Introduction
Hypertension is a complex clinical syndrome that is
usually characterized by abnormally elevated systolic blood
pressure (SBP) and/or diastolic blood pressure (DBP) and comprises
a wide range of patients and various clinical complications.
Various pathological mechanisms have been indicated to be closely
linked to the occurrence and progression of hypertension and target
organ injury mediated by hypertension, among which inflammation is
one of the most important mechanisms (1-3).
Interleukin-37 (IL-37) belongs to the IL-1 family
and is the only anti-inflammatory factor in this family (4). IL-37 is expressed in tissues and
organs of a variety of mammals but is not observed in mice; in
addition, IL-37 is mainly secreted by immune cells such as
macrophages and dendritic cells and may also be secreted in small
amounts by endothelial cells and smooth muscle cells (4,5). IL-37
is able to regulate the differentiation of various immune cells,
affect the expression of downstream inflammatory factors and
participate in the development and progression of a variety of
diseases by binding with IL-18 receptor (IL-18R)α and IL-1R8
receptors and further activating the Toll-like receptor 4 pathway
(6-8).
IL-37 has been the focus of recent research and
increasing evidence has confirmed that it is closely associated
with the progression of cardiovascular disease (9,10).
IL-37 has been indicated to alleviate the progression of
atherosclerosis and enhance the stability of plaques in
apolipoprotein E knockout mice fed a high-fat diet by inhibiting
T-lymphocyte differentiation, dendritic cell maturation and
vascular smooth muscle cell apoptosis (11-13).
In another study, IL-37 was reported to have no effect on
atherosclerosis development in low-density lipoprotein
receptor-deficient mice receiving a high-fat diet, although the
local inflammation in the blood vessels was reduced (14). In addition, IL-37 may reduce the
area of myocardial infarction and reverse cardiac remodeling by
inhibiting the maturation of dendritic cells (15). Furthermore, treatment with exogenous
IL-37 alleviates myocardial injury induced by ischemia/reperfusion
via the upregulation of IL-10 expression (16). However, the association between
IL-37 and hypertension has remained elusive and the purpose of the
present study was to observe IL-37 expression in the context of
hypertension.
Materials and methods
Blood sample collection
As described in a previous study, patients with
secondary hypertension, coronary artery disease, heart failure,
stroke, valvular heart disease, collagen disease, advanced liver
disease, renal failure, malignant disease, septicemia, or other
inflammatory diseases were excluded from the present study
(17). The study was approved by
the ethics committee of the People's Hospital of Guangxi Zhuang
Autonomous Region (Nanning, China; approval no. 2015-16 of the
National Natural Science Foundation of China), and written informed
consent was obtained from the participants.
The diagnosis of hypertension was based on the
results of ambulatory blood pressure monitoring (ABPM). In brief,
blood pressure values for 48 time-points were collected over a 24-h
period and patients with abnormally high blood pressure (SBP/DBP
≥140/90 mmHg from 7 am to 0 am and SBP/DBP ≥125/85 mmHg from 0 am
to 7 am) detected at >25% of the time-points were diagnosed with
hypertension (18,19). Secondary hypertension,
hyperthyroidism, white-coat syndrome, elevated blood pressure due
to emotional agitation, and all other diseases and conditions that
may affect blood pressure, were excluded (19).
Within 2 months from October 2018 to January 2020, a
total of 110 subjects who were admitted for physical examination at
the medical center were enrolled. The subjects were divided in to a
non-hypertension group (control subjects, n=40) and a hypertension
group (n=70) based on the results of ABPM. The monocytes and
T-lymphocytes were separated from the fresh blood samples and then
stimulated to differentiate into macrophages, dendritic cells and
mature T-lymphocytes. In brief, peripheral blood mononuclear cells
were isolated from each blood sample after the red blood cells were
lysed. Subsequently, the T-lymphocytes and monocytes were
positively selected using human CD4 (cat. no. 130-045-101) and
CD11b (cat. no. 130-049-601) magnetic beads and an autoMACS
separator (cat. no. 130-049-601; all from Miltenyi Biotech)
(20). The monocytes were treated
with human macrophage colony-stimulating factor (M-CSF; 50 ng/ml;
cat. no. AF-300-25; PeproTech) or human granulocyte-M-CSF (50
ng/ml; cat. no. 900-K30; PeproTech) for 9 days to stimulate their
differentiation into macrophages and dendritic cells, respectively.
The T lymphocytes were incubated with Cell Stimulation Cocktail (2
µl/ml; cat. no. 00-4975-93; eBioscience), which was composed of
40.5 µmol/l phorbol myristate acetate, 670 µmol/l ionomycin, 5.5
µmol/l brefeldin and 1 µmol/l monensin for 4 h to promote
maturation. IL-37 mRNA expression in each isolate of immune cells
from all subjects was detected.
From March 2017 to January 2020, 491 hospitalized
subjects were enrolled in the present study. According to the
above-mentioned criteria, 67 subjects were excluded from the study.
Blood samples were collected from the remaining 424 subjects
(including 90 subjects without hypertension and 334 patients with
hypertension) in a fasted state in the morning following admission
by experienced nurses for the collection of plasma samples and
measurement of IL-37 levels.
Reverse transcription-quantitative
(RT-q)PCR detection of IL-37 mRNA expression
The macrophages, T-lymphocytes and dendritic cells
were lysed using TRIzol reagent (cat no. T9424; Sigma-Aldrich;
Merck KGaA) according to the manufacturer's protocol, and the total
mRNA of each sample was individually extracted. Subsequently, the
complementary (c)DNA was obtained by RT using 2 µg of total mRNA
and a Transcriptor First Strand cDNA Synthesis kit (cat. no.
04896866001; Roche Molecular Systems, Inc.) at 92˚C for 5 min. The
IL-37 mRNA levels were detected by PCR amplification using SYBR
Green (cat no. 04707516001; Roche Molecular Systems, Inc.) and were
normalized to GAPDH levels using the 2-ΔΔCq method
(21). The following thermocycling
conditions were used for the PCR: 35 cycles at 92˚C for 30 sec,
58˚C for 40 sec and 72˚C for 35 sec. The primers (Qingke
Technology) used in the present study were as follows: IL-37
forward, 5'-AGTGCTGCTTAGAAGACCCGG-3' and reverse,
5'-AGAGTCCAGGACCAGTACTTTGTGA-3'; GAPDH forward,
5'-GAGTCAACGGATTTGGTCGT-3' and reverse,
5'-GACAAGCTTCCCGTTCTCAG-3'.
Measurement of plasma IL-37
levels
The samples were obtained in sodium heparin
vacutainers and centrifuged at 5,000 x g for 15 min. Plasma was
obtained from each sample after centrifugation and was stored at
-80˚C until further detection. Prior to investigation, the frozen
plasma samples were thawed at 4˚C and an ELISA (cat. no.
88-52103-22; Invitrogen; Thermo Fisher Scientific, Inc.) was
performed to determine the IL-37 levels in each sample. All assays
were performed in duplicate.
