Introduction
Multiple sclerosis (MS) is an inflammatory,
demyelinating and neurodegenerative disease of the central nervous
system (CNS) (1,2). It is estimated that a total of 2.8
million people live with MS worldwide (35.9 cases per 100,000
globally); representing a significant socioeconomic impact
(3). MS is the main cause of
disability in young adults (20-40 years old) and is one of the
leading causes of neurological disability in this age group
(4). Depression is a common and
significant comorbidity in MS (5),
whereby ~50% of patients with MS will experience major depression
and disease-related psychosocial challenges (6). Depression and the associated
symptoms, including fatigue and anxiety, are strong determinants of
a patients quality-of-life with MS (6), with depression in patients increasing
the risk of suicidal ideation and death by suicide in this
population (7,8). Treating depression in patients with
MS is a challenge and there is therefore an urgent need for
effective, safe and better-tolerated therapies for depression in
patients with MS (9).
Moreover, another disabling consequence of MS is
cognitive impairment (CI). This can affect 40-70% of patients at
any time throughout disease progression (10,11).
Attention, working memory and executive function are commonly
affected in patients with MS (12,13).
Disease-modifying therapies, memory-enhancing agents, physical
exercise and cognitive restraint have demonstrated limited and
inconsistent benefits (11,14).
As a result, efficient treatments for depression and CI in patients
with MS are urgently needed.
Rodent models to study MS are generally divided into
autoimmune, viral-induced and toxin-induced models (15). The cuprizone (CPZ) model has been
widely used to study demyelination and remyelination in the context
of MS (16), as it is capable of
recapitulating the demyelination-induced reorganization of brain
circuitry observed in patients with MS (16,17).
Previous CPZ studies have demonstrated memory impairment and
depression like-symptoms following a CPZ diet of 5-6 weeks
(18-20).
Hence, strategies that promote intrinsic repair of myelin may help
restore brain function and consequently alleviate depression and
improve cognition in animal models, and ultimately, patients with
MS.
In the present study the effects of a novel
technology that delivers low-intensity magnetic stimulation to
multiple cortical areas (21),
called low-field magnetic stimulation (LFMS), were investigated.
Over 25 years ago, a population of individuals with bipolar
disorder, reported mood improvements following a magnetic resonance
spectroscopic imaging procedure (22). Since then, numerous studies have
sought to investigate the ability of magnetic stimulation on
neuromodulation (23-26).
LFMS is of interest as a non-invasive neuromodulation therapy,
which has been shown to rapidly improve mood in patients diagnosed
with depression (27). In our
previous study, we demonstrated that 40 Hz LFMS restored cognitive
and motor functions in an animal model of traumatic brain injury
(28) and enhanced the
differentiation of oligodendrocyte progenitor cells in vitro
(29). More recently, LFMS
stimulation has been reported to promote myelin repair in the
prefrontal cortex (PFC) and improves cognition and depression-like
symptoms in a chronic CPZ mouse model (30). Together, these findings support the
hypothesis that LFMS will improve depressive symptoms and CI, while
restoring demyelination in an acute CPZ-induced mouse model of
MS.
In the present study, we aimed to investigate
locomotor activity, working memory, depression-like behavior and
adaptability following a 3- or 6-week CPZ diet with concurrent sham
or LFMS treatment. Following the behavioral analysis, the levels of
demyelination within the PFC and hippocampus (HPC) were examined
through immunostaining and immunoblotting for myelin-basic protein
(MBP).
Materials and methods
Animals
Female C57BL/6 mice (age, 7 weeks; weight, 18-20 g)
were purchased from Charles River Laboratories, Inc. and were
acclimatized for one week in the vivarium (12 h light-dark cycles;
22±0.5˚C at 60% humidity; ad libitum access to food and
water). As MS is predominantly diagnosed in women, we chose to
complete our experiments in female mice (31,32).
Furthermore, a previous study examining sex differences in a CPZ
model of C57BL/6 mice demonstrated no differences in terms of
demyelination (33). All animal
procedures were performed in accordance with the guidelines of the
Canadian Council on Animal Care (34,35)
and approved by the University of Saskatchewan's Animal Research
Ethics Board in 2016 (Saskatoon, Canada; approval no.
20160103).
Experimental design and CPZ
treatment
After the acclimatization period, mice were randomly
divided into four groups in a 2x2 experimental design: Normal diet
healthy mice with either sham treatment (CTL) or LFMS treatment
(LFMS) and CPZ-treated mice received either the sham treatment
(CPZ) or LFMS treatment (CPZ + LFMS). CPZ was purchased from
Sigma-Aldrich (Merck KGaA). The CPZ groups received rodent chow
supplemented with 0.2% CPZ for 3 or 6 weeks to induce acute
demyelination. The effect of LFMS treatment was studied at two time
points: i) After 3 weeks CPZ diet, marking the peak inflammatory
response within the brain; and ii) at the end of 6 weeks, when
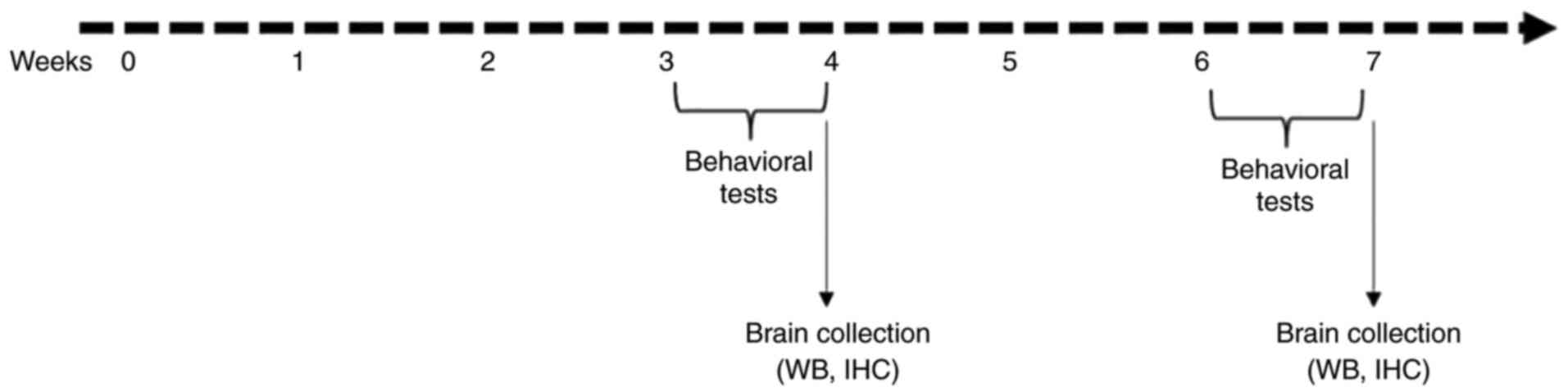
acute demyelination is fully established (36). At the end of week 3, half of the
mice from each group underwent behavioral tests followed by
euthanasia, the remaining animals were treated until the end of
week 6, at which point they underwent behavioral analysis and were
euthanized (the experimental design is presented in Fig. 1A). For immunohistochemistry, a deep
plane of surgical anesthesia was induced at 5% isoflurane and the
mice were maintained in this state with 2% isoflurane. Deep
anesthesia was confirmed by loss of pedal reflexes prior to the
perfusion of 1X PBS followed by 4% paraformaldehyde via
transcardiac injection through the left ventricle of the heart. The
animals were maintained in a deep surgical plane of anesthesia
until death was confirmed by cessation of breathing, no palpable
heartbeat and pale/blue/grey colored mucous membranes. For protein
extraction and western blotting, a deep plane of surgical
anesthesia was reached as described prior to decapitation, tissue
dissection and collection of the specified brain regions.
LFMS treatment
The LFMS device is composed of two 360 mm-diameter
coils and a control center that generates intermittent gamma
stimulation waves (Beijing Antis Biotech Co., Ltd.). The LFMS
parameter settings were based on our previous in vivo and
in vitro studies (28-30,37).
