Introduction
Endometriosis is a gynecological disease affecting
fertile women, with a prevalence of 2-10% in the general female
population (1), which can reach up
to 50% in patients with pain, infertility or abdomino-pelvic
surgery (2-4).
First described by Rokitansky in 1860(2), endometriosis is characterized by the
presence of endometrial stroma outside the uterus, with the same
reactivity to hormonal stimuli as the normal stroma (3,5). The
disease can be primary, where the endometrial tissue is found
outside the uterus without any external intervention and secondary
or iatrogenic, following obstetric and gynecological procedures
(uterine wall opening), of which Caesarean-section (C-section) is
the most frequent (4).
In patients with C-sections, the incidence can be as
high as 1% (5). Usually, the
diagnosis is made several months or up to several years following
the procedure (5). The clinical
appearance varies with the depth and localization of the tumor
(6). The symptomatology is not
always present, and the diagnosis is difficult. However, the most
frequent symptom is cyclic pain and a positive history for surgery
may be a clue for the correct diagnosis (5,7).
Herein, we present the case of skin endometriosis
presenting as a subcutaneous nodule in the proximity of a C-section
surgical scar and review existing literature in order to increase
the index of suspicion in the case of painful lesions appearing
close to surgical scars following gynecological or obstetrical
procedures.
Case report
A 29-year-old female presented to the Dermatology
Department of ‘Prof. Dr. Nicolae C. Paulescu’ National Institute of
Diabetes, Nutrition and Metabolic Diseases, Bucharest, Romania for
the investigation of a painful nodule located in the inferior
abdominal wall. Written informed consent was provided and the
patient agreed to undergo diagnostic and therapeutic procedures
included in the study protocol that was conducted in accordance
with the Declaration of Helsinki with approval of the Ethics
Committee of the Colentina Clinical Hospital (approval no.
25/27.11.2017).
The patient medical history was significant for a
right ovarian endometrial cyst, laparoscopically removed 4 years
prior to this presentation, and pathologically confirmed as primary
endometriosis. The patient presented menstrual cycle-dependent
lower abdominal pain after the first surgery. A second laparoscopy
was performed, showing multiple sites of endometriosis. The lesions
were thermocoagulated followed by treatment with triptorelin 3.75
mg, 1 monthly injection for 5 months. After hormonal treatment
withdrawal, the patient became pregnant. During pregnancy, the
patient experienced diffuse low abdominal pain, but without any
complications and she delivered by term C-section. Two years after
giving birth, she presented at our department for a painful nodule
(the pain was worsening prior menses), located in the lower
abdominal wall. The patient also described an intermittent, rather
than cyclic, C-section scar pain which started one year after she
gave birth.
The physical examination showed a palpable
subcutaneous nodule of approximately 1.5 cm in diameter, round,
well defined and mobile, tender on palpation, located 2 cm superior
to the left side of the C-section scar (Pfannenstiel incision). The
suprajacent skin was normal. The C-section scar was well-healed,
supple and whitish, without any pathological finding upon
palpation. A diagnosis of cutaneous endometriosis was suspected
given the patient's history of laparoscopic intervention to the
ovary and flares of pain with menstrual periods.
The lesion was surgically excised. The macroscopic
aspect of the nodule excluded the diagnosis of a cystic lesion or
lipoma and the specimen was referred to the pathology department
for microscopic examination.
