Introduction
Non-alcoholic fatty liver disease (NAFLD) is the
most common broad spectrum liver disease in developed countries.
The potential evolution from simple steatosis (commonly referred as
nonalcoholic fatty liver-NAFL) to steatohepatitis (NASH), advanced
fibrosis, cirrhosis and, ultimately, hepatocellular carcinoma is
one of the main reasons why NAFLD has gained much research
attention in the last few years (1-3).
A follow-up study by Ekstedt et al (4) addressed NASH as ‘NAFLD and elevated
liver enzymes’. Other follow-up studies showed that patients with
NASH have a reduced survival compared to patients with simple
steatosis (4,5).
Hepatocyte damage induced by hepatic lipotoxicity is
one of the main causes among the plethora of factors involved in
NAFLD pathogenesis (6). Oxidative
stress and endoplasmic reticulum stress can be triggered even as an
adaptive response to lipotoxicity, which is a hepatic overflow of
fatty acids, triglycerides, cholesterol, biliary acids and
ceramides, among other active lipid metabolites (6-8).
These lipids act as promoters of steatosis, reactive oxygen species
(ROS) accumulation and alterators of liver signaling pathways
(9). In addition, cytokine
production and lipid peroxidation caused by ROS promote the
progression of liver fibrosis and further hepatocellular injury
(7,10). It is currently accepted that an
imbalance between anti-inflammatory cytokines [such as interleukin
(IL)-10] and pro-inflammatory cytokines [IL-6 and tumor necrosis
factor (TNF-α)] could play an important role in the promotion of
inflammation and progression of fibrosis in patients with NAFLD and
particularly in NASH (10-12).
High serum levels of TNF-α and IL-6 and low serum levels of IL-10
and adiponectin exert deleterious effects on NASH progression
towards severe fibrosis (6,7,13).
Liver fibrosis progression, independent of the
presence of NASH, is the most crucial predictor of liver-related
complications and overall mortality (14). Thus, it is important to establish an
early diagnosis of advanced fibrosis in patients with NAFLD, likely
using validated panels of serum biomarkers [e.g. Fibrosis-4 Index
(FIB-4) or the NAFLD Fibrosis Score (NFS)] (15). In addition, transient elastography
or newer techniques may be used to identify fibrosis, even if they
overestimate the liver fibrosis in cases of severe steatosis
(detected by ultrasonography or histology) (16).
Paraoxonase-1 (PON1) is an enzyme synthesized in the
liver. PON1 exerts an important antioxidant, anti-inflammatory and
anti-atherogenic effect by its main roles of protecting
LDL-cholesterol from oxidation, reducing the transformation of
macrophages into ‘foam cells’ and the catabolism of homocysteine
thiolactone, among other activities (17,18).
PON1 activity is modulated by PON1 gene polymorphisms, but
also by non-genetic factors (chemicals, drugs, smoking or diet,
among others) (19). In chronic
liver diseases (including NAFLD), PON1 activity is usually
decreased, and this was found to be associated with alterations in
HDL particles (20), peroxisome
proliferator-activated receptor (PPAR)δ expression and upregulation
of monocyte chemoattractant protein-1 (MCP-1) (21), together with an increase in TNF-α
and IL-6(22). All these
associations suggest that low PON1 levels could be considered as a
marker of lipid peroxidation, and a potential surrogate marker for
enhanced oxidative stress and fibrosis in patients with NAFLD
(23,24).
Periostin (POSTN) is an extracellular matrix protein
mainly secreted by osteoblasts, which exerts a pro-fibrotic effect
in repairing damaged tissues (25).
POSTN expression has been found to be associated with many diseases
(including cancer), and recently it was suggested that this enzyme
has a potential pro-fibrotic effect in the liver, mainly due to
activation of lysyl-oxidase in hepatic stellate cells (HSCs)
(26). Several studies have shown
that serum POSTN levels are higher in patients with NAFLD compared
to controls, but a potential causal relationship of POSTN and NAFLD
has not been confirmed (25).
The aim of this pilot study was to evaluate PON1 and
POSTN serum concentrations, together with the cytokine status
(TNF-α, IL-6, IL-10), in a cohort of patients with NAFLD and
persistently elevated serum aminotransferases, and to correlate the
findings with the liver fibrosis validated previously using
non-invasive scores (FIB-4/NFS).
Patients and methods
The study was an observational, analytical,
prospective, transversal and cohort type study. It was conducted at
the Clinical CF University Hospital, Cluj-Napoca, Romania, between
January 2016 and July 2019. The study was conducted according to
the Declaration of Helsinki and was previously approved by the
Ethics Committee of the ‘Iuliu Hațieganu’ University of Medicine
and Pharmacy (no. 404/02/Jul/2015). All patients signed an informed
consent form prior to study inclusion.
We consecutively enrolled 52 patients (mean age, 50
years; range, 18-70) diagnosed with NAFLD (either NAFL or NASH),
with an equal distribution of men and women. Inclusion criteria
included patients diagnosed with liver steatosis [by
ultrasonography (US)] and moderately elevated aminotransferase
levels at two or more prior visits and screened for a minimum of
six months before study inclusion. All the enrolled subjects had
negative biomarkers for viral hepatitis, autoimmune hepatitis,
primary biliary cirrhosis or cholangitis, Wilson's disease or
hemochromatosis. Liver cirrhosis or liver tumors were excluded
clinically, biologically (normal coagulation parameters, normal
albumin serum levels), ultrasonographically and by exclusion of
portal hypertension (ultrasonographic signs, absence of
splenomegaly and upper digestive endoscopy without gastroesophageal
varices). Exclusion criteria consisted of significant chronic
alcohol consumption, as defined as ≥30 g/day for men and ≥20 g/day
for women (27); pregnancy; chronic
use of medication with hepatotoxic potential and presence of any
other disease proven to have an influence on POSTN and PON1
concentrations (active cancer or positive personal history of
malignancy, asthma, thyroid gland dysfunctions, autoimmune
disorders, psoriasis, allergies and psychiatric disorders).
