Introduction
Researchers consider the heart to be an organ that
regenerates among the last in mammalian systems. It was previously
considered as a post-mitotic organ (1,2).
Recent theories state that the heart is characterized by
cardiomyocyte turnover throughout life, although these properties
are insufficient for restoration in cases of heart disease or after
heart injury (3).
Once the cardiomyocytes complete their
differentiation and interrupt their mitotic activity, which occurs
in the first days after birth, the regenerative capacity of the
heart suddenly decreases (4-7).
The extracellular matrix (ECM) is composed of
fibrillary (fibrillar collagen) and non-fibrillary components
(basement membrane, proteoglycans and glycoproteins), which all
have structural and signaling functions. The ECM provides support
and anchorage for the shape of the cells and regulates cell
dynamics (8).
From a spatial point of view, ECM contains two main
areas, the basement membrane/pericellular matrix and the
interstitial matrix. The interstitial matrix is organized into
three interconnected levels as follows: the epimysium that wraps
the entire organ, the perimysium that forms major bundles of
myofibers and the endomysium that surrounds individual
cardiomyocytes (3,9).
The collagen matrix is mainly composed of collagen
type I (Col-1; >80%) and Col-3 (>10%), which are fixed to the
basement membranes of the cardiomyocytes via Col-4 and fibronectin
(FN) (10). The ECM anchors
chemokines, cytokines, growth factors, proteases [such as matrix
metallopeptidases (MMPs)], proteases inhibitors [such as tissue
inhibitors of metalloproteinases (TIMPs)], and noncoding RNAs (such
as microRNAs) (3,11).
The cardiac interstitium is mainly composed of
rod-like thick fibers, located both in the epimysium and the
perimysium. These fibers are composed of Col-1, while a fine
network of fibers is formed by Col-3, which are more prominent in
the endomysium (9).
MMPs represent a large group of zinc-dependent
endopeptidases, which are initially synthesized as zymogens
(12). The gelatinases MMP-2 and
MMP-9 can be found in cardiac myocytes, cardiac fibroblasts and
endocardial cells. The activity of MMPs is inhibited by TIMPs
(9).
Following cardiac injury and cardiomyocyte death,
cardiac repair cascade is initiated by cellular responses,
secondary to dynamic changes in the composition of the ECM
(13). The restoration process
consists of the following three steps: A first phase of
inflammation, a second proliferative phase and a final maturation
phase. Inflammatory mediators, such as cytokines and chemokines, are
subsequently released from the serum into the interstitial matrix,
which leads to leukocyte recruitment and activation of the
neutrophils. Inflammatory mediators increase vascular permeability,
which is followed by extravasation of plasma proteins, including
fibrin, fibrinogen and FN. Simultaneously, MMPs expression and
activity are increased (3). The
consequence is the formation of provisional ECM with abundant
growth factors, to which fibroblasts adhere, inducing therefore
fibroblast proliferation and transdifferentiation.
Removal of dead cells and ECM residues by phagocytic
activity induces the spill of anti-inflammatory mediators, which is
useful in solving the inflammatory phase, thus delimiting the
transition to the proliferative phase (14).
Mononuclear cells and macrophages secrete growth
factors during the proliferative phase of infarct healing. Large
amounts of structural ECM proteins synthesized by myofibroblasts and
activated by growth factors modulate several important cell
functions such as cell survival, proliferation, polarity,
differentiation, adhesion and migration. These proteins also serve
key roles in matrix assembly and myocardial protection from adverse
remodeling. The best-known growth factors active during this phase
are the following: Tenascin-C (Tn-C), Tn-X, thrombospondin,
secreted protein acidic and rich in cysteine, osteopontin,
osteoglycin, periostin and cellular communication network (13,15,16).
Three forms of cardiac fibrosis are recognized,
reflecting distinct mechanisms of fibrotic remodeling. These are
the replacement fibrosis, interstitial fibrosis and perivascular
fibrosis (9). The replacement
fibrosis describes the formation of a scar in zones with myocardium
necrosis, representing the consequence of a replacement process
that is secondary to primary cardiomyocyte lesion, such as
following myocardial infarction. Interstitial fibrosis occurs in
the case of different injurious stimuli, including a pressure load,
metabolic dysfunction and aging (9).