The specimen was routinely processed for
paraffin-embedding; then 3-µm-thick sections were cut and routinely
stained with hematoxylin and eosin (H&E), and
immunohistochemistry analysis for CD10, estrogen receptor and Ki67
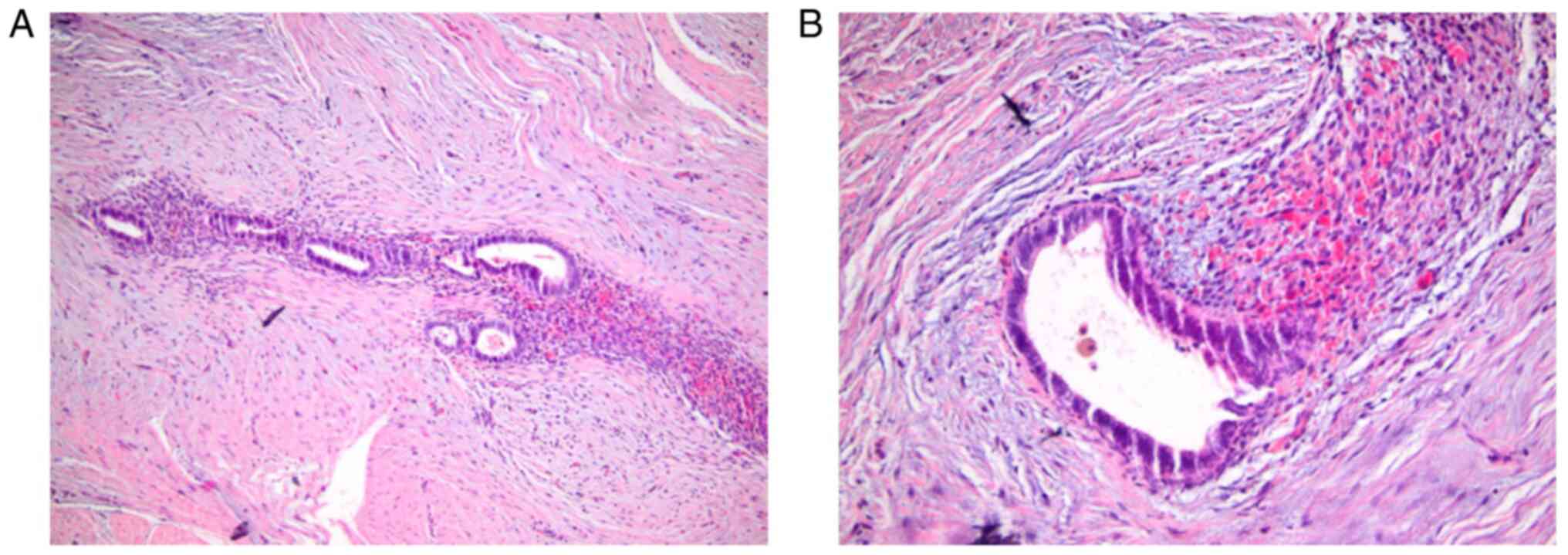
was performed. The pathologic report showed adipose connective
tissue, neuro-vascular and fascial tissue including multiple
glandular structures of variable dimensions with a simple columnar
focal ciliated epithelium, surrounded by an endometrial stroma
(Fig. 1) with multiple hematic
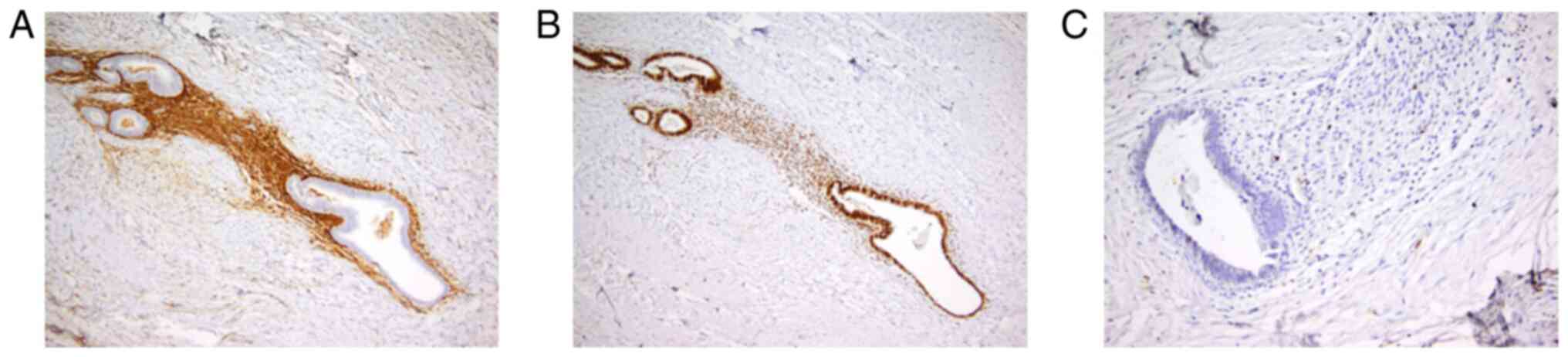
extravasations and rare siderophages. The immunohistochemical
testing showed positivity for the estrogen receptor in the nuclei
of epithelial cells lining glandular structure endometrial-like
cells, CD10 diffuse and intense positivity in the endometrial
stroma and Ki67 positivity below 1% of epithelial cell nuclei
(Fig. 2). These findings sustained
the diagnosis of skin endometriosis. The post-operatory evolution
was good, with disappearance of the nodule-associated symptoms.