Echocardiography and ABPM
All of the control subjects and patients with
hypertension received B-mode ultrasound examinations and underwent
ABPM to identify whether the patients suffered from carotid
atherosclerosis and/or non-dipper hypertension. The structural and
functional changes in the heart may affect interleukin secretion.
Therefore, all subjects underwent echocardiography, and subjects
with significant cardiac structural and functional changes were
excluded. Both of these tests were completed and analyzed by
doctors with >10 years of experience. In addition, the 24-h
coefficient of variation of SBP (CV-SBP), CV-DBP, night drop rate
of SBP (NDR-SBP), NDR-DBP, average SBP in 24 h (A24h-MSBP), 24
h-MDBP, average active period of SBP (AP-SBP), AP-DBP, average
passive period of SBP (PP-SBP) and PP-DBP were collected after the
ABPM of each patient was completed.
Collection of patient information
Information on age, sex, smoking, drinking, body
mass index (BMI) and medical treatments was obtained when the
patients' medical history was taken after admission. Information on
SBP, DBP and heart rate (HR) was collected by the attending
physician. The fasting glucose (Glu), hemoglobin A1c (HbAlc), total
cholesterol (TC), total triglycerides (TG), high-density
lipoprotein cholesterol (HDL-C), low-density lipoprotein
cholesterol (LDL-C), creatinine (CREA), C-reactive protein (CRP),
homocysteine (Hcy), sleep apnea hypopnea syndrome (SAHS) and
hypoxemia-associated data were obtained after laboratory tests.
Statistical analysis
All of the data in the present study are expressed
as the mean ± standard deviation and were further analyzed using
SPSS 26.0 statistical software (IBM Corp.). Discrete variables are
expressed as n (%) and were compared with Chi-square tests. For the
continuous variables, Student's t-test was performed to compare the
differences between 2 groups, and one-way analysis of variance
followed by the Tukey's post hoc test was used for comparisons
involving 3 groups. In addition, the correlations between IL-37
levels and SBP, DBP and clinical characteristics in patients with
hypertension were calculated using Pearson's correlation.
Furthermore, the influence of hypertension on IL-37 secretion and
IL-37 levels on the presence of carotid atherosclerotic plaque
(CAP) were identified by univariate analysis and subsequent
multivariate linear regression analysis. P<0.05 was considered
to indicate statistical significance in all analyses.
Results
Clinical characteristics
The clinical characteristics of each group are
listed in Table I. Compared with
the control group, the hypertension group exhibited a higher rate
of smoking, higher SBP and DBP and elevated levels of CRP. No
significant inter-group differences were observed in other clinical
characteristics, including age, sex, drinking, HR, presence of CAP,
BMI, Glu, HbAlc, blood lipids, CREA and Hcy.
 | Table IClinical characteristics of the
control and hypertension groups. |
Table I
Clinical characteristics of the
control and hypertension groups.
|
Characteristics | Normal range | Control (n=40) | Hypertension
(n=70) | P-value |
|---|
| Age (years) | - | 53.2±10.2 | 56.2±11.5 | 0.1735 |
| Male sex | - | 25 (62.5) | 40 (50.0) | 0.6877 |
| Smoking | - | 13 (32.5) | 45 (64.3) | 0.0016 |
| Drinking | - | 9 (22.5) | 20 (28.6) | 0.6533 |
| Obesity | - | 14 (35.0) | 30 (42.9) | 0.5433 |
| T2DM | - | 3 (7.5) | 4 (5.7) | 0.7033 |
| HLP | - | 10 (25.0) | 20 (28.6) | 0.8246 |
| CAP | - | 2 (5.0) | 13 (18.6) | 0.0800 |
| HR (bpm) | 60-100 | 72.5±10.6 | 75.3±12.1 | 0.2252 |
| SBP (mmHg) | 90-140 | 119±13 | 155±26 | <0.0001 |
| DBP (mmHg) | 60-90 | 76±12 | 95±19 | <0.0001 |
| BMI
(kg/m2) | 20-25 | 23.2±3.5 | 23.6±3.6 | 0.5724 |
| Glu (mmol/l) | 3.5-6.1 | 4.6±0.6 | 4.7±0.6 | 0.4023 |
| HbAlc (%) | 4-6 | 4.8±1.0 | 4.9±1.1 | 0.6366 |
| TC (mmol/l) | 3-5.2 | 4.5±0.8 | 4.7±1.0 | 0.2817 |
| TG (mmol/l) | 0.5-1.7 | 1.1±0.5 | 1.1±0.8 | 0.8724 |
| HDL-C (mmol/l) | 0.9-2.0 | 1.1±0.4 | 1.3±0.7 | 0.3523 |
| LDL-C (mmol/l) | 1.8-3.1 | 2.2±0.6 | 2.5±0.9 | 0.5262 |
| CREA (µmol/l) | 57-97 | 66±14 | 69±19 | 0.2152 |
| CRP (mg/l) | <5 | 0.6±0.8 | 6.4±4.7 | <0.0001 |