Briefly, the magnetic field alternated every 2 min between
approximate and linear gradients. Cycles consisting of 2 sec on and
8 sec off were applied for 20 min. Every 2-sec output was composed
of 80 trains of stimulation, producing a 40-Hz rhythm. Each train
had 6 pulses (6 msec) at 1,000 Hz frequency and 19 msec intervals.
The maximal magnetic flux density was less than 2 mT and the peaked
induced electric field was less than 0.5 V/m.
Following the removal of any metal components, mice
in their home cages were placed on the LFMS device and received
20-min of LFMS treatment daily, 5 days a week (Monday to Friday)
for 3 weeks and up to 6 weeks. Animals in the sham group underwent
the same treatment routine without LFMS stimulation.
Behavioral tests Open Field Test
(OFT)
The OFT is frequently used to assess a rodent's
locomotor activity and anxiety levels in an open space (38). Briefly, mice were placed in the
center of a white PVC plastic box (50x50x38 cm; built in house) and
allowed to freely explore the apparatus for 5 min. The surface of
the box was divided into 25 equal squares. In the middle, nine
squares were appointed as the central zone and the remaining
squares adjacent to the wall were designated the peripheral zone
using ANY-maze software (version 6.35; Stoelting Co.). The
following parameters were collected for each mouse during the test:
the time spent in the central and peripheral zones (sec), the total
distance travelled (m), the mean freezing score and the mean speed
velocity (mm/sec). The OFT data were quantified using ANY-maze
tracking system software.
Y-maze spontaneous alternation
test
The Y-Maze is widely used to evaluate spatial
working memory (39) and has been
previously used to detect memory deficits in CPZ models in numerous
studies (18,30,40,41).
Mice exhibiting normal behavior remember the arm they have already
explored and will enter one of the other arms of the maze (19). The Y maze apparatus was built in
house and previously described (19,30).
Briefly, each mouse was placed at the end of arm A and allowed to
freely explore all three arms for 5 min. The number and sequence of
arms entered was evaluated and the results were calculated as the
percentage of the spontaneous alternations (%)=(number of
alternations)/(total number of arm entries-2) x100(19). If a mouse re-entered an arm
immediately after exiting it but before entering another arm, it
was not counted as a separate entry and was not included in the
final analysis. If an animal entered <8 arms throughout the
duration of the test, the animal was excluded from statistical
analysis to avoid the potential effect that low numbers of entries
may have on the spontaneous alternation score (42). Distance travelled (m) was measured
during the test and used as a measure of mobility. The Y-maze data
were quantified using ANY-maze tracking system software.
Forced Swim Test (FST)
The FST is one of the most commonly used animal
models for assessing antidepression-like behavior (43) and has been used to test the
efficacy of existing and novel antidepressant drugs (44). The FST test is based on the
assumption that when a rodent is placed in a container filled with
water it will first attempt to escape, but will eventually stop or
become immobile, reflecting a measure of ‘behavioral despair’
(45). While there has been
controversy regarding the use of the forced swim test in mice
(46), numerous studies have
utilized this behavior assay in mice and have concluded that this
test can be used with good reliability (47,48).
Behavioral despair analysis is usually measured after one FST. The
FST can also be used to measure adaptive learned behavioral
responses, behaviors that promote survival and coping skill
abilities (49,50). Repeated exposure to the FST can
progressively increase the passive coping strategies evidenced by
floating behavior (immobility time) over time (49,50).
The shift from active (climbing and swimming) to passive (floating)
coping strategies is a normal response in rodents repeatedly
exposed to an inescapable water environment, where animals
deficient in their adaptive response will continue to struggle
(50).
On the third day of the week following the 3-week
treatment, mice were individually placed in a plexiglass cylinder
(10 cm internal diameter, 20 cm high) filled with water to a depth
of 10 cm (25-26˚C). Each mouse was allowed to swim for 6 min;
however, total immobility time was only recorded for the last 5 min
of the session to analyze depression-like behavioral despair.
Mobility time was measured using a stopwatch by researchers blinded
to experimental conditions. The mobile time was then subtracted
from the total test time of 300 seconds to determine the immobility
time. To analyze adaptive learned behavioral responses and
long-term memory coping skills, mice were tested 24 h after the
initial FST. A third and fourth test were run 24 h apart following
the completion of the 6-week treatment.
Immunohistochemistry
Perfused brains were cut into 30 µm sections
coronally using the Leica Vibrating Microtome (model VT1200; Leica
Microsystems, Inc.). Floating sections were quenched with 0.3%
hydrogen peroxide in 0.01 M PBS at room temperature (RT) for 30 min
to remove endogenous peroxidase activity. The sections were
subsequently incubated in 0.01 M PBS with 10% goat serum (Abcam)
blocking solution for 1 h at RT and then incubated overnight with
rabbit anti-myelin basic protein (MBP; 1:250; cat. no. 78896; Cell
Signaling Technology, Inc.) primary antibody diluted in the
blocking solution. After washing in 0.01 M PBS, the sections were
incubated with goat anti-rabbit IgG biotin-conjugated secondary
antibody (1:1,000; cat. no. BA-1000-1.5; Vector Laboratories, Inc.)
for 1 h at RT. The Avidin-Biotin Complex Kit (cat. no. PK-6100;
Vector Laboratories, Inc.) was used according to manufacturer's
instructions and visualized by incubating for 1-2 min at room
temperature in DAB chromogen (Sigma-Aldrich; Merck KGaA),
monitoring for color development.
Image analysis
All images were obtained using an Aperio Scanscope
CS Digital Pathology Scanner (Leica Microsystems Inc.). The
integrated optical density of MBP-positive staining was analyzed
using ImageJ software (version 1.51; National Institutes of
Health), which was calibrated using an optical density step tablet
(Stouffer Industries). Uniform areas within the images were
selected and the integrated density of the image was calculated and
compared between groups. A minimum of 20 coronal sections were
examined, with a minimum of 3 animals per group.
Western blotting
After the removal of the brain, one hemisphere from
randomly selected mice in each group was dissected into the PFC and
HPC using a brain matrix. Samples were then stored at -80˚C until
further use. Frozen brain samples were homogenized and total
protein was extracted using a Tris-EDTA lysis buffer (1% Triton
X-100, 10% glycerol, 20 mM Tris, pH 7.5, 1 mM EDTA) with a freshly
added protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA).
Protein quantification was completed using the Bio-Rad Protein
Assay (cat. no. 500-0006; Bio-Rad Laboratories, Inc.) as per
manufacturer's instructions. Equal quantities of protein (40 µg
protein/well) were separated on a 10% SDS-PAGE gel and transferred
onto a 0.45 µm nitrocellulose membrane (Bio-Rad Laboratories, Inc.)
prior to blocking in 5% milk in TBS for 1 h at room temperature.
β-actin was used as the internal protein loading control. Membranes
were incubated overnight at 4˚C with primary antibodies against
β-actin (1:1,000; cat. no. A3854; Sigma-Aldrich; Merck KGaA) and
MBP (1:1,000; cat. no. 78896; Cell Signaling Technology, Inc.)
diluted in 10% BSA in TBS + 0.1% Tween-20 (TBST; Sigma-Aldrich,
Merck KGaA). The membranes were washed in TBST, prior to incubation
for 1 h at room temperature with the appropriate HRP-conjugated
horse anti-mouse IgG (1:10,000; cat. no. 7076; Cell Signaling
Technology, Inc.) and goat anti-rabbit IgG (1:10,000; cat. no.
7074, Cell Signaling Technology, Inc.) secondary antibodies.
Protein bands were visualized using an ECL detection kit (Amersham;
Cytiva). Band densities were semi-quantified using ImageJ (version
1.51; National Institutes of Health), with MBP band intensities
normalized to the corresponding β-actin band intensity, prior to
being expressed as a ratio to the control (CTL) band.