After 1 year, the patient remains without recurrence of the disease
or appearance of endometriosis.
Discussion
Skin endometriosis is a rare disorder with variable
clinical and histopathological appearance that depends on hormonal
stimuli and it primarily affects women of reproductive age. More
than 300 cases of skin endometriosis have been described in the
literature and in a review published by Stojanovic et al,
they found 210 cases of skin endometriosis located on surgical
scars, whereof 119 cases followed a C-section procedure (8).
In regards to skin endometriosis, the most frequent
extrapelvic site affected is the abdominal wall and it is
associated with obstetric and gynecologic surgery (9). Abdominal wall endometriosis usually
presents at surgery departments, being misdiagnosed as incisional
hernia or granuloma (10). The
clinical differential diagnoses of skin endometriosis are
represented by incisional hernia, lipoma, dermoid cyst, abscess,
suture granuloma, keloid, melanoma, hematoma and others (11,12).
In our case, based on characteristic history and
examination findings, behind the most probable diagnosis of
endometriosis, other diagnoses including lipoma, granuloma and
desmoid tumor, were also deliberated.
Multiple diagnostic tools have been used in the
diagnosis of skin endometriosis. Examination techniques usually
used for diagnosis include ultrasonography, computed tomography
(CT), magnetic resonance imaging (MRI), and Doppler sonography.
Ultrasonography seems to be the first choice for evaluation of any
abdominal lesion, but CT and MRI may exclude other possible
diagnoses such as a lipoma, hernia or a tumor (13). Tumor markers such as CA19-9 and
CA125 may or may not be elevated; however histologic examination
after excision of the lesion can confirm the diagnosis (14). Kinkel et al reported the
sensitivity and specificity of MRI in diagnosing endometriomas to
be 90-92 and 91-98%, respectively (15).
In regards to skin imaging techniques, standardized
dermoscopic features of skin endometriosis have not yet been
established. Multiple factors such as the site of occurrence,
histological subtype, depth of the lesion or patient phototype may
influence the dermoscopic aspect of skin endometriosis (16). However, this examination technique
may provide additional information useful for clinical diagnosis. A
study published by de Giorgi et al revealed dominant
dermoscopic features in skin endometriosis such as homogeneous
reddish pigmentation, containing small red globular structures,
which they termed ‘red atolls’ (7).
Moreover, Costa et al described the dermoscopy pattern of
cutaneous endometriosis in the follicular phase as
erythematous-violaceous polypoid projections with light brown spots
and areas of active bleeding; further in the luteal phase the
dermoscopic feature of the lesion was as an erythematous-bluish
aspect (17).
Skin endometriosis usually affects women in their
reproductive age (mean age 30-40 years) and its clinical
presentation starts with a pigmented or skin-colored papule or
nodule with an average diameter of 2 cm. The symptoms associated
with this disease are pain, tenderness or bleeding during menstrual
cycle and their persistence ranges from 2 months to 2 years
(18). After surgery the average
period to the onset of symptoms is between 3 months to 18 years
(19).
