Introduction
Alzheimer's disease (AD) is a common
neurodegenerative disease that is characterized by cognitive
dysfunction (1). AD has become the
fourth most common lethal human disease following cardiovascular
disease, cancer and stroke (2).
In-depth study of the pathogenesis of AD has become one of the main
foci of neuroscience research.
In the variety of hypotheses concerning AD,
oxidative stress has received increasing attention. Oxidative
stress is a state in which an intracellular oxidative-antioxidative
imbalance leads to stress damage in the organism. It has been
confirmed that oxidative stress is directly associated with the
formation of conditions such as senescence (3), Parkinson's disease (4) and AD (5). The development of drugs targeting
antioxidant stress to treat AD has become a promising area of
research.
Recent studies have shown that pyroptosis is closely
associated with the pathogenesis of AD (6). Pyroptosis is a programmed cell death
that is mediated by caspase-1(7).
Cell swelling and rupture while releasing pro-inflammatory factors
leads to cell death. Several studies have revealed molecular
signaling pathways associated with pyroptosis, such as the nuclear
factor erythroid 2-related factor 2 (Nrf2) pathway (8) and the thioredoxin-interacting protein
(TXNIP) pathway (9), among which
Nrf2 is also considered to be a necessary factor for the regulation
of nanoparticles activate the NLR pyrin domain containing 3 (NLRP3)
inflammasome (10). NLRP3
inflammasome activation is the most important complex protein
formed during pryoptosis, which is considered to be a key factor
mediating the development of neuroinflammation (11).
The DJ-1 gene was first reported in 1997 by
Nagakubo et al (12) in
Japan and the human DJ-1 gene was mapped to 1p36. A number
of studies have shown that DJ-1 is associated with diseases
associated with the nervous system, such as Parkinson's,
Alzheimer's and depression. DJ-1 also serves a role in a
number of physiological processes such as anti-oxidative stress
(13) and anti-neuronal apoptosis
(14). As an important nuclear
transcription factor, Nrf2 is involved in the regulation of the
expression of various antioxidant enzymes in cells and serves an
important role in the prevention of oxidative stress (15). Studies have shown that DJ-1
can regulate the expression of Nrf2 signaling pathway in
Parkinson's disease and diabetic nephropathy (16-18).
DJ-1 inhibits dopaminergic denaturation by activating the
Nrf2 signaling pathway in Parkinson's disease (19). Nrf2 can affect the activation of the
NLRP3 inflammasome by regulating the TXNIP pathway (20,21).
In addition, activation of the NLLRP3 inflammasome further mediates
Caspase-1-based pyroptosis (22).
Based on these studies, it was hypothesized that DJ-1 may be
involved in the pathogenesis of AD by activating Nrf2 signaling
pathways to regulate oxidative stress and pyroptosis. To verify
this hypothesis, 5XFAD transgenic mice were used as AD model mice
to overexpress DJ-1 in mouse brain by lentiviral system
transfection to investigate the role of DJ-1/Nrf2 signaling
pathway in the pathogenesis of AD.
Materials and methods
Animals and materials
A total of 60 5XFAD transgenic mice (male, 3 months
old, 20-25 g) were provided by Nanyang Institute of Technology. The
mice received sterile rodent chow and water ad libitum, were
maintained on a 12 h light/dark cycle in controlled temperature
(24±1˚C) and at 55% humidity. All experimental procedures were
ethically approved by the Animal Use and Care Committee of the
Nanyang Institute of Technology (Nanyang, China) and were conducted
in accordance with the National Institute for Health ‘Guide for the
Care and Use of Laboratory Animals’ (23).
Lentiviral particles containing a specific targeting
DJ-1 gene and lentiviral particles containing non-specific
RNA sequences were constructed and provided by Guangzhou Qingyin
Biotechnology Co., Ltd.
A total RNA extraction kit (Tiangen Biotech Co.,
Ltd.), PCR primers (Shanghai Shenggong Biology Engineering
Technology Service, Ltd.), a protein concentration determination
kit (Beijing Solarbio Science & Technology Co., Ltd.) and
interleukin (IL)-1β (cat. no. hz-EL-6452), IL-18 (cat. no.
hz-EL-M0730c), β-amyloid protein (Aβ)40 (cat. no.
hz-EL-M0067) and Aβ42 ELISA kits (cat. no. hz-EL-M0068c;
Huzhen Industrial Co., Ltd.) were also used.
Experimental design
To begin, 10 of the 5XFAD transgenic mice were
randomly selected to receive the DJ-1 lentivirus injected
into the bilateral hippocampus (DJ-1 group) and another 10
mice were injected with lentiviruses of unrelated RNA sequences in
the bilateral hippocampus as the negative control (NC) group. Mice
in the sham group had the anterior fontanel exposed without
injection of lentivirus. Then, 2 months after the lentivirus
infection, the Morris water maze test was performed. After the
behavioral evaluation was completed, the mice were anesthetized by
intraperitoneal injection of 0.1% sodium pentobarbital (35 mg/kg,
Sigma-Aldrich, Merck KGaA) and were decapitated to obtain the brain
tissue of the mice. Sections of the brain tissues were subjected to
hematoxylin-eosin (HE) staining to observe pathological changes;
the other tissues were used for reverse transcription-quantitative
(RT-q) PCR, ELISA and western blotting.
Intracerebroventricular
injections
The mice were placed into a stereotaxic frame (RWD
Life Science) following anesthesia. The skin was incised under
aseptic conditions and the periosteum of the skull was isolated to
reveal the anterior fontanel. The co-ordinates of the anterior
fontanel sagittal axis (A), coronal axis (L) and vertical axis (V)
were recorded. The Paxinos map was referred to in order to
determine the right hippocampal dentate gyrus injection point
(A1=A-3.3, L1=L+1.8) (24). The
fixed micro-sampler was on the TAXIC-653 brain locator. DJ-1
(5 µl; 10,000 TU/µl) containing lentivirus or negative control was
injected; the tip of the micro syringe was inserted into the
dentate depth (V1=V+4.4) and the virus was slowly injected,
following which the needle was removed for 10 min. The left
hippocampal dentate gyrus injection point was determined by
reference to the Paxinos map (A1=A-3.3, L1=L-1.8) (24) and DJ-1 or control lentivirus
was injected in the same manner. In the sham group, only the
periosteum of the skull was isolated and the anterior fontanel was
revealed. After the animal exhibited no intracranial hemorrhage,
the bone suture was closed with bone wax and the skin sutured.
Following intramuscular injection of penicillin, the mouse was
returned to its cage.
RT-qPCR
After the hippocampal tissue (100 mg) was
sufficiently ground, 1 ml TRIzol® (Thermo Fisher
Scientific, Inc.) was added for lysis. Subsequently, total RNA was
extracted using the phenol-chloroform method (25). Ultraviolet spectrophotometry
(Nanodrop™ 2000; Thermo Fisher Scientific, Inc.) was used to
determine the purity of RNA by evaluating the A260/A280 ratio. cDNA
was obtained by reverse transcription (RT) using a GoScript™
Reverse Transcription System (Promega Corporation) from 2 µg RNA.
Each RT reaction mixture was prepared as follows: RNA template (5
µl), Oligo dT primers (2 µl), super pure dNTP (2 µl) and
H2O (5.5 µl). The reaction conditions involved heating
to 70˚C before rapid cooling on ice for 2 min at room temperature,
following which transient centrifugation was performed at 500 x g
for 30 sec at 4˚C. Subsequently, 4 µl 5X first-strand buffer, 0.5
µl RNasin and 1 µl M-MLV were added prior to gentle mixing with a
pipette. After being kept at 25˚C for 10 min, the sample was
incubated at 42˚C for 50 min before termination at 95˚C for 5 min.
qPCR was subsequently performed using SYBR® Premix Ex
Taq™ kit according to manufacturer's protocol (Takara Bio, Inc.).