Materials and methods
Patient selection
From a database of 100 patients with ischemic heart
diseases, a small study batch of 10 cases consisting of 5 men and 5
women (age range, 59-89 years; mean age, 74.8±7.62 years; sex
ratio, 1:1) with myocardial infarcts of various ages were selected
for microscopic investigation of the ECM between January 2016 and
December 2020. The tissues were collected at autopsy in ‘Sf.
Pantelimon’ Hospital. The present study fulfilled the ethical
criteria of the World Medical Association Declaration of Helsinki.
All tissue specimens were harvested in accordance with the
legislation of our country and the study protocol was previously
approved by the Bioethics Committee of St. Pantelimon Hospital. All
patients provided written informed consent at the admission in the
hospital.
Histopathology investigation
Heart tissue samples were subjected to
histopathological examination. The tissues were collected at
autopsy from different parts of the left ventricle, such as the
anterior and the lateral walls. The samples were fixed in 10%
neutral buffered formalin (pH 7) for 24-48 h at room temperature
and paraffin embedded. Tissues were cut into 5-µm sections that
were stained by hematoxylin and eosin and van Gieson. Hematoxylin
and eosin staining is the standard staining for tissues and was
performed in order to obtain the permanent microscopic slide. The
staining consists of 2 dyes, namely Meyer hemalaun (a base dye) and
Gelbich eosin (an acidic dye), which are used to stain in
blue-violet the cell nucleus and in red-pink the cell cytoplasm,
respectively. For standard hemalaun & eosin, the staining takes
place at room temperature and briefly, the main steps are the
following: staining with Meyer hemalaun (10 min), washing in tap
water, washing in 1-3 cm3 saturated solution of
LiCO3 (a few seconds), staining with eosin (2-3 min),
washing in distilled water and ethanol (90%), dehydration in
ethanol (95%), ethanol (100%), xylene and mounting the slides. Van
Gieson stain is a trichrome stain for tissues. It is composed of 3
dyes: Weigert hematoxylin, picric acid and acidic fuxin. The
nucleus stains black, cytoplasm stains yellow and collagen stains
red. The stain is used in light microscopy to highlight the
connective tissue, particularly collagen fibers. For Van Gieson,
the staining takes place at room temperature and briefly, the main
steps are the following: staining with Weigert hematoxylin (15
min), differentiation in chlorohydric alcohol (a few seconds),
washing in LiCO3, staining with pycrofucsin (30 sec-1
min), washing in acidic water (few seconds), washing in distilled
water, dehydration in ethanol, mounting the slides. Multiple
sections were prepared per sample and histopathologically examined.
Additional slices (3 µm) of tissue have also been prepared for IHC
analysis.
IHC analysis was performed for Col-1 (clone, Col-1;
1:100; Sigma-Aldrich; Merck KGaA), Tn-C (clone, 49; 1:100; Leica
Microsystems GmbH), MMP-9 (clone, 15W2; 1:400; Leica Microsystems
GmbH), CD34 (clone, Qbend; ready to use; CellMarque™), CD68 (clone,
KP-1; RTU; CellMarque™) using sections placed on glass slides that
were previously treated with poly-L-lysine. IHC was performed for 3
µm-thick sections (formalin-fixed paraffin-embedded). The method
used was an indirect tristadial Avidin-Biotin-Complex technique,
using a NovoLink Polymer detection system, which utilizes a novel
control polymerization technology to prepare polymeric HRP-linker
antibody conjugates, according to the manufacturer's specifications
(Novocastra). Antigen retrieval technique (enzymatic pre-treatment)
was performed, according to the technical specifications from the
producer. The steps were as follows: deparaffinization in xylene
for 15 min, rehydration in ethanol series (100%-5 min, 96%-5 min,
70%-5 min), washing in PBS, incubation with normal serum (200 µl,
Cell Marque) for 20 min, incubation with primary antibody
overnight, standard labeled streptavidin-biotin complex (ready to
use, Cell Marque), washing in carbonate buffer and development in
3-3'-DAB hydrochloride. All steps were performed at room
temperature.