| Hcy (µmol/l) | 0-15 | 10.3±6.4 | 11.1±7.1 | 0.1952 |
| Medical
treatments |
|
ACEI/ARB | - | 0 (0.0) | 39 (55.7) | <0.0001 |
|
β-blocker | - | 0 (0.0) | 23 (32.9) | <0.0001 |
|
CCB | - | 0 (0.0) | 51 (72.9) | <0.0001 |
|
Diuretics | - | 0 (0.0) | 30 (42.8) | <0.0001 |
|
α-blocker | - | 0 (0.0) | 6 (8.6) | 0.0845 |
|
Statin | - | 4 (10.0) | 36 (51.4) | <0.0001 |
IL-37 mRNA levels in patients with
hypertension and controls
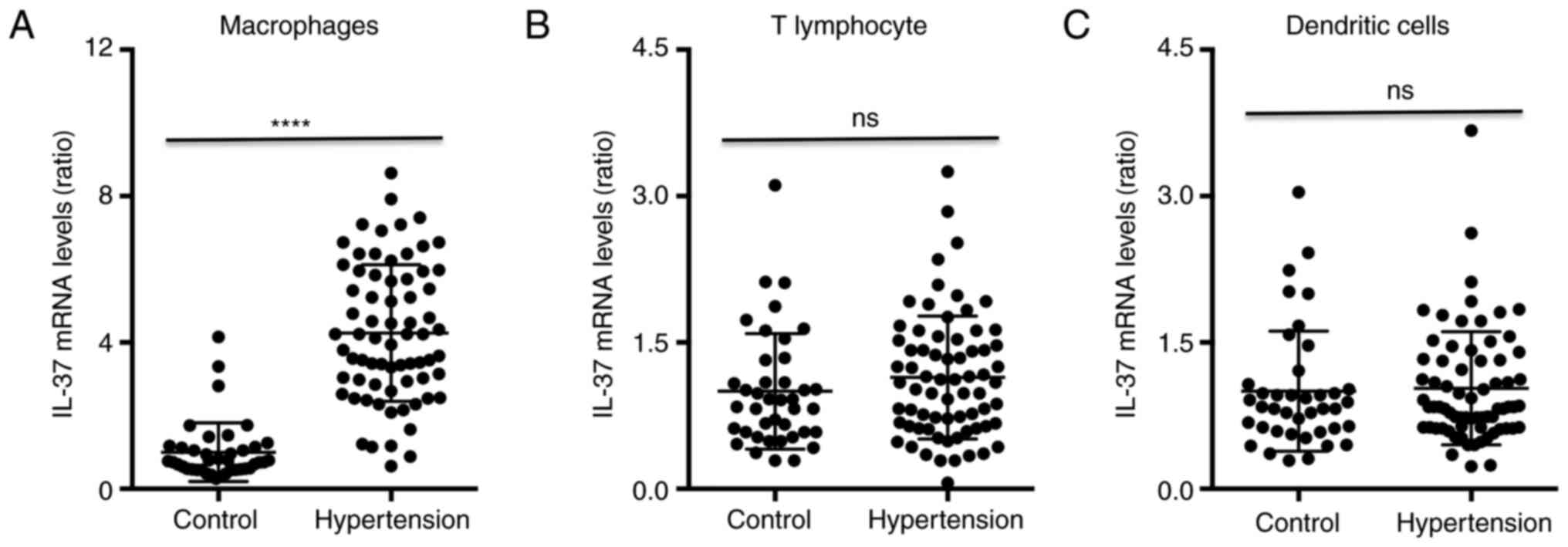
The RT-qPCR results indicated that the average IL-37
mRNA expression in macrophages from hypertensive patients was
increased ~3.5-fold compared with that in macrophages from control
subjects (Fig. 1A). The IL-37 mRNA
levels in T-lymphocytes and dendritic cells exhibited no
differences between control subjects and patients with hypertension
(Fig. 1B and C, respectively). In addition, a univariate
analysis was performed to identify any factors affecting IL-37
expression in the entire cohort. The β-values, 95% CI of β and
P-values are listed in Table II,
and factors including macrophages, T-lymphocytes, dendritic cells,
hypertension, smoking, drinking, obesity, CREA, CRP and Hcy in
Table II were adjusted. The
results suggested that macrophages, hypertension, smoking and CRP
significantly affected IL-37 expression. These variables were used
to further perform multivariate regression analysis and the results
indicated that hypertension and macrophages were closely and
independently associated with the expression of IL-37.
 | Table IILogistic regression analysis of the
influence of clinical factors on interleukin-37 mRNA expression in
the cohort. |
Table II
Logistic regression analysis of the
influence of clinical factors on interleukin-37 mRNA expression in
the cohort.
| | Univariate | Multivariate |
|---|
| Factor | Cut-off value | Adjusted β | 95% CI of β | P-value | Adjusted β | 95% CI of β | P-value |
|---|
| Macrophages | Yes | 0.491 | 0.352-0.630 | <0.001 | 0.305 | 0.226-0.484 | <0.001 |
| T-lymphocytes | Yes | 0.094 | 0.041-0.147 | 0.098 | | | |
| Dendritic
cells | Yes | 0.033 | 0.010-0.056 | 0.274 | | | |
| Hypertension | Yes | 0.392 | 0.267-0.517 | <0.001 | 0.196 | 0.101-0.291 | 0.009 |
| Smoking | Yes | 0.202 | 0.150-0.254 | 0.029 | 0.101 | 0.051-0.151 | 0.091 |
| Drinking | Yes | 0.088 | 0.037-0.139 | 0.339 | | | |
| Obesity | Yes | 0.042 | 0.019-0.065 | 0.618 | | | |
| CREA | >79 µmol/l | 0.007 | -0.012-0.026 | 0.512 | | | |
| CRP | >8.6 mg/l | 0.164 | 0.084-0.244 | 0.004 | 0.078 | 0.037-0.119 | 0.019 |
| Hcy | >13.5
µmol/l | -0.04 | -0.023-0.015 | 0.887 | | | |
Clinical characteristics of subgroups
of hypertension and controls
The clinical characteristics of each group are
listed in Table SI. Compared with
those in the control group, the hypertension group exhibited
significantly higher rates of obesity, type 2 diabetes mellitus
(T2DM), hyperlipidemia (HLP), SAHS and CAP, higher levels of SBP,
DBP, BMI, Glu, HbAlc, TG, CREA, CRP and Hcy, and lower levels of
HDL-C. A higher tendency of male sex, smoking and drinking was
observed in the hypertension group, but it was not statistically
significant compared with the control group. No differences in
other clinical factors were observed between the two groups. In
addition, the non-dipper group exhibited increased T2DM and CAP
rates compared with those in the dipper group, and the dipper group
exhibited a lower rate of CAP compared with the total group. No
differences in terms of any other clinical characteristics,
including age, sex, smoking, drinking, HR, TC or HDL-C, were
obtained among the control, dipper hypertension, non-dipper
hypertension and total hypertension group.
Information on ABPM
The ABPM data for each group are listed in Table SII. The hypertension group had a
higher NDR-SBP, NDR-DBP, A24h-SBP, A24h-DBP, AP-SBP, AP-DBP, PP-SBP
and PP-DBP than the control group. Compared with the dipper group,
the non-dipper group exhibited increases in the PP-SBP and PP-DBP
and decreases in the NDR-SBP and NDR-SBP. No differences in
A24h-SBP, A24h-DBP, AP-SBP and AP-DBP were observed between the
dipper group and the non-dipper group, and no differences in CV-SBP
and CV-DBP were observed among the four groups.