Statistical analysis
Each animal was considered to be a single biological
replicate (the number of animals analyzed are indicated in the
figure legend for each assay). Data are presented as the mean ±
SEM. Statistical analysis was completed using GraphPad PRISM
software (version 8.0; GraphPad Software, Inc.) using unpaired
student's t-test or one- and two-way ANOVAs followed by Tukey's
post hoc test. Two-way ANOVA was used to evaluate the behavioral
tasks with factors of treatment (CTL, LFMS, CPZ and CPZ + LFMS) and
time (3- and 6-weeks). For immunohistochemistry and western
blotting data, one-way ANOVA was used to compare differences among
more than two groups. The unpaired two-tailed t-test was used to
statistically compare the demyelination data between the CPZ and
CPZ + LFMS. Data that did not reach normality and/or equal
variances were analyzed using a nonparametric Kruskal-Wallis test,
which was followed by Dunn's post hoc test. All data were tested
for outliers using Grubb's test and identified outliers were
removed. P<0.05 was considered to indicate a statistically
significant difference.
Results
LFMS treatment has no effect on
locomotion or anxiety parameters in CPZ mice
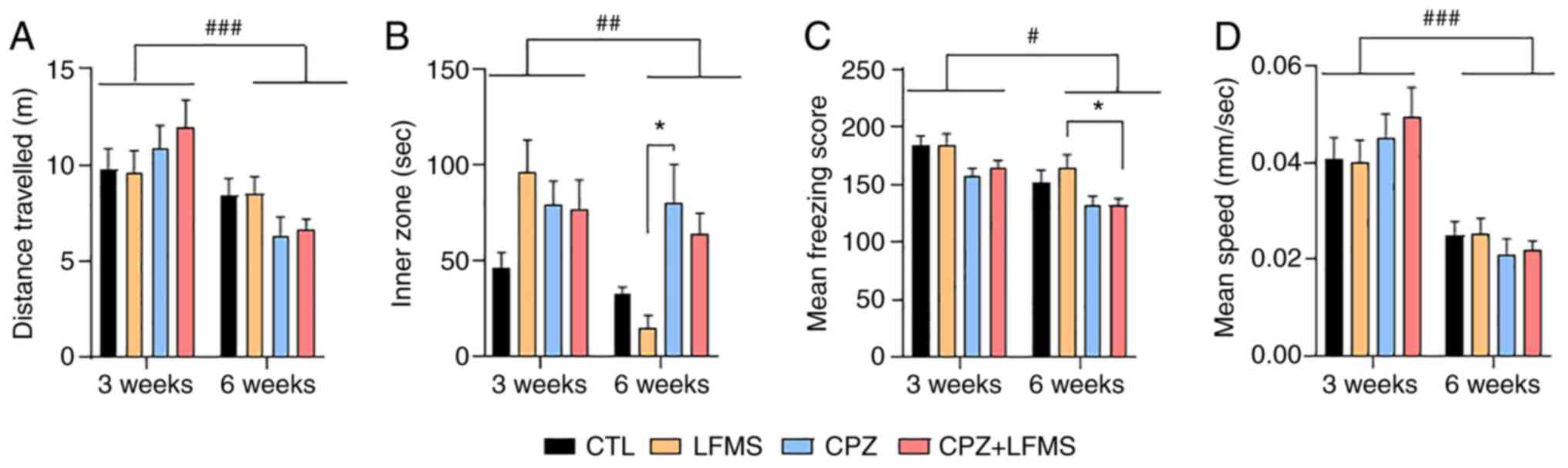
The impact of 3- and 6-week LFMS treatment on
locomotor activity was quantified using the OFT, which evaluated
the total distance travelled (Fig.
2A). The two-way ANOVA demonstrated a significant effect of
time (F(1,85)=16.26; P=0.0001). However, no significant
effect was seen with types of treatment (F(3,85)=0.18;
P=0.90) or treatment by time interaction (F(3,85)=1.97;
P=0.12) among the groups. Statistical analysis on the mean speed
velocity, demonstrated a significant effect of time
(F(1,84)=31.31; P=0.0001; Fig. 2D). However, there was no
statistically significant difference with treatment effect or
interaction. These results suggested that the animals remembered
the open field arena and therefore exhibited reduced exploration at
6 weeks, however, neither the CPZ diet nor LFMS treatment had an
impact on locomotor ability.
The OFT can also be used to assess anxiety-like
behaviors. Time spent in the inner zone of the arena demonstrated a
significant main effect of treatment (F(3,83)=3.60;
P=0.0167), without time differences or interaction between factors.
Tukey's post hoc test demonstrated that CPZ-treated mice spent
markedly more time (P<0.05) in the inner zone compared with the
LFMS group after 6 weeks (Fig.
2B), which suggested these mice potentially exhibited lower
anxiety levels than the controls. The two-way ANOVA also
demonstrated that the mean freezing score was significantly
affected by treatment (F(3,85)=6.06; P=0.0009) and time
(F(1,85)=20.58; P=0.0001), but not by interaction
(F(3,85)=6.24; P=0.86) The post hoc multiple comparisons
test demonstrated that mice fed the CPZ diet with LFMS treatment
exhibited significantly lower mean freezing scores (P<0.05)
compared with the LFMS mice (Fig.
2C). These findings indicated that the CPZ diet may inhibit
anxiety-like behaviors compared with the controls; however, LFMS
treatment alone has no impact on anxiety levels.
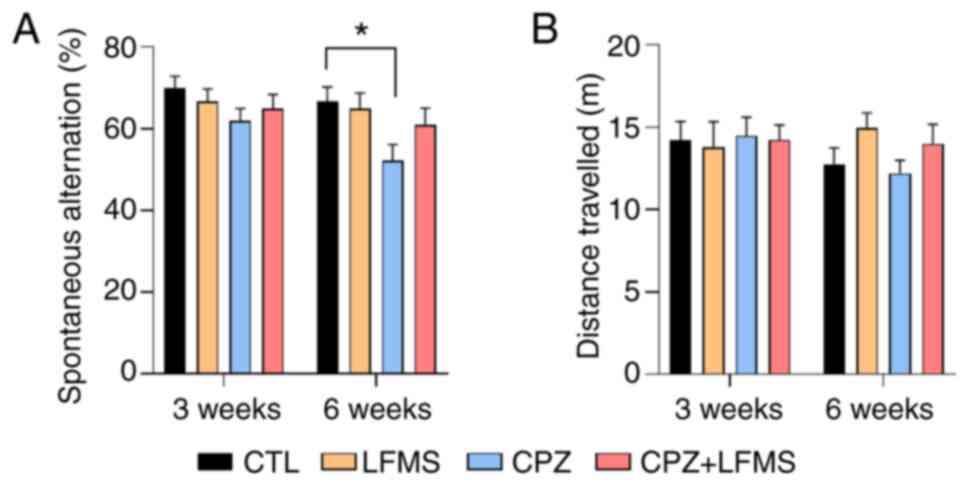
LFMS treatment improves spatial memory
in CPZ mice
In support of the OFT results, locomotor activity
was not significantly different between groups in the Y maze
(Fig. 3B). Analysis of the
spontaneous alternation revealed a statistically significant result
in treatment effect (F(3,80)=3.9574; P<0.0175).
However, there were no significant results for time
(F(1,80)=3.262; P=0.0777) or interaction
(F(3,80)=0.4641; P<0.7081). Moreover, while CPZ diet
mice demonstrated a significant spatial memory deficit (P<0.05)
compared with the control group at 6 weeks, LFMS treatment markedly
improved spontaneous alteration compared with the CPZ group;
however, this trend was not significant (Fig. 3A).
