that from a clinical,
intraoperative point of view, the retinal details can be observed
only when the whole vitreous mass has been removed. Adding
intraocular dyes increases ILM visualization, offering the surgeon
a better and safer surgical approach. In our study, we used two
different dyes, but no major differences were noted between the two
groups. In all cases, ILM was identified and removed. We may
consider that the protective screen formed by intraocular dyes
protects the retinal cells from the phototoxic effect.
The association between the inverted ILM flap
technique and intraocular dyes offers good results regarding VA and
the MH closure rate.
To sum up, our study confirmed that both the BBG and
L/Z groups of eyes exhibited in our retrospective study, were
equivalent in mean age affected with full-thickness MHs, exhibited
improved VA at 6-month after surgery confirming the fact that
intraocular dyes facilitate the surgical steps and have no toxic
effect on the retinal cells.
Acknowledgements
Professional editing, linguistic and technical
assistance were performed by Irina Radu, Individual Service
Provider.
Funding
No funding was received.
Availability of data and materials
All data and materials supporting the results of the
present study are available in the published article.
Authors' contributions
ISP designed the study and was responsible for the
acquisition and interpretation of the data. SN provided scientific
advice. OM, CCC, AT and CD, were involved in the design of the
study; they carefully inspected and also revised the manuscript.
SS, CP and HF were involved in the conception and drafting of the
study and revised the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Local Ethics Committee
of Dr. Carol Davila' Central Military Emergency University Hospital
Bucharest (no. 403). Informed consent was signed by all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
The first author, Ioana Stella (Patoni) Popescu, is
a PhD student at the Department of Ophthalmology of the ‘Victor
Babes’ University of Medicine and Pharmacy in Timisoara where she
is solely pursuing her PhD thesis.
References
|
1
|
Knapp H: About isolated ruptures of the
choroid as a result of trauma to the eyeball. Arch Augenheilkd.
1:6–29. 1869.(In German).
|
|
2
|
Noyes HD: Detachment of the retina with
laceration at the macula. Trans Am Ophthalmol Soc. 1:128–129.
1871.PubMed/NCBI
|
|
3
|
Bikbova G, Oshitari T, Baba T, Yamamoto S
and Mori K: Pathogenesis and management of macular hole: Review of
current advances. J Ophthalmol. 2019(3467381)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Christensen UC: Value of internal limiting
membrane peeling in surgery for idiopathic macular hole and the
correlation between function and retinal morphology. Acta
Ophthalmol. 87:1–23. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Almony A, Nudleman E, Shah GK, Blinder KJ,
Eliott DB, Mittra RA and Tewari A: Techniques, rationale, and
outcomes of internal limiting membrane peeling. Retina. 32:877–891.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gelman R, Stevenson W, Prospero Ponce C,
Agarwal D and Byron Christoforidis JB: Retinal damage induced by
internal limiting membrane removal. J Ophthalmol.
2015(939748)2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Walia HS, Shah GK and Hariprasad SM: ILM
peeling a vital intervention for many vitreoretinal disorders.
Ophthalmic Surg Lasers Imaging Retina. 45:92–96. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kelly NE and Wendel RT: Vitreous surgery
for idiopathic macular holes: Results of a pilot study. Arch
Ophthalmol. 109:654–659. 1991.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Morris R, Kuhn F and Witherspoon CD:
Hemorrhagic macular cysts. Ophthalmology. 101(1)1994.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lobeck E: Investigations into the role of
retinal tear in skin detachment. Experimental investigations on the
intraocular fluid change in artificial netskin detachment. Alb v.
Graefes Arch Ophthalmol. 128:513–573. 1932.(In German).
|
|
11
|
Abrams GW, Topping T and Machemer R: An
improved method for practice vitrectomy. Arch Ophthalmol.
96:521–525. 1978.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Farah ME, Maia M and Rodrigues EB: Dyes in
ocular surgery: Principles for use in chromovitrectomy. Am J
Ophthalmol. 148:332–340. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dubey AK: Trypan blue enhanced vitrectomy
in clear gel vitrectomy. Indian J Ophthalmol. 51:286–287.
2003.PubMed/NCBI
|
|
14
|
Peyman GA, Cheema R, Conway MD and Fang T:
Triamcinolone acetonide as an aid to visualization of the vitreous
and thre posteror hyaloid during pars plana vitrectomy. Retina.
20:554–555. 2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Neuman GO, Holbach H and Kruse FE: Applied
Pathology for Ophthalmic Microsurgens. 5.6 Retina and Vitreous.
Springer, pp300-301, 2008.
|
|
16
|
Totan Y, Güler E and Dervişoğulları MS:
Brilliant blue G assisted epiretinal membrane surgery. Sci Rep.
