Introduction
Kidney cancer is among the 10 most common cancers,
and it is estimated that 73,750 individuals were diagnosed with
kidney cancer in 2020 in the United States (1). Renal cell carcinoma (RCC), also known
as renal cancer, is the most common form of kidney cancer and is
responsible for up to 85% of kidney cancer cases in the United
States; it is more frequent in males than in females (ratio, 1.7:1)
(2), and the majority of patients
are of older age, with an average age of 64 years (3). The disease encompasses >10
histological and molecular subtypes, of which clear cell RCC
(ccRCC) is the most common and accounts for the majority of kidney
cancer-associated deaths (4).
Localized RCC can be successfully managed with surgery, whereas
metastatic RCC is refractory to conventional chemotherapy (5,6). The
tumor size, Fuhrman nuclear grade, tumor histology, performance
status and surrounding fat invasion are well-known prognostic
factors (7); however, lymph node
metastasis also serves a key role in the survival of patients with
locally advanced RCC, and patients with lymph node metastases often
have a poor prognosis (8). As RCC
advances, the 5-year survival rate from 93% decreases to 67% for
patients with regional metastases and 12% for those with distant
metastatic disease (9).
The generation of new lymphatic vessels through
lymphangiogenesis and the remodeling of existing lymphatics are
considered to be important steps in cancer metastasis (10). Tumor cells can either acquire access
to the lymphatic system by inducing intra-tumoral lymphangiogenesis
or by invading pre-existing lymphatics in the surrounding tissue
(11). Recent evidence indicates
that peri-tumoral or intra-tumoral lymphangiogenesis is a precursor
for lymphatic metastasis in the majority of carcinoma and melanoma
cases (12). The ability of a tumor
to induce and activate lymphatic growth has been positively
associated with metastasis (13).
Lymphangiogenesis is a complex process regulated by
a number of factors (14). It has
been reported that vascular endothelial growth factor-C (VEGF-C)
and its receptor, VEGFR-3, are the basis of lymphatic vessel
formation (15). VEGF-C/VEGFR-3
signaling is important for the progression of various types of
cancer, such as head and neck cancer, melanoma and breast cancer
(16-18).
These receptors are expressed mainly on endothelial cells, but are
also expressed on tumor cells (19). During tumor development, lymphatic
endothelial cells substantially expand in response to VEGFR-3
engagement by VEGF-C produced in the tumor microenvironment, a
process known as tumor-associated lymphangiogenesis (20). Therefore, VEGF-C can induce tumor
lymphangiogenesis and promote lymph node metastasis (21). During the process of tumor
lymphangiogenesis and lymphatic metastasis, the molecules
associated with the VEGF-C/VEGFR-3 signaling pathway, such as
Furin-like protease 1, contactin-1, prospero homeobox protein 1,
lymphatic vessel endothelial hyaluronic acid receptor 1,
podoplanin, SOX-18 and C-X-C chemokine receptor type 4, serve a
crucial role in the complex biological activities of tumor growth
and progression (22). In a variety
of experimental tumors, such as non-small cell lung cancer,
colorectal cancer and bladder cancer, VEGFR-3 inhibitory antibodies
or VEGF-C-targeted small interfering RNA molecules can decrease the
incidence of lymph node metastasis (19,23,24).
However, to the best of our knowledge, there are no studies
concerning the specific profile of lymphangiogenesis in RCC.
The aim of the present study was to investigate the
association between VEGF-C/VEGFR-3 and lymphangiogenesis, clinical
pathology and lymph node metastasis in RCC.
Materials and methods
Patients and samples
A total of 40 surgically resected samples of RCC
with single tumors were collected from the Affiliated Hospital of
Chengde Medical University (Chengde, China) between July 2016 and
September 2017. Among these, 18 cases were treated with
nephron-sparing surgery and 22 cases underwent radical nephrectomy,
with 11 of the aforementioned 22 patients exhibiting lymph node
metastasis and undergoing regional lymph node dissection. There
were 25 males and 15 females aged between 24 to 65 years with an
average age of 51.9 years. Pathological analysis confirmed the
diagnosis of RCC in all cases, including 37 cases of ccRCC, 2 cases
of papillary RCC and 1 case of chromophobe RCC. Patients did not
receive radiotherapy, chemotherapy or immunotherapy prior to
surgery. A total of 10 adjacent normal renal tissues (meeting the
requirement of >2 cm above the edge of the tumor) were selected
as the controls. The RCC tissues were excised rapidly for
histological investigation and RNA isolation. According to the WHO
criteria published in 2004(25), 20
cases were highly differentiated, 11 cases were moderately
differentiated and 9 cases were poorly differentiated or
undifferentiated. According to the clinical staging of the American
Joint Committee on Cancer for renal cell carcinoma in 2002(26), there were 22 cases of stage I, 6
cases of stage II, 11 cases of stage III and 1 case of stage IV.
Among all cases, there were 29 cases without lymph node metastasis
and 11 cases with renal hilar lymph node metastasis.
RNA extraction and reverse
transcription-semiquantitative PCR (RT-sqPCR)
Total RNA was isolated from freshly dissected
tissues using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Total
RNA was used as template to synthesize the first chain of cDNA
using PrimeScript™ 1st Strand cDNA Synthesis Kit (Takara
Bio, Inc.) according to the manufacturer's protocol. β-actin served
as the internal control. Primers were synthesized by Sangon Biotech
Co., Ltd., and were as follows: VEGF-C (target fragment, 373 bp)
forward, 5'-AGAGACGGCACAAGGATGAG-3' and reverse,
5'-ATCGGCAGGAAGTGTGATTG-3'; ACTB (target fragment, 453 bp) forward,
5'-AGCGGGAAATCGTGCGTGAC-3' and reverse, 5'-ACATCTGCTGGAAGGTGGAC-3'.
The thermocycling conditions were as follows: 3 min of
pre-denaturation at 95˚C, followed by 30 cycles of denaturation at
95˚C for 15 sec and annealing at 60˚C for 30 sec. Finally, the
mixture was incubated at 72˚C for 5 min and cooled to 4˚C.