All slides were examined and images were taken using
a Leica MC190 HD microscope (Leica Microsystems GmbH;
magnification, x100). Images were acquired using an incorporated
software program and were further processed and analyzed using
Microsoft Office Picture Manager running under Windows 10.
Results
The histopathological examinations demonstrated
various degrees of diffuse or focal interstitial and perivascular
fibrosis, due to collagen deposition, along with cardiomyocyte
degeneration.
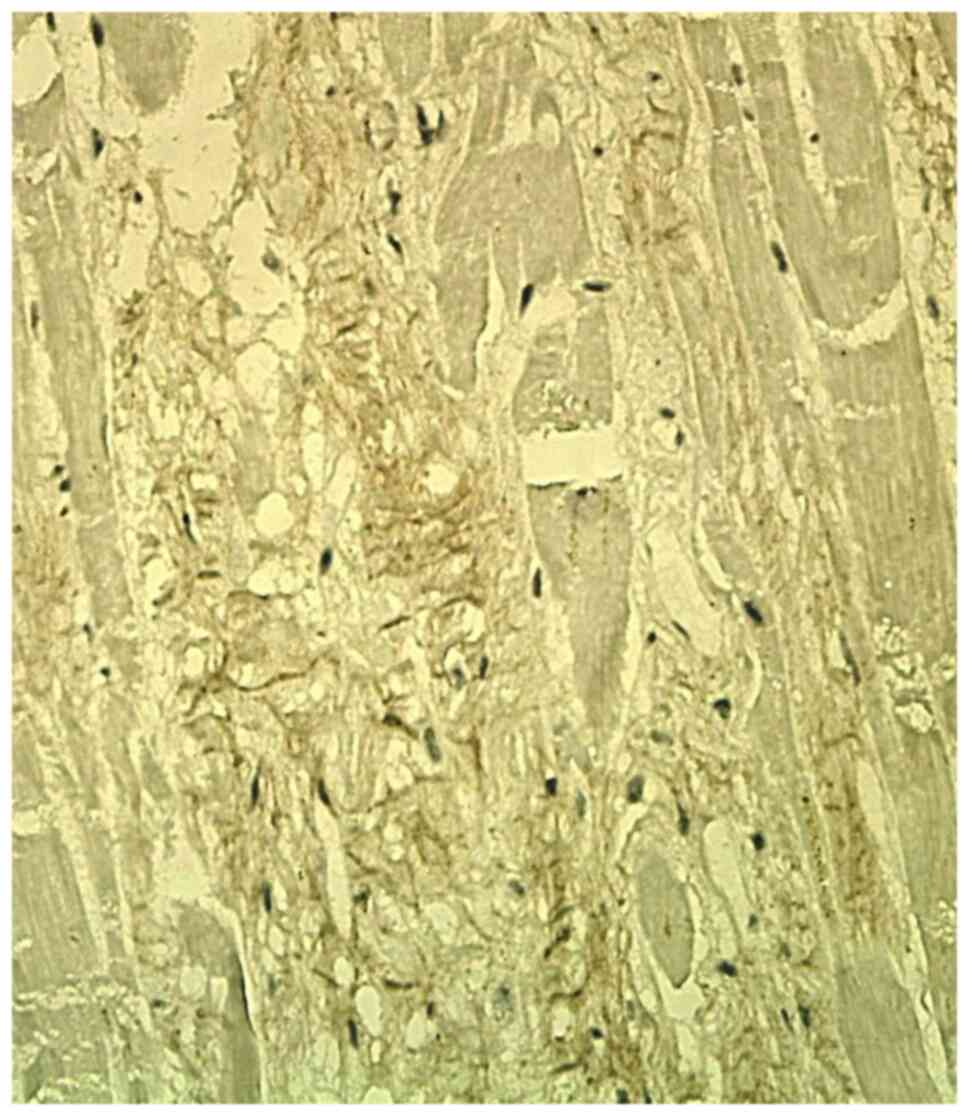
Col-1 staining was focally positive in the
interstitium, in the scarring areas and in the residual areas
adjacent to the myocardial scar. It showed a variable expression in
the ECM, with a continuous reticular pattern, in the form of a
fibrillar plexiform network (Fig.
