Introduction
Granular cell tumor (GCT) was initially discovered
by Weber in 1854(1), but more
accurately reported by Abrikossoff in 1926(2). GCT was initially called ‘granular cell
myoblastoma’ due to the suspected muscle origin, but electronic
microscopy and immunohistochemical (IHC) studies showed that GCTs
are of Schwann cell origin (3-5).
The most frequently affected regions are the tongue,
skin and subcutaneous tissues or breast (6). GCTs in the gastrointestinal (GI) tract
are uncommon (approximately 8% of all GCTs), mostly affecting the
esophagus or colon and less frequently the stomach (7,8). Most
GCTs are benign, with fewer than 2% of cases being malignant
(9).
Histologically, GCTs are made up of large polygonal
and fusiform cells, consisting of an expansive and granular
cytoplasm, arranged in nests. GCTs are almost always positive for
S100 protein, CD68 and CD56 antibodies. Endoscopic examination of
the lesion reveals more commonly a yellowish-white sessile mass,
covered by normal appearing mucosa (3).
Herein, we report an incidental GCT that had
developed in the gastric cardia and successfully removed by
endoscopic submucosal dissection (ESD). A review of the literature
concerning gastric GCTs is also presented.
Case report
A 52-year-old woman presented to a
gastroenterologist for routine colonoscopy on March, 2020. The
patient complained over the past few months of upper abdominal
discomfort after eating, without dysphagia or other distinct
symptoms and was unable to mention when the symptoms commenced.
Personal medical history was unremarkable and the family medical
history included a distant relative with colorectal
adenocarcinoma.
Upon examination, the colonoscopy showed no
abnormalities, but upper GI endoscopy revealed a polypoid lesion of
the gastric cardia. ESD of the lesion was performed and the
specimen was submitted for histopathological evaluation.
Gross examination of the resected specimen revealed
an elastic, tan, polypoid fragment of 12x10x8 mm, with a stalk of