PCR-amplified products were analyzed by electrophoresis on a 2%
agarose gel using ethidium bromide staining, and photographs were
captured with a UV transmission analyzer (Gene Company, Ltd.). The
density scanning of the electrophoresis strips of the amplified
products was performed using Quantity One software v4.6.6 (Bio-Rad
Laboratories, Inc.), and the results were expressed as the
absorbance (A) ratio of VEGF-C to β-actin
(AVEGF-C/Aβ-actin).
Immunohistochemistry (IHC)
Tissue samples were fixed with 10% formalin at 4˚C
for 12 h and embedded in paraffin. The paraffin-embedded samples
were cut into 4-µm-thick sections, which were then blocked with 3%
hydrogen peroxide for 60 min at room temperature. The sections were
dewaxed in toluene and rehydrated through sequential changes of
alcohol (100, 95 and 70%) and distilled water. For antigen
retrieval, the tissue sections were incubated with 0.01 M sodium
citrate (pH 6) in a microwave oven at 95˚C for 10 min, followed by
blocking with 5% normal goat serum (cat. no. ZLI-9021; OriGene
Technologies, Inc.) for 10 min at room temperature, the tissue
sections were incubated with rabbit anti-human VEGF-C monoclonal
antibody (cat. no. BA0548; 1:200; Wuhan Boster Biological
Technology Co., Ltd.), rabbit anti-human VEGFR-3 monoclonal
antibody (cat. no. A01276-3; 1:200; Wuhan Boster Biological
Technology Co., Ltd.) and mouse anti-human D2-40 monoclonal
antibody (cat. no. ZM-0465; undiluted; OriGene Technologies, Inc.)
for 12 h at 4˚C. Following primary antibody incubation, the tissue
sections were incubated with HRP-labeled secondary antibodies
[anti-rabbit (cat. no. BM3894, 1:1,000) and anti-mouse (cat. no.
BM3895; 1:1,000; both from Wuhan Boster Biological Technology Co.,
Ltd.)] for 1 h at room temperature. DAB (cat. no. AR1000; Wuhan
Boster Biological Technology Co., Ltd.) was used for coloration,
and hematoxylin was used for counterstaining at 37˚C for 2 min. PBS
buffer solution was used as a negative control to substitute the
primary antibody.
Histopathological evaluation
The results of VEGF-C and VEGFR-3 IHC staining were
determined. The cytoplasm and/or membrane of RCC cells that were
clear brown yellow particles was set as the standard, and the
results were analyzed using the double scoring method as previously
described by Volm et al (27). In the homogeneously dyed tumor area,
5 high magnification views (x200) of the light microscope were
selected according to the percentage of positive cells (A value)
and the staining intensity (B value). The percentage of positive
cells was scored as follows: 0, No obvious positive cells; 1,
<25% positive cells; 2, 25-50% positive cells; and 3, >50%
positive cells. The staining intensity was scored as follows: 0, No
coloring; 1, light brown-yellow; 2, brown-yellow; and 3, brown. The
final score was determined by adding the score for the percentage
of positive staining cells (A value) with that of the staining
intensity (B value). The final value thus ranged from 0 to 6, and
was as follows: 0, negative (-); 1-2, weak (+); 3-4, moderate (++);
and 5-6, strong expression (+++). The immunohistochemical staining
was grouped into two categories: Low expression (-/+) and high
expression (++/+++).
The IHC streptavidin-peroxidase conjugated method
(SP Ready-To-Use kit; cat. no. SP-9000; OriGene Technologies, Inc.)
was used to detect D2-40 expression. The positively stained D2-40
protein was mainly located in the cytoplasm and/or cell membrane of
the lymphatic endothelium, and was presented as a brown-yellow
color. The determination of lymphatic vessel density (LVD) was
according to the method previously described by Weidner et
al (28), which was used to
observe and select 5 regions with maximum LVD (hot spots) under a
light microscope (magnification, x100), and then 5 optic fields
were counted under x200 magnification (covering an area of 0.74
mm2) and the average LVD value was used. All these
assessments were made by two independent observers. LVD was defined
as the number of vessels/mm2. Intra-tumoral LVD was
defined as D2-40+ vessels that were in close contact
with tumor cells. Peritumoral LVD was defined as D2-40+
vessels in the fibrous capsule or at the interface of tumor and
adjacent kidney.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism v8.0.1 (GraphPad Software, Inc.). Continuous variables were
presented as the mean ± SD of three independent experiments, and
the difference between two groups was analyzed using unpaired
Student's t-test. One-way ANOVA with Bonferroni post-hoc test was
used to compare differences among multiple groups. The categorical
data was analyzed using χ2 test. Mann-Whitney U test was
used to evaluate significant differences for the RT-sqPCR data.