1). In recent myocardial infarction, there was no IHC
expression of the Col-1 in the ECM.
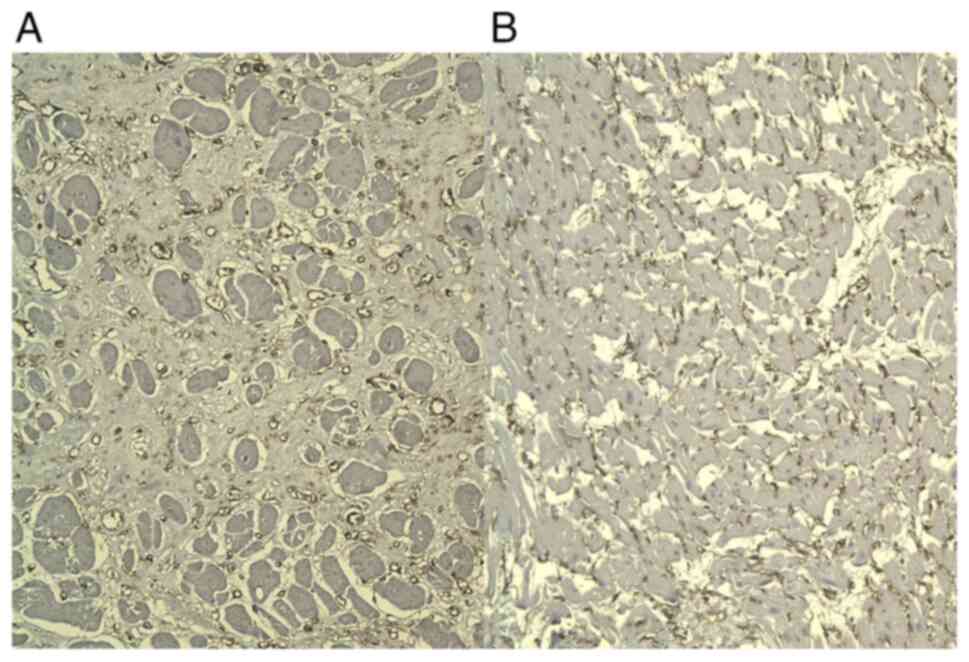
Tn-C staining was focally positive in subendocardial
or subepicardial areas of the ECM. Tn-C was expressed in the ECM in
recently infarcted areas and in adjacent residual areas of
myocardium. It showed a discontinuous variable network with a
reticular pattern (Fig. 2).
MMP9 staining was negative in the ECM, in all layers
of the heart, regardless of the age of the infarction.
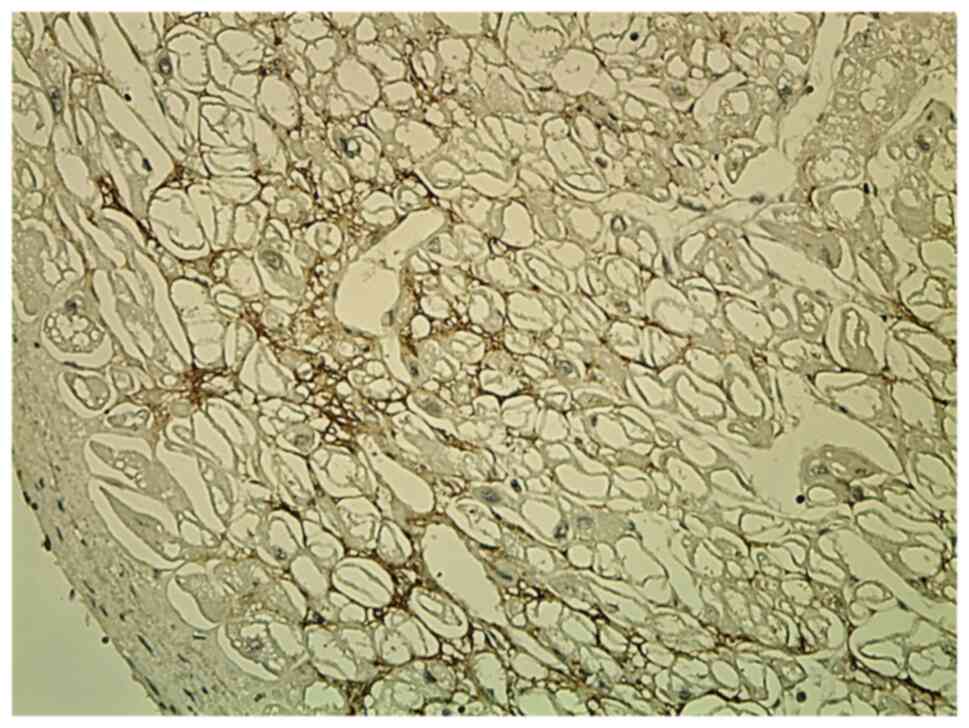
CD34 staining showed diffuse strong immunoreaction
in newly formed capillary vessels, in the adjacent areas of the
infarction and in the scarring areas, thus demonstrating a high
micro-vascular density (Fig.