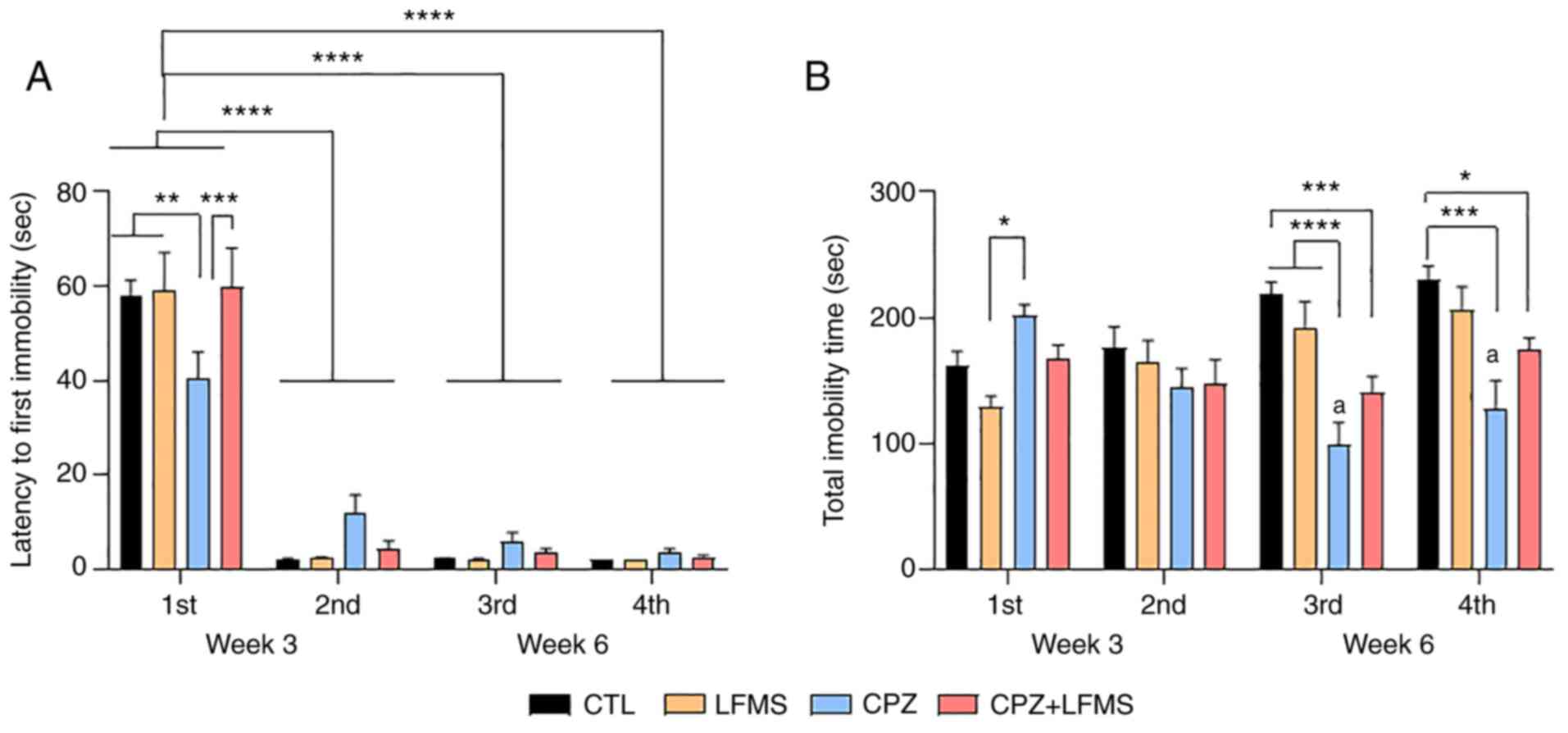
LFMS treatment significantly improves
depression-like symptoms and impacts the adaptive learning response
in CPZ mice
The FST was used to assess depression-like symptoms
and evaluate adaptive learning behavior. Analysis of the latency to
first immobility (time to start floating) revealed a significant
time effect (F(3,164)=185.9; P<0.0001) without
treatment effect (F(3,164)=0.2419; P=0.8670) but with
interaction (F(3,164)=0.2419; P=0.8670; Fig. 4A). Post hoc analysis revealed that
the CPZ diet mice exhibited a significant lower latency to start
floating (P<0.01) when compared to the CTL, LFMS and CPZ + LFMS
groups (Fig. 4A; 1st swim at 3rd
week). LFMS treatment significantly reversed the CPZ effect on the
latency to the first immobility instance compared with the CPZ only
group (P<0.001), suggesting that LFMS may have a potential
antidepressant effect on the animal demyelination model. Repeated
exposure to the FST (2nd, 3rd and 4th swim at week 3 and 6)
revealed a significant reduction in the latency to the first
instance of immobility (P<0.0001) as determined by two-way ANOVA
with Tukey's post hoc multiple comparisons, suggesting that all
animals remembered their previous experience.
Results from total immobility time revealed a
significant main treatment effect (F(3,168)=9.927;
P<0.0001) and significant differences with interaction
(F(9,168)=6.095; P<0.0001), but not with time
(F(3,168)=2.423; P=0.10). The CPZ group exhibited a
significantly higher total immobility time compared with the LFMS
treatment group (P<0.05) in the first FST (Fig. 4B), which indicated a
depressive-like behavior in CPZ mice. Total immobility time did not
show significant differences on the second trial day; however, in
the third trial, the CPZ diet mice presented reduced immobility
time compared with the CTL and LFMS groups (P<0.0001). This
trend continued in the fourth trial, although the CPZ differed from
the CTL group only (P<0.001; Fig.
4B). LFMS treatment demonstrated a slight improvement on the
passive behavior compared with the CPZ fed mice during the third
and fourth FST trials; however, this effect was not significant.
Furthermore, there was a significant difference between the CPZ +
LFMS and CTL groups in the third (P<0.001) and fourth trials
(P<0.05) (Fig. 4B), suggesting
that LFMS was unable to completely ameliorate this adaptive
learning impairment.
LFMS treatment significantly impacts
myelination in the PFC in CPZ mice
CPZ has previously been demonstrated to cause
oligodendrocyte death leading to demyelination (51). Using western blotting, the relative
protein expression levels of MBP, a major protein involved in
myelin function, within the PFC was compared following 3 or 6 weeks
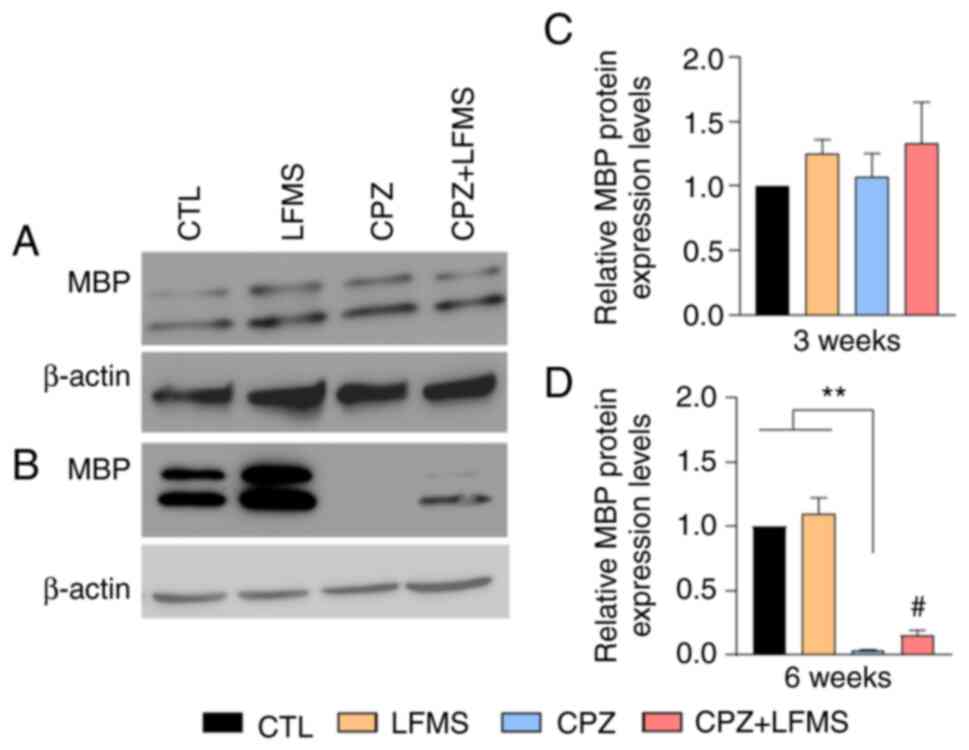
of treatment. The results demonstrated that MBP protein expression
levels were not significantly difference between groups after 3
weeks (Fig. 5A and C), whereas statistically significant
differences were observed following 6 weeks on the CPZ diet
(Kruskal-Wallis 4,19; P<0.0003; Fig. 5D). The CPZ diet mice exhibited a
decrease (P<0.001) in relative MBP protein expression levels
compared with the control groups (CTL and LFMS treatment only),
which demonstrated that there was potentially a significant loss of
myelin in the PFC of CPZ diet mice after 6 weeks. The two-tailed
unpaired t-test (t=2.665; degrees of freedom=10; P<0.0237)
revealed a significant difference between CPZ and CPZ + LFMS mice,
whereby LMFS treatment significantly increased the relative protein
expression levels of MBP in CPZ treated mice. These results
therefore indicated that LFMS treatment either protected the PFC
from demyelination or promoted remyelination following 6 weeks of
treatment.