Spearman's correlation analysis was used for correlation analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
VEGF-C mRNA expression
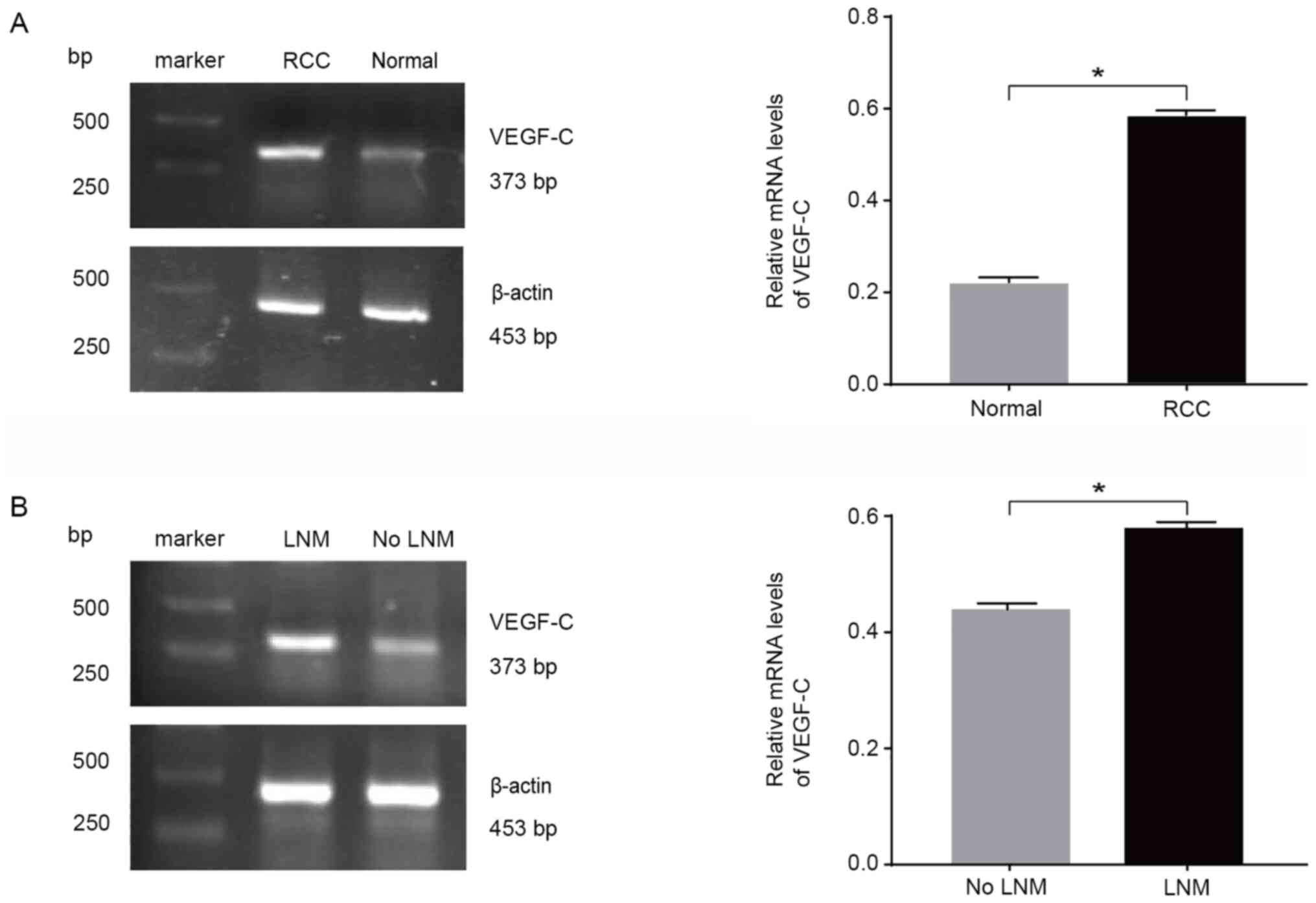
The results of the RT-sqPCR analysis revealed a
small amount of VEGF-C mRNA in the 10 normal renal tissues, with a
relative expression level of 0.250±0.104; however, in the 40 RCC
tissues, the relative VEGF-C expression was 0.576±0.191, which was
significantly higher than that in normal tissues (Fig. 1A). The relative mRNA expression
levels of VEGF-C were significantly higher in the lymph node
metastasis group compared with in the non-lymph node metastasis
group, with relative expression levels of 0.693±0.174 and
0.532±0.181, respectively (Fig.
1B). There were no significant differences in VEGF-C mRNA
expression according to age, sex and differentiation, but VEGF-C
mRNA expression was significantly higher in the group with
lymphatic metastasis compared with in the group without lymphatic
metastasis, as well as in patients with higher stages compared with
in patients with lower stages (Table
I). These data indicated that VEGF-C may serve an important
role in RCC progression.
 | Table IDifferences in VEGF-C mRNA expression
among different clinicopathological parameters in patients with RCC
(n=40). |
Table I
Differences in VEGF-C mRNA expression
among different clinicopathological parameters in patients with RCC
(n=40).
|
Characteristics | N | Relative mRNA
expression |
P-valuea |
|---|
| Kidney tissues | | | 0.001b |
|
RCC | 40 | 0.576±0.191 | |
|
Normal | 10 | 0.250±0.104 | |
| Sex | | | 0.372 |
|
Male | 25 | 0.596±0.215 | |
|
Female | 15 | 0.544±0.146 | |
| Age, years | | | 0.388 |
|
<55 | 18 | 0.606±0.229 | |
|
≥55 | 22 | 0.552±0.156 | |
|
Differentiation | | | 0.932 |
|
Good or
moderate | 31 | 0.575±0.210 | |
|
Poor | 9 | 0.581±0.113 | |
| Lymphatic
metastasis | | | 0.018b |
|
Negative | 29 | 0.532±0.181 | |
|
Positive | 11 | 0.693±0.174 | |
| Clinical
stages | | | 0.002b |