3).
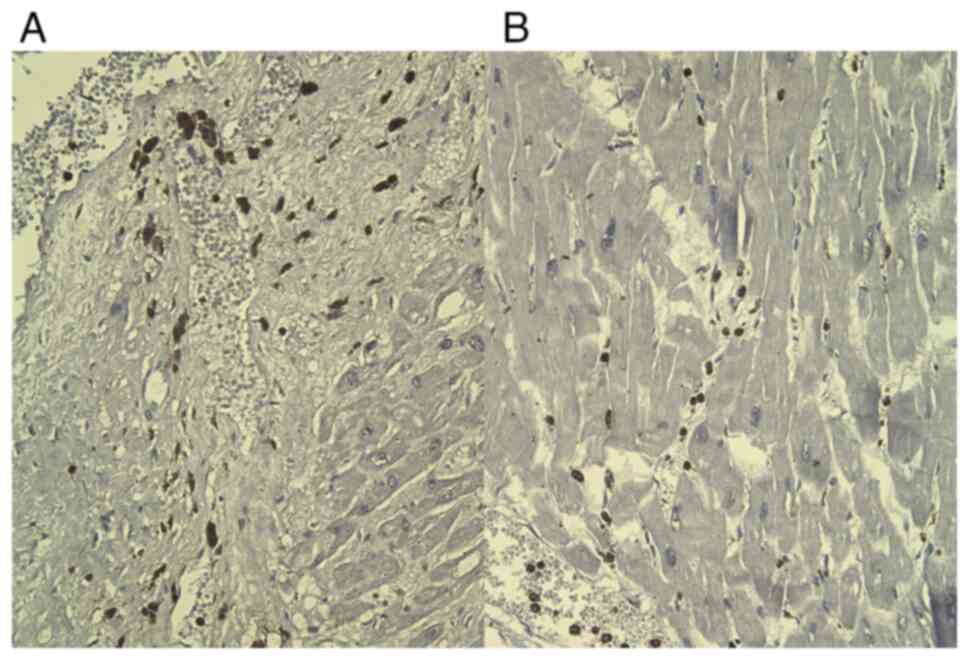
CD68 staining was positive in frequent reactive
histiocytes, located in interstitial and perivascular areas of the
necrotic areas (Fig. 4), but also
into the interstitium of the adjacent normal myocardium.
There was no correlation between the aforementioned
markers, which were therefore independent from each other.
Discussion
Col-1 deposition is difficult to be repaired in
conditions of myocardial infarction, as it provides a mechanically
strong network for maintaining integrity, minimizing infarct
extension and resistance to maladaptive remodeling. The synthesis
of Col-1 requires the expression of pro-α1 and pro-α2 collagen
chains encoding genes, intracellular assembly of the protein and
secretion of procollagen I, which will be cleaved outside the cell
and assembled into triple helical fibrils (Col-1). Accumulation of
Col-1 can be degraded by degradation of interstitial collagenases
and MMP-1, MMP-8, MMP13, MMP2, and membrane type 1
MMP/MMP-14(17).