Plasma IL-37 concentration in patients
with hypertension
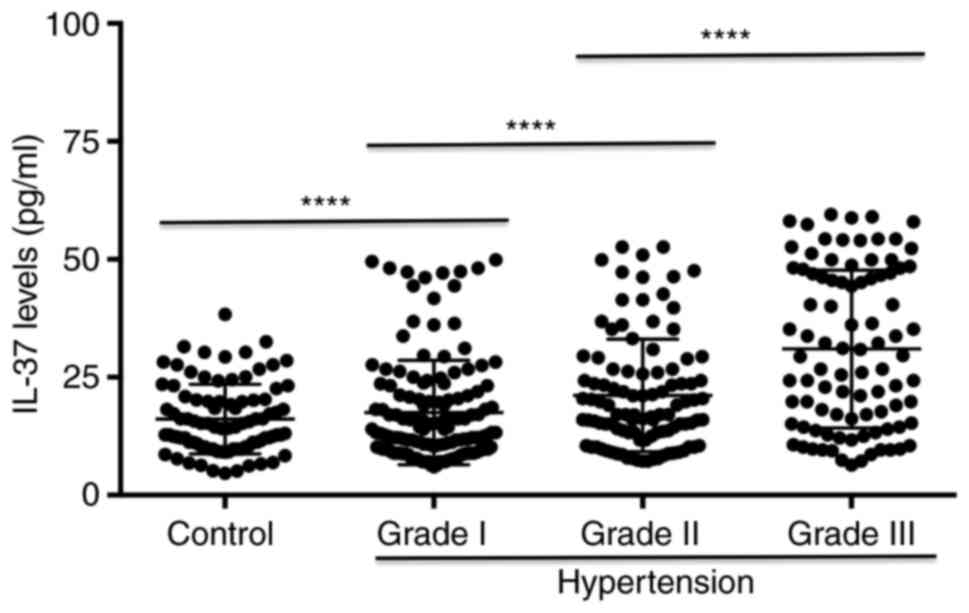
Regarding the ELISA results, the IL-37 levels
gradually increased in patients with grade I, II and III
hypertension and those in all groups were higher than those in the
control group (Fig. 2). In
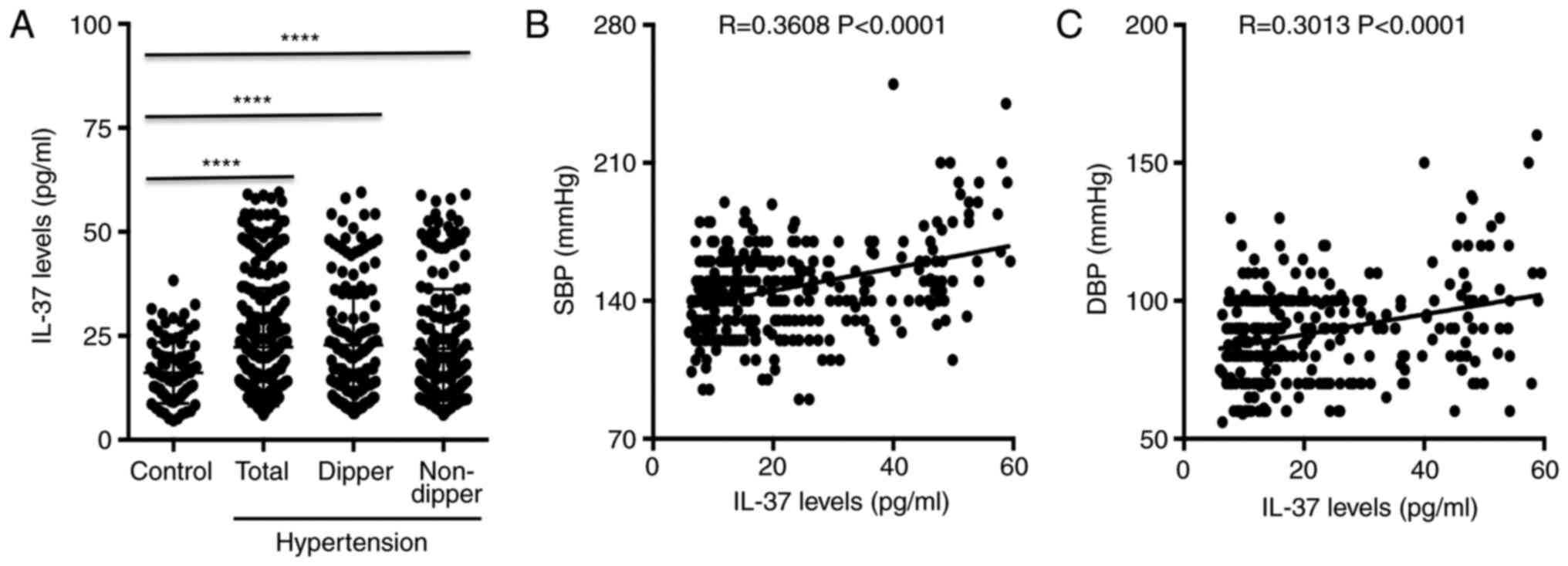
addition, no differences in plasma IL-37 levels were observed among
the hypertension group, the dipper group and the non-dipper group,
while the levels in all of these groups were significantly elevated
compared with the levels in the control group (Fig. 3A). Furthermore, IL-37 levels were
positively correlated with SBP and with DBP in patients with
hypertension (Fig. 3B and C, respectively). A univariate analysis of
variables affecting the plasma levels of IL-37 was then performed.
The β values, 95% CI of β, and P-values are listed in Table III. The results suggested that
CAP, hypertension, non-dipper hypertension, smoking, T2DM, HLP,
CREA, CRP and Hcy had a significant influence on the plasma levels
of IL-37. These variables were subjected to further multivariate
regression analysis and the results suggested that CAP,
hypertension, smoking, T2DM, HLP, CREA, CRP and Hcy were closely
independently associated with the secretion of IL-37 into the
plasma.
 | Table IIILogistic regression analysis of the
effects of clinical factors on plasma interleukin-37 levels. |
Table III
Logistic regression analysis of the
effects of clinical factors on plasma interleukin-37 levels.
| | Univariate | Multivariate |
|---|
| Factor | Cut-off value | Adjusted β | 95% CI of β | P-value | Adjusted β | 95% CI of β | P-value |
|---|
| CAP | Yes | 0.377 | 0.219-0.535 | <0.001 | 0.195 | 0.125-0.265 | <0.001 |
| Hypertension | Yes | 0.272 | 0.156-0.388 | <0.001 | 0.152 | 0.106-0.197 | <0.001 |
| Non-dipper | Yes | 0.293 | 0.180-0.406 | 0.031 | 0.123 | 0.088-0.158 | 0.129 |
| Age | >55 years | 0.244 | 0.127-0.361 | 0.058 | | | |
| Sex | Yes | 0.041 | -0.061-0.155 | 0.459 | | | |
| Smoking | Yes | 0.313 | 0.191-0.435 | <0.001 | 0.149 | 0.103-0.195 | <0.001 |
| Drinking | Yes | 0.048 | -0.071-0.167 | 0.752 | | | |
| Obesity | Yes | 0.196 | 0.113-0.189 | 0.279 | | | |
| T2DM | Yes | 0.155 | 0.056-0.254 | 0.009 | 0.088 | 0.064-0.112 | 0.021 |
| HLP | Yes | 0.137 | 0.034-0.220 | 0.026 | 0.063 | 0.052-0.074 | 0.049 |
| SAHS | Yes | 0.198 | 0.091-0.305 | 0.055 | | | |
| Hypoxemia | Yes | 0.177 | 0.074-0.280 | 0.063 | | | |
| CREA | >92 µmol/l | 0.257 | 0.143-0.371 | 0.004 | 0.102 | 0.071-0.133 | 0.012 |
| CRP | >6.8 mg/l | 0.134 | 0.031-0.237 | 0.008 | 0.061 | 0.050-0.072 | 0.023 |
| Hcy | >17.9
µmol/l | 0.124 | 0.022-0.228 | 0.017 | 0.051 | 0.039-0.063 | 0.046 |
Smoking, T2DM and CAP influence
circulating IL-37 levels in patients with hypertension
In addition, all patients with hypertension were
divided into two groups according to sex, smoking, drinking,
obesity, T2DM, HLP, SAHS, hypoxemia and CAP and the serum levels of
IL-37 were compared between them (Fig.