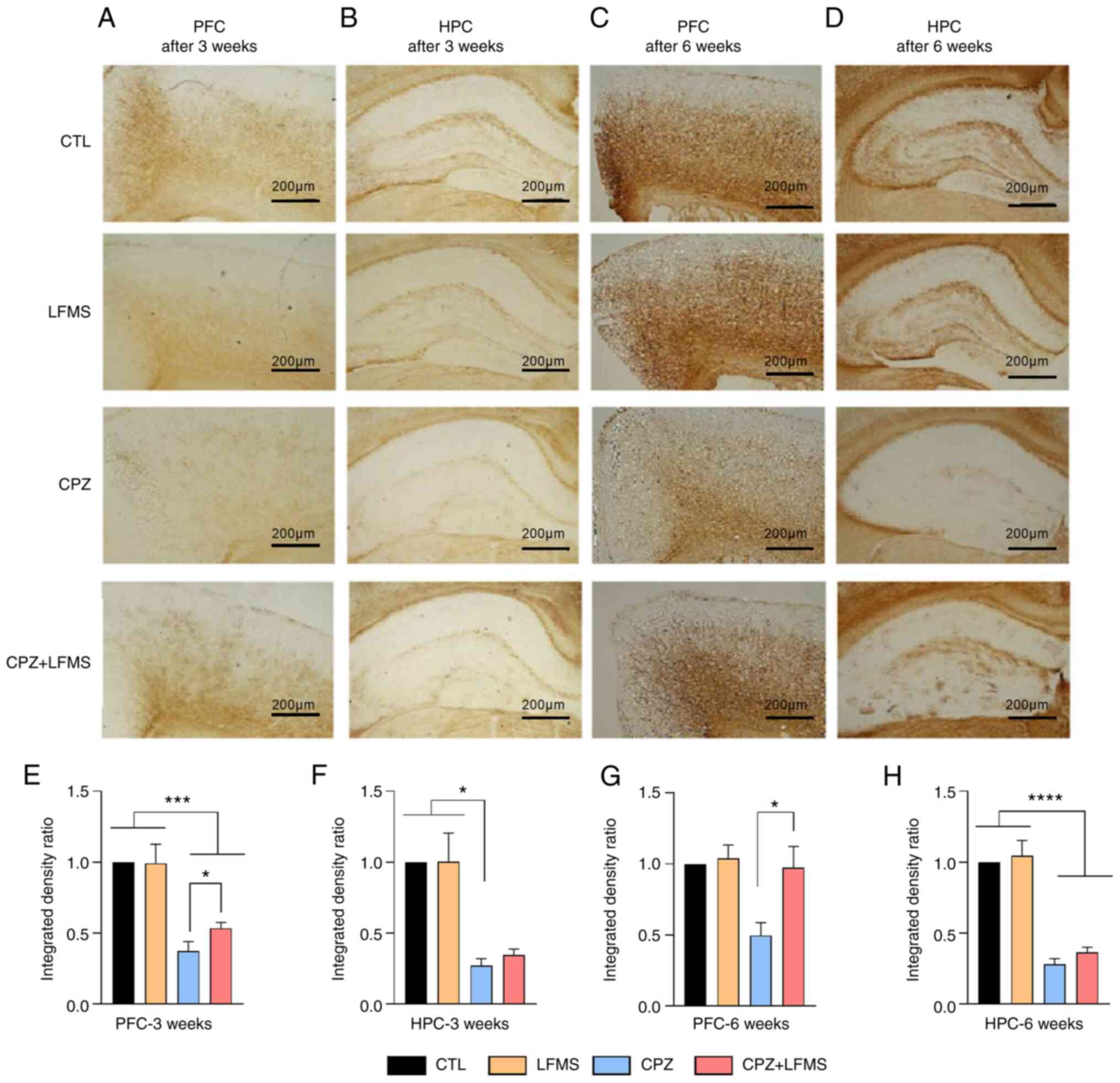
One-way ANOVA demonstrated significant differences
in MBP staining density between diet in the PFC
(F(3,20)=19.25; P<0.0001) and HPC
(F(3,16)=18.67; P<0.0001) at 3 weeks (Fig. 6A and B). The CPZ diet mice exhibited
significantly decreased MBP staining density compared with the CTL
or LFMS treatment group in both the PFC and HPC (Fig. 6E-G), which represented a potential
loss of myelin in mice fed with the CPZ diet. Mice that received
the LFMS treatment (CPZ + LFMS) exhibited significantly increased
MBP staining density in the PFC compared with the CPZ group
(t=2.246; degrees of freedom=15; P<0.0402) and HPC
(P<0.0025).
Following 6 weeks of treatment, one-way ANOVA
demonstrated significant differences between groups in the PFC
(F(3,21)=4.472; P<0.0141) and HPC
(F(3,19)=60.57; P<0.0001). Reduction of MBP staining
was sustained at 6 weeks in the mice on the CPZ diet, with
significant reductions within the HPC (P<0.001) compared with
the CTL and LFMS groups, but not in the PFC. Moreover, LFMS
treatment did not impact MBP staining density in CPZ diet mice
within the HPC, whereas LFMS treatment significantly increased MBP
staining density in CPZ diet mice within the PFC compared with the
CPZ only group (P<0.05).
Discussion
In the present study, it was demonstrated, to the
best of our knowledge for the first time, the beneficial effects of
LFMS, a non-invasive and deep brain stimulation, after 3 or 6 weeks
of treatment in a CPZ-induced demyelination animal model. Mice
treated with LFMS exhibited significantly improved depression-like
symptoms and demonstrated modest enhancements in cognitive function
and adaptive learning skills. Furthermore, in the demyelination
model, LFMS treatment was able to potentially protect from or
reverse the demyelination processes, evidenced by the markedly
increased immunostaining density of MBP in the PFC after 3 and 6
weeks of treatment.
It was also observed that following 3 weeks on the
CPZ diet, mice demonstrated depressive-like behaviors, while
cognitive deficits were observed following 6 weeks on the CPZ diet.
These behavioral changes were associated with demyelination, as
quantified by significantly reduced immunostaining of MBP in the
PFC and HPC evident at 3 and 6 weeks and confirmed by western blot
analysis in the PFC at 6 weeks. These results indicated that the
CPZ-induced acute demyelination model may be useful for studying
the effects of LFMS on myelination.
To the best of our knowledge, this is the first
study to have demonstrated early depression-like behavior following
3 weeks of CPZ feeding. Previous studies have demonstrated an
increase in depression-like symptoms in CPZ models after 5, 6 and
12 weeks on a CPZ diet (19,30,52).