9x8 mm. The cut surface showed a solid, yellowish nodular tumor of
6x5 mm. Relevant sections including tumor with the stalk were
sampled for histological examination.
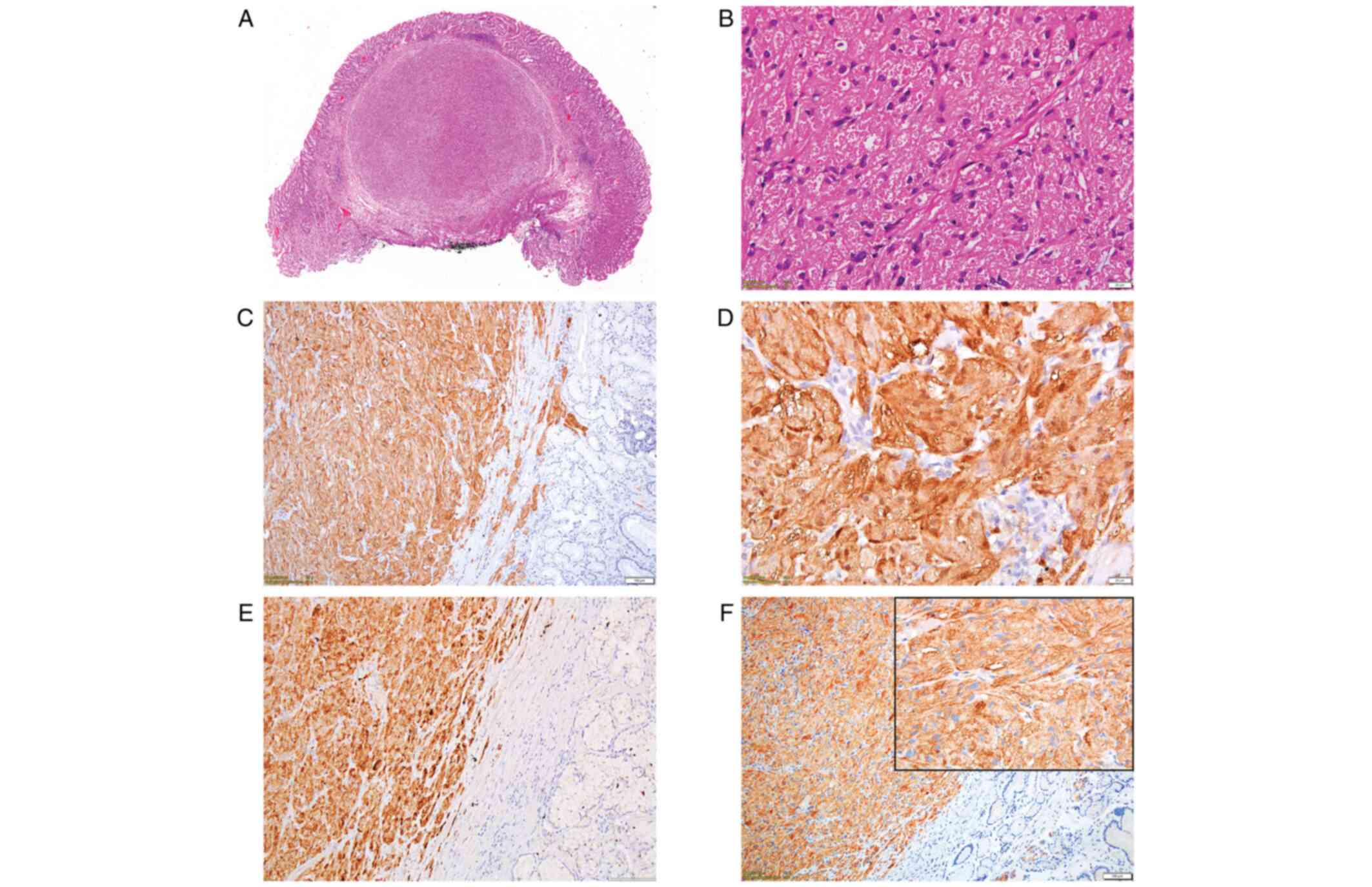
Microscopic examination using hematoxylin and eosin
(H&E) staining showed a well-defined, nodular tumor, which
developed into the submucosal layer and was covered by normal
gastric mucosa. It was composed of spindle and polygonal cells,
arranged in strands, with abundant eosinophilic granular cytoplasm.
Nuclei were slightly pleomorphic and vesicular, without mitotic
activity (Fig. 1A and B).
Tumor cells were positive for S-100 protein
(Fig. 1C and D), inhibin alpha (Fig. 1E), CD56 (Fig. 1F) and CD68. CD34 expression was
noted within the tumor, more prominent towards tumor borders, while
the Ki-67 index was lower than 1%. The entire panel of markers used
for IHC analysis of this case is presented in Table I.
 | Table IAntibodies used to establish the IHC
profile of the tumor. |
Table I
Antibodies used to establish the IHC
profile of the tumor.
| Antibody | IHC expression | Dilution | Clone | Company |
|---|
| S100 | Positive | RTU | Polyclonal | Novocastra |
| Inhibin alpha | Positive | RTU | R1 | Dako/Agilent
Technologies, Inc. |
| CD56 | Positive | 500:1 | CD564 | Novocastra |
| CD68 | Positive | RTU | PG-M1 | Dako/Agilent
Technologies, Inc. |
| Ki-67 | <1% | RTU | MIB 1 | Dako/Agilent
Technologies, Inc. |
| CD34 | Negative | RTU | QBEnd 10 | Novocastra |
| CD117 | Negative | RTU | EP10 | Master
Diagnostica |
| DOG1 | Negative | RTU | K9 | Novocastra |
| PanCK | Negative | RTU | AE1/AE3 | Dako/Agilent
Technologies, Inc. |
| Calretinin | Negative | RTU | DAK-Calret 1 | Dako/Agilent
Technologies, Inc. |
Surgical margins were tumor-free, with neoplastic
cells located at 0.4 mm of the closest limit.
Based on the H&E examination and IHC profile,
the tumor was diagnosed as a benign GCT. The ESD was uneventful and
the patient was discharged on the same day. At 5 months of
follow-up, there were no signs of recurrence or other related
events.