4). The results suggested that patients who smoked, those who
had T2DM and those with CAP exhibited higher plasma IL-37 levels
than patients who did not smoke or have T2DM or CAP (Fig. 4B, E
and I). Other factors, including
sex, drinking, obesity, hyperlipidemia, SAHS and hypoxemia, had no
effects on the plasma levels of IL-37 in patients with hypertension
(Fig. 4A, C, D,
F and H).
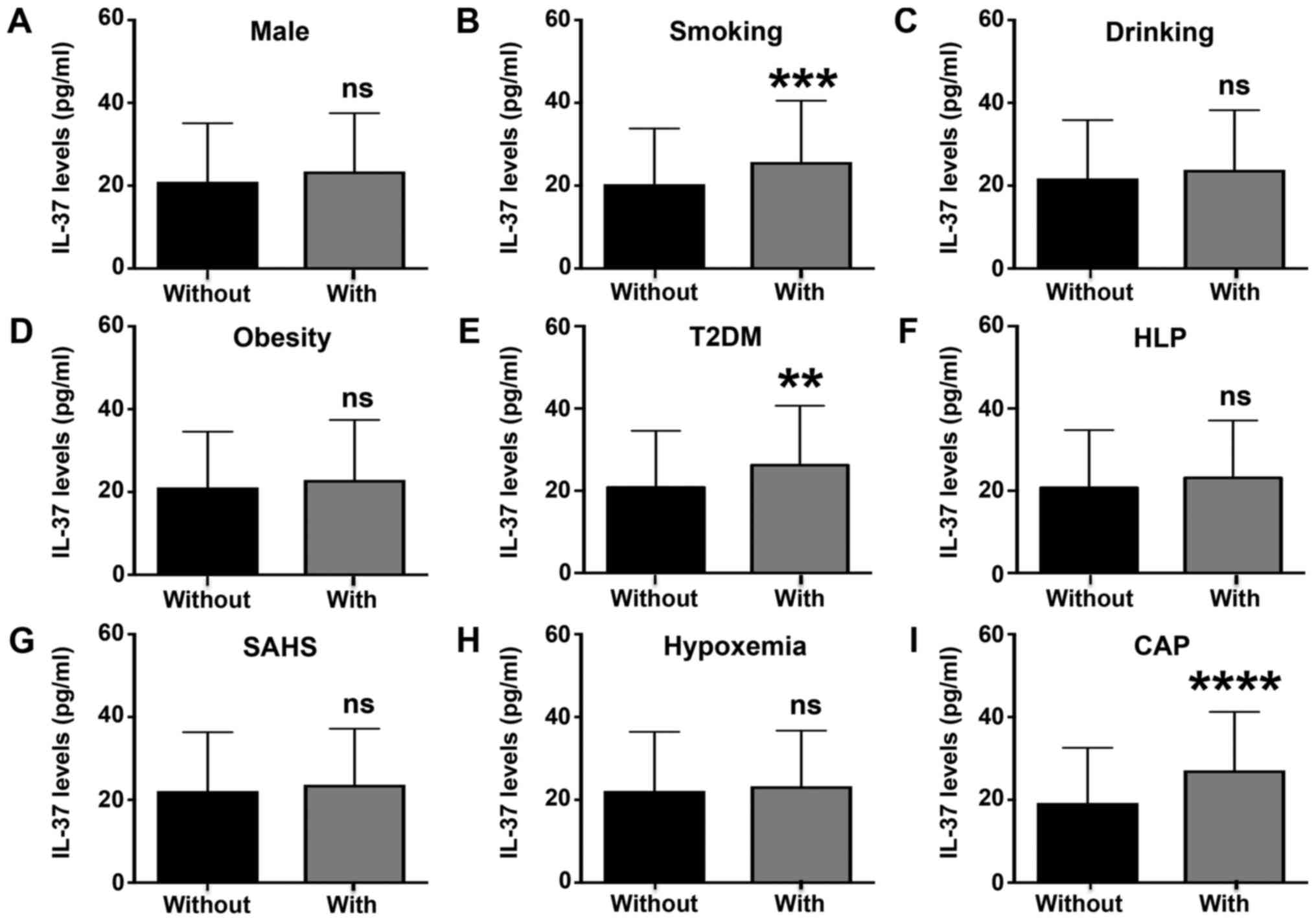 | Figure 4Circulating IL-37 levels in patients
with different characteristics. IL-37 levels were determined in
patients with hypertension with or without the following
characteristics: (A) Male sex, (B) smoking, (C) drinking, (D)
obesity, (E) T2DM, (F) HLP, (G) SAHS, (H) hypoxemia and (I) CAP.
**P<0.01 vs. the without T2DM group;
***P<0.001 vs. the without smoking group;
****P<0.0001 vs. the without CAP group. ns, no
significance; IL, interleukin; T2DM, type 2 diabetes mellitus; HLP,
hyperlipidemia; SAHS, sleep apnea hypopnea syndrome; CAP, carotid
atherosclerotic plaque. |
Effects of IL-37 on the presence of
CAP
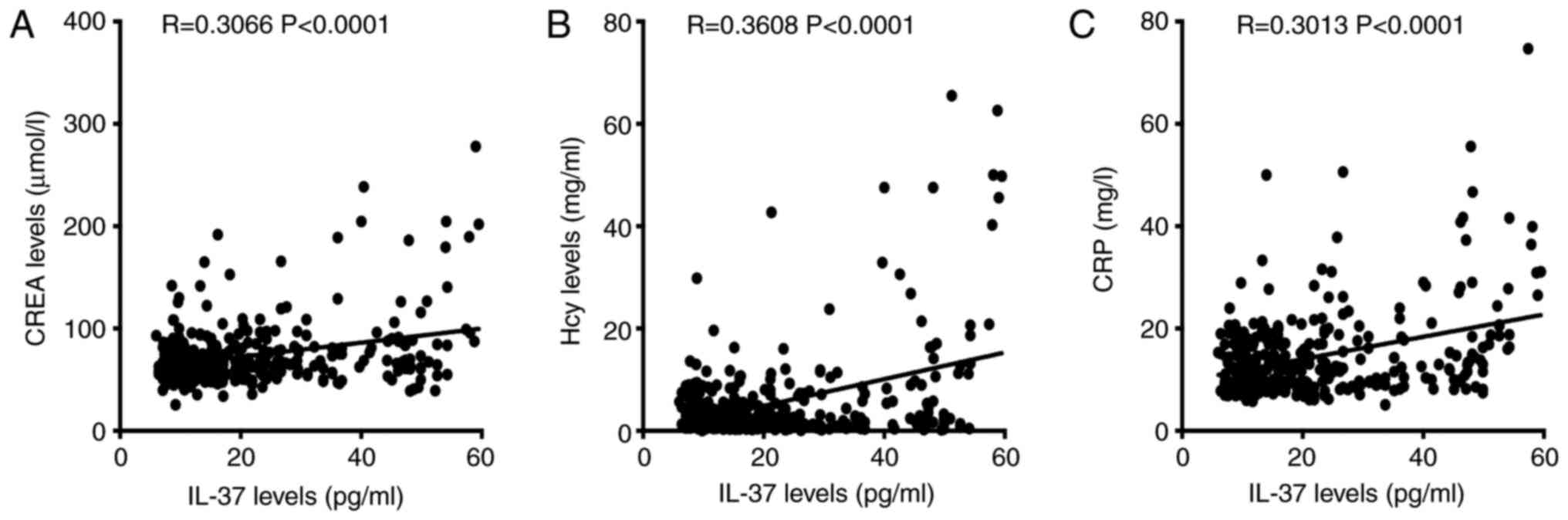
The correlation between IL-37 levels and clinical
characteristics was then analyzed. The results suggested that CREA,
Hcy and CRP were all positively correlated with plasma IL-37 levels
in individuals with hypertension (Fig.
