Introduction
Paragangliomas, like pheochromocytomas, are
neuroendocrine tumors originating in the chromaffin cells of the
sympathetic system's adrenal medulla or lymph nodes due to their
common embryonic origin (1). They
can secrete catecholamines, neurotransmitters which are responsible
for various symptoms, including diaphoresis, cardiovascular
symptoms (hypertension, tachycardia, hypertensive cardiomyopathy),
or psychiatric disorders (2).
Failure of adequate treatment can lead to acute aortic dissection
and cerebral catastrophes (3,4).
Genetically, a paraganglioma or a pheochromocytoma may be part of
various tumor genetic syndromes associated with other potentially
malignant tumors such as multiple endocrine neoplastic syndromes
and Von Hippel-Lindau disease (5).
In the embryonic period, neuroendocrine ridge cells (the
histological origin of pheochromocytoma and paraganglioma) migrate
from the neural tube to the periaortic region and region of
mesenchymal cells that form the fetal cortex of the adrenal gland,
forming the medullary adrenal gland (1). Thus, the tumor that develops from the
adrenal medulla is called pheochromocytoma, and one that originates
in the sympathetic ganglia is called a paraganglioma. Paraganglioma
can be located in the cervical, mediastinal, and abdominal
sympathetic ganglion chain. It is most often found in patients aged
20-50 years, with a prevalence of 1:10 of the total number of
neurotransmitter-secreting neuroendocrine tumors and 1:10 of the
total number of malignant paragangliomas with a gender ratio of
1:1(2). Paragangliomas can be
sympathetic, parasympathetic, or dopamine-secreting.
Parasympathetic paragangliomas are more common than sympathetic and
are most commonly found at the skull base along the cranial nerves
IX and X, usually inactive (6).
Sympathetic paragangliomas are rare but with a better clinical
picture, being secretory, and the rarest form secretes dopamine
that occurs due to a deficiency of the enzyme β-hydroxylase that
metabolizes dopamine into norepinephrine (7). This report describes a novel case of a
functional paraaortic paraganglioma discovered after laparoscopic
cholecystectomy performed due to abdominal pain.
Case presentation
A 44-year-old male was first examined at the
Cardiology Department of a regional hospital. He was admitted after
having multiple episodes of hypertension [peak systolic blood
pressure (SBP) >250 mmHg] immediately after laparoscopic
cholecystectomy was performed.
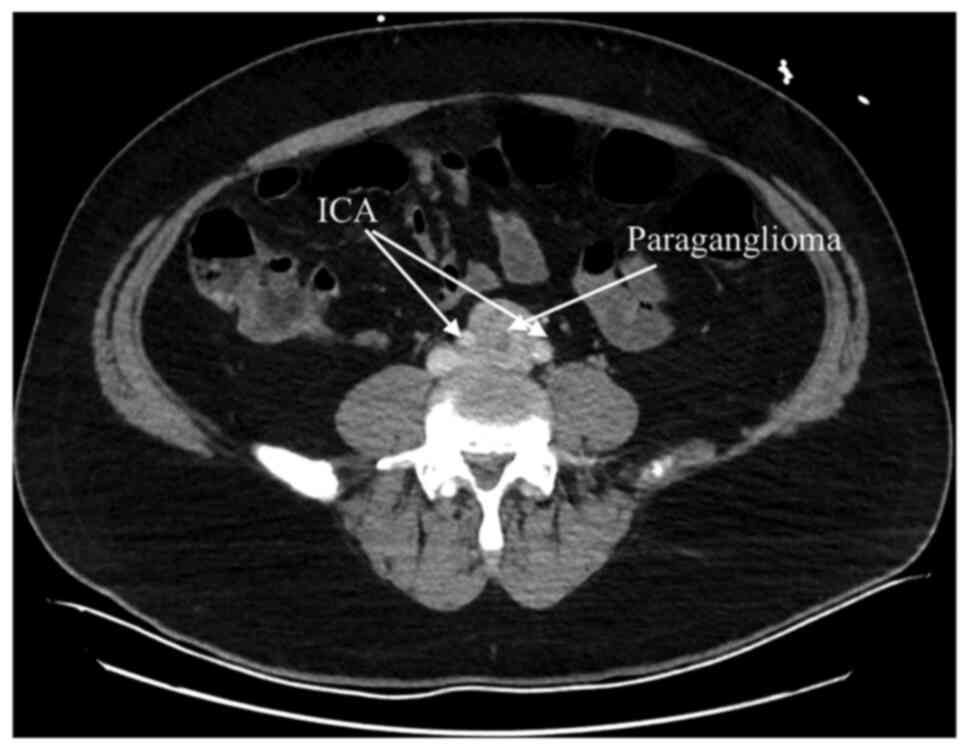
Abdominal computed tomography angiography (CTA) scan
revealed a 33x31 mm sized solitary heterogeneous mass at the aortic
bifurcation and between the two common iliac arteries (Fig. 1), which was consistent with