Discussion
GCT was initially reported by Weber in 1854(1), but only 70 years later, in 1926, was
this type of tumor properly described by Abrikosoff (2). Abrikossoff initially described GCT as
a ‘granular cell myoblastoma’ because of suspected muscle origin
(3). Until only recently, the
histogenesis of GCTs were controversial, but due to electronic
microscopy and immunohistochemistry (IHC), it was possible to
consider a peripheral nerve-related cell origin for these tumors
(4,5).
GCTs are rare lesions which can occur anywhere in
the body. Lack et al conducted a retrospective study over a
32-year period and found that GCTs accounted for only 0.03% of more
than 410,000 surgical specimens examined in their facility. They
also found that these tumors involved more frequently the oral
cavity, skin and subcutaneous tissue (6).
One year later, Johnston and Helwig published a
study of 75 GCTs found in the GI tract, most of them being located
in the esophagus, followed by the colon, perianal region, stomach,
small intestine and appendix (7).
Recently, Yasuda et al (2020) conducted a
literature search in PubMed and Ichushi-Web for the term ‘gastric
granular cell tumor’ and found 71 cases reported from inception to
2019(8). This reconfirms the rare
appearance of gastric GCTs and, to the best of our knowledge, this
is the 72nd case described in the literature.
According to Patti et al gastric GCTs are
more commonly diagnosed between the fourth to sixth decade of life,
with no gender predisposition, but with ethnic concerns, with most
cases reported in the Japanese population (10). In our case, the patient was a
52-year-old woman from Eastern Europe, whose age corresponds to the
most common age range described in the literature for this type of
tumor.
Most GI-GCTs are discovered incidentally on
endoscopy. Some authors consider that it is doubtful whether
symptoms such as dysphagia or abdominal discomfort are caused by
GCTs due to their small size, with the exception of large gastric
GCTs, which may be directly responsible for specific symptoms such
as gastric obstruction or upper GI hemorrhage (10). Regarding our case, the localization
of the tumor in the gastric cardia could have caused the abdominal
discomfort, without dysphagia or other distinct symptoms; a
hypothesis confirmed by the remission of the symptoms after tumor
excision.
Conventional endoscopy is the most frequent
technique performed for the evaluation of the GI tract. For this
reason, GI-GCT is usually discovered upon endoscopy and it is often
described as a submucosal mass with overlying normal mucosa, on
occasion with yellow discoloration (11). On the other hand, if available,
endoscopic ultrasound (EUS) is the best procedure to evaluate
GI-GCTs which are usually described as hypoechoic, homogeneous and
smooth-edged lesions. Nonetheless, GI-GCTs can be hyperechoic,
heterogeneous and have irregular margins as well. Most importantly,
EUS can show whether the tumor involves or originates from
muscularis propria (9).
Histologically, most gastric GCTs develop in the
submucosal layer, being composed of sheets or compact nests of
polygonal and fusiform cells with abundant, eosinophilic or
amphophilic granular cytoplasm, with Periodic acid-Schiff
(PAS)-positive granules and uniform pyknotic nuclei. The granular
aspect of these tumors, positivity for CD68 and PAS, are due to the
accumulation of lysosomes in the cell cytoplasm (12,13).
Lazar and Fletcher showed that CD68 is positive in nonneural GCTs
as well (12).
We noted strong expression of S100 protein and CD56
which was concordant with results from previous studies and
supports the neural origin of this tumor (11,14).
Inhibin is a hormone secreted primarily by ovarian
granulosa cells and testicular Sertoli cells, with involvement in
the regulation of the pituitary-gonadal feedback system. Inhibin
alpha is a useful marker for ovarian sex cord-stromal tumors and
adrenocortical neoplasm. Some studies have reported expression of
inhibin alpha in GCTs with different locations, including those
with GI origin (11,13,14).