The diagnosis of NAFLD was thus based on: i) The
presence of liver steatosis evaluated by US; ii) exclusion of other
liver conditions that may be evaluated with steatosis and
persistently elevated aminotransferases; iii) exclusion of patients
with significant alcohol consumption.
We recorded general information concerning each
patient: Age, sex, body-mass index (BMI, calculated as the body
mass divided by the square of the body height), and other
comorbidities [pre-diabetes-impaired fasting glucose or/and
impaired glucose tolerance, type 2 diabetes mellitus (T2DM) and
metabolic syndrome]. A blood sample was obtained from each patient
for routine assessments: Glycemia, aspartate aminotransferase
(AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP),
γ-glutamyl transferase (GGT), platelet count (PLT), serum
bilirubin, total cholesterol, HDL-cholesterol, triglycerides, and
albumin. A separate blood sample was used for testing IL-6, IL-10,
TNF-α, high-sensitivity C-reactive protein (Hs-CRP), PON1 and POSTN
serum concentrations.
Routine laboratory testing was performed using
different commercial kits for use with a Konelab Prime 60i analyzer
(Thermo Fisher Scientific, Inc.). Hs-CRP, IL-6, IL-10 and TNF-α
values were assessed using ELISA kits (Abbexa). POSTN and PON1
serum levels were determined by ELISA (Abbexa, USA) according to
the manufacturer's instructions. For each assay, samples were
diluted as needed and protein levels were calculated based on a
four-parameter logistic (4-PL) curve-fit.
Abdominal ultrasound (US) was performed on each
subject by the same experienced physician using a convex transducer
on an Aloka Prosound Alpha 7 Premier ultrasound machine
(Hitachi-Aloka Medical). Severity of steatosis was assessed by US,
as described in detail in 2015 by Petta et al, and was
established either as mild, moderate or severe NAFLD (16).
For each patient, we calculated the NAFLD fibrosis
score (NFS), FIB-4 score, AST to platelet ratio index (APRI) and
BARD score, using available free online calculators (www.mdcalc.com) and the NAFLD fibrosis score
formula:
Formula=1.675+0.037 x age (years) + 0.094 x BMI
(kg/m2) + 1.13 x IFG/diabetes (yes=1, no=0) + 0.99 x
AST/ALT ratio-0.013 x platelets (109/l)-0.66 x albumin
(g/dl).
For NFS, a low cutoff (lower than -1.455) excluded
severe fibrosis, while a high score (>0.676) was a predictor of
severe fibrosis (28).
Statistical analysis
Statistical analysis was performed using MedCalc
Statistical Software version 19.1.5 (MedCalc Software bv, Ostend,
Belgium; https://www.medcalc.org; 2020). The
quantitative data was tested for normality of the distribution
(Shapiro Wilk test) and was characterized by median and 25-75
percentiles. The qualitative variables were described by absolute
and relative frequencies. Comparisons between groups were performed
using the Man-Whitney or Kruskal-Wallis tests, whenever
appropriate. Correlations between quantitative variables were
verified using the Spearman's rank correlation coefficient. The
independent association between variables and fibrosis scores was
assessed by multivariate linear regression. The model included the
variables that achieved a P-value <0.2 in the univariate
analysis. A P-value <0.05 was considered statistically
significant.
Results
The clinical, biochemical and ultrasonographical
data recorded for each subject are shown in Table I.
 | Table IDescriptive variables of the study
group. |
Table I
Descriptive variables of the study
group.
| Variables (unit of
measurement; reference values) | Patients with NAFLD
(N=52) |
|---|
| Age
(years)a | 50 (37.25;
60.75) |
| BMI
(kg/m2)a | 30.23 (27.49;
32.68) |
| T2DMb | 12(23) |
|
Pre-diabetesb | 15 (28.8) |
| Metabolic
syndromeb | 31 (59.6) |
| AST (UI/l;
5-37)a | 52 (45; 59) |
| ALT (UI/l;
5-40)a | 70 (62.25;
84.75) |
| ALP (U/l;
98-279)a | 215.5 (171;
272.5) |
| GGT (U/l;
7-32)a | 38.5 (32; 59) |
| Total cholesterol
(mg/dl; 100-200)a | 204.5 (175.25;
237) |
| HDL-cholesterol
(mg/dl; >40)a | 38 (35; 50.75) |
| Triglycerides
(mg/dl; 45-140)a | 176.5 (110;
211.75) |
| Total bilirubin
(mg/dl; 0.3-1.2)a | 0.7 (0.5;
0.87) |
| PLT
(103/µl; 150-350)a | 231 (186; 268) |
| Serum albumin
(g/dl; 3.5-5.2)a | 4.4 (4.1; 4.9) |
| Hs-CRP (ng/ml;
0.391-25)a | 3.5 (1.67;
5.2) |
| IL-10 (pg/ml;
3.12-200)a | 7.48 (3.89;
9.06) |
| IL-6 (pg/ml;
0.78-50)a | 3.21 (2.47;
4.41) |
| TNF-α (pg/ml;
15.6-1000)a | 19.69 (0;
95.5) |
| POSTN (ng/ml;
7.8-500)a | 43.52 (6.68;
114.55) |
| PON1 concentration
(ng/ml; 3.12-200)a | 11.81 (11.23;
12.37) |
| NFSa | -1.55 (-2.9;
-0.33) |
| FIB-4
scorea | 1.32 (0.91;
2.04) |
| APRI
scorea | 0.63 (0.49;
0.88) |
| BARD
scorea | 2 (1; 2) |
We did not find a statistically significant
difference between sex in regards to the NFS (P=0.700), FIB-4 score
(P=0.080), APRI score (P=0.200) and BARD score (P=0.800). The
presence of metabolic syndrome was associated with significantly
higher values for NFS [-0.4 (-1.6; 0) vs. -2.78 (-3.4; -2.1);
P<0.001], FIB-4 score [1.5 (0.9; 1.6) vs. 1.1 (0.7; 1.5);
P=0.030], BARD score [2 (2; 2) vs. 1 (0.5; 1); P<0.001]. APRI
score was not significantly higher in patients with metabolic
syndrome (P=0.100).