A significantly increased latency to the first immobile episode was
observed in the CPZ group compared to the control and LFMS groups
after only 3 weeks on the CPZ diet (FST1), which was ameliorated by
LFMS treatment. This therefore demonstrated that LFMS has an
antidepressant-like effect in this demyelination model.
Almost one-half of patients with MS display symptoms
of depression (53). The
depressive symptoms observed in patients with MS can precede the
onset of neurological symptoms, suggesting that depression may be
related to early disease-specific processes (54). Both MS and major depressive
disorder share the common pathophysiology of demyelination of CNS
regions and are associated with neuro-inflammation processes
(55,56). Indeed, previous CPZ mouse models
have reported depression-like symptoms and myelin deficiency
(19,30) demonstrating a possible correlation
between depression and demyelination of the CNS. In the present
study, it was demonstrated that mice fed with CPZ exhibited
markedly reduced MBP staining density within the PFC and HPC after
only 3 weeks, which suggested that there was a possible link
between early depression and myelin damage in this demyelinating
disease model. Most importantly, the LFMS antidepressant-like
effect observed after 3 weeks of treatment during the FST1 may be
associated with markedly greater levels of MBP in the PFC. The
significant increase in MBP levels following LFMS treatment after 6
weeks suggested that myelin is either protected by or its loss is
reversed by LFMS treatment. These findings indicated that
myelination is an important factor to improve depression in
patients with MS and possibly in other demyelinating diseases.
Future studies are needed to elucidate the mechanism by which LFMS
impacts myelination, either via protecting myelin from
demyelination or stimulating remyelination, and to examine the
integrity of the protected/restored myelin, including investigating
the re-establishment of the nodes of Ranvier.
Demyelination is associated with axon damage,
leading to cognitive deficits, including in memory and attention
(52). In the present study, CPZ
fed mice demonstrated a significantly lower percentage of
spontaneous alternations in the Y maze after 6 weeks, which
demonstrated a working memory deficit. Spontaneous alternations
were not altered after 3 weeks of CPZ feeding, which indicated that
short-term CPZ exposure may not impair working memory. In support
of this finding, previous studies have reported no changes in the
Y-maze test after 0.4% CPZ-feeding for 3 weeks or 0.2% CPZ for 1
week (57,58). Together, these findings suggested
that short-term exposure (1-3 weeks) to the CPZ diet may not cause
working memory dysregulation. LFMS treatment demonstrated a trend
in repairing the cognitive impairment caused by the 6-week CPZ
diet. A previous study demonstrated that LFMS treatment improved
cognition in the CPZ mouse model after twelve weeks of 0.2% CPZ
exposure followed by four weeks of CPZ withdrawal with sham or LFMS
treatment (29). The difference
between treatment time and procedure may explain the discrepancy
between the observed effect of LFMS treatment on working
memory.
Evidence has also suggested that patients with MS
have demonstrated higher levels of anxiety (59,60).
In the present study, we used the time spent in the central area of
the OFT arena to measure anxiety-like behavior. The results
demonstrated that mice treated with CPZ for 6 weeks had a
significantly higher center-area activity compared with the LFMS
group, which indicated diminished anxiety. Previous studies have
demonstrated similar behavior after CPZ treatment for 3 and 4 weeks
(61,62). This result can be associated with
an inhibited anxiogenic response to novel environments or increased
impulsiveness and could be related to white matter alterations
(61). Further studies
investigating anxiety, such as the elevated plus maze or the
light-dark box, will need to be conducted to obtain a clearer
understanding of the impact of CPZ on anxiety levels.
CPZ mice displayed significantly decreased
immobility at the FST3 and FST4 timepoints, whereas the control
groups exhibited an adaptative learning behavior response or intact
coping strategies following exposure to a stressful situation.
These results suggested that the CPZ mice failed to display normal
adaptation from active to passive coping. To the best of our
knowledge this is the first study to have demonstrated that the CPZ
model impairs adaptive coping strategies. LFMS treatment resulted
in a marked improvement in the CPZ mice coping strategy but was not
sufficient to reverse this effect. Coping strategies serve an
important role in challenging conditions ensuring the ability to
adapt to stressful life conditions (63,64).
Patients with MS and other neuropsychiatric conditions are less
able to integrate adaptive coping abilities compared with healthy
individuals (65,66). As coping strategies are important
factors that can affect a patients' quality of life (67) further investigation in this area is
required.
Depression and defective working memory (68), have both been shown to have a
negative impact on the ability to utilize coping strategies
(69,70). Furthermore, the PFC coordinates
processes which enable effective coping skills (71). Considering these factors, the
results of the present study indicated that failure to engage in
coping strategies by mice exposed to CPZ, may be associated with
the depressive-like symptoms and white matter impairment and
demyelination observed in the PFC. It can therefore be hypothesized
that these elements are required for a healthy coping
mechanism.
The results of the present study have provided the
first evidence for the short-term effect of LFMS in attenuating
early-stage depressive-like behavior in a demyelination animal
model. It was also demonstrated that LFMS treatment either provided
protection against demyelination or promoted remyelination with
increased immunostaining of MBP in the PFC after 3 and 6 weeks of
treatment, confirmed by western blot analysis at 6 weeks.
Therefore, these results indicated that LFMS may have the potential
to be a novel therapy to manage depression in patients with MS. The
present study has raised the possibility for the future application
of this non-pharmacological therapy for MS and has encouraged the
exploration of technological advances in manipulating brain
activity in a non-invasive manner to treat different neurological
and neuropsychiatric diseases. The present study's design did not
determine the mechanism by which LFMS increased MBP levels and
whether the treatment protected oligodendrocytes from damage or
whether the treatment stimulated remyelination. The ability for
LFMS to trigger remyelination has been previously reported
(30), but it is unknown if LFMS
treatment is sufficient to mitigate the toxic effects of CPZ in
oligodendrocytes, protecting them from damage, or if the LFMS
treatment could stimulate repair at a rate faster than CPZ
destruction. Further investigation into the mechanism by which LFMS
acts is needed to better understand the impact on myelination and
how this treatment can be used in clinical conditions.
Acknowledgements
The authors would like to thank Dr Francisco
Cayabyab (University of Saskatchewan, Saskatoon, Canada) and Dr
Valerie Verge (University of Saskatchewan, Saskatoon, Canada), for
their technical assistance. The authors also would like to thank Dr
Changiz Taghibiglou (University of Saskatchewan, Saskatoon, Canada)
for providing access to his lab's equipment for protein
quantification and brain sectioning.
Funding
Funding: This study was supported by start-up funding from the
University of Saskatchewan, the Iver and Joyce Graham Indiana Small
Professorship, the Saskatchewan Health Research Foundation
Establishment Grant (grant no. 4640) and the Intramural fund from
the Department of Psychiatry, University of Saskatchewan.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AM developed the project, collected the data and
performed formal analysis. TS analyzed and interpreted the data and
wrote and edited the manuscript. RVB assisted with data
interpretation, writing, review and editing of the manuscript. HL
and ZW contributed towards research data collection. XML made
substantial contributions to the conception and design of the
study. YZ provided funding acquisition, project development and
conception, supervision, review and editing. AM and YZ confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal
Research Ethics Board from University of Saskatchewan (Saskatoon,
Canada) in 2016 (approval no. 20160103).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dendrou CA, Fugger L and Friese MA:
Immunopathology of multiple sclerosis. Nat Rev Immunol. 15:545–558.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Islas MÁM and Ciampi E: Assessment and
impact of cognitive impairment in multiple sclerosis: An overview.
Biomedicines. 7(22)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Walton C, King R, Rechtman L, Kaye W,
Leray E, Marrie RA, Robertson N, La Rocca N, Uitdehaag B, van der
Mei I, et al: Rising prevalence of multiple sclerosis worldwide:
Insights from the ATLAS of MS, third edition. Mult Scler.