In our case report, there are many similarities to
previously reported cases. Our female patient was 29 years of age,
thus she was in her reproductive age; the site of the lesion was
close to the C-section scar; the lesion appeared approximately 2
years after surgery as a subcutaneous nodule; and associated
symptomatology included abdominal pain that started one year after
C-section procedure. In this case, characteristic symptoms of
endometriosis such as bleeding or monthly swelling were not present
and considering previously described differentials near a scar
(20,21), this lesion was not easy to
diagnose.
The surgical excision revealed a non-cystic
appearance, and the specimen was referred to the
anatomopathologist. The characteristic features of ectopic
endometrial tissue include glands with cylindrical epithelium and
endometrial stroma which may be influenced by cyclic hormonal
changes. This is one of the reasons why all stages of the menstrual
cycle could be found in the ectopic tissue. Immunohistochemical
testing in our case showed positive CD10, a similarity found with
other reported cases (13,22). Studies have reported that CD10 as a
useful marker for diagnosis as it is strongly expressed in
endometrial stromal nodules (23).
Furthermore, in our case, Ki67 positivity was found
in less than 1% of the nuclei of the cells. According to the
literature, an increased Ki67 and CD10 positivity indicates that
the stroma is decidualized (24).
Another finding of our case was the positivity for the estrogen
receptor in endometroid-like cells. This was also previously
reported (25-28).
The production of estrogen can be stimulated in endometrial lesions
by aromatase activity (29).
Concerning the possible pathophysiological
mechanisms involved, one accepted theory suggests that primitive
pluripotent mesenchymal cells that have undergone differentiation
metaplasia are one of the cause of abdominal wall endometriosis
(30). In addition, some authors
propose that retrograde menstruation could be a cause for the
implantation of endometrial cells in the peritoneal area (31), while others advocate the theory of
lymphatic or hematogenic dissemination (32), the role of genetics (33) and other probable pathophysiological
mechanisms (34,35). Another theory explains the
possibility that during a C-section procedure endometrial tissue
could be iatrogenically grafted into ectopic sites such as the
skin, muscles or other layers of the abdominal wall, this being the
reason why the nodule appears frequently above the scar. In
addition, these grafted endometrial cells are able to proliferate
due to hormonal stimulation (36).
Therefore, one recommendation according to various authors is to
irrigate the abdominal wall with saline solution before closing the
abdominal layers (33). Other
recommendations published in the literature include isolation of
the surgical scar, changing needles during the closure of the
superficial layer of the abdomen wall, and replacing the
instruments used during C-section to prevent the iatrogenic
grafting of cells (37).
A retrospective study of 198 Caesarean scar-related
endometriosis cases by Zhang et al found more than 70% of
the endometrial cases in superficial regions of the abdominal wall;
5.7% were found in the adipose layer and 64.6% between the adipose
layer and the fascia layer, and 83% were located in a corner of the
Pfannenstiel incision scar (38).
Ding and Zhu conducted another retrospective study with similar
results; 77.1% of the cases were located in the corners of the
scars (39). One argument for this
particular location may be that endometrial cells are less easily
detached from the corners of the incisions during C-section
(38).
The most frequently used abdominal skin incisions
are Pfannenstiel incision and vertical midline; therefore C-section
seems to be one of the most popular surgical procedures utilized on
the female population (38,39). According to Zhang et al, more
blood loss in the Pfannenstiel incision would supply a relatively
rich nutritional background for the implantation and expansion of
residual endometrial cells, facilitating the development of skin
endometriosis. Therefore, this type of incision would increase the
risk for skin endometriosis (38).
In our patient, the cause for this ectopic tissue
may have been the iatrogenic transportation of endometrial cells
during the C-section incision. The site of the lesion was found in
the superficial abdominal wall, in the proximity of the left side
of the scar, after Pfannenstiel incision, as similarly mentioned in
the studies above.