The sequences of the primers used were as follows: DJ-1
forward, 5'-UGGAGACGGUCAUCCCUGU-3' and reverse,
5'-ACCUCUGCCAGUAGGGACA-3; β-actin forward,
5'-ACACTGTGCCCATCTAGGAGG-3' and reverse,
5'-AGGGGCCGGACTCGTCATACT-3'. The thermocycling conditions were:
Initial denaturation at 94˚C for 1 min, followed by 35 cycles of
94˚C for 30 sec, 55˚C for 30 sec and 72˚C for 1 min, a final
extension at 72˚C for 2 min and hold at 4˚C. β-actin was used as
the internal reference. Data were analyzed using the
2-ΔΔCq method (26).
Western blotting
The hippocampal tissue samples were lysed by RIPA
lysate (Beyotime Institute of Biotechnology) to extract total
protein. Nuclear and cytosolic proteins were extracted by a
Nuclear/Cytosol Fractionation kit (BioVision, Shanghai, China).
Protein concentration was determined using a BCA Protein Assay Kit
(Beijing Solarbio Science & Technology Co., Ltd.). Protein (80
or 120 µg) was separated on a denatured 12% polyacrylamide gel and
transferred onto a polyvinylidene fluoride membrane. Membranes were
blocked in TBS with 0.1% Tween-20 containing 5% non-fat milk and
then incubated overnight at 4˚C with the corresponding primary
antibodies. The primary antibodies used were as follows:
Anti-DJ-1 (1:500 dilution; cat. no. SAB4500248;
Sigma-Aldrich, Merck KGaA), anti-Nrf2 (1:500 dilution; cat. no.
SAB4501984; Sigma-Aldrich, Merck KGaA), anti-NLRP3 (1:500 dilution;
cat. no. ERP20425; Abcam), anti-apoptosis-associated speck-like
protein containing a CARD (ASC;1:500 dilution; cat. no. SAB4501315;
Sigma-Aldrich, Merck KGaA), anti-caspase-1 p10 (1:1,000 dilution;
cat. no. AF1681; Beyotime Institute of Biotechnology), anti-cleaved
caspase-3 (1:1,000 dilution; cat. no. AF1150; Beyotime Institute of
Biotechnology), anti-β-actin (1:500 dilution; cat no sc-7210; Santa
Cruz Biotechnology, Inc.), and anti-histone H3 (1:500 dilution;
cat. no. ab176842; Abcam). Membranes were then incubated with
horseradish peroxidase-conjugated secondary antibody IgG (1:1,000
dilution; cat. no. 61-1620; Invitrogen, Thermo Fisher Scientific,
Inc.) for 1 h at room temperature. The gels were developed using an
enhanced chemiluminescent detection kit (Beyotime Institute of
Biotechnology). The relative expression levels of the protein of
interest were analyzed using Quantity One gel image analysis
software (version 3.0; Thermo Fisher Scientific, Inc.).
H&E staining
The hippocampus tissue was fixed in 4%
paraformaldehyde solution overnight at room temperature and
sectioned (5 µm) following paraffin embedding. Paraffin sections
were deparaffinized using xylene and rehydrated using a graded
alcohol series (100-70% v/v), following which they were then
stained with 5% hematoxylin solution for 15 min at temperature and
counterstained with 0.5% eosin solution for 5 min prior to
dehydration with a graded alcohol series (100-70% v/v). Following
clearing and mounting, the sections were observed using light
microscopy (magnification x400).
ELISA
NP-40 buffer (cat. no. P0013F; Beyotime Institute of
Biotechnology) was added to the hippocampus tissue. Following
sufficient grinding, the tissue homogenate was centrifuged at 5,000
x g for 15 min at 4˚C and the supernatant was collected. ELISA was
performed according to the manufacturer's instructions for
interleukin (IL)-1β, IL-18, Aβ40 or Aβ42.
Finally, the absorbance value at 450 nm was measured with a
microplate reader and the corresponding concentration was
calculated.
Morris water maze test
The Morris water maze test was performed as
described previously (27). In
brief, each training was performed in 4 steps, each step beginning
from a new entry point. The mice faced the pool wall prior to
entering the water and the time from the entry of the water to the
climbing of the platform (latency) was recorded. If the mouse did
not find the platform within 90 sec, the experimenter guided it
onto the platform and recorded it as 90 sec. After the mice climbed
the platform and rested for 30 sec, the next training step began.
The average value of 4 training results was recorded as the latency
of the day. After 4 days of training, the platform was removed on
the 5th day. The mice were allowed to swim for 90 sec in
the pool and their trajectory recorded. The percentage of total
time and total distance traveled by the mouse in the quadrant of
the platform was analyzed.
Terminal transferase-mediated biotin
dUTP nick end labeling (TUNEL) assay
Hippocampus tissue was cut into 6 µm sections using
a Mcllwain microtome (Science Products GmbH) and fixed overnight in
4% paraformaldehyde at 4˚C. In situ detection of hippocampal
neuronal apoptosis was performed using the ApopTag Fluorescein
in situ Apoptosis Assay kit (EMD Millipore; cat. no. S7110)
by modification of genomic DNA using terminal deoxynucleotidyl
transferase according to the manufacturer's instructions. Following
TUNEL labeling, the nuclei were labeled with DAPI (Abcam), and the
neurons labeled with NeuN and examined in at ≥5 fields under a
fluorescence microscope (magnification x200). The number of TUNEL
positive cells in the hippocampus was counted.
Oxidative stress marker detection
Fresh hippocampus tissue was removed and rinsed with
PBS, homogenized in NP-40 buffer (cat. no. P0013F; Beyotime
Institute of Biotechnology) and centrifuged at 12,000 x g at 4˚ for
15 min. Protein quantification was subsequently performed as
described previously (28).
Reactive oxygen species (ROS) content in the brain tissue was
detected by fluorescent probe 2',7'-dichlorofluorescin diacetate
(DCFH-DA) method. In brief, 100 mg hippocampal tissue was fully
lysed with 300 µl lysis buffer at room temperature. After the
sample was centrifuged at 12,000 x g for 10 min at 4˚C, the
supernatant was collected. The sample was placed with 50 µl
supernatant in a 96-well plate, and 10 µl/well DCFH-DA solution was
added and incubated in the dark at 37˚C for 30 min. Samples were
measured by using a fluorescence microplate reader (DTX800, Beckman
Coulter, Inc.). The content of malondialdehyde (MDA) in brain
tissue was detected by the thiobarbituric acid method. In brief,
100 mg of hippocampal tissue was lysed and homogenized with 250 µl
pre-chilled buffer at 4˚C. The samples were mixed with 10 µl
butylated hydroxytoluene, 250 µl 1 M phosphoric acid, 250 µl
2-thiobarbiruric acid and incubated at 60˚C for 1 h. Subsequently,
the sample was centrifuged at 12,000 x g for 5 min at 4˚C and the
supernatant was collected. The supernatant (50 µl) was placed in a
96-well plate and measured on a microplate reader (BioTek
Instruments Inc.). Superoxide dismutase (SOD) activity was
determined by the xanthine oxidase method. In brief, hippocampal
tissue was homogenized in lysis buffer. The lysate was centrifuged
at 12,000 x g for 10 min at 4˚C and the supernatant collected.
Samples and SOD standards were placed in 96-well plates at 20
µl/well and 160 µl of working reagent was added to each well.
Xanthine oxidase enzyme was added at 20 µl/well and incubated at
37˚C for 1 h in the dark. Measurements were performed using a
microplate reader at 450 nm. All operations were performed in
strict accordance with the manufacturer's protocols.