paraganglioma. The laboratory results showed that plasma
metanephrines and normetanephrine were both elevated, respectively,
68.2 pg/ml (normal local laboratory range <65 pg/ml) and 2,194
pg/ml (normal local laboratory range <195 pg/ml). Serum
chromogranin A level was elevated at 189 µg/l (local normal range
27-94 µg/l). Simultaneously, urinary vanyl mandelic acid (VMA) was
increased at 12 mg/24 h (normal local laboratory range <8.0
mg/24 h). The patient was treated with diltiazem 60 mg per day, 5
mg of doxazosin per day, and 25 mg of carvedilol per day;
hypertension episodes were managed with a mean arterial pressure
(MAP) of 80 mmHg. At this point, the patient was referred to our
clinic for surgery. Upon admission, the patient was clinically
stable with no fever, no peripheral edema, hypertensive with a
blood pressure of 145/80 mmHg in both upper limbs. The
electrocardiogram (ECG) revealed sinus rhythm with a heart rate of
75 beats/min. The chest X-ray was normal. Laboratory tests revealed
a mildly elevated C-reactive protein (CRP) with normal white blood
cell count, hypercholesterolemia (222 mg/dl) with low-density
lipoprotein (LDL) level of 160 mg/dl, hyperchloremia (109 mmol/l)
and creatinine levels of 0.64 mg/dl. Cardiac biomarkers were also
mildly elevated with creatine-kinase (CK) of 244 U/l and creatine
kinase myocardial band (CK-MB) of 29 U/l. Transthoracic
echocardiography (TTE) performed showed left ventricular
hypertrophy with normal ejection fraction. Coronary angiography
revealed normal coronary arteries. A diagnosis of paraganglioma was
confirmed, and surgical treatment was then decided upon.
Routine monitoring included ECG, invasive arterial
line (left radial artery), a central venous catheter (CVC) in the
left internal jugular vein, dialysis catheter in the right internal
jugular vein, 2 peripheral venous catheters 16 G, pulse oximetry,
temperature, and urinary catheter. An automated external
defibrillator and a rapid fluid infuser were available.
Rapid-acting cardiovascular drugs were also prepared. Standard
preoperative antibiotic prophylaxis was given with cefuroxime.
Extracorporeal membrane oxygenation (ECMO) on
standby was prepared. Using the Seldinger technique, both the right
common femoral artery and vein were catheterized.
After preoxygenation, general anesthesia was induced
with fentanyl 0.5 mg/kg, rocuronium 60 mg and midazolam 5 mg with
hemodynamic stability and orotracheal intubation was accomplished.
Maintenance of anesthesia was accomplished with fentanyl,
rocuronium and sevoflurane. Midline laparotomy incision was
performed with an additional dose of fentanyl and rocuronium.