The NFS and the FIB-4 score were strongly correlated
with the age of the patients (r=0.501, P<0.001 and r=0.709,
P<0.001, respectively), and moderately correlated with the IL-6
serum values (r=0.336, P=0.010 and r=0.297, P=0.030, respectively)
(Table II). The NFS was moderately
correlated with patient BMI and weakly correlated with the TNF-α
serum levels. The BARD score was strongly correlated with patient
BMI and weakly correlated with the IL-6 serum levels (Table II).
 | Table IICorrelations between fibrosis scores
and clinical and biochemical markers of the study group. |
Table II
Correlations between fibrosis scores
and clinical and biochemical markers of the study group.
| | NFS | FIB-4 score | APRI score | BARD score |
|---|
| Variables | r | P-value | r | P-value | r | P-value | r | P-value |
|---|
| Age (years) | 0.501 | <0.001 | 0.709 | <0.001 | 0.171 | 0.200 | 0.187 | 0.100 |
| BMI
(kg/m2) | 0.413 | 0.002 | 0.110 | 0.400 | 0.007 | 0.900 | 0.645 | <0.001 |
| Hs-CRP (ng/ml) | 0.093 | 0.510 | 0.031 | 0.800 | 0.027 | 0.800 | 0.17 | 0.200 |
| IL-10 (pg/ml) | 0.092 | 0.500 | 0.051 | 0.700 | 0.170 | 0.200 | 0.134 | 0.300 |
| IL-6 (pg/ml) | 0.336 | 0.010 | 0.297 | 0.030 | 0.255 | 0.060 | 0.288 | 0.030 |
| TNF-α (pg/ml) | 0.266 | 0.050 | 0.226 | 0.100 | 0.155 | 0.270 | 0.178 | 0.200 |
| POSTN (ng/ml) | -0.017 | 0.900 | -0.037 | 0.700 | 0.096 | 0.400 | -0.129 | 0.300 |
| PON1 concentration
(ng/ml) | 0.004 | 0.900 | 0.040 | 0.700 | 0.084 | 0.500 | 0.062 | 0.600 |
In order to evaluate the independent association of
the clinical and laboratory variables with the fibrosis scores, we
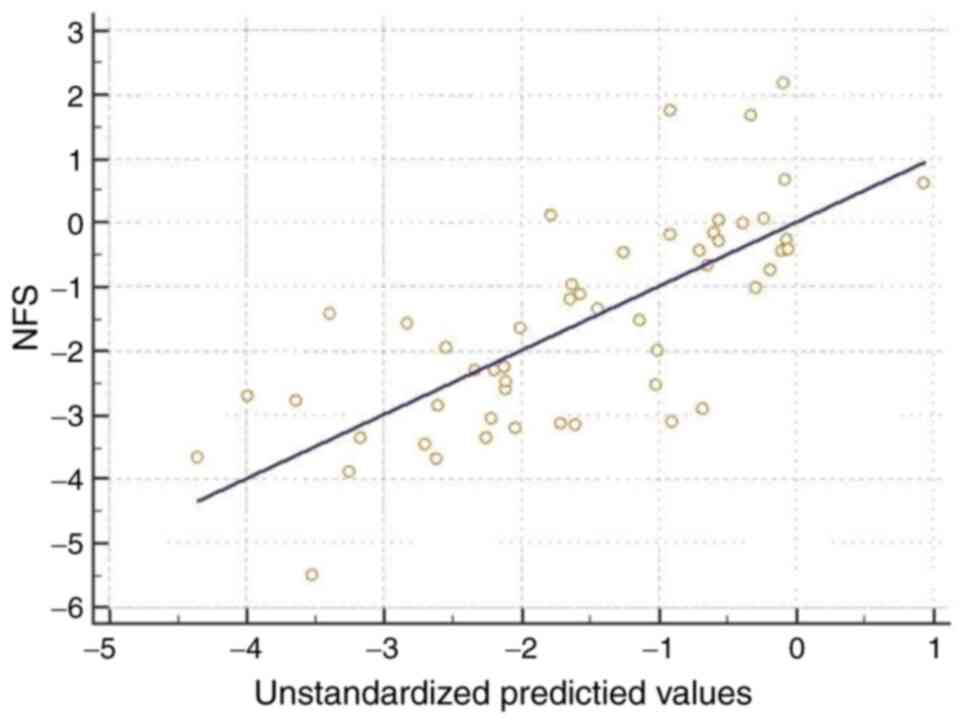
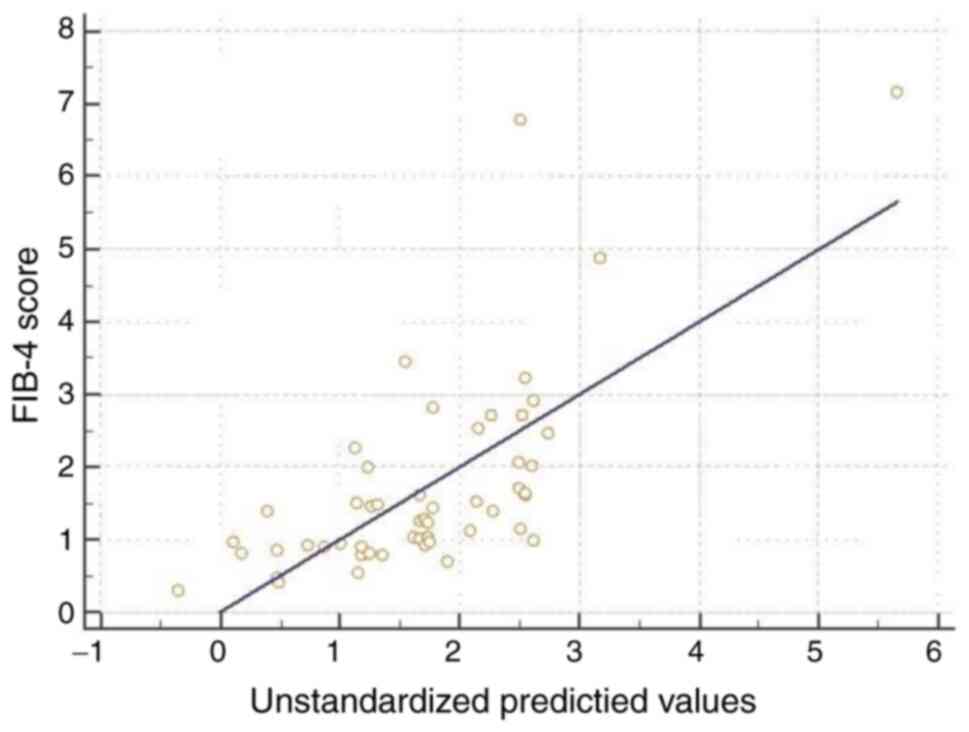
used multivariate linear regressions (Table III; Figs. 1 and 2). We obtained an R2 of 0.545
for the NFS, and R2 of 0.594 for the FIB-4 score and an
R2 of 0.489 for the BARD score. Age, metabolic syndrome
and TNF-α serum values were significantly correlated with the NFS.
Furthermore, age and TNF-α serum values were independently linked
to the FIB-4 score. The metabolic syndrome was the only independent