Statistical analysis
All statistical analyses were performed using SPSS
software (v.16.0; SPSS Inc.). Data are expressed as the mean ±
standard deviation. Comparisons among three or more groups were
conducted using a one-way analysis of variance followed by Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of DJ-1 overexpression in brain
on cognitive function and brain tissue damage in 5XFAD transgenic
mice
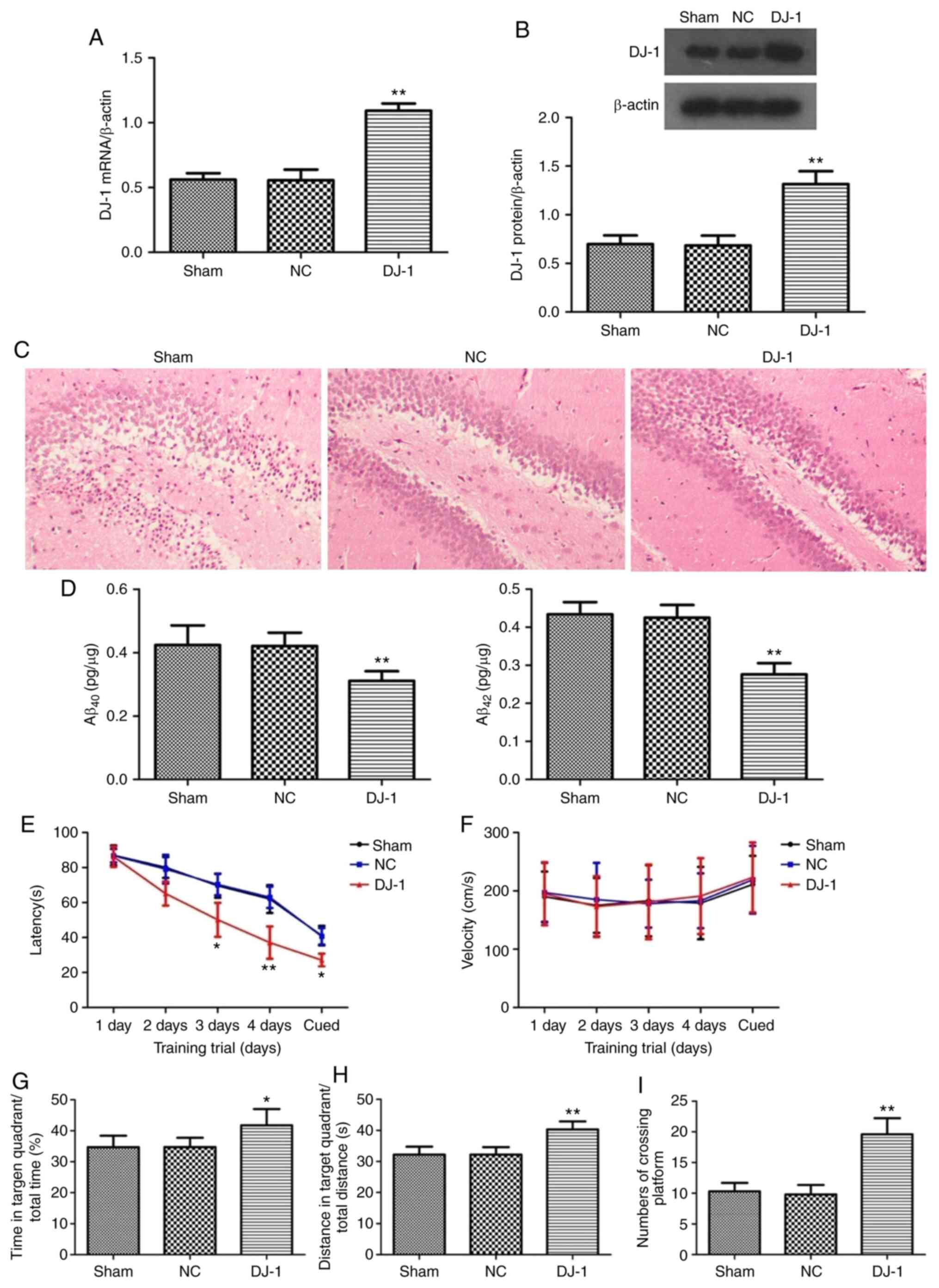
The results of RT-qPCR and western blotting showed
that the expression levels of DJ-1 mRNA and protein in the
hippocampus of 5XFAD transgenic mice injected with DJ-1
lentivirus in bilateral hippocampus were significantly increased
compared with the sham group (P<0.05; Fig. 1A and B). There was no significant difference in
the expression of DJ-1 mRNA and protein in hippocampus of
5XFAD mice between the NC and sham groups (P>0.05).
HE staining was used to observe the pathological
changes of hippocampus and neurons (Fig. 1C). It was identified that
hippocampal neurons in the sham group were disordered in
hippocampus and a large number of necrotic neurons appeared in the
hippocampus and the nucleus staining was deep and the nucleus
exhibited pyknosis. The above pathological changes were improved in
the DJ-1 group.
The deposition of a large amount of Aβ outside the
neurons is one of the typical pathological features of AD. The
content of Aβ40 and Aβ42 in the hippocampus
of AD mice was analyzed by ELISA. It was identified that
Aβ40 and Aβ42 levels in the DJ-1 group
were significantly decreased compared with the sham group
(P<0.05; Fig. 1D), suggesting
that DJ-1 overexpression in brain can ameliorate Aβ
deposition in hippocampus of AD mice.
The Morris water maze test demonstrated that the
latency of finding the platform during the acquired training in the
DJ-1 group was significantly shorter compared with that in
the sham group (P<0.05; Fig.
1E). There was no significant difference in the swimming speed
of the visual platform test between the DJ-1 and sham groups
(P>0.05; Fig. 1F). During the
exploratory training period, the percentage of total time in the
target quadrant (Fig. 1G) and the
percentage of the total distance (Fig.
1H) of the DJ-1 group were significantly increased
compared with the sham group (P<0.05). Simultaneously, the
number of mice crossing the platform in the DJ-1 group was
significantly increased compared with the sham group (P<0.05;
Fig. 1I).
Effect of brain DJ-1 silencing on
oxidative stress in 5XFAD transgenic mice
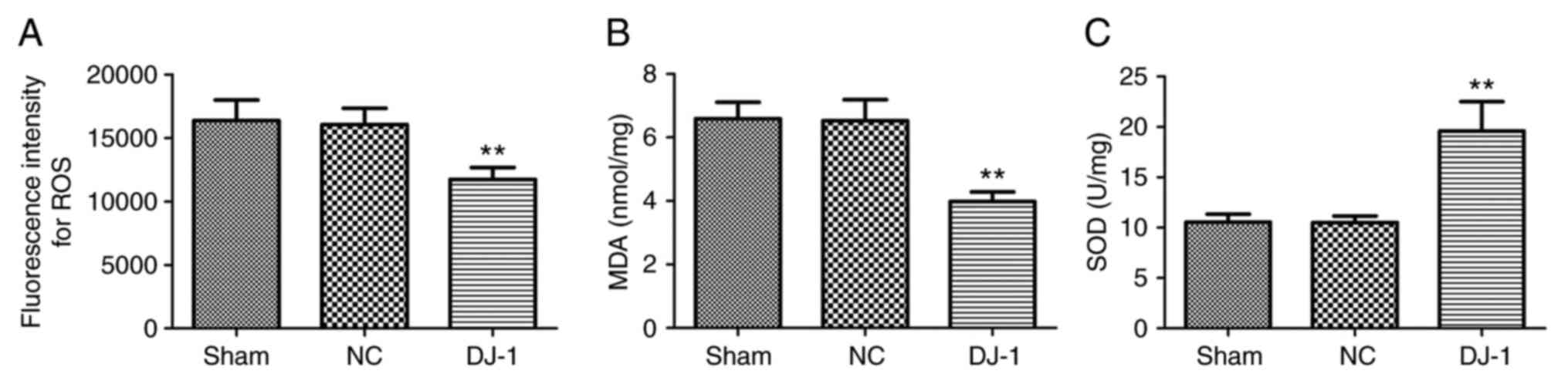
Oxidative stress is an important pathological
manifestation of AD. DJ-1 is considered to be an oxidative
stress response protein (29) and
is closely associated with the regulation of oxidative stress in
nerve cells (30). Therefore, the
effect of DJ-1 overexpression in brain on oxidative stress
in brain tissue of 5XFAD mice was analyzed. It was identified that
compared with the sham group, the ROS activity and MDA content in
the brain tissue of the DJ-1 group were significantly
decreased, while the SOD activity was significantly increased
(P<0.05; Fig. 2). There was no
significant difference in the content of ROS, MDA and SOD between
the sham and NC groups. These results suggested that overexpression
of DJ-1 in the brain may reduce oxidative stress damage in
the brain of 5XFAD mice.