|
I+II | 28 | 0.517±0.177 | |
|
III+IV | 12 | 0.714±0.153 | |
VEGF-C expression in normal renal and
RCC tissues
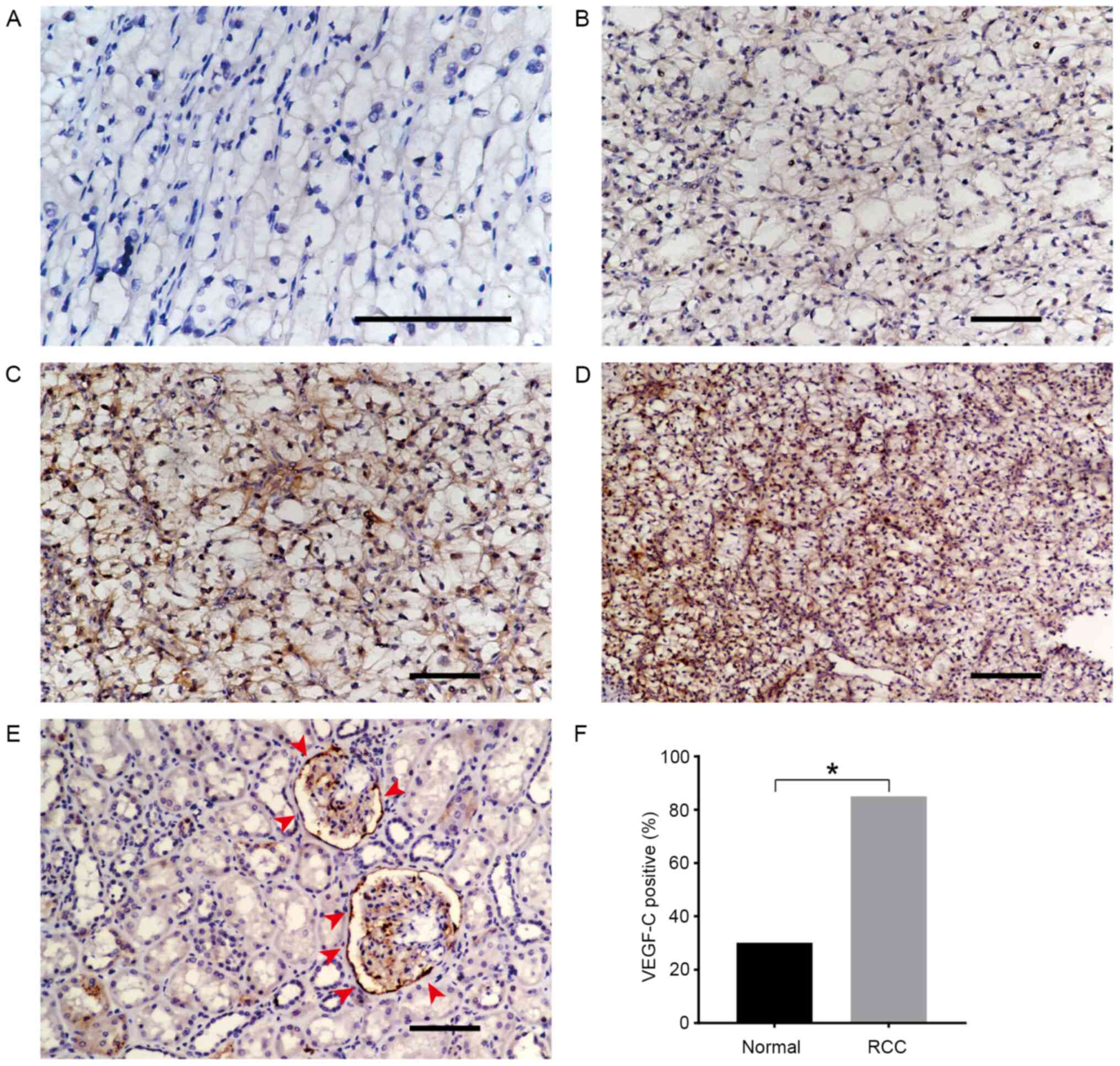
VEGF-C expression in renal tissues was further
observed via IHC. According to the percentage of positively stained
cells and staining intensity as described in the materials and
methods section, a total of 40 RCC samples were divided into the
low VEGF-C expression (n=20) and high VEGF-C expression (n=20)
groups. Specific and representative IHC-staining intensity patterns
for the VEGF-C protein in the RCC samples are presented in Fig. 2A-D. Only 3/10 normal renal tissue
samples exhibited weak VEGF-C expression (Fig. 2E). The positive expression rate
(including weak, moderate and strong staining intensity) of VEGF-C
in the RCC group was significantly higher than that in the normal
renal tissue group (85 vs. 30%, respectively; Fig. 2F). VEGF-C expression was independent
of age, sex and differentiation, but was significantly associated
with lymph node metastasis and clinical staging of RCC (Table II). These results indicated that
VEGF-C expression was upregulated in RCC tissues and associated
with tumor progression.
 | Table IIAssociation between VEGF-C expression
and clinicopathological parameters of patients with renal cell
carcinoma (n=40). |
Table II
Association between VEGF-C expression
and clinicopathological parameters of patients with renal cell
carcinoma (n=40).
| | VEGF-C
expression | |
|---|
|
Characteristics | N | Low | High |
P-valuea |
|---|
| Sex | | | | 0.327 |
|
Male | 25 | 11 | 14 | |
|
Female | 15 | 9 | 6 | |
| Age, years | | | | 0.525 |
|
<55 | 18 | 8 | 10 | |
|
≥55 | 22 | 12 | 10 | |
|
Differentiation | | | | 0.449 |
|
Good or
moderate | 31 | 17 | 14 | |
|
Poor | 9 | 3 | 6 | |
| Lymphatic
metastasis | | | | 0.013b |
|
Negative | 29 | 18 | 11 | |
|
Positive | 11 | 2 | 9 | |
| Clinical
stages | | | | 0.038b |
|
I+II | 28 | 17 | 11 | |
|
III+IV | 12 | 3 | 9 | |
VEGFR-3 expression