26:1816–1821. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Eijlers AJC, Van Geest Q, Dekker I,
Steenwijk MD, Meijer KA, Hulst HE, Barkhof F, Uitdehaag BMJ,
Schoonheim MM and Geurts JJG: Predicting cognitive decline in
multiple sclerosis: A 5-year follow-up study. Brain. 141:2605–2618.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Patten SB, Marrie RA and Carta MG:
Depression in multiple sclerosis. Int Rev Psychiatry. 29:463–472.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Salehpoor G, Rezaei S and Hosseininezhad
M: Quality of life in multiple sclerosis (MS) and role of fatigue,
depression, anxiety, and stress: A bicenter study from north of
Iran. Iran J Nurs Midwifery Res. 19:593–599. 2014.PubMed/NCBI
|
|
7
|
Kalb R, Feinstein A, Rohrig A, Sankary L
and Willis A: Depression and suicidality in multiple sclerosis: Red
flags, management strategies, and ethical considerations. Curr
Neurol Neurosci Rep. 19(77)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shen Q, Lu H, Xie D, Wang H, Zhao Q and Xu
Y: Association between suicide and multiple sclerosis: An updated
meta-analysis. Mult Scler Relat Disord. 34:83–90. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Carta MG, Paribello P, Anastasia A, De
Berardis D, Nardi AE and Fornaro M: Pharmacological management of
depression in patients with multiple sclerosis. Expert Opin
Pharmacother. 19:1533–1540. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vanotti S and Caceres FJ: Cognitive and
neuropsychiatric disorders among MS patients from Latin America.
Mult Scler J Exp Transl Clin. 3(2055217317717508)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sumowski JF, Benedict R, Enzinger C,
Filippi M, Geurts JJ, Hamalainen P, Hulst H, Inglese M, Leavitt VM,
Rocca MA, et al: Cognition in multiple sclerosis: State of the
field and priorities for the future. Neurology. 90:278–288.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nebel K, Wiese H, Seyfarth J, Gizewski ER,
Stude P, Diener HC and Limmroth V: Activity of attention related
structures in multiple sclerosis patients. Brain Res. 1151:150–160.
2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Guimarães J and Sá MJ: Cognitive
dysfunction in multiple sclerosis. Front Neurol.
3(74)2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Feinstein A, Freeman J and Lo AC:
Treatment of progressive multiple sclerosis: What works, what does
not, and what is needed. Lancet Neurol. 14:194–207. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Procaccini C, De Rosa V, Pucino V,
Formisano L and Matarese G: Animal models of multiple sclerosis.
Eur J Pharmacol. 759:182–191. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhan J, Mann T, Joost S, Behrangi N, Frank
M and Kipp M: The cuprizone model: Dos and do nots. Cells.
9(843)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hübner NS, Mechling AE, Lee HL, Reisert M,
Bienert T, Hennig J, von Elverfeldt D and Harsan LA: The
connectomics of brain demyelination: Functional and structural
patterns in the cuprizone mouse model. Neuroimage. 146:1–18.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hibbits N, Pannu R, Wu TJ and Armstrong
RC: Cuprizone demyelination of the corpus callosum in mice
correlates with altered social interaction and impaired bilateral
sensorimotor coordination. ASN Neuro. 1(e00013)2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang Y, Bi Y, Adebiyi O, Wang J,
Mooshekhian A, Cohen J, Wei Z, Wang F and Li XM: Venlafaxine
improves the cognitive impairment and depression-like behaviors in
a cuprizone mouse model by alleviating demyelination and
neuroinflammation in the brain. Front Pharmacol.
10(332)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mi G, Gao Y, Liu S, Ye E, Li Y, Jin X,
Yang H and Yang Z: Cyclin-dependent kinase inhibitor flavopiridol
promotes remyelination in a cuprizone induced demyelination model.
Cell Cycle. 15:2780–2791. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shafi M, Stern AP and Pascual-Leone A:
Adding low-field magnetic stimulation to noninvasive
electromagnetic neuromodulatory therapies. Biol Psychiatry.
76:170–171. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Posse S, Dager SR, Richards TL, Yuan C,
Ogg R, Artru AA, Müller-Gärtner HW and Hayes C: In vivo measurement
of regional brain metabolic response to hyperventilation using
magnetic resonance: Proton echo planar spectroscopic imaging
(PEPSI). Magn Reson Med. 37:858–865. 1997.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Becker JE, Shultz EKB and Maley CT:
Transcranial magnetic stimulation in conditions other than major
depressive disorder. Child Adolesc Psychiatr Clin N Am. 28:45–52.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Machado S, Arias-Carrion O, Paes F, Vieira
RT, Caixeta L, Novaes F, Marinho T, Almada LF, Silva AC and Nardi
AE: Repetitive transcranial magnetic stimulation for clinical
applications in neurological and psychiatric disorders: An
overview. Eurasian J Med. 45:191–206. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Singh A, Erwin-Grabner T, Goya-Maldonado R
and Antal A: Transcranial magnetic and direct current stimulation
in the treatment of depression: Basic mechanisms and challenges of
two commonly used brain stimulation methods in interventional
psychiatry. Neuropsychobiology. 79:397–407. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Camprodon JA: Therapeutic neuromodulation
for bipolar disorder-The case for biomarker-driven treatment
development. JAMA Netw Open. 4(e211055)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rohan ML, Yamamoto RT, Ravichandran CT,
Cayetano KR, Morales OG, Olson DP, Vitaliano G, Paul SM and Cohen
BM: Rapid mood-elevating effects of low field magnetic stimulation
in depression. Biol Psychiatry. 76:186–193. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sekar S, Zhang Y, Mahabadi HM, Parvizi A
and Taghibiglou C: Low-field magnetic stimulation restores
cognitive and motor functions in the mouse model of repeated
traumatic brain injury: Role of cellular prion protein. J
Neurotrauma. 36:3103–3114. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dolgova N, Wei Z, Spink B, Gui L, Hua Q,
Truong D, Zhang Z and Zhang Y: Low-field magnetic stimulation
accelerates the differentiation of oligodendrocyte precursor cells
via non-canonical TGF-β signaling pathways. Mol Neurobiol.
58:855–866. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang Z, Baharani A, Wei Z, Truong D, Bi X,
Wang F, Li XM, Verge VMK and Zhang Y: Low field magnetic
stimulation promotes myelin repair and cognitive recovery in
chronic cuprizone mouse model. Clin Exp Pharmacol Physiol.
48:1090–1102. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Harbo HF, Gold R and Tintoré M: Sex and
gender issues in multiple sclerosis. Ther Adv Neurol Disord.
6:237–248. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Voskuhl RR, Sawalha AH and Itoh Y: Sex
chromosome contributions to sex differences in multiple sclerosis
susceptibility and progression. Mult Scler. 24:22–31.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Taylor LC, Gilmore W, Ting JPY and
Matsushima GK: Cuprizone induces similar demyelination in male and
female C57BL/6 mice and results in disruption of the estrous cycle.
J Neurosci Res. 88:391–402. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Schiefer HB: Guide to the care and use of
experimental animals, volume 2. Can J Comp Med. 49(49)1985.
|
|
35
|
Canadian Council on Care (CCAC): Guide to
the Care and Use of Experimental Animals. Vol 1. 2nd edition.