5A-C). No significant correlations between plasma IL-37 levels
and BMI, Glu, TC, LDL-C or HDL-C were observed (data not shown).
Subsequently, a univariate linear regression analysis regarding the
influence on the presence of CAP was performed for the variables
which exhibited differences between the control group and the
hypertension group or were demonstrated to be associated with the
presence of CAP, including IL-37, hypertension, non-dipper status,
age, male sex, smoking, drinking, obesity, T2DM, HLP, SAHS,
hypoxemia, CREA, CRP and Hcy. The β-values, 95% CI of β and
P-values are listed in Table IV.
The results suggested that IL-37, hypertension, non-dipper status,
age, smoking, T2DM, HLP, SAHS, hypoxemia and CRP had an influence
on the occurrence of CAP. These variables provided in Table IV, including IL-37, hypertension,
non-dipper status, age, sex, smoking, drinking, obesity, T2DM, HLP,
SAHS, hypoxemia, CREA, CRP and Hcy, were all adjusted and used to
further perform multivariate regression analysis and the results
suggested that the presence of CAP was independently associated
with IL-37, hypertension, non-dipper state, age, sex and
smoking.
 | Table IVInfluence of various factors
including serum IL-37 on the presence of carotid atherosclerotic
plaque assessed by univariate analysis and subsequent multivariate
linear regression analysis. |
Table IV
Influence of various factors
including serum IL-37 on the presence of carotid atherosclerotic
plaque assessed by univariate analysis and subsequent multivariate
linear regression analysis.
| | Univariate | Multivariate |
|---|
| Variable | Cut-off value | Adjusted β | 95% CI of β | P-value | Adjusted β | 95% CI of β | P-value |
|---|
| IL-37 | >30.9 mg/ml | 0.180 | 0.078-0.280 | 0.018 | 0.110 | 0.014-0.206 | 0.024 |
| Hypertension | Yes | 0.183 | 0.081-0.285 | <0.001 | 0.103 | 0.001-0.205 | 0.047 |
| Non-dipper | Yes | 0.191 | 0.090-0.293 | <0.001 | 0.123 | 0.024-0.223 | 0.016 |
| Age | >55 years | 0.216 | 0.115-0.317 | <0.001 | 0.187 | 0.088-0.286 | <0.001 |
| Sex | Yes | 0.024 | -0.079-0.126 | 0.646 | | | |
| Smoking | Yes | 0.282 | 0.183-0.381 | <0.001 | 0.239 | 0.142-0.336 | <0.001 |
| Drinking | Yes | 0.003 | -0.100-0.107 | 0.950 | | | |
| Obesity | Yes | 0.083 | -0.020-0.186 | 0.115 | | | |
| T2DM | Yes | 0.147 | 0.044-0.249 | 0.005 | 0.036 | -0.066-0.137 | 0.491 |
| HLP | Yes | 0.124 | 0.022-0.227 | 0.018 | 0.037 | -0.060-0.134 | 0.452 |
| SAHS | Yes | 0.185 | 0.084-0.287 | <0.001 | 0.211 | -0.045-0.467 | 0.106 |
| Hypoxemia | Yes | 0.156 | 0.054-0.258 | 0.003 | -0.084 | -0.340-0.173 | 0.522 |
| CREA | >92 µmol/l | 0.039 | -0.065-0.142 | 0.464 | | | |
| CRP | >6.8 mg/l | -0.069 | -0.172-0.034 | 0.191 | -0.068 | -0.164-0.027 | 0.160 |
| Hcy | >17.9
µmol/l | 0.016 | -0.088-0.119 | 0.767 | | | |
Discussion
The present study was the first to report that IL-37
mRNA expression was significantly increased in macrophages in
patients with hypertension, and that circulating IL-37 levels were
significantly increased in patients with hypertension and were
positively correlated with SBP and DBP. Although non-dipper
hypertension did not affect plasma IL-37 levels, smoking, T2DM and
CAP were able to promote IL-37 expression. IL-37 levels were also
positively correlated with CREA, CRP and Hcy levels in patients
with hypertension, and elevated IL-37 levels were independently
associated with the presence of CAP.
Numerous clinical studies have confirmed that IL-37
expression is elevated in a variety of cardiovascular diseases. In
an early study, IL-37 levels were observed to be increased in the
plasma and arteries of patients with arterial calcification
(22). Subsequently, plasma IL-37
levels were indicated to be increased in patients with aortic
calcification and aortic valve calcification (23,24).
In addition, IL-37 levels were elevated in patients with chronic
heart failure and higher IL-37 levels suggested poor prognosis
(25). Furthermore, substantial
evidence has indicated that the plasma concentration of IL-37 was
significantly increased in patients with ischemic cardiomyopathy,
including acute coronary syndrome and acute ST-segment elevation
myocardial infarction after percutaneous coronary intervention
(26-29).
Plasma levels of IL-37 were gradually increased in patients with
paroxysmal atrial fibrillation, persistent atrial fibrillation and
permanent atrial fibrillation, while the IL-37 levels in all groups
were significantly higher than those in the normal group (30). In the present study, the circulating
IL-37 concentration was significantly increased in patients with
hypertension. These studies may indicate that IL-37 is involved in
the development of hypertension. The results also reaffirm the
close association between IL-37 and cardiovascular disease.
It is well known that there is a close association
between hypertension and immune cells (20,31).
Similarly, the differentiation and maturation of immune cells may
promote the secretion of various inflammatory factors, which is
closely linked to hypertension development (15,31,32).
Combined with a previous result by our group indicating that IL-37
may have a regulatory role in the differentiation and maturation of
various immune cells and the release of inflammation-associated
factors (13,33), it was hypothesized that IL-37 may be
involved in the process of hypertension through regulation of
inflammatory effects, similar to those that occur in other
cardiovascular diseases. The specific role and mechanisms remain to
be fully elucidated and still require to be determined in both
animal hypertension studies and clinical experiments.