Dilatated intestinal loops were observed at gross inspection. To
gain access to the retroperitoneal space, the intestines were
mobilized (Figs. 2 and 3). Subsequently, the patient experienced
severe hypotension and became mildly bradycardic followed rapidly
by electromechanical dissociation. Resuscitation protocol was
initiated. Chest compressions were started, 2 mg of atropine and 2
mg of titrate intravenous adrenalin were administered with the
onset of ventricular fibrillation. A 200 J electrical shock was
used and the patient experienced asystole. Chest compressions were
continued and 150 mg of amiodarone, 100 mg of lidocaine 1% and
titrate intravenous adrenalin reaching 20 mg were administered with
the reoccurrence of ventricular fibrillation. Another 200 J shock
was used with sinus rhythm establishment at 70 beats/min with
systolic blood pressure (SBP) of 120 mmHg with vasopressor support,
noradrenaline 50 ng/kg/min. After 15 min of cardiac arrest, ST
elevation was observed in DII, DIII, aVF and V5-V6 leads.
Transesophageal echocardiography (TEE) was performed which showed
diffuse hypokinesis of both ventricles. Hemodynamic stability was
achieved with the normalization of the ECG and complete remission
of hypokinesis 15 min after the cardiac arrest. Considering the
hemodynamic stability, there was no need to initiate extracorporeal
circulation by ECMO. The serum lactate peaked at 5.4 mmol/l. It was
decided by the surgical/anesthesiology/cardiology team to continue
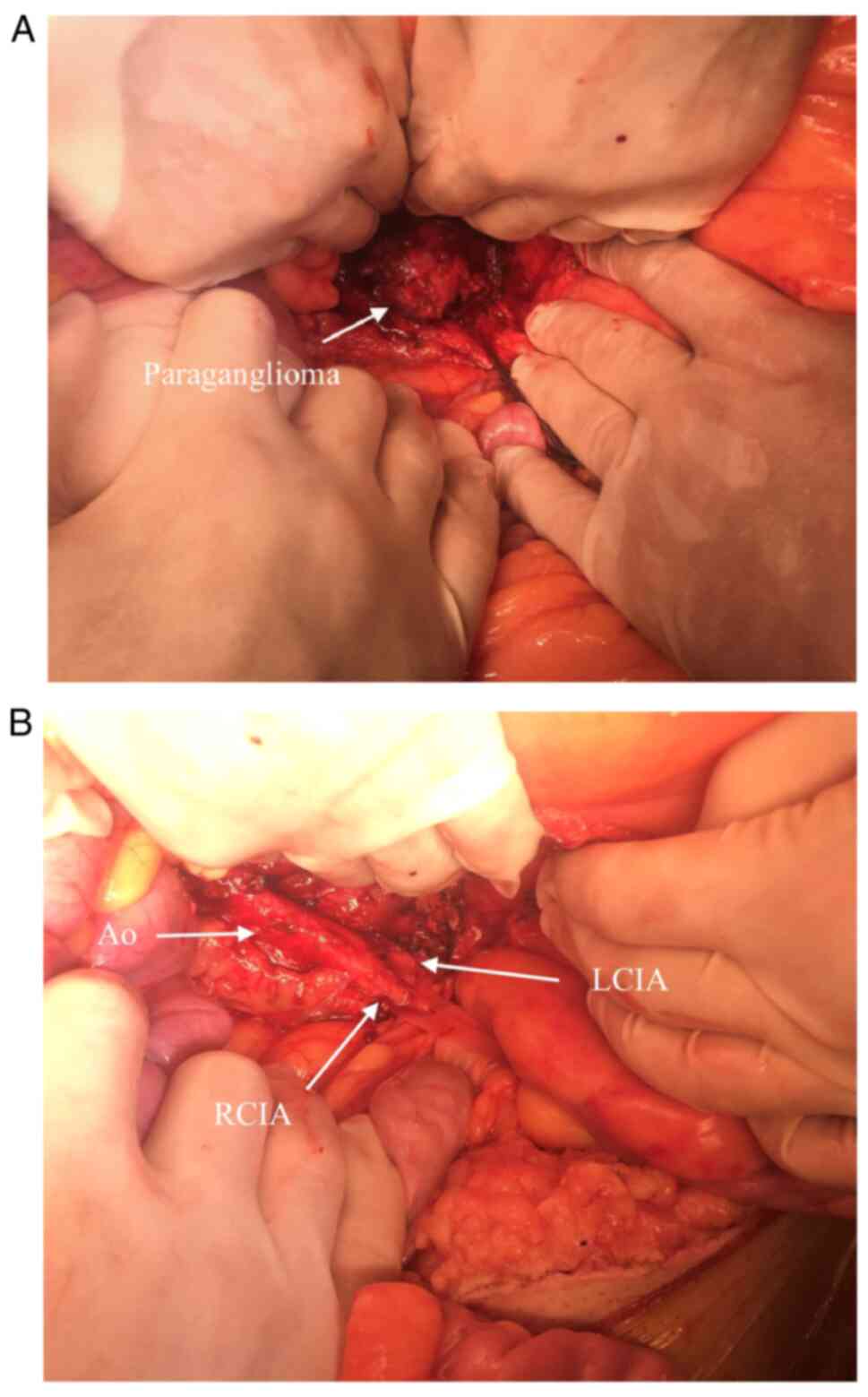
the procedure. The retroperitoneal space was opened at the aorta
bifurcation. The aorta was cross-clamped at an SBP of 100 mmHg. The
tumor was identified and the tissue around it was gradually
dissected (Fig. 2). During this
maneuver, the SBP reached 280 mmHg with 115 beats/min controlled
with continuous infusion of urapidil and metoprolol adjusted
according to heart rate reaching an SBP of 140 mmHg and a heart
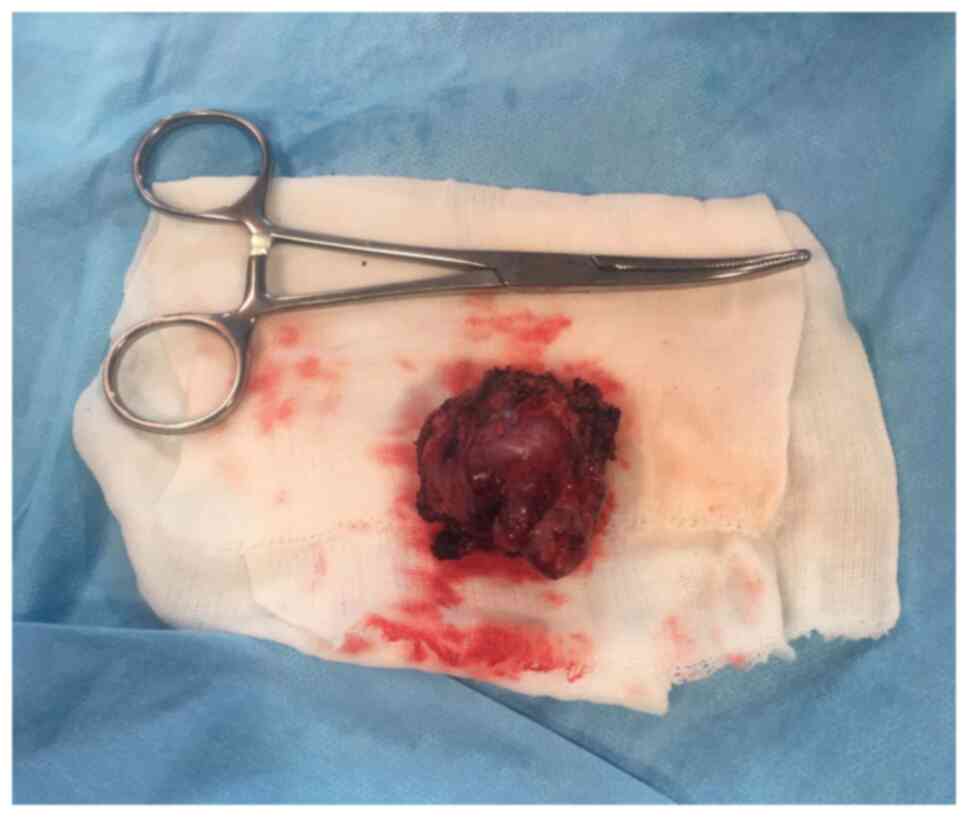
rate of 80 beats/min, sinus rhythm. The successful removal of the
mass was accomplished with no injury to the aorta, iliac arteries
or iliac veins (Fig. 3). The clamp
was removed and hemodynamic stability was obtained with
noradrenaline 100 ng/kg/min with a MAP of 70 mmHg. The mass was
sent for pathology and immunohistochemistry exams. During the
procedure, continuous fluid replacement was achieved with 3,500 ml
of Hartmann's solution, 2000 ml of Gelofusine and 5 units of fresh
frozen plasma (FFP).