The ECM glycoprotein Tn-C is found only in the first
stages of embryonic development. Usually, Tn-C is not expressed in
the adult heart, but it reappears transiently in conditions of
active tissue remodeling in distinct areas of the heart (18). Tn-C is considered to be involved in
improper left ventricular remodeling, although the exact underlying
mechanism of cardiac dysfunction involving Tn-C remains unclear
(19).
Cardiac tissue remodeling following myocardial
ischemia may be accompanied by an overexpression of Tn-C variants.
Serum level of Tn-C was reported to be significantly increased in
patients with acute myocardial infarction compared with healthy
subjects. High serum levels of Tn-C are also correlated with
unfavorable prognostic outcome, in the case of left ventricular
hypertrophy and major adverse cardiac events (20). A high tissue level of Tn-C was
demonstrated in thrombosis, atherosclerotic plaques or stenosis of
coronary artery, and bypass-grafts (21-24).
MMP-7 is the metalloproteinase expressed in
cardiomyocytes, endothelial cells and macrophages. In animal models
of myocardial infarction, the level of MMP-7 increases three-fold
at 7 days following infarction, both in ischemic and remote regions
(25,26). The high level of MMP-7 activity is
associated with an increased risk for major adverse cardiac events,
including low survival rate post-myocardial infarction and
increased hospitalization period for patients with congestive heart
failure (27). Furthermore, high
serum MMP-7 level has been demonstrated to be associated with left
ventricular structural remodeling in 144 patients with left
ventricular hypertrophy (28).
MMP-7 includes a wide variation of target
substrates, such as Col-4, FN, Tn-C, connexin-43, peroxiredoxin,
laminin and tumor necrosis factor-α (29). MMP-7 can also degrade other MMPs,
including MMP-1, MMP-2, and MMP-9, leading to their activation and
thus suggesting that MMP-7 might be a direct and indirect regulator
for left ventricular remodeling (27). In addition, MMP-7 has major effects
on connexin-43 and plays an important role in arrhythmias that
appear post infarction (30). Both
collagenases (MMP-1) and gelatinases, such as MMP-2 and MMP-9,
present high levels in patients with acute ischemic myocardium
(31).
Other targets for MMPs also exist that are located
intracellularly and are involved in protein degradation, including
α-actinin, titin and myosin (16).
In suffering or remodeled tissues, fragmentation of matrix proteins
leads to the release of matrikines. Rapid activation of MMPs in
ischemic conditions leads to a rapid matrix fragmentation. However,
the functional role of these fragments, which act as bioactive
proinflammatory matrikines, remains unclear.
Previous studies demonstrated that in the first 30
min following coronary ischemia, the serum level of Col-1 fragments
increases (32,33). In the infarcted myocardium,
fragmentation of constituents of the basement membrane, such as
Col-4, and of non-collagenous matrix components also takes place
(16,34).
The CD68+ macrophages serve two roles in
heart remodeling under ischemic conditions, a fibrogenic role and
an angiogenic role. Furthermore, macrophages could also contribute
to ECM remodeling by producing MMPs (35).
At an early stage of ischemia, macrophages express
certain heterogeneity, gaining regulatory, fibrogenic or angiogenic
phenotypes. At a later stage, turnover of macrophages in the
ischemic zones depends on proliferation. Subsequent expansion of
macrophage population in viable zones is stimulated by chemokines,
as a consequence of a high wall stress. Activation of macrophages
in the vascularized aria of the myocardium may lead to development
and progression of heart failure (36,37).
In summary, the ECM represents a polymorphic
microenvironment with its own dynamics that is in a continuous
change, involving a large spectrum of heterogeneous molecules,
which play different roles in myocardium remodeling under hypoxic
ischemic conditions.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data used and/or analyzed in the current study
are available from the corresponding author on reasonable
request.