variable significantly associated with the BARD score. The TNF-α
was closely linked with the BARD score, but the statistical
threshold was slightly passed.
 | Table IIIMultivariate linear regressions for
fibrosis scores. |
Table III
Multivariate linear regressions for
fibrosis scores.
| | NFS | FIB-4 score | BARD score |
|---|
| Variables | B | P-value | B | P-value | B | P-value |
|---|
| Age (years) | 0.043 | 0.002 | 0.014 | <0.001 | 0.002 | 0.800 |
| Metabolic
syndrome | 1.593 | <0.001 | 0.097 | 0.080 | 1.062 | <0.001 |
| IL-6 (pg/ml) | 0.126 | 0.200 | 0.080 | 0.400 | 0.066 | 0.200 |
| TNF-α (pg/ml) | 0.003 | 0.040 | 0.001 | 0.001 | 0.001 | 0.100 |
Discussion
Non-alcoholic fatty liver disease (NAFLD) is a
disease spectrum that is gaining more and more research interest,
particularly due to its potential evolution from NAFL towards
steatohepatitis (NASH), cirrhosis and hepatocellular carcinoma
(1). As a specific treatment for
NAFLD does not exist and the accuracy of liver biopsy for NASH
diagnosis is not yet matched by other methods, current efforts are
now focused on discovering non-invasive NAFL and/or NASH diagnostic
scoring systems and targeted therapies (1,29,30).
The concept of this study came from the pragmatic
review of Dyson et al, which concluded that alanine
aminotransferase (ALT) is a poor predictor of NAFLD presence, US is
the first-line imaging technique and liver fat decreases as
fibrosis increases (31). Thus, we
designed a study which would include US evaluation of liver
steatosis [as utilized by Petta et al (16)], validated non-invasive liver
fibrosis markers (trying to replace the need for a liver biopsy)
and serum assessment of potential biomarkers of liver impairment
(either inflammation via oxidative stress [(PON1), cytokine
activation (IL-6, TNF-α) and promotion of fibrosis (POSTN)]. To our
knowledge, this is the first study to evaluate a potential relation
between PON1 serum concentration and POSTN serum level and
non-invasive liver fibrosis scores.
The median score for NFS in our patients was-1.55.