Our treatment for this patient was surgical excision
of the lesion with clear margins to prevent recurrence. Generally,
surgical excision is the first-line treatment as it is the best way
to establish a clear diagnosis and to ensure a low rate of
recurrence. The recurrence rate is generally low, but other
publications have described recurrence in 6-11% of patients
(40,41). The malignancy rate is also extremely
low with less than 1% of endometriosis cases reported in
association with cancer; one of the most common types is clear-cell
carcinoma with a survival rate of 80% (39,42).
Hormonal therapy with gonadotropin releasing hormone
(GnRH) agonists, danazol, preoperative or postoperative
progesterone is also advocated in the literature. Preoperative
therapy is effective in ameliorating symptoms such as pain and
minimizing the lesion size. The goal of postoperative hormonal
therapy is to prevent recurrence, but its use is still under debate
as the overall result has been poor (43). Moreover, considering the
psychological and social impact of the disease, patient counseling
should also be considered (44,45).
In conclusion, skin endometriosis is a rare and
benign condition, with an unknown mechanism and a very low rate of
malignancy. Its clinical appearance is very unspecific which may
hinder the dermatologist's diagnosis, delaying the right management
of the lesion. The rate of C-section is increasing in the female
population and the associated incidence in skin endometriosis
(found on or in close proximity to a scar associated with this
procedure) may increase in the future. Dermatologists should be
aware of this condition in any women with pain and a lump close to
an incisional scar after pelvic surgery.
The first-line treatment of skin endometriosis is
surgical excision and the gold standard for its diagnosis is
histopathologic and, if necessary, immunohistochemical
examination.
Acknowledgements
Not applicable.
Funding
This research and review was funded by a grant from the Romanian
Ministry of Research and Innovation, CCCDI-UEFISCDI (project no.
61PCCDI⁄2018 PN-III-P1-1.2-PCCDI-2017-0341) within PNCDI-III.
Availability of data and materials
All findings generated or analyzed during this study
are included in this published article and the literature findings
are documented by relevant references.
Authors' contributions
AMM and AMDI contributed equally to the
conceptualization, data analysis, and writing of the manuscript.
MC, SAZ, DB, IS and LFG conducted the literature research and data
analysis. CC and MAI conducted the data analysis, critical review,
editing of the manuscript and supervision of the project. All
authors read and gave approval for publication of the final
manuscript.
Ethics approval and consent to
participate
Ethics approval was obtained from the Ethics
Committee of the Colentina Clinical Hospital (approval no.
25/27.11.2017). Written informed consent of the patient was
obtained.
Patient consent for publication
Written informed consent of the patient was obtained
for publication of the information.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dunselman GAJ, Vermeulen N, Becker C,
Calhaz-Jorge C, D'Hooghe T, De Bie B, Heikinheimo O, Horne AW,
Kiesel L, Nap A, et al: ESHRE guideline: Management of women with
endometriosis. Hum Reprod. 29:400–412. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Giudice LC and Kao LC: Endometriosis.
Lancet. 364:1789–1799. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Blanco RG, Parithivel VS, Shah AK, Gumbs
MA, Schein M and Gerst PH: Abdominal wall endometriomas. Am J Surg.
185:596–598. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Scholefield HJ, Sajjad Y and Morgan PR:
Cutaneous endometriosis and its association with caesarean section
and gynaecological procedures. J Obstet Gynaecol. 22:553–554.
2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ozel L, Sagiroglu J, Unal A, Unal E, Gunes
P, Baskent E, Aka N, Titiz MI and Tufekci EC: Abdominal wall
endometriosis in the cesarean section surgical scar: A potential
diagnostic pitfall. J Obstet Gynaecol Res. 38:526–530.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tognetti L, Cinotti E, Tonini G, Habougit
C, Cambazard F, Rubegni P and Perrot JL: New findings in
non-invasive imaging of cutaneous endometriosis: Dermoscopy,
high-frequency ultrasound and reflectance confocal microscopy. Ski
Res Technol. 24:309–312. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
De Giorgi V, Massi D, Mannone F, Stante M
and Carli P: Cutaneous endometriosis: Non-invasive analysis by
epiluminescence microscopy. Clin Exp Dermatol. 28:315–317.