Vered et al studied the expression of inhibin alpha (ABD
Serotec, Clone R1, dilution 1:50) in 42 oral GCTs and 38 of them
were diffusely positive (13). An
et al analyzed the inhibin alpha expression (ABD Serotec,
MCA951S, dilution 1:100) in 58 cases of GI-GCTs, 30 of them being
positive (52%), with more common expression in colorectal than
esophageal GCTs. Only 1 out of 3 gastric GCTs was positive
(11). In contrast, Parfitt et
al reported no expression of inhibin alpha (Cell Marque,
dilution 1:25) in 7 GI-GCTs (15).
Fanburg-Smith et al six histologic criteria
of malignancy in their 73 cases of GCT which included: necrosis,
spindling of tumor cells, increased nuclear-to-cytoplasmic ratio,
nuclear pleomorphism, vesicular nuclei with prominent nucleoli and
an increased mitotic rate with more than two mitoses/10 high-power
fields (x200 magnification). Based on these criteria, they
classified GCTs into benign (none of the criteria or focal
pleomorphism), atypical (1 or 2 criteria) and malignant (3 to 6
criteria) (16). Even if in our
case there was a small population of spindle cells within the
tumor, it was insufficient in our opinion to define it as atypical;
thus, we reported the tumor as a benign GCT. To the best of our
knowledge, only two cases of malignant gastric GCT have been
reported in the English literature; both patients were diagnosed in
Japan (8).
The Ki-67 proliferation index and p53 expression
have been correlated with histologic classification and clinical
course. Benign GCTs are commonly negative for p53 and the Ki-67
index is under 10%, while malignant GCTs show upregulation of p53
as well as a Ki-67 index higher than 10% (10). In our case presented above, the
Ki-67 index was lower than 1%, which supported the benign character
of the tumor.
The best course of treatment for GI-GCTs is
resection by ESD. Lu et al published a study on 14 patients
with esophageal GCTs treated by ESD. This research showed that this
procedure removes the tumor entirely and also provides all the
means necessary for pathological assessment. Moreover, patient
follow-up ranged from 4 to 40 months, during which no local
recurrence was observed (17).
In conclusion, a GCT arising in the stomach is a
rare condition, mostly benign, which should be taken into
consideration when a gastric polypoid lesion is examined. Inhibin
alpha, along with S100 protein, prove to be valuable in the
differential diagnosis of GCTs from other tumors, but further
studies should be performed to determine why tumor cells show
immunohistochemical expression for inhibin alpha.
Acknowledgements
Not applicable.
Funding
Article processing charge for publication was supported by
‘Victor Babeș’ University of Medicine and Pharmacy (Timișoara,
Romania).
Availability of data and materials
All information in this article is documented by
relevant references, and further information concerning the case
report is available upon request.
Authors' contributions
SMT contributed to the conception of the study,
collected, analyzed and interpreted data from the literature and
critically revised the manuscript. RAB contributed to the
conception of the study, performed the literature research, drafted
the manuscript and is responsible for confirming the authenticity
of all the raw data. ADP contributed to the conception of the
study, performed the literature research, drafted the manuscript
and is responsible for confirming the authenticity of all the raw
data ALCD contributed to the interpretation of the data from the
literature, collected, analyzed and interpreted the data
corresponding to the patient and critically revised the manuscript.
IMR collected, analyzed and interpreted the data corresponding to
the patient and critically revised the manuscript. OP performed the
literature research, selected the included studies, analyzed and
interpreted the data and drafted the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Ethics approval is not required for case reports at
our institution. Informed consent from the patient was obtained to
utilize images of the tissue for scientific purposes, with the
condition of not disclosing identifiable information.
Patient consent for publication
The patient provided consent for publication.
Competing interests
The authors declare that they have no conflicts of
interest or competing interests.
Authors' information
Sorina Maria Taban: ORCID: 0000-0002-3971-2756
Robert Alexandru Barna: ORCID: 0000-0003-4634-969X Alis Liliana
Carmen Dema: ORCID: 0000-0003-0767-2718 Iulia Maria Ratiu: ORCID:
0000-0002-5622-9926 Oana Popa: ORCID: 0000-0003-3883-2438 Andrei
Dorel Plopeanu: ORCID: 0000-0002-4900-4809.