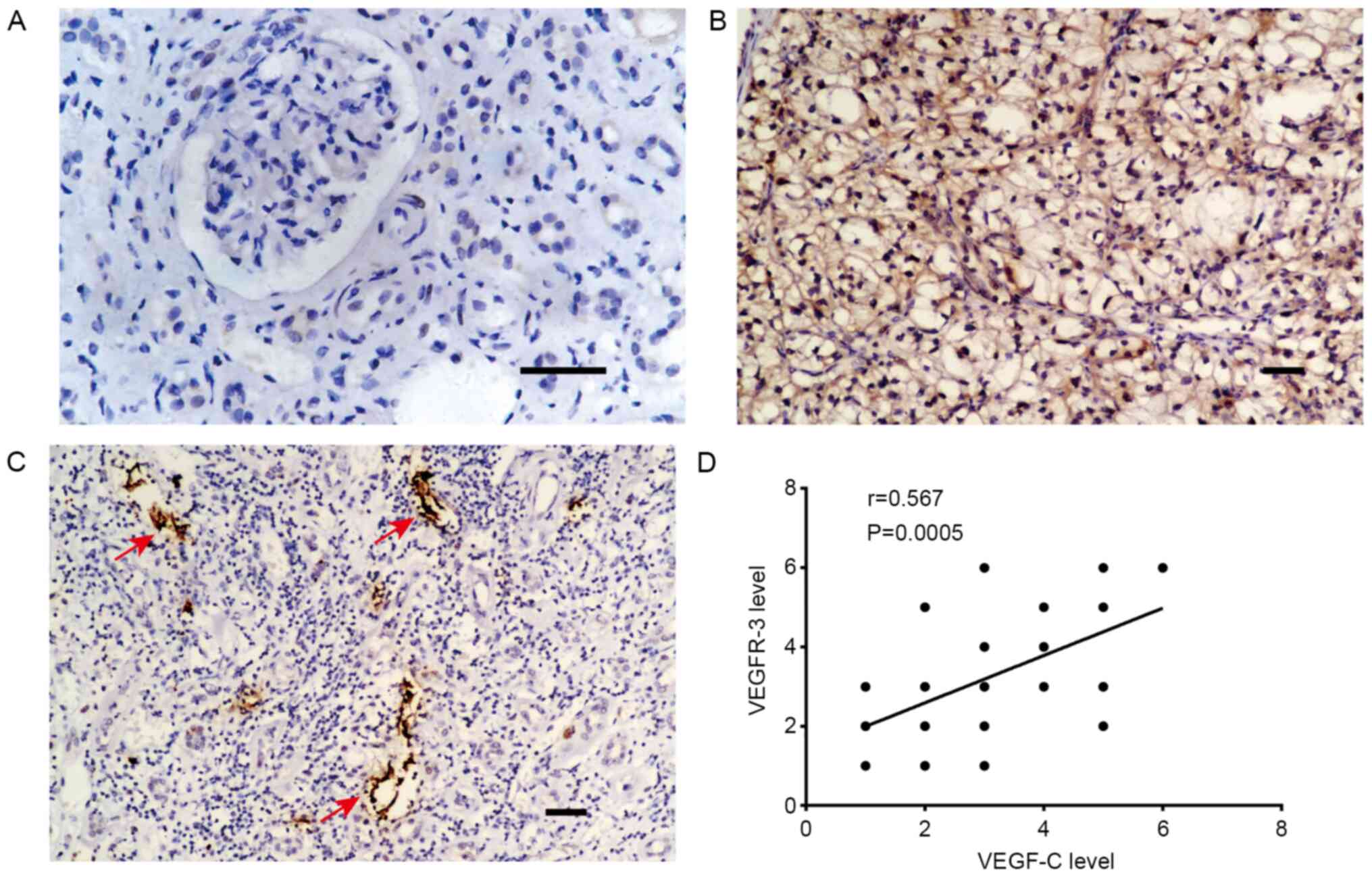
Considering that VEGFR-3, as a receptor of VEGF-C,
is widely expressed on lymphatic endothelial cells (29), the association between VEGF-C and
VEGFR-3 expression in RCC tissues was further investigated. As
shown in Fig. 3A and B, compared with in normal renal tissues,
VEGFR-3 expression was markedly higher in the tumor tissues.
Additionally, VEGFR-3+ lymphatic vessels were detected
in RCC tissues (Fig. 3C).
Furthermore, Spearman's correlation analysis demonstrated a
positive correlation between VEGF-C and VEGFR-3 expression in RCC
tissues (Fig. 3D). These results
support the notion that VEGF-C/VEGFR-3 serve a crucial role in RCC
progression.
LVD in RCC and surrounding tissues,
and its association with lymph node metastasis
Given that lymphangiogenesis serves an important
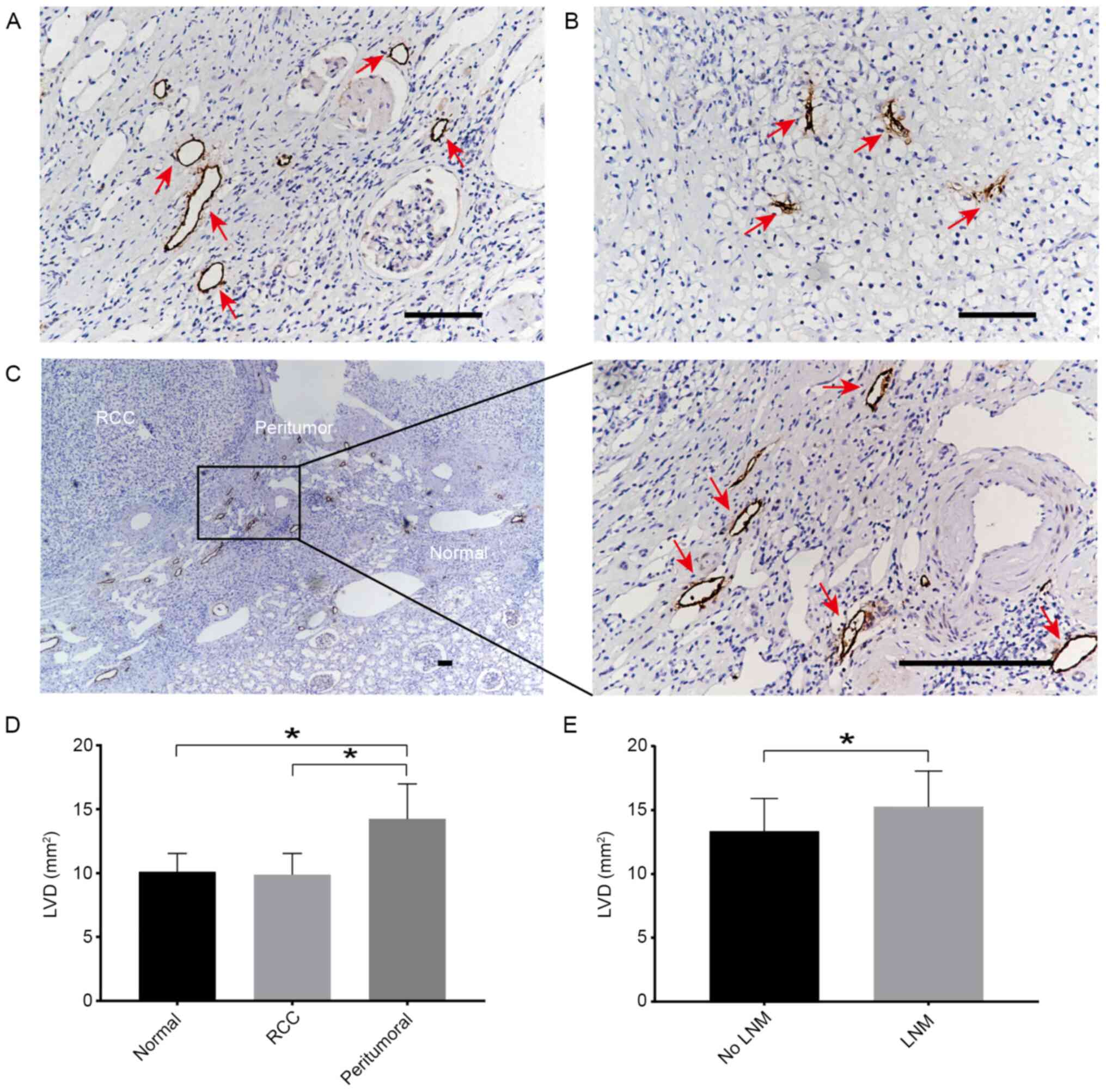
role in the process of lymph node metastasis (30), the lymphatic marker D2-40 was used
to stain the lymphatic tubes, and LVD was analyzed. IHC staining
revealed that the staining for D2-40+ cells was mainly
located in the cell membrane of lymphatic endothelial cells, and
was presented as a brown-yellow color (Fig. 4A). It was observed that the lumen of
the lymphatic vessels in the tumor appeared narrow (Fig. 4B) and the lymphatic vessels around
the tumor were functionally dilated with an enlarged diameter
(Fig. 4C). The peritumoral LVD was
14.16±2.58, which was significantly higher than that in the RCC
(9.74±2.48) and in the normal renal tissues (10.27±2.92) (Fig. 4D). However, the LVD between the
tumor and normal renal tissues did not exhibit a significant
difference (Fig. 4D). The LVD of
the peritumoral tissues in 11 cases in the lymph node metastasis
group was 15.24±1.86, which was significantly higher than that in
the 29 cases without lymph node metastasis (13.37±2.03) (Fig. 4E). These data indicated that the
lymphatic vessels around the tumor may be important in promoting
lymph node metastasis in RCC.