2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Stojanovic M, Brasanac D and Stojicic M:
Cutaneous inguinal scar endosalpingiosis and endometriosis: Case
report with review of literature. Am J Dermatopathol. 35:254–260.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bektaş H, Bilsel Y, Sar YS, Ersöz F, Koç
O, Deniz M, Boran B and Huq GE: Abdominal wall endometrioma; a
10-year experience and brief review of the literature. J Surg Res.
164:e77–e81. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Loh SH, Lew BL and Sim WY: Primary
cutaneous endometriosis of umbilicus. Ann Dermatol. 29:621–625.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Curry TW: Subcutaneous endometriomas: Two
case reports and review of the literature. J Gynecol Surg.
14:31–34. 1998.
|
|
12
|
Tatu AL: Umbilicated blue-black lesion on
the lateral thorax. J Cutan Med Surg. 21(252)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Neri I, Tabanelli M, Dika E, Valeria G and
Patrizi A: Diagnosis and treatment of post-Caesarean scar
endometriosis. Acta Derm Venereol. 87:428–429. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mechsner S, Bartley J, Infanger M,
Loddenkemper C, Herbel J and Ebert AD: Clinical management and
immunohistochemical analysis of umbilical endometriosis. Arch
Gynecol Obstet. 280:235–242. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kinkel K, Frei KA, Balleyguier C and
Chapron C: Diagnosis of endometriosis with imaging: A review. Eur
Radiol. 16:285–298. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jaime TJ, Jaime TJ, Ormiga P, Leal F,
Nogueira OM and Rodrigues N: Endometriose umbilical: Relato de um
caso e seus achados dermatoscópicos. An Bras Dermatol. 88:121–124.
2013.
|
|
17
|
Costa IMC, Gomes CM, Morais OO, Costa MC,
Abraham LS and Argenziano G: Cutaneous endometriosis: Dermoscopic
findings related to phases of the female hormonal cycle. Int J
Dermatol. 53:e130–e132. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Friedman PM and Rico MJ: Cutaneous
endometriosis. Dermatol Online J. 6(8)2000.PubMed/NCBI
|
|
19
|
Kazakov DV, Ondic O, Zamecnik M,
Shelekhova KV, Mukensnabl P, Hes O, Dvorak V and Michal M:
Morphological variations of scar-related and spontaneous
endometriosis of the skin and superficial soft tissue: A study of
71 cases with emphasis on atypical features and types of müllerian
differentiations. J Am Acad Dermatol. 57:134–146. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tatu AL, Kluger N and Nwabudike LC: Pain
and shingles on an old scar. Int J Dermatol. 59:1158–1159.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ardeleanu V, Jecan CR, Tatu AL and Motoc
AG: A recurrent solitary glomus tumor of the forearm. Rom J Morphol
Embryol. 60:1019–1023. 2019.PubMed/NCBI
|
|
22
|
Kerr OA, Mowbray M and Tidman MJ: An
umbilical nodule due to endometriosis. Acta Derm Venereol.
86:277–278. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Van den Nouland D and Kaur M: Primary
umbilical endometriosis: A case report. Facts Views Vis Obgyn.
9:115–119. 2017.PubMed/NCBI
|
|
24
|
Val-Bernal JF, Val D, Gómez-Aguado F,
Corcuera MT and Garijo MF: Hypodermal decidualized endometrioma
with aberrant cytokeratin expression. A lesion mimicking
malignancy. Am J Dermatopathol. 33:e58–e62. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kholová I, Ryska A and Dedic K: Composite
tumor consisting of dermatofibrosarcoma protuberans and giant cell
fibroblastoma associated with intratumoral endometriosis. Report of
a case. Pathol Res Pract. 197:263–267, 269-270. 2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dragoumis K, Mikos T, Zafrakas M,
Assimakopoulos E, Stamatopoulos P and Bontis J: Endometriotic
uterocutaneous fistula after cesarean section. A case report.