Authors' contributions
ZC and MiC performed the histological examinations
and immunohistochemistry, and provided major contributions in
writing the manuscript. BS and MaC analyzed and interpreted the
data from patient. GPG and MP searched the literature for similar
work and articles and contributed to writing the manuscript. ZC and
BS confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study fulfilled the ethical criteria of
the World Medical Association Declaration of Helsinki. This study
was approved by the local Bioethics Committee from ‘Sf. Pantelimon’
Emergency Clinical Hospital (Bucharest, Romania). All patients have
previously signed the hospital's standard written informed consent
about admission, treatment and a possible future publication of
their data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bergmann O, Zdunek S, Felker A, Salehpour
M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T,
Dos Remedios C, et al: Dynamics of cell generation and turnover in
the human heart. Cell. 161:1566–1575. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Laflamme MA and Murray CE: Heart
regeneration. Nature. 473:326–335. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Silva AC, Pereira C, Fonseca ACRG,
Pinto-do-Ó P and Nascimento DS: Bearing my heart: The role of
extracellular matrix on cardiac development, homeostasis, and
injury response. Front Cell Dev Biol. 8(621644)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Quaini F, Urbanek K, Beltrami AP, Finato
N, Beltrami CA, Nadal-Ginard B, Kajstura J, Leri A and Anversa P:
Chimerism of the transplanted heart. New Engl J Med. 346:5–15.
2002.PubMed/NCBI
|
|
5
|
Porrello ER, Mahmoud AI, Simpson E, Hill
JA, Richardson JA, Olson EN and Sadek HA: Transient regenerative
potential of the neonatal mouse heart. Science. 331:1078–1080.
2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Notari M, Ventura-Rubio A, Bedford-Guaus
SJ, Jorba I, Mulero L, Navajas D, Marti M and Raya A: The local
microenvironment limits the regenerative potential of the mouse
neonatal heart. Sci Adv. 4(eaao5553)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhu W, Zhang E, Zhao M, Chong Z, Fan C,
Tang Y, Hunter JD, Borovjagin AV, Walcott GP, Chen JY, et al:
Regenerative potential of neonatal porcine hearts. Circulation.
138:2809–2816. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chute M, Aujla P, Jana S and Kassiri Z:
The non-fibrillar side of fibrosis: Contribution of the basement
membrane, proteoglycans, and glycoproteins to myocardial fibrosis. J
Cardiovasc Dev Dis. 6(35)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Frangogiannis N: The extracellular matrix
in ischemic and nonischemicheart failure. Circ Res. 125:117–146.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bashey RI, Martinez-Hernandez A and
Jimenez SA: Isolation, characterization, and localization of
cardiac collagen type VI. Associations with other extracellular
matrix components. Circ Res. 70:1006–1017. 1992.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fan D, Creemers EE and Kassiri Z: Matrix
as an interstitial transport system. Circ Res. 114:889–902.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
Structure, function, and biochemistry. Circ Res. 92:827–839.
2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dobaczewski M, Bujak M, Zymek P, Ren G,
Entman ML and Frangogiannis NG: Extracellular matrix remodeling in
canine and mouse myocardial infarcts. Cell Tissue Res. 324:475–488.
2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Murphy-Ullrich JE and Sage EH: Revisiting
the matricellular concept. Matrix Biol. 37:1–14. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kong P, Christia P and Frangogiannis NG:
The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 71:549–574.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Frangogiannis NG: The extracellular matrix
in myocardial injury, repair, and remodeling. J Clin Invest.
127:1600–1612. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Nong Z, O'Neil C, Le M, Gros R, Watson A,
Rizkalla A, Mequanint K, Li S, Frontini MJ, Feng Q and Pickering
JG: Type I collagen cleavage is essential for effective fibrotic
repair after myocardial infarction. Am J Pathol. 179:2189–2198.