Hypertension may be divided into dipper hypertension
and non-dipper hypertension depending on how much the blood
pressure drops at night. Usually, a blood pressure drop of >10%
at night is referred to as dipper hypertension, which is normal,
while a blood pressure drop of <10% at night is called
non-dipper hypertension, which is abnormal. Several studies have
demonstrated that patients with non-dipper hypertension exhibit
severe heart, brain and kidney injury, more clinical complications
and higher all-cause mortality than patients with dipper
hypertension, and this distinction may help predict the prognosis
of patients with hypertension (32,34).
In the present study, the incidence of CAP was indicated to be
significantly lower in patients with dipper hypertension than in
patients with non-dipper hypertension. The plasma IL-37 levels in
patients with dipper hypertension and non-dipper hypertension were
also compared. Of note, there was no significant difference in
IL-37 levels between the two groups. These results may suggest that
the presence of non-dipper hypertension is associated not with the
elevation in circulating IL-37 levels but with other causes. In
fact, a previous study reported that the occurrence of non-dipper
hypertension was linked to the abnormal increase in blood pressure
caused by nocturnal sympathetic nerve excitation (35). These results partially support the
hypothesis of the present study.
Numerous clinical characteristics, including
smoking, drinking, diabetes, obesity and hyperlipidemia, have been
demonstrated to be associated with inflammatory responses and have
been indicated to regulate the release of various cytokines,
including IL-37 (36,37). These clinical characteristics may
promote complex inflammatory responses that may mediate the release
of IL-37 by immune cells. To investigate the effects of these
clinical characteristics on IL-37 release in patients with
hypertension, the patients were divided into two groups based on
whether they had these clinical factors and the results suggested
that the IL-37 levels were higher in hypertensive patients who
smoked, as well as those with T2DM and with CAP. The IL-37 levels
were also closely linked to the onset of CAP. These results suggest
that elevated IL-37 may have a critical role in inflammatory
responses mediated by factors such as smoking and T2DM. As an
anti-inflammatory cytokine, IL-37 is increased in a variety of
chronic inflammatory diseases. One possible explanation is that
IL-37 is increased as a form of feedback to prevent damage mediated
by the inflammatory response, just as brain natriuretic peptide is
increased in patients with chronic heart failure (38).
There are several limitations to the present study.
First, the present study had a single-center design and in the
future, studies including samples from multiple centers should be
performed. Furthermore, no follow-up was performed to observe the
occurrence of complications and determine the impact of IL-37 on
prognosis in these patients with hypertension.
In conclusion, the present study indicated that
plasma IL-37 levels were increased in patients with hypertension
and were positively correlated with blood pressure. IL-37 was also
independently associated with the presence of CAP. IL-37 may be a
novel therapeutic target for preventing and treating hypertension
and prevent organ damage.
Supplementary Material
Clinical characteristics of subgroups
of patients with hypertension and control subjects.
Data from ambulatory blood pressure
monitoring in patients with hypertension and control subjects.
Acknowledgements
The authors would like to thank Dr Jiao Lan from the
Molecular Biology Laboratory of Guangxi Zhuang Autonomous Region
People's Hospital for their technical support on dendritic cells
stimulation and differentiation.
Funding
Funding: This work was supported by the National Natural Science
Foundation of China (grant nos. 81560085 and 81770472 to QJ and
grant no. 81760051 to YL).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL, JW and QJ conceived and designed the study; JY
and YW collected the samples; JY, YW, ZW, YL, LL, QZ, MW, YX, DY,
JZ, JW and QJ (all authors) performed the experiments; ZW and QZ
analyzed the data; MW and YX were involved in drafting the
manuscript or revising it critically for important intellectual
content; DY and JZ reviewed and edited the manuscript; QJ and JW
gave final approval of the version to be published. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study conformed to the guidelines approved by
the Ethics Committee of the People's Hospital of Guangxi Zhuang
Autonomous Region (Nanning, China; approval no. 2015-16 of the
National Natural Science Foundation of China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Laurent S and Boutouyrie P: The structural
factor of hypertension: Large and small artery alterations. Circ
Res. 116:1007–10021. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Guzik TJ and Touyz RM: Oxidative stress,
inflammation, and vascular aging in hypertension. Hypertension.
70:660–667. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shirakabe A, Ikeda Y, Sciarretta S,
Zablocki DK and Sadoshima J: Aging and autophagy in the heart. Circ
Res. 118:1563–1576. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Boraschi D, Lucchesi D, Hainzl S, Leitner
M, Maier E, Mangelberger D, Oostingh GJ, Pfaller T, Pixner C,
Posselt G, et al: IL-37: A new anti-inflammatory cytokine of the
IL-1 family. Eur Cytokine Netw. 22:127–147. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Busfield SJ, Comrack CA, Yu G, Chickering
TW, Smutko JS, Zhou H, Leiby KR, Holmgren LM, Gearing DP and Pan Y:
Identification and gene organization of three novel members of the
IL-1 family on human chromosome 2. Genomics. 66:213–216.
2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dinarello CA, Nold-Petry C, Nold M, Fujita
M, Li S, Kim S and Bufler P: Suppression of innate inflammation and
immunity by interleukin-37. Eur J Immunol. 46:1067–1081.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nold-Petry CA, Lo CY, Rudloff I, Elgass
KD, Li S, Gantier MP, Lotz-Havla AS, Gersting SW, Cho SX, Lao JC,
et al: IL-37 requires the receptors IL-18Rα and IL-1R8 (SIGIRR) to
carry out its multifaceted anti-inflammatory program upon innate
signal transduction. Nat Immunol. 16:354–365. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Nold MF, Nold-Petry CA, Zepp JA, Palmer
BE, Bufler P and Dinarello CA: IL-37 is a fundamental inhibitor of
innate immunity. Nat Immunol. 11:1014–1022. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Zhuang X, Wu B, Li J, Shi H, Jin B and Luo
X: The emerging role of interleukin-37 in cardiovascular diseases.
Immun Inflamm Dis. 5:373–379. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Jia H, Liu J and Han B: Reviews of
interleukin-37: Functions, receptors, and roles in diseases. Biomed
Res Int. 2018(3058640)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ji QW, Meng K, Yu KW, Huang S, Huang Y,
Min XH, Zhong YC, Wu BW, Liu YZ, Nie SP, et al: Exogenous
interleukin 37 ameliorates atherosclerosis via inducing the Treg
response in ApoE-deficient mice. Sci Rep. 7(3310)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu J, Lin J, He S, Wu C, Wang B, Liu J,
Duan Y, Liu T, Shan S, Yang K, et al: Transgenic overexpression of
IL-37 protects against atherosclerosis and strengthens plaque
stability. Cell Physiol Biochem. 45:1034–1050. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu T, Liu J, Lin Y, Que B, Chang C, Zhang
J, Liang Z, Gao X, Liu S, Liu L, et al: IL-37 inhibits the
maturation of dendritic cells through the IL-1R8-TLR4-NF-κB
pathway. Biochim Biophys Acta Mol Cell Biol Lipids. 1864:1338–1349.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hoeke G, Khedoe PP, van Diepen JA,
Pike-Overzet K, van de Ven B, Vazirpanah N, Mol I, Hiemstra PS,
Staal FJ, Stienstra R, et al: The effects of selective
hematopoietic expression of human IL-37 on systemic inflammation
and atherosclerosis in LDLr-deficient mice. Int J Mol Sci.