Effect of DJ-1 overexpression in brain
on neuronal pyroptosis in 5XFAD transgenic mice
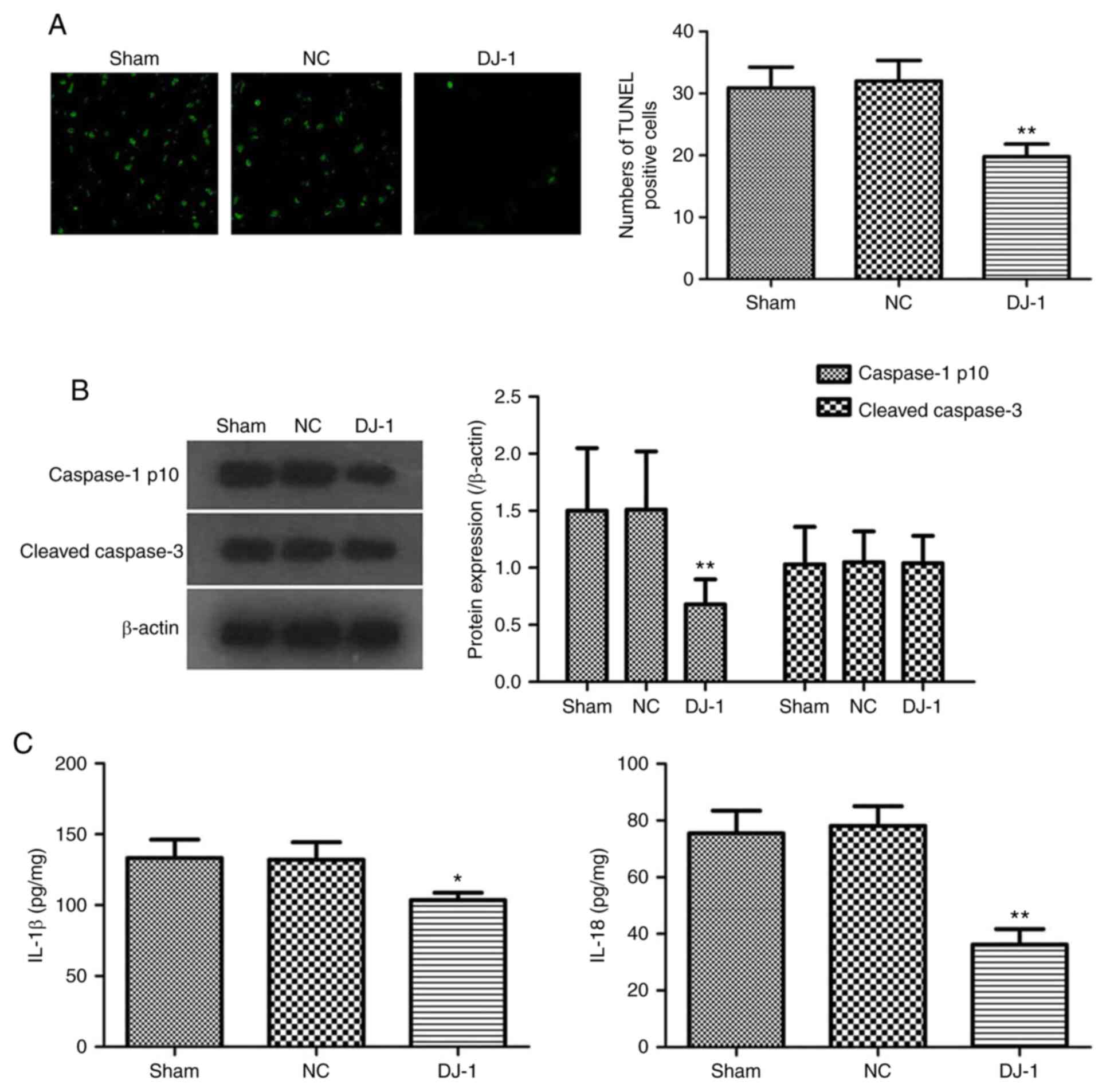
Neuronal death is another important feature of AD.
The effect of DJ-1 overexpression in the brain on neuronal
cell death in 5XFAD mice was analyzed. The TUNEL assay results
showed that the fluorescent spots of cell death primarily
corresponded to the distribution of neuronal cell bodies (Fig. 3A). A large number of TUNEL-positive
cells were observed in the hippocampus of the sham group, while the
number of TUNEL-positive cells in the DJ-1 group was
decreased. Quantitative analysis also confirmed that the number of
TUNEL-positive cells in the hippocampus of DJ-1 group was
significantly decreased compared with that of the sham group
(P<0.05; Fig. 3A). The results
suggested that overexpression of DJ-1 may significantly
improve neuronal cell death in 5XFAD mice.
Caspase-1 and caspase-3 are important molecules
regulating cell death; caspase-3 is an important executive molecule
of apoptosis (31) and caspase-1 is
mainly involved in the mediation of pyroptosis (32). Therefore, the expression levels of
caspase-1 p10 and cleaved caspase-3 in hippocampus of 5XFAD mice
were analyzed. It was identified that the overexpression of
DJ-1 in the brain significantly decreased the activation of
caspase-1 in the hippocampus of 5XFAD mice without affecting the
activation of caspase-3 (Fig.
3B).
Loss of cell membrane integrity and release of
inflammatory cell contents to induce inflammatory responses are
important features of pyroptosis, unlike apoptosis (33). It was identified that DJ-1
overexpression significantly decreased the IL-1β and IL-18 levels
in the hippocampus tissues of the 5XFAD mice (Fig. 3C). These results revealed that the
effect of DJ-1 overexpression in brain on the death of
hippocampal neurons in AD mice may be associated with the
regulation of pyroptosis.