This value is below the low cut-off score (-1.455) proposed by
Angulo et al (28). The high
cut-off value (>0.676), indicating a potentially advanced
fibrosis, was found only in three patients. As for the FIB-4 score,
which is considered to be one of the most useful and simple
non-invasive tests to assess advanced fibrosis in NAFLD (32), the median score was 1.32. As it was
proven that an FIB-4 score of <1.3 has a 90% negative predictive
value (NPV) for advanced fibrosis, our cohort seemed to be a rather
‘non-advanced liver fibrosis’ one. The median APRI score was 0.63
in our study group, while the median BARD score was 2. A recently
published meta-analysis compared all the 4 scores used by us in a
population of 13,046 patients with NAFLD based on 64 studies
(33). The FIB-4 and NFS performed
better than the others, both with NPV >90% in ruling out
advanced liver fibrosis (33), and
have been lately proposed as first-line instruments for identifying
patients that seem unlikely to need further assessment (34). A recently published study suggested
a different approach (a step layered combination of non-invasive
liver fibrosis markers to improve the accuracy of predicting
advanced liver fibrosis), and showed that APRI, BARD, NIKEI
(non-invasive Koeln-Essen-index) and FibroMeter NAFLD could be
preferred to FIB-4 and NFS for the diagnosis of advanced fibrosis
in NAFLD, due to a better diagnostic accuracy for liver fibrosis
(35). In our study, the NAFLD
fibrosis score was the only one to have multiple positive
correlations with the other parameters. It was strongly correlated
with patient age, moderately correlated with patient BMI and serum
levels of IL-6, and weakly correlated with the serum values of
TNF-α.
We did not find a statistically significant
correlation between PON1 serum concentration (which was rather low
in our cohort, taking into consideration the detection range
between 3.12-200 ng/dl: Median=11.81 ng/dl) and the non-invasive
fibrosis scores. Due to its protective effect against oxidative
stress (36), we expected PON1
concentration to be correlated with the estimated degree of liver
fibrosis in patients with NAFLD (especially in our cohort with
persistently elevated aminotransferase levels). As we recently
reported, PON1 serum concentration was found to be decreased in the
serum of patients with NAFLD compared to subjects without NAFLD
(37). In addition, in previous
studies, we showed an association between PON1 and obesity and
metabolic syndrome (38,39). Still, in the present study, our
cohort had a prevalence of metabolic syndrome of only 59.6%, and
this might partially explain the lack of correlation between PON1
serum levels and the non-invasive fibrosis scores. Another
potential explanation of the lack of correlations between PON1 and
fibrosis could have its origins in modulators of PON1. Activity of
this enzyme is influenced by PON1 gene polymorphisms, and by
other non-genetic factors. The L55M polymorphism seems to be
associated with NAFLD (37), but
its variants cannot fully explain the lack of correlation between
PON1 and fibrosis in the present study. A recent study showed that
PON1 activity is modulated only by resistin, among other cytokines
and adipokines evaluated (IL-6, IL-8, TNF-α, leptin, adiponectin)
(40). Furthermore, it is currently
accepted that high levels of peroxynitrite can 0lead to
modification of PON1 activity (41). This parameter could not be evaluated
in our study. Another factor that was not evaluated in the present
study was the diet of the subjects. As we previously mentioned,
diet is an important modulator of PON1 activity. In the present
study, it was difficult to record all of our patient dietary
habits, although we observed that many of them were followers of a
Western diet (known to have a negative impact on NAFLD). Finally,
to the best of our knowledge, there is no published study which has
evaluated the possible association between PON1 and liver fibrosis
in NAFLD patients (either assessed by liver biopsy or by other
methods such as fibrosis scores or imaging methods), thus the
results of our pilot study cannot be compared with data from the
literature.
As for POSTN, we also did not observe a
statistically significant correlation between its serum levels and
non-invasive fibrosis scores. In 2015, Amara et al showed
that liver fibrogenesis is induced by TNF-α and IL-17, through
enhanced expression of POSTN (42).
Still, the main mechanism by which POSTN exerts its pro-fibrotic
action in the liver is the activation of HSCs. POSTN was
demonstrated to be at high levels in the serum of patients with
cirrhosis, compared to controls, and at even higher values in
patients with hepatocellular carcinoma (43). However, few subsequent clinical
studies have suggested that NAFLD patients could benefit from
treatment with POSTN antagonists and that POSTN could become a
liver fibrosis biomarker in NAFLD (25). In our study, we found a lack of
correlation between POSTN and non-invasive fibrosis scores, as,
even if we did not find a similar study in the literature, we
expected a direct relationship between this pro-fibrotic enzyme and
NAFLD non-invasive fibrosis scores. A potential explanation for
this lack of correlation comes from a study published after the
writing of our study protocol. In that study, Takeda et al
showed that POSTN cross-reacts with the renin-angiotensin system,
and that blockade of the angiotensin-II receptor with losartan
improved liver fibrosis (44). With
many of our subjects being hypertensive and treated with
angiotensin-II receptor blockers, the results may have been altered
by this parameter.
In the multivariate linear regression model, we
found that TNF-α was linked with the non-invasive fibrosis scores,
mainly with FIB-4 and NFS. We also found an association between
IL-6 and the noninvasive fibrosis scores, an association which was
not confirmed in the linear regression model. We then focused to
find a potential explanation for the relationship between TNF-α and
the non-invasive liver fibrosis scores in NAFLD. In a 4-year
follow-up study, high serum levels of TNF-α were found to be
associated with NAFLD development in subjects without NAFLD
(45). In another study, TNF-α was
associated with the likelihood of NAFLD presence, but also with
high levels of IL-6 and visfatin, and decreased levels of
adiponectin (46). In a population
of pediatric patients with NAFLD, TNF-α serum levels were
correlated with the histologic liver injury scores (47). Still, we were not able to identify a
study that evaluated a potential direct relationship between serum
TNF-α levels and non-invasive liver fibrosis scores, although TNF-α
is known to play an important role in NAFLD, both in promoting
steatosis and liver fibrosis (48,49).
This role is acknowledged mainly due to the enhancement of survival
of HSCs, which was proven to be the main determinant of liver
fibrosis (50). In addition, TNF-α
promotes liver injury by enhancing hepatocyte apoptosis and
activation of B cells, which further produce proinflammatory
cytokines, mainly TNF-α and IL-6(51). This is a potential explanation for
the correlations between IL-6 and the non-invasive fibrosis markers
in our study (except for APRI score), even if a clear association
was not found in the multivariate linear regressions. The
usefulness of therapies with TNF-α antagonists (e.g. infliximab,
adalimumab) is addressed by a recent review, which concludes that
these agents may become useful medication for NASH (51).