4(3956)2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Musat O, Stefan C, Boariu AM, Colta D,
Cernat C, Alexandru L, Georgescu RD, Patoni IS, Timaru CM and De
Algerino S: Chromovitrectomy. Rom J Ophthalmol. 60:59–62.
2016.PubMed/NCBI
|
|
18
|
Preda MA, Popa G, Karancsi OL, Musat O,
Popescu SI, Munteanu M and Popa Z: Effectiveness of subconjunctival
bevacizumab associated with a laser-based procedure in the
treatment of neovascular glaucoma. Farmacia. 66:621–626. 2018.
|
|
19
|
Boruga O, Bălăşoiu AT, Giuri S, Munteanu
M, Stanca HT, Iovănescu G and Preda MA: Caruncular late-onset
junctional nevus: Apropos of an anatomo-clinical observation. Rom J
Morphol Embryol. 58:1461–1464. 2017.PubMed/NCBI
|
|
20
|
Balica NC, Poenaru M, Preda MA, Boia RE,
Burlacu ON, Horhat ID, Mogoanță CA, Vlăescu AN, Baderca F, Jifcu EM
and Sarău CA: Primary tonsillar tuberculosis-case report. Rom J
Morphol Embryol. 60:267–271. 2019.PubMed/NCBI
|
|
21
|
Popa G, Karancsi OL, Preda MA, Suta MC,
Stelea L, Musat O, Popescu SI, Balica NC, Bogdanici C and Munteanu
M: Assessment of Pain during laser-based procedures in the
treatment of glaucoma. Rev Chim. 70:2105–2107. 2019.
|
|
22
|
Preda MA, Karancsi OL, Munteanu M and
Stanca HT: Clinical outcomes of micropulse transscleral
cyclophotocoagulation in refractory glaucoma-18 months follow-up.
Lasers Med Sci. 35:1487–1491. 2020.
|
|
23
|
Stanca HT, Munteanu M, Jianu DC, Motoc
AGM, Tăbăcaru B, Stanca S, Ungureanu E, Boruga VM and Preda MA: New
perspectives in the use of laser diode transscleral
cyclophotocoagulation. A prospective single center observational
cohort study. Rom J Morphol Embryol. 59:869–872. 2018.PubMed/NCBI
|
|
24
|
Stanca HT, Munteanu M, Jianu DC, Motoc
AGM, Jecan CR, Tăbăcaru B, Stanca S and Preda MA: Femtosecond-LASIK
outcomes using the. VisuMax®-MEL® 80 platform
for mixed astigmatism refractive surgery, Rom J Morphol Embryol.
59:277–283. 2018.PubMed/NCBI
|
|
25
|
Munteanu M, Rosca C and Stanca HT:
Sub-inner limiting membrane hemorrhage in a patient with Terson
syndrome. Int Ophthalmol. 39:461–464. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Brandão-Lencart J and Sousa-Martins D:
New lutein-based dyes for ophthalmic surgery use. Kemin Inspired
Molecular Solutions 2012. http://peschkemed.com/wp-content/uploads/2013/10/Retina-.pdf.
|
|
27
|
Sousa-Martins D, Maia M, Moraes M,
Lima-Filho AA, Rodrigues EB, Chen J, Farah ME, Santos LB and
Belfort R Jr: Use of lutein and zeaxanthin alone or combined with
Brilliant Blue to identify intraocular structures intraoperatively.
Retina. 32:1328–1336. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Badaro E, Furlani B, Prazeres J, Maia M,
Alves Souza Lima A, Souza-Martins D, Muccioli C, Adami Lucatto LF
and Belfort R Jr: Soluble lutein in combination with brilliant blue
as a new dye for chromovitrectomy. Graefes Arch Clin Exp
Ophthalmol. 252:1071–1078. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Casaroli-Marano RP, Sousa-Martins D,
Martínez-Conesa EM, Badaró E, Nunes RP, Lima-Filho AA, Rodrigues
EB, Belfort R Jr and Maia M: Dye solutions based on lutein and
zeaxanthin: In vitro and in vivo analysis of ocular toxicity
profiles. Curr Eye Res. 40:707–718. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Maia M, Furlani BA, Souza-Lima AA, Martins
DS, Navarro RM and Belfort R Jr: Lutein: A new dye for
chromovitrectomy. Retina. 34:262–272. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Singh P, Deuchler S, Mueller M, Kohnen T,
Sousa-Martins D, Pinheiro-Torres B and Koch FHJ: Chromovitrectomy
with vitreodyne™. Invest Ophthalmol Vis Sci. 57(4452)2016.
|