Effect of DJ-1 overexpression in the
brain on the expression of Nrf2-NLRP3 axis in the hippocampus of
5XFAD transgenic mice
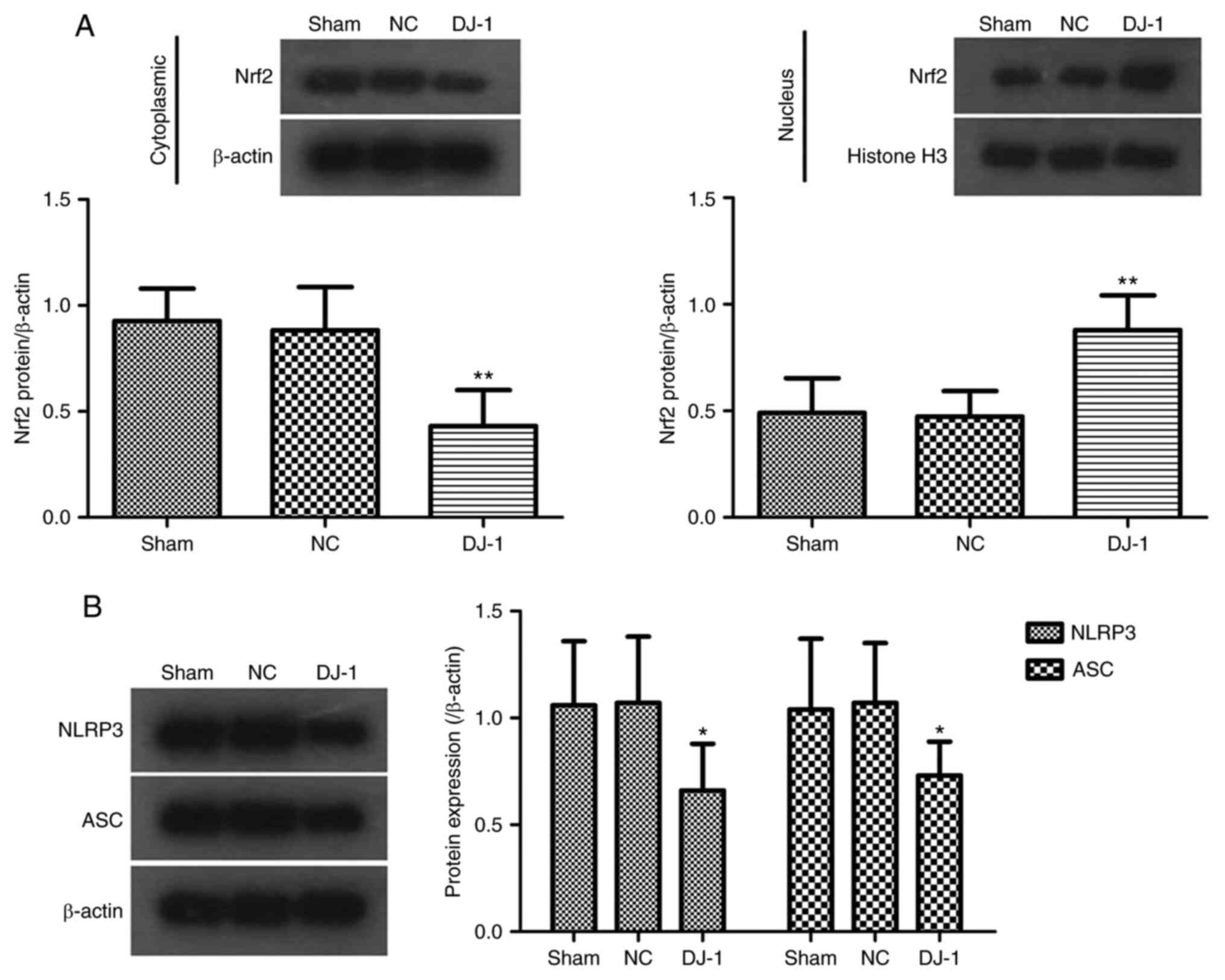
The Nrf2 pathway, as the most important endogenous
antioxidant stress pathway identified at present, serves a key role
in the development of AD (34).
Simultaneously, Nrf2 activation may be involved in the regulation
of pyroptosis by regulating the NLRP3 inflammasome (10,35).
In addition, DJ-1 regulates the expression of the Nrf2
signaling pathway in a variety of diseases (16,17,20).
To further investigate the potential mechanism by which DJ-1
overexpression improves brain tissue damage in 5XFAD mice, the
expression of associated proteins in the Nrf2-NLRP3 axis of brain
tissue associated with oxidative stress and pyroptosis was
analyzed. Western blotting data indicated that DJ-1
overexpression significantly decreased the expression of Nrf2
protein in the cytoplasm and increased the expression of Nrf2 in
the nucleus (Fig. 4A). The results
suggested that overexpression of DJ-1 can promote the
transfer of Nrf2 into the nucleus to exert an anti-oxidative stress
effect.
A previous study has shown that the Nrf2 pathway
serves an important role in the activation of NLRP3 inflammasome
(35), while NLRP3 inflammasome
activation is the most important complex protein formed during
pyroptosis (7). It was identified
that DJ-1 overexpression in the brain significantly
inhibited the expression of NLRP3 and ASC proteins in brain tissue
(Fig. 4B), suggesting that
DJ-1 overexpression inhibits the activation of NLRP3
inflammasome.
Discussion
5XFAD transgenic mice, carrying 3 APP mutant genes
and 2 PS1 mutant gene, have been used as AD model mice (36). The transgenic mice have been shown
to have similar functional and pathological changes to AD. The most
typical change in AD is the formation of senile plaques (SP) and
the main cause of SP formation is the deposition of Aβ. As an
important part of AD pathological changes, the hippocampus is
closely associated learning and memory and cognitive function, and
also participates in the regulation of the autonomic nervous system
through the nuclei, endocrine system and neurotransmitters of the
hypothalamus and brainstem (37).
In the present study, it was identified that after overexpression
of DJ-1 in the brain, cognitive impairment, hippocampal
tissue damage and Aβ deposition were significantly improved in
5XFAD transgenic mice and the level of neuronal apoptosis was
significantly decreased. These results suggested that DJ-1
serves an important protective role in the development of AD.
Oxidative stress is an important pathological
manifestation of AD. Decreased antioxidant capacity and increased
oxidative stress products disrupt the dynamic balance of
antioxidants and oxidation in the brain (5). SOD is an important antioxidant and MDA
is an end product of cytotoxicity produced by lipid peroxidation. A
previous study confirmed that abnormal changes in serum SOD and MDA
have a higher sensitivity and specificity for predicting AD
(38). Oxidative stress can induce
mitochondrial function and damage of blood-brain barrier caused by
cerebral vascular cell damage (39), whilst also cause clinical
pathological symptoms of AD such as amyloid deposition,
neurofibrillary tangles and cognitive dysfunction (40). Therefore, preventing oxidative
stress has become an important strategy for the treatment of AD.
DJ-1 is considered to be an oxidative stress response
protein (29) and is closely
associated with the regulation of oxidative stress in nerve cells
(30). Baulac et al
(41) demonstrated that DJ-1
is upregulated in the brain of patients with AD and is associated
with elevated levels of oxidative stress. Mullett et al
(42) suggested that DJ-1
safeguards astrocyte-mediated protection against neuronal oxidative
stress. Zhang et al (43)
identified that the high expression of wild-type DJ-1 gene
in SH-SY 5Y cells cultured in vitro has an inhibitory effect
on oxidative damage, which may be associated with the decrease of
intracellular ROS. Kim et al (44) reported that
DJ-1-/- embryonic cortical neurons showed
increased sensitivity to oxidative stress and neurons
overexpressing DJ-1 were protected from oxidative stress
in vitro. The present study identified that DJ-1
overexpression in the brain of AD mice could increase SOD activity
and decrease ROS activity and MDA content in the hippocampus,
suggesting that DJ-1 overexpression can decrease the degree
of oxidative stress in AD mice. Therefore, it was hypothesized that
DJ-1 may inhibit Aβ deposition by decreasing ROS activity,
thereby lessening brain damage in AD mice. ROS increased the
production of Aβ by promoting the expression of amyloid precursor
protein and β-secretase. The production and aggregation of Aβ
further induces the formation of more oxygen free radicals, forming
a vicious circle leading to neuronal degeneration and necrosis
(24). Additionally, this positive
feedback activity also promotes hyperphosphorylation of Tau protein
and induces the accumulation of soluble Tau protein to form fibrils
to promote the production of intracellular neurofibrillary tangles
(NFTs) (26,39). NFTs are also one of the
characteristic pathological features of AD. However, whether
DJ-1 can affect the deposition of Tau by inhibiting
oxidative stress requires further investigation.