Discussion
VEGF-C is a newly discovered lymphatic growth factor
that belongs to the VEGF family. It was first cloned from the cDNA
library of the PC-3 human prostate cancer cell line in 1996, and is
mainly expressed in lymph nodes, the heart and placenta (31). A previous study demonstrated that
VEGF-C has two existing forms, Mr31000 and
Mr21000(32). The latter is
a mature form of VEGF-C that is produced during the process of
protein hydrolysis, and acts as a ligand to bind to VEGFR-3,
thereby inducing the proliferation and migration of lymphatic
endothelial cells by activating VEGFR-3 and promoting lymphatic
duct hyperplasia (33). VEGFR-3,
also known as Flt4, is a member of the tyrosine kinase receptor
family and it consists of extracellular (including 7 immunoglobulin
homologous domains), transmembrane and intracellular domains
(31). VEGFR-3 is widely expressed
in vascular endothelial cells of early embryos, as well as being
expressed on lymphatic endothelial cells in the late embryonic
development stage and in healthy adults (34,35).
In the present study, positive VEGFR-3 expression in the lymphatic
endothelium of RCC was observed. However, it has been revealed that
a small amount of VEGFR-3 is expressed in microcapillaries and
renal tubular endothelial cells; thus, VEGFR-3 may be associated
with chronic kidney disease and tissue inflammation (36), and its role warrants further
investigation. D2-40 is a highly specific marker of the lymphatic
endothelium that is not expressed by the blood vessel endothelium
(37). Thus, the morphological
structure of the lymphatic capillary wall can be visually displayed
and distinguished from capillary vessels using D2-40.
Lymphatic metastasis is achieved through the
invasion of cells of mature lymphatic vessels, and subsequent
metastasis to the lymph nodes (38). Therefore, the more tumor cells
become dissociated from the primary tumor, the greater the
possibility of metastasis. Reports on the presence of lymphatic
vessels in tumors remain inconsistent. Some studies have indicated
the presence of lymphatic vessels in tumors, while others have
demonstrated contrasting results (10,39).
Although it remains controversial whether tumors can induce the
genesis of lymphatic vessels, it has been demonstrated that the
excessive expression of lymphogenerative factors, such as VEGF-C,
can induce more lymphatic vessels and facilitate metastasis through
tumor lymphatic vessels (14). In
experiments using mouse tumor models, it has been demonstrated that
VEGF-C induces lymphangiogenesis and that tumor cells enter the
capillary lymphatic vessels (40).
Ruddell et al (41) has
revealed that VEGF-C induces the expansion of collecting lymphatic
vessels to facilitate lymph flow, which in turn transports a large
number of tumor cells to the lymph nodes and contributes to the
distant metastasis of tumors.
At present, VEGF-C expression in the majority of
human malignant tumors, such as esophageal (42), colorectal (24) and gastric cancer (43), is known to be markedly higher than
that in normal tissues. However, to the best of our knowledge,
there are no studies available on VEGF-C expression and its
association with lymphatic metastasis in RCC. The present study
used RT-sqPCR to detect VEGF-C mRNA expression in RCC tissues,
which was significantly higher than that in normal renal tissues.
VEGF-C mRNA expression in the lymph node metastasis group was also
higher than that in the non-lymph node metastasis group. In
addition, VEGF-C mRNA expression in patients with stage III and IV
RCC was higher than that in patients with stage I and II RCC (the
majority of cases with stage III and IV RCC were accompanied by
lymph node metastasis). Moreover, the present study demonstrated a
correlation between VEGF-C and VEGFR-3 expression in tumor tissues.
The current results suggested that the VEGF-C/VEGFR-3 axis may
serve an important role in the progression of RCC.
It has been demonstrated that VEGFR-3+
and dilated functional lymphatic vessels are located around tumors
in mouse models of sarcoma, but functional lymphatic vessels are
absent within solid tumors (44).
Furthermore, despite the presence of VEGF-C and other
lymphangiogenesis-associated factors, the formation of functional
lymphatic vessels in a murine sarcoma model was prevented (40). The present study used D2-40 as a
marker to observe the morphology and structure of microlymphatic
vessels, revealing that there were microlymphatic vessels in RCC
tissues; however, there were no statistically significant
differences in LVD between RCC and normal renal tissues.
Additionally, the present results demonstrated that the lymphatic
vessels in the tumor were small and irregular, and most were
collapsed, which may not be functional. This may be due to the
rapid proliferation of cancer cells in tumor tissues and increased
interstitial hydrostatic pressure, resulting in the occlusion of
the lumen of neoplastic lymphatic vessels (45). The current results were consistent
with those of the aforementioned studies (40,44).
However, the present study indicated that there were functionally
dilated lymphatic vessels in the tissues surrounding the tumor. The
LVD in peritumoral tissues in the lymph node metastasis group was
higher than that in the non-lymph node metastasis group. Voss et
al (46) observed a significant
inflammatory response around ccRCC tissues, while macrophages
secreted VEGF-C, which may lead to the proliferation of peritumoral
lymphatic vessels.