18(1672)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhu R, Sun H, Yu K, Zhong Y, Shi H, Wei Y,
Su X, Xu W, Luo Q, Zhang F, et al: Interleukin-37 and dendritic
cells treated with interleukin-37 plus Troponin I ameliorate
cardiac remodeling after myocardial infarction. J Am Heart Assoc.
5(e004406)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wu B, Meng K, Ji Q, Cheng M, Yu K, Zhao X,
Tony H, Liu Y, Zhou Y, Chang C, et al: Interleukin-37 ameliorates
myocardial Ischaemia/reperfusion injury in mice. Clin Exp Immunol.
176:438–451. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ji Q, Cheng G, Ma N, Huang Y, Lin Y, Zhou
Q, Que B, Dong J, Zhou Y and Nie S: Circulating Th1, Th2, and Th17
levels in hypertensive patients. Dis Markers.
2017(7146290)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Postel-Vinay N, Steichen O, Pébelier E,
Persu A, Berra E, Bobrie G, Savard S, Nogueria J and Azizi M: Home
blood pressure monitoring and e-Health: Investigation of patients'
experience with the Hy-Result system. Blood Press Monit.
25:155–161. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rimoldi SF, Scherrer U and Messerli FH:
Secondary arterial hypertension: When, who, and how to screen? Eur
Heart J. 35:1245–1254. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ye J, Que B, Huang Y, Lin Y, Chen J, Liu
L, Shi Y, Wang Y, Wang M, Zeng T, et al: Interleukin-12p35 knockout
promotes macrophage differentiation, aggravates vascular
dysfunction, and elevates blood pressure in angiotensin II-infused
mice. Cardiovasc Res. 115:1102–1113. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yu K, Min X, Lin Y, Huang Y, Huang S, Liu
L, Peng Y, Meng K, Li D, Ji Q and Zeng Q: Increased IL-37
concentrations in patients with arterial calcification. Clin Chim
Acta. 461:19–24. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chai M, Zhang HT, Zhou YJ, Ji QW, Yang Q,
Liu YY, Zhao YX, Shi DM, Liu W, Yang LX, et al: Elevated IL-37
levels in the plasma of patients with severe coronary artery
calcification. J Geriatr Cardiol. 14:285–291. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kapelouzou A, Kontogiannis C, Tsilimigras
DI, Georgiopoulos G, Kaklamanis L, Tsourelis L and Cokkinos DV:
Differential expression patterns of toll like receptors and
interleukin-37 between calcific aortic and mitral valve cusps in
humans. Cytokine. 116:150–160. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shou X, Lin J, Xie C, Wang Y and Sun C:
Plasma IL-37 elevated in patients with chronic heart failure and
predicted major adverse cardiac events: A 1-year follow-up study.
Dis Markers. 2017(9134079)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ji Q, Zeng Q, Huang Y, Shi Y, Lin Y, Lu Z,
Meng K, Wu B, Yu K, Chai M, et al: Elevated plasma IL-37, IL-18,
and IL-18BP concentrations in patients with acute coronary
syndrome. Mediators Inflamm. 2014(165742)2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang X, Cai X, Chen L, Xu D and Li J: The
evaluation of plasma and leukocytic IL-37 expression in early
inflammation in patients with acute ST-Segment elevation myocardial
infarction after PCI. Mediators Inflamm.
2015(626934)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yang T, Fang F, Chen Y, Ma J, Xiao Z, Zou
S, Zheng N, Yan D, Liao S, Chen S, et al: Elevated plasma
interleukin-37 playing an important role in acute coronary syndrome
through suppression of ROCK activation. Oncotarget. 8:9686–9695.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mao X, Zhu R, Zhang F, Zhong Y, Yu K, Wei
Y, Sun H, Xu W, Luo Q, Wang Y, et al: IL-37 plays a beneficial role
in patients with acute coronary syndrome. Mediators Inflamm.
2019(9515346)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li W, Li S, Li X, Jiang S and Han B:
Interleukin-37 elevation in patients with atrial fibrillation. Clin
Cardiol. 40:66–72. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kirabo A, Fontana V, de Faria AP, Loperena
R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, et
al: DC isoketal-modified proteins activate T cells and promote
hypertension. J Clin Invest. 124:4642–4656. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Aksit E, Gursul E, Aydin F, Samsa M and
Ozcelik F: Non-dipper hypertension is associated with slow coronary
flow among hypertensives with normal coronary angiogram. Cardiovasc
J Afr. 28:14–18. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ji Q, Meng K, Yu K, Huang S, Huang Y, Min
X, Zhong Y, Wu B, Liu Y, Nie S, et al: Exogenous interleukin 37
ameliorates atherosclerosis via inducing the Treg response in
ApoE-deficient mice. Sci Rep. 7(3310)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen Y, Liu JH, Zhen Z, Zuo Y, Lin Q, Liu
M, Zhao C, Wu M, Cao G, Wang R, et al: Assessment of left
ventricular function and peripheral vascular arterial stiffness in
patients with dipper and non-dipper hypertension. J Investig Med.
66:319–324. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ragot S, Herpin D, Siché JP, Ingrand P and
Mallion JM: Autonomic nervous system activity in dipper and
non-dipper essential hypertensive patients. What about sex
differences? J Hypertens. 17:1805–1811. 1999.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Di Stefano A, Caramori G, Barczyk A,
Vicari C, Brun P, Zanini A, Cappello F, Garofano E, Padovani A,
Contoli M, et al: Innate immunity but not NLRP3 inflammasome
activation correlates with severity of stable COPD. Thorax.
69:516–524. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Borzouei S, Sheikh V, Ghasemi M, Zamani A,
Telikani Z, Zareighane Z, Salehi I, Mozayanimonfared A, Amirzargar
MA and Alahgholi-Hajibehzad M: Anti-inflammatory effect of combined
sitagliptin and vitamin D3 on cytokines profile in patients with
type 2 diabetes mellitus. J Interferon Cytokine Res. 39:293–301.
2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ye J, Wang Z, Ye D, Wang Y, Wang M, Ji Q,
Huang Y, Liu L, Shi Y, Shi L, et al: Increased interleukin-11
levels are correlated with cardiac events in patients with chronic
heart failure. Mediators Inflamm. 2019(1575410)2019.PubMed/NCBI View Article : Google Scholar
|