Nrf2 is considered to be the main active factor of
cellular responses to oxidative stress (45). As the most important endogenous
antioxidant stress pathway identified at present, the Nrf2 pathway
serves a key role in the development of AD (34). Under physiological conditions, Nrf2
binds to Kelch-like ECH-associated protein 1 (Keap1) to treat
non-activated and rapidly degraded ubiquitin-proteasome (46). Under the stimuli of oxidative
stress, the dissociation of Nrf2 and its inhibitory protein Keap1
inhibit the ubiquitination of Nrf2 to increase its stability.
Stable Nrf2 is transferred to the nucleus and binds to an
antioxidant response element (ARE) to initiate expression of a
series of antioxidant proteins regulated by ARE (47). Clements et al (48) reported that DJ-1 serves a key
role in the activation and regulation of the Nrf2 pathway. Sun
et al (18) demonstrated
that oxidative stress can activate DJ-1and its downstream
Nrf2 pathway for endogenous protection in a diabetic rat model.
High expression of DJ-1 promotes the dissociation of Nrf2
from Keap1 and prevents the binding of Nrf2 to Keap1, and the
ubiquitination of Nrf2, to restore the stability of Nrf2 against
cellular oxidative stress and apoptosis damage (49). The present study also identified
that overexpression of DJ-1 in the brain of AD mice can
significantly decrease the expression of Nrf2 protein in the
cytosol and promote the entry of Nrf2 into the nucleus. It was
hypothesized that overexpression of DJ-1 in hippocampus of
AD mice can effectively activate the Nrf2 signaling pathway to
induce the expression of a series of antioxidant enzyme proteins to
exert anti-oxidative stress.
Neuronal death is another important feature of AD.
The death of nerve cells is regulated by a variety of factors, such
as oxidative stress and inflammatory response. There are a number
of methods of cell death, some of which are caspase-dependent
(50). Caspase-1 and caspase-3 are
important molecules that regulate cell death and apoptosis.
Caspase-3 is an important executive molecule of apoptosis. A
previous study has shown that caspase-3 activation is the ultimate
mediator of apoptosis in brain homogenates of dementia rats, which
leads to the loss of neurons in AD model rats (31). Caspase-1 is primarily associated
with cell death caused by inflammation (32). Bergsbaken et al (7) reported that host cells undergo
caspase-1-dependent death from apoptosis when stimulated by
pathogenic microorganisms or endogenous risk signals, called
pyroptosis. Like apoptosis, pyroptosis is characterized by nuclear
shrinkage, chromatin DNA fragmentation and TUNEL positive staining.
Additionally, loss of cell membrane integrity and release of
inflammatory cellular contents to induce an inflammatory response
is an important feature of pyroptosis other than apoptosis
(33). A large number of necrotic
neurons in the hippocampus of AD mice and overexpression of
DJ-1 was observed to reduce the death of hippocampal neurons
in AD mice. Simultaneously, it was identified that the
overexpression of DJ-1 could significantly decrease the
expression of capsase-1 in hippocampus of AD mice, but had no
significant effect on the expression of caspase-3. Further analysis
indicated that overexpression of DJ-1 significantly
decreased the levels of IL-1β and IL-18 in the inflammatory
mediators of hippocampus. Therefore, it was hypothesized that the
improvement of DJ-1 overexpression on cognitive function and
brain damage in AD mice may involve caspase-1 mediated
pyroptosis.
Inflammasome is an important complex protein formed
during the activation of pyroptosis. NLRP3 inflammasome is
currently the most widespread inflammasome, consisting of NLRP3,
adaptor protein ASC and the effector protein pro-caspase-1. IL-1β
and IL-18 are the end products of pyroptosis, which are formed by
the activation of caspase-1 to cleave pro-IL-1β and
pro-IL-18(51). It is generally
accepted that the neuroinflammatory response regulated by the
pyroptosis key protein inflammasome and the neuronal loss caused by
pyroptosis are associated with the pathogenesis of AD (52,53).
The NLRP3 inflammasome may be involved in the promotion of Aβ
deposition by microglia (54).
NLRP3 inhibitors can decrease Aβ deposition and improve cognitive
function in AD mice, while inhibiting pyroptosis by the activation
of microglia and inflammasome in AD mice (55). NLRP3 knockout mice exhibit decreased
levels of caspase-1 expression and decreased Aβ aggregation,
suggesting that NLRP3 is closely associated with the pathology of
AD (56). To the best of our
knowledge, the activation mechanism of NLRP3 has not yet been
clarified. A previous study suggested that mitochondrial damage
produces a large amount of ROS that activates NLRP3 inflammasome
and produces an inflammatory response, suggesting that oxidative
stress may be associated with pyroptosis and inflammatory response
(57). Choi et al (58) observed severe mitochondrial
oxidative damage and brain inflammatory response in APP/SP1
transgenic AD accompanied by activation of NLRP3 inflammasome.
Notably, NLRP3 activation involves the production of ROS (59). Aβ stimulates microglia to produce
reactive oxygen species via mitochondria and NAD(P)H oxidase and
also activates NLRP3 inflammasome to generate caspase-1 to increase
the neurotoxicity of microglia (60). These studies suggest that oxidative
stress may lead to neurosynaptic dysfunction and neuronal loss by
activating the inflammasome-induced neuroinflammatory response.
Simultaneously, ROS produced by oxidative stress may act as a
signaling molecule to initiate pyroptosis to induce
neuroinflammation and neuronal death (61). Therefore, anti-oxidation may inhibit
the activation of inflammasome to decrease the production of
pyroptosis. Previousstudies have confirmed that the Nrf2 pathway
serves an important role in the activation of NLRP3 inflammatory
bodies. Zhao et al (10)
identified that Nrf2-deficient macrophages exhibited a decrease in
the maturation and secretion of caspase-1 and IL-1β and a decrease
in ASC formation and suggested that Nrf2 is a necessary factor for
the activation of NLRP3 inflammasome. However, Liu et al
(62) reported that Nrf2 activation
inhibits NLRP3 expression, caspase-1 cleavage and subsequent IL-1β
production, and suggested that the Nrf2 pathway serves a negative
regulatory role in ROS-induced NLRP3 inflammatory body activation.
Wang et al (20) identified
that, in the AD mouse model, upregulation of Nrf2 can inhibit the
activation of NLRP3 inflammatory bodies by modulating the TXNIP
pathway. The present study identified that DJ-1
overexpression in the brain activates the Nrf2 pathway in the AD
mouse model and also decreases the expression of NLRP3 and ASC in
hippocampus. In combination with data from previous studies, we
hypothesized that DJ-1 may be involved in the protection of
brain damage in AD mice by regulating Nrf2-NLRP3 axis to regulate
oxidative stress and pyroptosis.
In summary, overexpression of DJ-1 in the
brain can improve the cognitive function and Aβ deposition of 5XFAD
mice and serve an important protective role in the development of
AD. Its mechanism may be associated with the inhibition of
oxidative stress and neuropyroptosis by regulating the Nrf2
signaling pathway. In pharmacological studies, stimulation of
DJ-1 by activators, which in turn regulates a variety of
complex mechanisms including oxidative stress and cell coke, may
lead to new breakthroughs in the prevention and improvement of
AD.
Acknowledgements
Not applicable.
Funding
Funding: The authors were supported financially by Science and
Technology Projects in Henan Province (grant no. 162102310258).