The patient was extubated at 6 h after admission to
the intensive care unit and was weaned off the vasopressor support
the next day. No neurologic deficit was observed. Postoperative
24-h urine catecholamine studies and plasma metanephrines and
normetanephrine were in the normal range. During the intensive care
stay, the patient had a mean blood pressure of 70 mmHg and was
transferred to the ward after 2 days of intensive care treatment.
Bowel function was recovered on the 4th day. The patient had an
uneventful recovery and was discharged on the 7th postoperative
day.
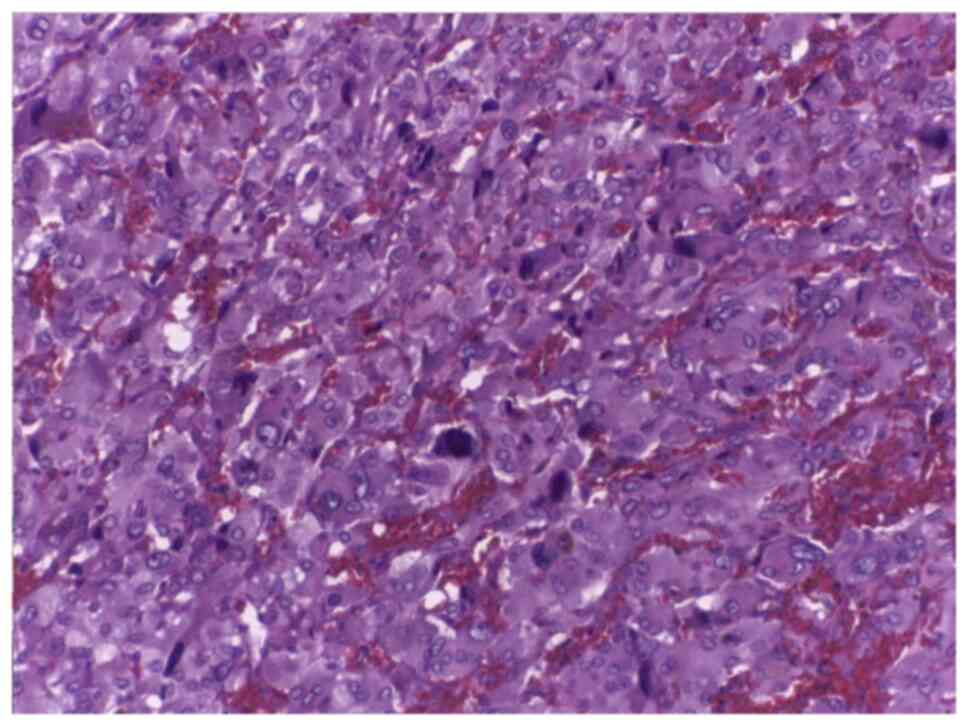
The microscopic anatomopathological examination was
performed using hematoxylin and eosin staining and showed a highly
vascular appearance, with chief cells and sustentacular cells
arranged in clusters called a zellballen pattern consistent with a
paraganglioma (Fig. 4).