The other two variables found to be associated with
the non-invasive fibrosis scores were age and metabolic syndrome.
Our result is consistent with the results of a recent study, in
which the authors proved that the risk for severe fibrosis
presented variability among age groups and that risk was higher in
patients who presented more components of the metabolic syndrome
(52).
Our study has several limitations. Firstly, the
study included a moderate number of patients, mainly due to strict
exclusion criteria. Secondly, although liver biopsy remains the
definitive diagnosis of NAFLD and especially NASH, we were not able
to perform it in all our patients, mainly due to their reluctance;
also, we had to take into consideration its invasiveness and the
sampling biases of this test (35).
After exclusion of any other causes of liver impairment or
significant alcohol consumption, in the context of persistently
elevated aminotransferases, we supposed that our cohort might have
been comprised of NASH patients (probably most of them). Still, we
were unable to fully evaluate them (e.g. by using transient
elastography or liver biopsy) because of method unavailability or
because of patient reluctance to undergo an invasive test (like
liver biopsy). Finally, because our subjects were recruited
consecutively, the number of patients with presumed advanced liver
fibrosis (as estimated by the non-invasive tests) was small.
Our study also has strengths. To the best of our
knowledge, it is the first study which evaluates the association
between PON1 and liver fibrosis, quantified by non-invasive
fibrosis scores, in patients with NAFLD. Although our study sample
is relatively small, the results showed that a correlation between
PON1 or POSTN and non-invasive fibrosis scores is highly unlikely
in patients with NAFLD, even within a larger study group.
Younossi et al suggested that, for the
detection of advanced liver fibrosis, the existing biomarkers did
not achieve the validity to be called ‘surrogate endpoints’
(32). Recent studies focus on
finding non-invasive indexes useful in NAFLD (53). The further search for the ideal
panel of biomarkers, able to distinguish ‘benign’ NAFL from NASH
and cirrhosis, is the ultimate purpose of the focused efforts on
NAFLD clinical research, because these markers could facilitate the
finding of a pathogenetic treatment for this condition. In
conclusion, serum levels of TNF-α, age and metabolic syndrome, were
associated with the non-invasive liver fibrosis scores. POSTN serum
levels and PON-1 serum concentrations were not correlated with the
non-invasive fibrosis scores. Thus, TNF-α, age and the presence of
metabolic syndrome might be considered for incorporation into a
future comprehensive score for non-invasive evaluation of liver
fibrosis in patients with NAFLD.
Acknowledgements
Not applicable.
Funding
This research study was partially financed by an internal grant
(nos. 7690/72/15.04.2016 and 5200/62/01.03.2017) from the ‘Iuliu
Hațieganu’ University of Medicine and Pharmacy Cluj-Napoca.
Availability of data and materials
The generated and analyzed data are included in this
published article.
Authors' contributions
Conceptualization of the research study was carried
out by MVM, ICB, LC, ȘCV and MA. Methodology was achieved by MVM,
LC, OS, ȘCV and MA and validation by MA, LC, DMM and VN. Formal
analysis was conducted by ȘCV; investigation by MVM, LC, RMP, ICB
and OS. Resources were accrued by ICB, ȘCV, RMP, DMM and ADB;
writing-original draft preparation was carried out by MVM.
Writing-review and editing was performed by ȘCV, LC, ICB and MA;
visualization by MVM; supervision by VN, ADB and MA. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was conducted according to the Declaration
of Helsinki and was approved by the Ethics Committee of the ‘Iuliu
Hațieganu’ University of Medicine and Pharmacy (no.
404/02/Jul/2015). All patients signed an informed consent form
prior to study inclusion.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
References
|
1
|
Sanyal AJ: Past, present and future
perspectives in nonalcoholic fatty liver disease. Nat Rev
Gastroenterol Hepatol. 16:377–386. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Friedman SL, Neuschwander-Tetri BA,
Rinella M and Sanyal AJ: Mechanisms of NAFLD development and
therapeutic strategies. Nat Med. 24:908–922. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
European Association for the Study of the
Liver (EASL); European Association for the Study of Diabetes
(EASD); European Association for the Study of Obesity (EASO).
EASL-EASD-EASO clinical practice guidelines for the management of
non-alcoholic fatty liver disease. J Hepatol. 64:1388–1402.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ekstedt M, Franzén LE, Mathiesen UL,
Thorelius L, Holmqvist M, Bodemar G and Kechagias S: Long-term
follow-up of patients with NAFLD and elevated liver enzymes.
Hepatology. 44:865–873. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ekstedt M, Nasr P and Kechagias S: Natural
history of NAFLD/NASH. Curr Hepatol Rep. 16:391–397.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Suzuki A and Diehl AM: Nonalcoholic
steatohepatitis. Annu Rev Med. 68:85–98. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang J, Fernández-Galilea M,
Martínez-Fernández L, González-Muniesa P, Pérez-Chávez A, Martínez
JA and Moreno-Aliaga MJ: Oxidative stress and non-alcoholic fatty
liver disease: Effects of omega-3 fatty acid supplementation.