Availability of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LC designed the study, conducted the experiments and
analyzed the data. WZ conducted most of the experiments and wrote
the manuscript. Both authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experimental procedures were ethically approved
by the Animal Use and Care Committee of the Nanyang Institute of
Technology and were conducted in accordance with the National
Institute for Health ‘Guide for the Care and Use of Laboratory
Animals’ (23).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cummings J, Lee G, Mortsdorf T, Ritter A
and Zhong K: Alzheimer's disease drug development pipeline: 2017.
Alzheimer's Dement (N Y). 3:367–384. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Graham WV, Bonito-Oliva A and Sakmar TP:
Update on Alzheimer's disease therapy and prevention strategies.
Annu Rev Med. 68:413–430. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Polettini J, Richardson LS and Menon R:
Oxidative stress induces senescence and sterile inflammation in
murine amniotic cavity. Placenta. 63:26–31. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sankhla CS: Oxidative stress and
Parkinson's disease. Neurol India. 65:269–270. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Collin F, Cheignon C and Hureau C:
Oxidative stress as a biomarker for Alzheimer's disease. Biomark
Med. 12:201–203. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tan MS, Tan L, Jiang T, Zhu XC, Wang HF,
Jia CD and Yu JT: Amyloid-Beta induces NLRP1-dependent neuronal
pyroptosis in models of Alzheimer's disease. Cell Death Dis.
2014(e1382)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bergsbaken T, Fink SL and Cookson BT:
Pyroptosis: Host cell death and inflammation. Nat Rev Microbiol.
7:99–109. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hu Q, Zhang T, Yi L, Zhou X and Mi M:
Dihydromyricetin inhibits NLRP3 inflammasome-dependent pyroptosis
by activating the Nrf2 signaling pathway in vascular endothelial
cells. Biofactors. 44:123–136. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Heo MJ, Kim TH, You JS, Blaya D,
Sancho-Bru P and Kim SG: Alcohol dysregulates miR-148a in
hepatocytes through FoxO1, facilitating pyroptosis via TXNIP
overexpression. Gut. 68:708–720. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhao C, Gillette DD, Li X, Zhang Z and Wen
H: Nuclear factor E2-related factor-2 (Nrf2) is required for NLRP3
and AIM2 inflammasome activation. J Biol Chem. 289:17020–17029.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Song L, Pei L, Yao S, Wu Y and Shang Y:
NLRP3 inflammasome in neurological diseases, from functions to
therapies. Front Cell Neurosci. 11(63)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nagakubo D, Taira T, Kitaura H, Ikeda M,
Tamai K, Iguchi-Ariga SM and Ariga H: DJ-1, a novel oncogene which
transforms mouse NIH3T3 cells in cooperation with ras. Biochem
Biophys Res Commun. 231:509–513. 1997.PubMed/NCBI View Article : Google Scholar
|
|
13
|
McCoy MK and Cookson MR: DJ-1 regulation
of mitochondrial function and autophagy through oxidative stress.
Autophagy. 7:531–532. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xu J, Zhong N, Wang H, Elias JE, Kim CY,
Woldman I, Pifl C, Gygi SP, Geula C and Yankner BA: The Parkinson's
disease-associated DJ-1 protein is a transcriptional co-activator
that protects against neuronal apoptosis. Hum Mol Genet.
14:1231–1241. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gan L, Johnson DA and Johnson JA:
Keap1-Nrf2 activation in the presence and absence of DJ-1. Eur J
Neurosci. 31:967–977. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wu L, Xu H, Cao L, Li T, Li R, Feng Y,
Chen J and Ma J: Salidroside protects against mpp+-induced neuronal
injury through DJ-1-Nrf2 antioxidant pathway. Evid Based Complem
Alternat Med. 2017(5398542)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sun Q, Shen ZY, Duan WN, Meng QT and Xia
ZY: Mechanism of myocardial ischemia/reperfusion-induced acute
kidney injury through DJ-1/Nrf2 pathway in diabetic rats. Exp Ther
Med. 14:4201–4207. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sun Q, Shen ZY, Meng QT, Liu HZ, Duan WN
and Xia ZY: The role of DJ-1/Nrf2 pathway in the pathogenesis of
diabetic nephropathy in rats. Ren Fail. 38:294–304. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lev N, Barhum Y, Ben-Zur T, Aharony I,
Trifonov L, Regev N, Melamed E, Gruzman A and Offen D: A DJ-1 based
peptide attenuates dopaminergic degeneration in mice models of
parkinson's disease via enhancing Nrf2. PLoS One.
10(e0127549)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang CY, Xu Y, Wang X, Guo C, Wang T and
Wang ZY: Dl-3-n-butylphthalide inhibits NLRP3 inflammasome and
mitigates Alzheimer's-Like pathology via Nrf2-TXNIP-TrX axis.
Antioxid Redox Signal. 30:1411–1431. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hou Y, Wang Y, He Q, Li L, Xie H, Zhao Y
and Zhao J: Nrf2 inhibits NLRP3 inflammasome activation through
regulating Trx1/TXNIP complex in cerebral ischemia reperfusion
injury. Behav Brain Res. 336:32–39. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wree A, Eguchi A, McGeough MD, Pena CA,
Johnson CD, Canbay A, Hoffman HM and Feldstein AE: NLRP3
inflammasome activation results in hepatocyte pyroptosis, liver
inflammation, and fibrosis in mice. Hepatology. 59:898–910.
2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bayne K: Revised guide for the care and
use of laboratory animals available. Physiologist. 39:208–211.
1996.PubMed/NCBI
|
|
24
|
Lamberty Y, Gower AJ, Gobert J, Hanin I
and Wulfert E: Behavioural, biochemical and histological effects of
AF64A following injection into the third ventricle of the mouse.
Behav Brain Res. 51:165–177. 1992.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ahmad J, Baig MA, Ali AA, Al-Huqail A,
Ibrahim MM and Qureshi MI: Comparative assessment of four RNA
extraction methods and modification to obtain high-quality RNA from
leaf. 3 Biotech. 7(373)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Vorhees CV and Williams MT: Morris water
maze: Procedures for assessing spatial and related forms of
learning and memory. Nat Protoc. 1:848–858. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li HN, Jiang YM, Zhao P, Zhou S and Yu JQ:
Effects of oxymatrine on oxidative stress in brain tissue of
neonatal rats with hypoxic-ischemic brain damage. J Ningxia Med
Uni. 7:743–745. 2016.(In Chinese).
|
|
29
|
Lakshminarasimhan M, Maldonado MT, Zhou W,
Fink AL and Wilson MA: Structural impact of three
Parkinsonism-associated missense mutations on human DJ-1.
Biochemistry. 47:1381–1392. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Joselin AP, Hewitt SJ, Callaghan SM, Kim
RH, Chung YH, Mak TW, Shen J, Slack RS and Park DS: ROS-dependent
regulation of parkin and DJ-1 localization during oxidative stress
in neurons. Hum Mol Genet. 21:4888–4903. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
D'Amelio M, Cavallucci V, Middei S,
Marchetti C, Pacioni S, Ferri A, Diamantini A, De Zio D, Carrara P,
Battistini L, et al: Caspase-3 triggers early synaptic dysfunction
in a mouse model of Alzheimer's disease. Nat Neurosci. 14:69–76.
2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Miao EA, Rajan JV and Aderem A:
Caspase-1-induced pyroptotic cell death. Immunol Rev,. 243:206–14.
2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fink SL and Cookson BT:
Caspase-1-Dependent pore formation during pyroptosis leads to
osmotic lysis of infected host macrophages. Cell Microbiol.
8:1812–1825. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mota SI, Costa RO, Ferreira IL, Santana I,
Caldeira GL, Padovano C, Fonseca AC, Baldeiras I, Cunha C, Letra L,
et al: Oxidative stress involving changes in Nrf2 and ER stress in
early stages of Alzheimer's disease. Biochim Biophys Acta.