Differential diagnosis of paraganglioma from liposarcoma or germ
cell tumor was considered. Immunohistochemistry showed strong
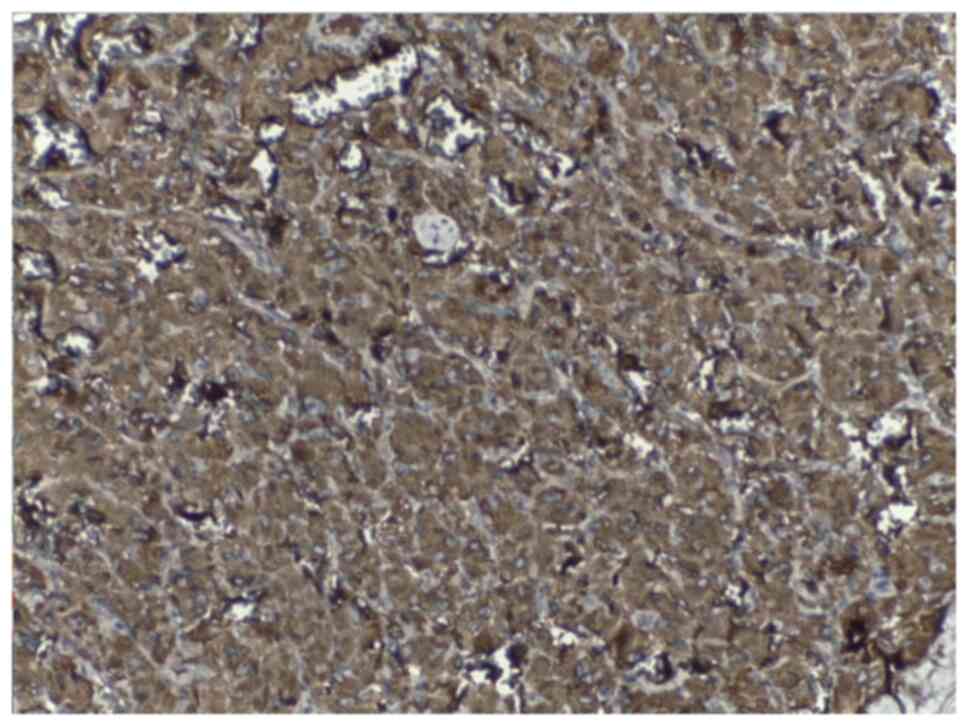
positivity for chromogranin (Fig.
5) and synaptophysin and negativity for vimentin, smooth muscle
actin, desmin, pancytokeratin, and S100 which confirmed the
diagnosis of paraganglioma.
The patient continued to be followed up; after one
year, he did not present with loco-regional recurrence or distant
metastasis, and blood pressure was normal without treatment. At six
months, 24-h urine catecholamine studies and plasma metanephrines
were negative.
Discussion
Only 15-20% of chromaffin-cell tumors are
paragangliomas (8); 1% of these
tumors are functional and produce catecholamines, the remainder are
asymptomatic or cause vague abdominal pain (9). Patients with functional paragangliomas
may experience headaches, palpitations, tachycardia, sweating,
episodic hypertension, flushing, anxiety, or profuse diaphoresis
(6). To the best of our knowledge,
this case report is the first to describe the sudden onset of the
described symptoms immediately after laparoscopic cholecystectomy
was performed. Computed tomography (CT) with contrast provides an
initial method for the localization of paragangliomas (sensitivity
88-100%), and also magnetic resonance imaging (MRI) can be useful
when a CT is contraindicated (10).