Nutrients. 11(872)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mendez-Sanchez N, Cruz-Ramon VC,
Ramirez-Perez OL, Hwang JP, Barranco-Fragoso B and Cordova-Gallardo
J: New aspects of lipotoxicity in nonalcoholic steatohepatitis. Int
J Mol Sci. 19(2034)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kim KH and Lee MS: Pathogenesis of
nonalcoholic steatohepatitis and hormone-based therapeutic
approaches. Front Endocrinol (Lausanne). 9(485)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Masarone M, Rosato V, Dallio M, Gravina
AG, Aglitti A, Loguercio C, Federico A and Persico M: Role of
oxidative stress in pathophysiology of nonalcoholic fatty liver
disease. Oxid Med Cell Longev. 2018(9547613)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bocsan IC, Milaciu MV, Pop RM, Vesa SC,
Ciumarnean L, Matei DM and Buzoianu AD: Cytokines
genotype-phenotype correlation in nonalcoholic steatohepatitis.
Oxid Med Cell Longev. 2017(4297206)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tarantino G, Citro V and Capone D:
Nonalcoholic fatty liver disease: A challenge from mechanisms to
therapy. J Clin Med. 9(15)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Stojsavljević S, Gomerčić Palčić M,
Virović Jukić L, Smirčić Duvnjak L and Duvnjak M: Adipokines and
proinflammatory cytokines, the key mediators in the pathogenesis of
nonalcoholic fatty liver disease. World J Gastroenterol.
20:18070–18091. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lindenmeyer CC and McCullough AJ: The
natural history of nonalcoholic fatty liver disease-an evolving
view. Clin Liver Dis. 22:11–21. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Stål P: Liver fibrosis in non-alcoholic
fatty liver disease-diagnostic challenge with prognostic
significance. World J Gastroenterol. 21:11077–11087.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Petta S, Maida M, Macaluso FS, Di Marco V,
Camma C, Cabibi D and Craxı A: The severity of steatosis influences
liver stiffness measurement in patients with nonalcoholic fatty
liver disease. Hepatology. 62:1101–1110. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Furlong CE, Marsillach J, Jarvik GP and
Costa LG: Paraoxonases-1, -2 and -3: What are their functions? Chem
Biol Interact. 259:51–62. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shokri Y, Variji A, Nosrati M,
Khonakdar-Tarsi A, Kianmehr A, Kashi Z, Bahar A, Bagheri A and
Mahrooz A: Importance of paraoxonase 1 (PON1) as an antioxidant and
antiatherogenic enzyme in the cardiovascular complications of type
2 diabetes: Genotypic and phenotypic evaluation. Diabetes Res Clin
Pract. 161(108067)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ciumarnean L, Milaciu MV, Macarie AE,
Sampelean DP and Achimas-Cadariu A: Non-genetic factors influencing
serum PON1 levels. HVM Bioflux. 6:20–24. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Marsillach J, Aragonès G, Mackness B,
Mackness M, Rull A, Beltrán-Debón R, Pedro-Botet J,
Alonso-Villaverde C, Joven J and Camps J: Decreased paraoxonase-1
activity is associated with alterations of high-density lipoprotein
particles in chronic liver impairment. Lipids Health Dis.
9(46)2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Marsillach J, Camps J, Ferre N, Beltran R,
Rull A, Mackness B, Mackness M and Joven J: Paraoxonase-1 is
related to inflammation, fibrosis and PPAR delta in experimental
liver disease. BMC Gastroenterol. 9(3)2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fuhrman B: Regulation of Hepatic
Paraoxonase-1 expression. J Lipids. 2012(684010)2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Samy W and Hassanian MA: Paraoxonase-1
activity, malondialdehyde and glutathione peroxidase in
non-alcoholic fatty liver disease and the effect of atorvastatin.
Arab J Gastroenterol. 12:80–85. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lou-Bonafonte JM, Gabás-Rivera C, Navarro
MA and Osada J: PON1 and mediterranean diet. Nutrients.
7:4068–4092. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jia Y, Zhong F, Jiang S, Guo Q, Jin H,
Wang F, Li M, Wang L, Chen A, Zhang F, et al: Periostin in chronic
liver diseases: Current research and future perspectives. Life Sci.
226:91–97. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kumar P, Smith T, Raeman R, Chopyk DM,
Brink H, Liu Y, Sulchek T and Anania FA: Periostin promotes liver
fibrogenesis by activating lysyl oxidase in hepatic stellate cells.
J Biol Chem. 293:12781–12792. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ratziu V, Bellentani S, Cortez-Pinto H,
Day C and Marchesini G: A position statement on NAFLD/NASH based on
the EASL 2009 special conference. J Hepatol. 53:372–384.
2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Angulo P, Hui JM, Marchesini G, Bugianesi
E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, et
al: The NAFLD fibrosis score: A noninvasive system that identifies
liver fibrosis in patients with NAFLD. Hepatology. 45:846–854.
2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Drescher HK, Weiskirchen S and Weiskirchen
R: Current status in testing for nonalcoholic fatty liver disease
(NAFLD) and nonalcoholic steatohepatitis (NASH). Cells.
8(845)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ratziu V: A critical review of endpoints
for non-cirrhotic NASH therapeutic trials. J Hepatol. 68:353–361.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dyson JK, Anstee QM and McPherson S:
Non-alcoholic fatty liver disease: A practical approach to
diagnosis and staging. Frontline Gastroenterol. 5:211–218.
2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Younossi ZM, Loomba R, Anstee QM, Rinella
ME, Bugianesi E, Marchesini G, Neuschwander-Tetri BA, Serfaty L,
Negro F, Caldwell SH, et al: Diagnostic modalities for nonalcoholic
fatty liver disease, nonalcoholic steatohepatitis, and associated
fibrosis. Hepatology. 68:349–360. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xiao G, Zhu S, Xiao X, Yan L, Yang J and
Wu G: Comparison of laboratory tests, ultrasound, or magnetic
resonance elastography to detect fibrosis in patients with
nonalcoholic fatty liver disease: A meta-analysis. Hepatology.