1852:1428–1441. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Liu X, Zhang X, Ding Y, Zhou W, Tao L, Lu
P, Wang Y and Hu R: Nuclear factor E2-related factor-2 negatively
regulates NLRP3 inflammasome activity by inhibiting reactive oxygen
species-induced NLRP3 priming. Antioxidants Redox Signal. 26:28–43.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Oakley H, Cole SL, Logan S, Maus E, Shao
P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik
L, et al: Intraneuronal beta-amyloid aggregates, neurodegeneration,
and neuron loss in transgenic mice with five familial Alzheimer's
disease mutations: Potential factors in amyloid plaque formation. J
Neurosci. 26:10129–10140. 2006.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu WH, Shi LS, Chung MC, Chang TC and Lee
SY: Antcamphin M inhibits TLR4-mediated inflammatory responses by
upregulating the Nrf2/HO-1 pathway and suppressing the nlrp3
inflammasome pathway in macrophages. Am J Chin Med. 47:1611–1626.
2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lopez N, Tormo C, De Blas I, Llinares I
and Alom J: Oxidative stress in Alzheimer's disease and mild
cognitive impairment with high sensitivity and specificity. J
Alzheimers Dis. 33:823–829. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Aliev G, Priyadarshini M, Reddy VP, Grieg
NH, Kaminsky Y, Cacabelos R, Ashraf GM, Jabir NR, Kamal MA,
Nikolenko VN, et al: Oxidative stress mediated mitochondrial and
vascular lesions as markers in the pathogenesis of Alzheimer
disease. Curr Med Chem. 21:2208–2217. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bonda DJ, Wang X, Perry G, Nunomura A,
Tabaton M, Zhu X and Smith MA: Oxidative stress in Alzheimer
disease: A possibility for prevention. Neuropharmacology.
59:290–294. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Baulac S, Lu H, Strahle J, Yang T,
Goldberg MS, Shen J, Schlossmacher MG, Lemere CA, Lu Q and Xia W:
Increased DJ-1 expression under oxidative stress and in Alzheimer's
disease brains. Mol Neurodegener. 4(12)2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Mullett SJ, Di Maio R, Greenamyre JT and
Hinkle DA: DJ-1 expression modulates astrocyte-mediated protection
against neuronal oxidative stress. J Mol Neurosci. 49:507–511.
2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhang Z, Wang Q and Pu XP: Inhibition of
oxidative damage by high expression of wild-type DJ-1 gene in
SH-SY5Y cells cultured in vitro. Chin J New Drugs. 16:1854–1857.
2007.(In Chinese).
|
|
44
|
Kim RH, Smith PD, Aleyasin H, Hayley S,
Mount MP, Pownall S, Wakeham A, You-Ten AJ, Kalia SK, Horne P, et
al: Hypersensitivity of DJ-1-deficient mice to
1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative
stress. Proc Natl Acad Sci USA. 102:5215–5220. 2005.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yagishita Y, Uruno A, Fukutomi T, Saito R,
Saigusa D, Pi J, Fukamizu A, Sugiyama F, Takahashi S and Yamamoto
M: Nrf2 improves leptin and insulin resistance provoked by
hypothalamic oxidative stress. Cell Rep. 18:2030–2044.
2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kaspar JW, Niture SK and Jaiswal AK:
Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol
Med. 47:1304–1309. 2009.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Nguyen T, Nioi P and Pickett CB: The
Nrf2-antioxidant response element signaling pathway and its
activation by oxidative stress. J Biol Chem. 284:13291–13295.
2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Clements CM, McNally RS, Conti BJ, Mak TW
and Ting JP: DJ-1, a cancer- and Parkinson's disease-associated
protein, stabilizes the antioxidant transcriptional master
regulator Nrf2. Proc Natl Acad Sci USA. 103:15091–15096.
2006.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Liu C, Chen Y, Kochevar IE and Jurkunas
UV: Decreased DJ-1 leads to impaired Nrf2-regulated antioxidant
defense and increased UV-A-induced apoptosis in corneal endothelial
cells. Invest Ophthalmol Vis Sci. 55:5551–5560. 2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhang L, Zhang T and Tan N: Programmed
cell death independent of caspases. Pro Mod Biomed. 66:3760–3763.
2009.
|
|
51
|
Liu D, Zeng X, Li X, Mehta JL and Wang X:
Role of NLRP3 inflammasome in the pathogenesis of cardiovascular
diseases. Basic Res Cardiol. 113(5)2018.
|
|
52
|
Bossu P, Ciaramella A, Moro ML,
Bellincampi L, Bernardini S, Federici G, Trequattrini A, Macciardi
F, Spoletini I, Di Iulio F, et al: Interleukin 18 gene
polymorphisms predict risk and outcome of Alzheimer's disease. J
Neurol Neurosurg Psychiatry. 78:807–811. 2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ojala J, Alafuzoff I, Herukka SK, van
Groen T, Tanila H and Pirttila T: Expression of interleukin-18 is
increased in the brains of Alzheimer's disease patients. Neurobiol
Aging. 30:198–209. 2009.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Feng J, Wang JX, Du YH, Liu Y, Zhang W,
Chen JF, Liu YJ, Zheng M, Wang KJ and He GQ: Dihydromyricetin
inhibits microglial activation and neuroinflammation by suppressing
NLRP3 inflammasome activation in APP/PS1 transgenic mice. CNS
Neurosci Ther. 24:1207–1218. 2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Dempsey C, Rubio Araiz A, Bryson KJ,
Finucane O, Larkin C, Mills EL, Robertson AAB, Cooper MA, O'Neill
LAJ and Lynch MA: Inhibiting the NLRP3 inflammasome with MCC950
promotes non-phlogistic clearance of amyloid-β and cognitive
function in APP/PS1 mice. Brain Behav Immun. 61:306–316.
2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Heneka MT, Kummer MP, Stutz A, Delekate A,
Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, et
al: NLRP3 is activated in Alzheimer's disease and contributes to
pathology in APP/PS1 mice. Nature. 493:674–678. 2013.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Sokolovska A, Becker CE, Ip WK, Rathinam
VA, Brudner M, Paquette N, Tanne A, Vanaja SK, Moore KJ, Fitzgerald
KA, et al: Activation of caspase-1 by the NLRP3 inflammasome
regulates the NADPH oxidase NOX2 to control phagosome function. Nat
Immunol. 14:543–553. 2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Choi AJ and Ryter SW: Inflammasomes:
Molecular regulation and implications for metabolic and cognitive
diseases. Mol Cells. 37:441–448. 2014.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Lu L, Lu Q, Chen W, Li J, Li C and Zheng
Z: Vitamin D protects against diabetic retinopathy by inhibiting
high-glucose-induced activation of the ros/txnip/nlrp3 inflammasome
pathway. J Diabetes Res. 22(8193523)2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Parajuli B, Sonobe Y, Horiuchi H, Takeuchi
H, Mizuno T and Suzumura A: Oligomeric amyloid β induces IL-1β
processing via production of ROS: Implication in Alzheimer's
disease. Cell Death Dis. 4(e975)2013.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Wali JA, Gurzov EN, Fynch S, Elkerbout L,
Kay TW, Masters SL and Thomas HE: Activation of the NLRP3
inflammasome complex is not required for stress-induced death of
pancreatic islets. PLoS One. 9(e113128)2014.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Liu X, et al: Nuclear Factor E2-Related
Factor-2 Negatively Regulates NLRP3 Inflammasome Activity by
Inhibiting Reactive Oxygen Species-Induced NLRP3 Priming.[J].
Antioxid Redox Signal. 26(28)2017.PubMed/NCBI View Article : Google Scholar
|