MRI has the highest sensitivity in regards to the detection of
extra-adrenal paragangliomas and pheochromocytomas (11). Ten percent of paragangliomas are
malignant (12). Approximately
20-42% of extra-adrenal sympathetic paragangliomas metastasize. The
sites of metastasis include lymph nodes, bone, liver, and lung. The
survival rate reported at 5-year for metastatic lesions is nearly
36% (13). Clinical, biochemical,
and radiological features, local invasion, and various
histopathological features can be suggestive but are inadequate to
predict malignancy. In addition, malignancy cannot be reliably
diagnosed histologically (14).
Surgical excision remains the treatment of choice for resectable
paragangliomas (15). Preoperative
cardiovascular symptoms must be controlled with alpha and
beta-blockers; appropriate patient preparation is crucial to
decrease the intraoperative hypertensive spikes and death. Complete
surgical excision is the only mode of therapy that has been shown
to improve symptoms, and to prolong survival, but, it depends on
the tumor's location, and the extent of the involvement of adjacent
structures. Therefore, in certain cases complex surgical procedures
might be needed; meanwhile improvement in the field of vascular
surgery alone or in association with various associated visceral
resection might be safely and efficiently performed (15). In our case, involvement was limited
to the supra adventitial layer of the aorta and iliac arteries, and
we were able to perform a complete resection of the tumor without
resection of arteries or veins and without compromising the
vascularization of the limbs. Following an R0 resection, patient
surveillance requires plasma catecholamines and free metanephrines,
urinary levels of catecholamines, VMA, total and fractionated
metanephrines. These should be measured every three months during
the first year, every six months until the third year, and then
annually for up to 10 years (8). CT
abdominal scan, abdominal MRI, and scintigraphy performed with
123-I labeled metaiodobenzylguanidine (MIBG) is essential in the
assessment surveillance of the patients for metastatic disease,
tumor recurrence, or delayed appearance of multiple primary tumors
(15). Histopathology examination
may reveal chief cells and sustentacular cells arranged in
clusters. Chief cells are positive upon immunohistochemistry for
synaptophysin, NSE, chromogranin, while sustentacular cells are
positive for S-100 protein (16).
These findings led to a histological diagnosis of retroperitoneal
paraganglioma.
In conclusion, paraaortic paragangliomas pose a
therapeutic challenge because of their location, which is in close
contact with the aorta, common iliac arteries and veins, and also
due to the fact that an R0 resection must be performed while
maintaining intact vascularization of the limbs. Furthermore,
management expertise is required when the paraganglioma is a
secreting tumor with potentially life-threatening intraoperative
complications. Having ECMO protection on standby is advisable in
order to deal with unpredictable alterations in blood pressure that
can occur during the surgical procedure, and may lead to
intraoperative death. In this case, complete surgical resection,
normalized plasma catecholamines and urinary levels of
catecholamines and VMA, the absence of local and general recurrence
one year after the surgical resection are signs that predict a
favorable patient outcome.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Further information regarding the case presentation
is available upon request.
Authors' contributions
OS, AD, CA and PRD performed the surgical procedure.
AD, CA, AT, NB, PRD and CSt reviewed the literature data. AD, CA,
CD and CSa carried out the preoperative investigation of the
patient and intraoperative management. OS, PRD, CS, IB, RCG and NB
prepared the draft of the article. VAI was the advisor of the
surgical procedures. OS, NB and VAI reviewed the final draft of the
manuscript. All authors read and approved the final version of the
article.
Ethics approval and consent to
participate
The Ethical Committee of ‘Prof. Dr. C. C. Iliescu’
Institute of Emergency for Cardiovascular Diseases approved the
study.
Patient consent for publication
Patient consent for publication was obtained and
signed by the patient on 16/07/2019.
Competing interests
There are no competing interests to declare
regarding this study.