Although the lymphatic vessels within the tumor
tissues may be dysfunctional, the increased and dilated lymphatic
vessels around the tumor increase the probability of lymphatic
metastasis of tumor cells. According to the current study, there
may be three reasons for this: i) The neoplastic lymphatic vessels
around the tumor may assist the transportation of oxygen and
nutrients in the blood flow, thus promoting the proliferation of
tumor cells and increasing the probability of the metastasis of
tumor cells through lymphatic vessels (47); ii) the abnormal proliferation and
expansion of lymphatic vessels around the tumor may make it easier
for tumor cells to metastasize through lymphatic vessels (48); iii) the overexpression of VEGF-C in
cancer cells may act as a chemokine, guiding cancer cells to
migrate to VEGFR-3-expressing lymphatic endothelial cells (21). These reasons suggest that, to a
certain extent, although there were no functionally enlarged
lymphatic vessels in RCC tissues, there may be a close association
with lymph node metastasis.
In conclusion, high VEGF-C expression in RCC may
serve a role in targeting its specific receptor, VEGFR-3, which
thus may promote the formation of peritumoral lymphatic vessels.
The increase in LVD and the expansion of the lumen diameter in the
surrounding tissues of the tumors may be crucial for lymphatic
infiltration and lymph node metastasis. Therefore, targeted therapy
for lymphangiogenesis in RCC may be a novel treatment strategy.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by The Science and
Technology Research and Development Project of Chengde, Hebei
province (grant no. 201801A101).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XL and YZe designed the study and confirmed the
authenticity of all the raw data. DS, HL and GM performed the
experiments. ZW, MY and YZh analyzed the data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Chengde Medical University
(Chengde, China). All participants provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Barata PC and Rini BI: Treatment of renal
cell carcinoma: Current status and future directions. CA Cancer J
Clin. 67:507–524. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Linehan WM, Srinivasan R and Schmidt LS:
The genetic basis of kidney cancer: A metabolic disease. Nat Rev
Urol. 7:277–285. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hsieh JJ, Purdue MP, Signoretti S, Swanton
C, Albiges L, Schmidinger M, Heng DY, Larkin J and Ficarra V: Renal
cell carcinoma. Nat Rev Dis Primers. 3(17009)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Choueiri TK and Motzer RJ: Systemic
therapy for metastatic renal-cell carcinoma. N Engl J Med.
376:354–366. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yu KJ, Keskin SK, Meissner MA, Petros FG,
Wang X, Borregales LD, Gu C, Tamboli P, Matin SF, Wood CG and Karam
JA: Renal cell carcinoma and pathologic nodal disease: Implications
for American joint committee on cancer staging. Cancer.
124:4023–4031. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bazzi WM, Sjoberg DD, Feuerstein MA,
Maschino A, Verma S, Bernstein M, O'Brien MF, Jang T, Lowrance W,
Motzer RJ and Russo P: Long-term survival rates after resection for
locally advanced kidney cancer: Memorial Sloan kettering cancer
center 1989 to 2012 experience. J Urol. 193:1911–1916.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tannir NM, Pal SK and Atkins MB:
Second-line treatment landscape for renal cell carcinoma: A
comprehensive review. Oncologist. 23:540–555. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Stacker SA, Williams SP, Karnezis T,
Shayan R, Fox SB and Achen MG: Lymphangiogenesis and lymphatic
vessel remodelling in cancer. Nat Rev Cancer. 14:159–172.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Padera TP, Kadambi A, di Tomaso E,
Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark
EJ, et al: Lymphatic metastasis in the absence of functional
intratumor lymphatics. Science. 296:1883–1886. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lala PK, Nandi P and Majumder M: Roles of
prostaglandins in tumor-associated lymphangiogenesis with special
reference to breast cancer. Cancer Metastasis Rev. 37:369–384.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Karaman S and Detmar M: Mechanisms of
lymphatic metastasis. J Clin Invest. 124:922–928. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Yu P, Wu G, Lee HW and Simons M:
Endothelial metabolic control of lymphangiogenesis. Bioessays.
40(e1700245)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Alitalo K, Tammela T and Petrova TV:
Lymphangiogenesis in development and human disease. Nature.
438:946–953. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Beasley NJ, Prevo R, Banerji S, Leek RD,
Moore J, van Trappen P, Cox G, Harris AL and Jackson DG:
Intratumoral lymphangiogenesis and lymph node metastasis in head
and neck cancer. Cancer Res. 62:1315–1320. 2002.PubMed/NCBI
|
|
17
|
Dadras SS, Paul T, Bertoncini J, Brown LF,
Muzikansky A, Jackson DG, Ellwanger U, Garbe C, Mihm MC and Detmar
M: Tumor lymphangiogenesis: A novel prognostic indicator for
cutaneous melanoma metastasis and survival. Am J Pathol.
162:1951–1960. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nathanson SD: Insights into the mechanisms
of lymph node metastasis. Cancer. 98:413–423. 2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen JC, Chang YW, Hong CC, Yu YH and Su
JL: The role of the VEGF-C/VEGFRs axis in tumor progression and
therapy. Int J Mol Sci. 14:88–107. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Garnier L, Gkountidi AO and Hugues S:
Tumor-associated lymphatic vessel features and immunomodulatory
functions. Front Immunol. 10(720)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Su JL, Yen CJ, Chen PS, Chuang SE, Hong
CC, Kuo IH, Chen HY, Hung MC and Kuo ML: The role of the
VEGF-C/VEGFR-3 axis in cancer progression. Br J Cancer. 96:541–545.
2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang J, Huang Y, Zhang J, Wei Y, Mahoud S,
Bakheet AM, Wang L, Zhou S and Tang J: Pathway-related molecules of
VEGFC/D-VEGFR3/NRP2 axis in tumor lymphangiogenesis and lymphatic
metastasis. Clin Chim Acta. 461:165–171. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Takahashi S: Vascular endothelial growth
factor (VEGF), VEGF receptors and their inhibitors for
antiangiogenic tumor therapy. Biol Pharm Bull. 34:1785–1788.