References
|
1
|
Weber CO: Anatomical examination of a
hypertrophic tongue along with remarks on the formation of striated
muscle fibers. Virchows Arch A Pathol Anat. 7:115–125. 1854.(In
German).
|
|
2
|
Abrikossoff A: Concerning myomas starting
from the striated voluntary musculature. Virchows Arch Pathol Anat.
260:215–233. 1926.(In German).
|
|
3
|
Kim DJ, Kim HW, Park SB, Choi CW, Kang DH,
Hong JB, Ji BH and Lee CS: A case of gastric granular cell tumor:
Review of literature and features of endoscopic ultrasonography.
Korean J Helicobacter Up Gastrointest Res. 15(59)2015.
|
|
4
|
Fisher ER and Wechsler H: Granular cell
myoblastoma-a misnomer. Electron microscopic and histochemical
evidence concerning its schwann cell derivation and nature
(granular cell schwannoma). Cancer. 15:936–954. 1962.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mazur MT, Shultz JJ and Myers JL: Granular
cell tumor. Immunohistochemical analysis of 21 benign tumors and
one malignant tumor. Arch Pathol Lab Med. 114:692–696.
1990.PubMed/NCBI
|
|
6
|
Lack EE, Worsham RGF, Callihan MD,
Crawford BE, Klappenbach S, Rowden G and Chun B: Granular cell
tumor: A clinicopathologic study of 110 patients. J Surg Oncol.
13:301–316. 1980.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Johnston MJ and Helwig EB: Granular cell
tumors of the gastrointestinal tract and perianal region A study of
74 cases. Dig Dis Sci. 26:807–816. 1981.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yasuda A, Yasuda T, Imamoto H, Hiraki Y,
Momose K, Kato H, Iwama M, Shiraishi O, Shinkai M, Imano M, et al:
A case of a gastric granular cell tumor preoperatively diagnosed
and successfully treated by single-incision laparoscopic surgery.
Surg Case Rep. 6(44)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Barakat M, Kar AA, Pourshahid S, Ainechi
S, Lee HJ, Othman M and Tadros M: Gastrointestinal and biliary
granular cell tumor: Diagnosis and management. Ann Gastroenterol.
31:439–447. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Patti R, Almasio PL and Di Vita G:
Granular cell tumor of stomach: A case report and review of
literature. World J Gastroenterol. 12:3442–3445. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
An S, Jang J, Min K, Kim MS, Park H, Park
YS, Kim J, Lee JH, Song HJ, Kim KJ, et al: Granular cell tumor of
the gastrointestinal tract: Histologic and immunohistochemical
analysis of 98 cases. Hum Pathol. 46:813–819. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lazar AJF and Fletcher CDM: Primitive
nonneural granular cell tumors of skin: Clinicopathologic analysis
of 13 cases. Am J Surg Pathol. 29:927–934. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vered M, Carpenter WM and Buchner A:
Granular cell tumor of the oral cavity: Updated immunohistochemical
profile. J Oral Pathol Med. 38:150–159. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fine SW and Li M: Expression of calretinin
and the alpha-subunit of inhibin in granular cell tumors. Am J Clin
Pathol. 119:259–264. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Parfitt JR, McLean CA, Joseph MG,
Streutker CJ, Al-Haddad S and Driman DK: Granular cell tumours of
the gastrointestinal tract: Expression of nestin and
clinicopathological evaluation of 11 patients. Histopathology.
48:424–430. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fanburg-Smith JC, Meis-Kindblom JM, Fante
R and Kindblom LG: Malignant granular cell tumor of soft tissue:
Diagnostic criteria and clinicopathologic correlation. Am J Surg
Pathol. 22:779–794. 1998.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lu W, Xu MD, Zhou PH, Zhang YQ, Chen WF,
Zhong YS and Yao LQ: Endoscopic submucosal dissection of esophageal
granular cell tumor. World J Surg Oncol. 12(221)2014.PubMed/NCBI View Article : Google Scholar
|