References
|
1
|
Lumb R and Schwarz Q: Sympathoadrenal
neural crest cells: The known, unknown and forgotten? Dev Growth
Differ. 57:146–157. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tabakin AL, Weintraub MA, Radadia KD,
Salazar CG, Sadimin E and Singer EA: Metastatic retroperitoneal
paraganglioma: Case report and review of the literature. Clin Oncol
(Belmont). 4(1589)2019.PubMed/NCBI
|
|
3
|
Stiru O, Gorduza EV, Dorobantu FL, Parasca
CA, Chioncel O, Bubenek Turconi SI, Filipescu DC and Iliescu VA:
Surgical management of type a acute aortic dissection in patients
with marfan syndrome: A single center experience. Rev Med Chir Soc
Med Nat Iasi. 120:611–618. 2016.PubMed/NCBI
|
|
4
|
Abderrahim SB, Meddeb MA, Marrakchi J,
Besbes G, Rammah-Rommani S, Hamdoun M and Khelil MB: Sudden death
due to neck paraganglioma: A pediatric case report and review of
the literature. Am J Forensic Med Pathol. 41:199–202.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gaal J, van Nederveen FH, Erlic Z,
Korpershoek E, Oldenburg R, Boedeker CC, Kontny U, Neumann HP,
Dinjens WN and de Krijger RR: Parasympathetic paragangliomas are
part of the Von Hippel-Lindau syndrome. J Clin Endocrinol Metab.
94:4367–4371. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Manger W and Gifford RJ: Pheochromocytoma:
A clinical review. In: Hypertension: Pathophysiology, diagnosis,
and management. Vol 2. 2nd edition. Laragh J and Brenner B (eds).
Raven Press, New York, NY, pp2225-2244, 1995.
|
|
7
|
Feldman JM, Blalock JA, Zern RT, Shelburne
JD, Gaede JT, Farrell RE and Wells SA Jr: Deficiency of
dopamine-beta-hydroxylase. A new mechanism for normotensive
pheochromocytomas. Am J Clin Pathol. 72:175–185. 1979.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Samara AA, Diamantis A, Symeonidis D,
Anagnostou A, Diamantis AM, Mavrovounis G and Tepetes K:
Asymptomatic presacral paraganglioma: Management of an
unpredictable intraoperative finding. Surg J (NY). 6:e131–e134.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mantas D, Kandilis A and Charalampoudis P:
Nonfunctioning symptomatic paraganglioma: Is there an optimal
follow-up for patients with extra-adrenal benign paragangliomas. J
Surg Case Rep. 2014(rju092)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lenders JW, Duh QY, Eisenhofer G,
Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K and
Young WF Jr: Pheochromocytoma and paraganglioma: An endocrine
society clinical practice guideline. J Clin Endocrinol Metab.
99:1915–1942. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Archontovasilis F, Markogiannakis H,
Dikoglou C, Drimousis P, Toutouzas KG, Theodorou D and Katsaragakis
S: Paraganglioma of the greater omentum: Case report and review of
the literature. World J Surg Oncol. 5(87)2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Plouin PF, Amar L, Dekkers OM, Fassnacht
M, Gimenez-Roqueplo AP, Lenders JW, Lussey-Lepoutre C and Steichen
O: European society of endocrinology clinical practice guideline
for long-term follow-up of patients operated on for a
phaeochromocytoma or a paraganglioma. Eur J Endocrinol. 174:G1–G10.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sclafani LM, Woodruff JM and Brennan MF:
Extraadrenal retroperitoneal paragangliomas: Natural history and
response to treatment. Surgery. 108:1124–1129. 1990.PubMed/NCBI
|
|
14
|
Mannina EM, Xiong Z, Self R and Kandil E:
Resection of a catecholamine-elaborating retroperitoneal
paraganglioma invading the inferior vena cava. Case Rep Surg.
2014(837054)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Disick GI and Palese MA: Extra-adrenal
pheochromocytoma: Diagnosis and management. Curr Urol Rep. 8:83–88.
2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lack EE, Cubilla AL, Woodruff JM and
Lieberman PH: Extra-adrenal paragangliomas of the retroperitoneum:
A clinicopathologic study of 12 tumors. Am J Surg Pathol.
4:109–120. 1980.PubMed/NCBI View Article : Google Scholar
|