2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tacconi C, Correale C, Gandelli A,
Spinelli A, Dejana E, D'Alessio S and Danese S: Vascular
endothelial growth factor C disrupts the endothelial lymphatic
barrier to promote colorectal cancer invasion. Gastroenterology.
148:1438–1451.e8. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Moch H, Cubilla AL, Humphrey PA, Reuter VE
and Ulbright TM: The 2016 WHO classification of tumours of the
urinary system and male genital organs-part A: Renal, penile, and
testicular tumours. Eur Urol. 70:93–105. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Howard GE and Wood CG: Staging refinements
in renal cell carcinoma. Curr Opin Urol. 16:317–320.
2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Volm M, Koomagi R and Mattern J:
Prognostic value of vascular endothelial growth factor and its
receptor Flt-1 in squamous cell lung cancer. Int J Cancer.
74:64–68. 1997.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis-correlation in invasive breast
carcinoma. N Engl J Med. 324:1–8. 1991.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Schwager S and Detmar M: Inflammation and
lymphatic function. Front Immunol. 10(308)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yamakawa M, Doh SJ, Santosa SM, Montana M,
Qin EC, Kong H, Han KY, Yu C, Rosenblatt MI, Kazlauskas A, et al:
Potential lymphangiogenesis therapies: Learning from current
antiangiogenesis therapies-a review. Med Res Rev. 38:1769–1798.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Joukov V, Pajusola K, Kaipainen A, Chilov
D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N and Alitalo K: A
novel vascular endothelial growth factor, VEGF-C, is a ligand for
the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases.
EMBO J. 15:290–298. 1996.PubMed/NCBI
|
|
32
|
Kukk E, Lymboussaki A, Taira S, Kaipainen
A, Jeltsch M, Joukov V and Alitalo K: VEGF-C receptor binding and
pattern of expression with VEGFR-3 suggests a role in lymphatic
vascular development. Development. 122:3829–3837. 1996.PubMed/NCBI
|
|
33
|
Katsuta M, Miyashita M, Makino H, Nomura
T, Shinji S, Yamashita K, Tajiri T, Kudo M, Ishiwata T and Naito Z:
Correlation of hypoxia inducible factor-1alpha with lymphatic
metastasis via vascular endothelial growth factor-C in human
esophageal cancer. Exp Mol Pathol. 78:123–130. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Oliver G: Lymphatic vasculature
development. Nat Rev Immunol. 4:35–45. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
35
|
Jacquemier J, Mathoulin-Portier MP,
Valtola R, Charafe-Jauffret E, Geneix J, Houvenaeghel G, Puig B,
Bardou VJ, Hassoun J, Viens P and Birnbaum D: Prognosis of
breast-carcinoma lymphagenesis evaluated by immunohistochemical
investigation of vascular-endothelial-growth-factor receptor 3. Int
J Cancer. 89:69–73. 2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kinashi H, Ito Y, Sun T, Katsuno T and
Takei Y: Roles of the TGF-β-VEGF-C pathway in fibrosis-related
lymphangiogenesis. Int J Cancer. 19(2487)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kaiserling E: Immunohistochemical
identification of lymph vessels with D2-40 in diagnostic pathology.
Pathologe. 25:362–374. 2004.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
38
|
Zavyalova MV, Denisov EV, Tashireva LA,
Savelieva OE, Kaigorodova EV, Krakhmal NV and Perelmuter VM:
Intravasation as a key step in cancer metastasis. Biochemistry
(Mosc). 84:762–772. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tammela T, He Y, Lyytikka J, Jeltsch M,
Markkanen J, Pajusola K, Ylä-Herttuala S and Alitalo K: Distinct
architecture of lymphatic vessels induced by chimeric vascular
endothelial growth factor-C/vascular endothelial growth factor
heparin-binding domain fusion proteins. Circ Res. 100:1468–1475.
2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Leu AJ, Berk DA, Lymboussaki A, Alitalo K
and Jain RK: Absence of functional lymphatics within a murine
sarcoma: A molecular and functional evaluation. Cancer Res.
60:4324–4327. 2000.PubMed/NCBI
|
|
41
|
Ruddell A, Harrell MI, Minoshima S,
Maravilla KR, Iritani BM, White SW and Partridge SC: Dynamic
contrast-enhanced magnetic resonance imaging of tumor-induced lymph
flow. Neoplasia. 10:706–713. 2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li J, Xie Y, Wang X, Jiang C, Yuan X,
Zhang A, Liu C, Pang L, Li F and Hu J: Overexpression of VEGF-C and
MMP-9 predicts poor prognosis in Kazakh patients with esophageal
squamous cell carcinoma. PeerJ. 7(e8182)2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen H, Guan R, Lei Y, Chen J, Ge Q, Zhang
X, Dou R, Chen H, Liu H, Qi X, et al: Lymphangiogenesis in gastric
cancer regulated through Akt/mTOR-VEGF-C/VEGF-D axis. BMC Cancer.
15(103)2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Tammela T, Zarkada G, Wallgard E,
Murtomäki A, Suchting S, Wirzenius M, Waltari M, Hellström M,
Schomber T, Peltonen R, et al: Blocking VEGFR-3 suppresses
angiogenic sprouting and vascular network formation. Nature.
454:656–660. 2008.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ma Q, Dieterich LC and Detmar M: Multiple
roles of lymphatic vessels in tumor progression. Curr Opin Immunol.
53:7–12. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Voss M, Steidler A, Grobholz R, Weiss C,
Alken P, Michel MS and Trojan L: The lymphatic system and its
specific growth factor vascular endothelial growth factor C in
kidney tissue and in renal cell carcinoma. BJU Int. 104:94–99.
2009.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yue H, Wang J, Chen R, Hou X, Li J and Lu
X: Gene signature characteristic of elevated stromal infiltration
and activation is associated with increased risk of hematogenous
and lymphatic metastasis in serous ovarian cancer. BMC Cancer.
19(1266)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ma Q, Dieterich LC, Ikenberg K, Bachmann
SB, Mangana J, Proulx ST, Amann VC, Levesque MP, Dummer R, Baluk P,
et al: Unexpected contribution of lymphatic vessels to promotion of
distant metastatic tumor spread. Sci Adv.
4(eaat4758)2018.PubMed/NCBI View Article : Google Scholar
|