Gynecol Obstet Invest. 57:90–92. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Frischknecht F, Raio L, Fleischmann A,
Dreher E, Lüscher KP and Mueller MD: Umbilical endometriosis. Surg
Endosc. 18(347)2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Farooq U, Laureano AC, Miteva M and Elgart
GW: Cutaneous endometriosis: Diagnostic immunohistochemistry and
clinicopathologic correlation. J Cutan Pathol. 38:525–528.
2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bulun SE, Imir G, Utsunomiya H, Thung S,
Gurates B, Tamura M and Lin Z: Aromatase in endometriosis and
uterine leiomyomata. J Steroid Biochem Mol Biol. 95:57–62.
2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dwivedi AJ, Agrawal SN and Silva YJ:
Abdominal wall endometriomas. Dig Dis Sci. 47:456–461.
2002.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Vinatier D, Orazi G, Cosson M and Dufour
P: Theories of endometriosis. Eur J Obstet Gynecol Reprod Biol.
96:21–34. 2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Horton JD, DeZee KJ, Ahnfeldt EP and
Wagner M: Abdominal wall endometriosis: A surgeon's perspective and
review of 445 cases. Am J Surg. 196:207–212. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Witz CA: Pathogenesis of endometriosis.
Gynecol Obstet Invest. 53 (Suppl 1):S52–S62. 2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Nwabudike LC and Tatu AL: Reply to Happle
R et al. Koebner's sheep in Wolf's clothing: Does the
isotopic response exists as a distinct phenomenon? J Eur Acad
Dermatology Venereol. 32:e336–e337. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Aida Maranduca M, Liliana Hurjui L,
Constantin Branisteanu D, Nicolae Serban D, Elena Branisteanu D,
Dima N and Lacramioara Serban I: Skin-a vast organ with
immunological function (Review). Exp Ther Med. 20:18–23.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Seydel AS, Sickel JZ, Warner ED and Sax
HC: Extrapelvic endometriosis: Diagnosis and treatment. Am J Surg.
171:239–241. 1996.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Agarwal A and Fong YF: Cutaneous
endometriosis. Singapore Med J. 49:704–709. 2008.PubMed/NCBI
|
|
38
|
Zhang P, Sun Y, Zhang C, Yang Y, Zhang L,
Wang N and Xu H: Cesarean scar endometriosis: Presentation of 198
cases and literature review. BMC Womens Health.
19(14)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ding Y and Zhu J: A retrospective review
of abdominal wall endometriosis in Shanghai, China. Int J Gynecol
Obstet. 121:41–44. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chatterjee SK: Scar endometriosis: A
clinicopathologic study of 17 cases. Obstet Gynecol. 56:81–84.
1980.PubMed/NCBI
|
|
41
|
Steck WD and Helwig EB: Cutaneous
endometriosis. JAMA. 191:167–170. 1965.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Petca A, Radu N and Petca R: Insights into
malignant potential of ovarian endometriomas. In: The 17th National
Congress of the Romanian Society of Obstetrics and Gynecology and
First Advanced Colposcopy Course. pp614-619, 2019.
|
|
43
|
Raffi L, Suresh R, McCalmont TH and Twigg
AR: Cutaneous endometriosis. Int J Womens Dermatol. 5:384–386.
2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Rebegea L, Firescu D, Baciu G and Ciubara
A: Psycho-oncology support. BRAIN Broad Res Artif Intell Neurosci.
10:77–88. 2019.
|
|
45
|
Aerts L, Grangier L, Streuli I, Dällenbach
P, Marci R, Wenger JM and Pluchino N: Psychosocial impact of
endometriosis: From co-morbidity to intervention. Best Pract Res
Clin Obstet Gynaecol. 50:2–10. 2018.PubMed/NCBI View Article : Google Scholar
|