2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Taki J, Inaki A, Wakabayashi H,
Imanaka-Yoshida K, Ogawa K, Hiroe M, Shiba K, Yoshida T and Kinuya
S: Dynamic expression of tenascin-C after myocardial ischemia and
reperfusion: Assessment by 125I-anti-tenascin-C antibody imaging. J
Nucl Med. 51:1116–1122. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gonçalves I, Acar E, Costantino S, Szabo
PL, Hamza O, Tretter EV, Klein KU, Trojanek S, Abraham D, Paneni F,
et al: Epigenetic modulation of tenascin C in the heart:
Implications on myocardial ischemia, hypertrophy and metabolism. J
Hypertens. 37:1861–1870. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Franz M, Jung C, Lauten A, Figulla HR and
Berndt A: Tenascin-C in cardiovascular remodeling: Potential impact
for diagnosis, prognosis estimation and targeted therapy. Cell Adh
Migr. 9:90–95. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Willems IE, Arends JW and Daemen MJ:
Tenascin and fibronectin expression in healing human myocardial
scars. J Pathol. 179:321–325. 1996.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ballard VL, Sharma A, Duignan I, Holm JM,
Chin A, Choi R, Hajjar KA, Wong SC and Edelberg JM: Vascular
tenascin-C regulates cardiac endothelial phenotype and
neovascularization. FASEB J. 20:717–719. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kenji K, Hironori U, Hideya Y, Michinori
I, Yasuhiko H and Nobuoki K: Tenascin-C is associated with coronary
plaque instability in patients with acute coronary syndromes. Circ
J. 68:198–203. 2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wallner K, Li C, Shah PK, Fishbein MC,
Forrester JS, Kaul S and Sharifi BG: Tenascin-C is expressed in
macrophage-rich human coronary atherosclerotic plaque. Circulation.
99:1284–1289. 1999.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Christia P and Frangogiannis NG: Targeting
inflammatory pathways in myocardial infarction. Eur J Clin Invest.
43:986–995. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cauwe B and Opdenakker G: Intracellular
substrate cleavage: A novel dimension in the biochemistry, biology
and pathology of matrix metalloproteinases. Crit Rev Biochem Mol
Biol. 45:351–423. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
DeLeon-Pennell K, Meschiari C, Jung M and
Lindsey ML: Matrix metalloproteinases in myocardial infarction and
heart failure. Prog Mol Biol Transl Sci. 147:75–100.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zile MR, Desantis SM, Baicu CF, Stroud RE,
Thompson SB, McClure CD, Mehurg SM and Spinale FG: Plasma
biomarkers that reflect determinants of matrix composition identify
the presence of left ventricular hypertrophy and diastolic heart
failure. Circ Heart Fail. 4:246–256. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Frangogiannis NG: Matricellular proteins
in cardiac adaptation and disease. Physiol Rev. 92:635–688.
2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lindsey ML, Escobar GP, Mukherjee R,
Goshorn DK, Sheats NJ, Bruce JA, Mains IM, Hendrick JK, Hewett KW,
Gourdie RG, et al: Matrix metalloproteinase-7 affects connexin-43
levels, electrical conduction, and survival after myocardial
infarction. Circulation. 113:2919–2928. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Danielsen CC, Wiggers H and Andersen HR:
Increased amounts of collagenase and gelatinase in porcine
myocardium following ischemia and reperfusion. J Mol Cell Cardiol.
30:1431–1442. 1998.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Villarreal F, Omens J, Dillmann W, Risteli
J, Nguyen J and Covell J: Early degradation and serum appearance of
type I collagen fragments after myocardial infarction. J Mol Cell
Cardiol. 36:597–601. 2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shinde AV and Frangogiannis NG:
Fibroblasts in myocardial infarction: A role in inflammation and
repair. J Mol Cell Cardiol. 10:74–82. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ceausu Z, Socea B, Dimitriu MC, Predescu
D, Constantin VD, Bacalbaşa N, Cîrstoveanu C, Costache M and Ceausu
M: Dormant cardiac stem cells: A promising tool in cardiac
regeneration. Exp Ther Med. 20:3452–3457. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen B and Frangogiannis NG: The role of
macrophages in nonischemic heart failure. JACC Basic Transl Sci.
3:245–248. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ceauşu Z, Socea B, Costache M, Predescu D,
Şerban D, Smarandache CG, Pacu I, Alexandru HH, Daviţoiu A,
Jacotă-Alexe F, et al: Fibroblast involvement in cardiac remodeling
and repair under ischemic conditions. Exp Ther Med.
21(269)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen B and Frangogiannis NG: Macrophages
in the remodeling failing heart. Circ Res. 119:776–778.
2016.PubMed/NCBI View Article : Google Scholar
|