66:1486–1501. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Castera L, Friedrich-Rust M and Loomba R:
Noninvasive assessment of liver disease in patients with
nonalcoholic fatty liver disease. Gastroenterology.
156:1264–1281.e4. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yang M, Jiang L, Wang Y, Li X, Zou Z, Han
T, Nan Y, Lu F and Zhao J: Step layered combination of noninvasive
fibrosis models improves diagnostic accuracy of advanced fibrosis
in nonalcoholic fatty liver disease. J Gastrointestin Liver Dis.
28:289–296. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Levy D, Reichert CO and Bydlowski SP:
Paraoxonases activities and polymorphisms in elderly and old-age
diseases: An overview. Antioxidants (Basel). 8(118)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Milaciu MV, Vesa ȘC, Bocșan IC, Ciumărnean
L, Sâmpelean D, Negrean V, Pop RM, Matei DM, Pașca S, Răchișan AL,
et al: Paraoxonase-1 serum concentration and PON1 gene
polymorphisms: Relationship with non-alcoholic fatty liver disease.
J Clin Med. 8(2200)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ciumarnean L, Vesa SC, Dronca E, Sampelean
DP, Vlad VC, Moldovan MS and Achimaş CA: Distribution of
paraoxonase 1 polymorphisms and activities in obese patients. Rev
Romana Med Lab. 21:381–389. 2013.
|
|
39
|
Ciumarnean L, Dronca E, Vesa SC, Sampelean
D, Buzoianu AD and Achimas-Cadariu A: Paraoxonase 1
genotype-phenotype correlation in patients with metabolic syndrome.
Rom J Morphol Embryol. 56:387–392. 2015.PubMed/NCBI
|
|
40
|
Tisato V, Romani A, Tavanti E, Melloni E,
Milani D, Bonaccorsi G, Sanz JM, Gemmati D, Passaro A and
Cervellati C: Crosstalk between adipokines and paraoxonase 1: A new
potential axis linking oxidative stress and inflammation.
Antioxidants (Basel). 8(287)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Marín M, Moya C and Máñez S: Mutual
influences between nitric oxide and paraoxonase 1. Antioxidants
(Basel). 8(619)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Amara S, Lopez K, Banan B, Brown SK,
Whalen M, Myles E, Ivy MT, Johnson T, Schey KL and Tiriveedhi V:
Synergistic effect of pro-inflammatory TNFα and IL-17 in periostin
mediated collagen deposition: Potential role in liver fibrosis. Mol
Immunol. 64:26–35. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lv Y, Wang W, Jia WD, Sun QK, Huang M,
Zhou HC, Xia HH, Liu WB, Chen H, Sun SN and Xu GL: High
preoparative levels of serum periostin are associated with poor
prognosis in patients with hepatocellular carcinoma after
hepatectomy. Eur J Surg Oncol. 39:1129–1135. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Takeda K, Noguchi R, Kitade M, Namisaki T,
Moriya K, Kawaratani H, Okura Y, Kaji K, Aihara Y, Douhara A, et
al: Periostin cross-reacts with the renin-angiotensin system during
liver fibrosis development. Mol Med Rep. 16:5752–5758.
2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Seo YY, Cho YK, Bae JC, Seo MH, Park SE,
Rhee EJ, Park CY, Oh KW, Park SW and Lee WY: Tumor necrosis
factor-α as a predictor for the development of nonalcoholic fatty
liver disease: A 4-year follow-up study. Endocrinol Metab (Seoul).
28:41–45. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Jamali R, Arj A, Razavizade M and Aarabi
MH: Prediction of nonalcoholic fatty liver disease via a novel
panel of serum adipokines. Medicine (Baltimore).
95(e2630)2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Manco M, Marcellini M, Giannone G and
Nobili V: Correlation of serum TNF-alpha levels and histologic
liver injury scores in pediatric nonalcoholic fatty liver disease.
Am J Clin Pathol. 127:954–960. 2007.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Niederreiter L and Tilg H: Cytokines and
fatty liver disease. Liv Res. 2:14–20. 2018.
|
|
49
|
Copaci I, Micu L and Voiculescu M: The
role of cytokines in non-alcoholic steatohepatitis. A review. J
Gastrointestin Liv Dis. 15:363–373. 2006.PubMed/NCBI
|
|
50
|
Yang YM and Seki E: TNFα in liver
fibrosis. Curr Pathobiol Rep. 3:253–261. 2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lopetuso LR, Mocci G, Marzo M, D'Aversa F,
Rapaccini GL, Guidi L, Armuzzi A, Gasbarrini A and Papa A: Harmful
effects and potential benefits of anti-tumor necrosis factor
(TNF)-α on the liver. Int J Mol Sci. 19(2199)2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Petta S, Eslam M, Valenti L, Bugianesi E,
Barbara M, Camma C, Porzio M, Rosso C, Fargion S, George J and
Craxì A: Metabolic syndrome and severity of fibrosis in
nonalcoholic fatty liver disease: An age-dependent risk profiling
study. Liver Int. 37:1389–1396. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Culafic M, Vezmar Kovacevic S, Dopsaj V,
Stulic M, Vlaisavljevic Z, Miljkovic B and Culafic D: A simple
index for nonalcoholic steatohepatitis-HUFA-Based on routinely
performed blood tests. Medicina (Kaunas). 55(243)2019.PubMed/NCBI View Article : Google Scholar
|