Publication date:. 1993, Revision date: 2020. CCAC, Ottawa, ON,
2013. https://ccac.ca/Documents/Standards/Guidelines/Experimental_Animals_Vol1.pdf.
|
|
36
|
Gudi V, Moharregh-Khiabani D, Skripuletz
T, Koutsoudaki PN, Kotsiari A, Skuljec J, Trebst C and Stangel M:
Regional differences between grey and white matter in cuprizone
induced demyelination. Brain Res. 1283:127–138. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang Y, Adebiyi O, Wei Z, Mooshekhian A,
Truong D, Lavoie C, Cohen J, Zhang Z, Wang F, Bowen R and Li XM:
Low-field magnetic stimulation (LFMS) decreases cuprizone-induced
cognitive impairment and brain pathology in mice. Eur
Neuropsychopharmacol. 29:S227–S228. 2019.
|
|
38
|
Seibenhener ML and Wooten MC: Use of the
open field maze to measure locomotor and anxiety-like behavior in
mice. J Vis Exp. 6(e52434)2015.PubMed/NCBI View
Article : Google Scholar
|
|
39
|
Cleal M, Fontana BD, Ranson DC, McBride
SD, Swinny JD, Redhead ES and Parker MO: The Free-movement pattern
Y-maze: A cross-species measure of working memory and executive
functio. Behav Res Methods. 53:536–557. 2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yan G, Xuan Y, Dai Z, Shen Z, Zhang G, Xu
H and Wu R: Brain metabolite changes in subcortical regions after
exposure to cuprizone for 6 weeks: Potential implications for
schizophrenia. Neurochem Res. 40:49–58. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Xu H, Yang HJ, Rose GM and Li XM: Recovery
of behavioral changes and compromised white matter in C57BL/6 mice
exposed to cuprizone: Effects of antipsychotic drugs. Front Behav
Neurosci. 5(31)2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Rustay NR, Cronin EA, Curzon P, Markosyan
S, Bitner RS, Ellis TA, Waring JF, Decker MW, Rueter LE and Browman
KE: Mice expressing the Swedish APP mutation on a 129 genetic
background demonstrate consistent behavioral deficits and
pathological markers of Alzheimer's disease. Brain Res.
1311:136–147. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Slattery DA and Cryan JF: Using the rat
forced swim test to assess antidepressant-like activity in rodents.
Nat Protoc. 7:1009–1014. 2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kraeuter AK, Guest PC and Sarnyai Z: The
forced swim test for depression-like behavior in rodents. Methods
Mol Biol. 1916:75–80. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Porsolt PR, Le Pichon M and Jalfre M:
Depression: A new animal model sensitive to antidepressant
treatments. Nature. 266:730–732. 1977.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Borsini F and Meli A: Is the forced
swimming test a suitable model for revealing antidepressant
activity? Psychopharmacology (Berl). 94:147–160. 1988.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Petit-Demouliere B, Chenu F and Bourin M:
Forced swimming test in mice: A review of antidepressant activity.
Psychopharmacology (Berl). 177:245–255. 2005.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hascoët M and Bourin M: The forced
swimming test in mice: A suitable model to study antidepressants.
Neuromethods. 1:85–118. 2009.
|
|
49
|
Mul JD, Zheng L and Goodyear LJ: Validity
assessment of 5 day repeated forced-swim stress to model human
depression in young-adult C57BL/6J and BALB/CJ mice. eNeuro.
29(ENEURO.0201-16.2016)2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Commons KG, Cholanians AB, Babb JA and
Ehlinger DG: The rodent forced swim test measures stress-coping
strategy, not depression-like behavior. ACS Chem Neurosci.
8:955–960. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Titus HE, Chen Y, Podojil JR, Robinson AP,
Balabanov R, Popko B and Miller SD: Pre-clinical and clinical
implications of ‘Inside-Out’ vs. ‘Outside-In’ paradigms in multiple
sclerosis etiopathogenesis. Front Cell Neurosci.
14(599717)2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Liu Q, Lv HW, Yang S, He YQ, Ma QR and Liu
J: NEP1-40 alleviates behavioral phenotypes and promote
oligodendrocyte progenitor cell differentiation in the hippocampus
of cuprizone-induced demyelination mouse model. Neurosci Lett.
725(134872)2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Khalilian B, Madadi S, Fattahi N and
Abouhamzeh B: Coenzyme Q10 enhances remyelination and regulate
inflammation effects of cuprizone in corpus callosum of chronic
model of multiple sclerosis. J Mol Histol. 52:125–134.
2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Vattakatuchery JJ, Rickards H and Cavanna
AE: Pathogenic mechanisms of depression in multiple sclerosis. J
Neuropsychiatry Clin Neurosci. 23:261–276. 2011.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Shail MS: Neuropsychiatry in demyelination
disease: Using depression as a prodrome for early diagnosis and
treatment of multiple sclerosis. Cureus. 9(e1813)2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Shin JS, Kwon YN, Choi Y, Lee JY, Lee YI,
Hwang JH, Choi SH and Kim SM: Comparison of psychiatric
disturbances in patients with multiple sclerosis and neuromyelitis
optica. Medicine (Baltimore). 98(e1718)2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Shao Y, Peng H, Huang Q, Kong J and Xu H:
Quetiapine mitigates the neuroinflammation and oligodendrocyte loss
in the brain of C57BL/6 mouse following cuprizone exposure for one
week. Eur J Pharmacol. 765:249–257. 2015.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Chang H, Liu J, Zhang Y, Wang F, Wu Y,
Zhang L, Ai H, Chen G and Yin L: Increased central dopaminergic
activity might be involved in the behavioral abnormality of
cuprizone exposure mice. Behav Brain Res. 331:143–150.
2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Wood B, Van Der Mei IAF, Ponsonby AL,
Pittas F, Quinn S, Dwyer T, Lucas RM and Taylor BV: Prevalence and
concurrence of anxiety, depression and fatigue over time in
multiple sclerosis. Mult Scler. 19:217–224. 2013.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Serra-de-Oliveira N, Boilesen SN, de
França Carvalho CP, LeSueur-Maluf L, de Lima Zollner R, Spadari RC,
Medalha CC and de Castro GM: Behavioural changes observed in
demyelination model shares similarities with white matter
abnormalities in humans. Behav Brain Res. 287:265–275.
2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Franco-Pons N, Torrente M, Colomina MT and
Vilella E: Behavioral deficits in the cuprizone-induced murine
model of demyelination/remyelination. Toxicol Lett. 169:205–213.
2007.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Xu H, Yang HJ, Zhang Y, Clough R, Browning
R and Li XM: Behavioral and neurobiological changes in C57BL/6 mice
exposed to cuprizone. Behav Neurosci. 123:418–429. 2009.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Milanlioglu A, Özdemir PG, Cilingir V,
Gülec TÇ, Aydin MN and Tombul T: Coping strategies and mood
profiles in patients with multiple sclerosis. Arq Neuropsiquiatr.
72:490–495. 2014.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Holubova M, Prasko J, Hruby R, Latalova K,
Kamaradova D, Marackova M, Slepecky M and Gubova T: Coping
strategies and self-stigma in patients with schizophrenia-spectrum
disorders. Patient Prefer Adherence. 10:1151–1158. 2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Cotton SM, McCann TV, Gleeson JF, Crisp K,
Murphy BP and Lubman DI: Coping strategies in carers of young
people with a first episode of psychosis. Schizophr Res.
146:118–124. 2013.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Grech LB, Kiropoulos LA, Kirby KM, Butler
E, Paine M and Hester R: Target coping strategies for interventions
aimed at maximizing psychosocial adjustment in people with multiple
sclerosis. Int J MS Care. 20:109–119. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
McCabe MP, McKern S and McDonald E: Coping
and psychological adjustment among people with multiple sclerosis.
J Psychosom Res. 56:355–361. 2004.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Reising MM, Bettis AH, Dunbar JP, Watson
KH, Gruhn M, Hoskinson KR and Compas BE: Stress, coping, executive
function, and brain activation in adolescent offspring of depressed
and nondepressed mother. Child Neuropsychol. 24:638–656.
2018.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Arnett PA, Higginson CI, Voss WD, Randolph
JJ and Grandey AA: Relationship between coping, cognitive
dysfunction and depression in multiple sclerosis. Clin
Neuropsychol. 16:341–355. 2002.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Goretti B, Portaccio E, Zipoli V, Hakiki
B, Siracusa G, Sorbi S and Amato MP: Impact of cognitive impairment
on coping strategies in multiple sclerosis. Clin Neurol Neurosurg.
112:127–130. 2010.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Bradshaw SD, Shumway ST, Dsauza CM, Morris
N and Hayes ND: Hope, coping skills, and the prefrontal cortex in
alcohol use disorder recovery. Am J Drug Alcohol Abuse. 43:591–601.
2017.PubMed/NCBI View Article : Google Scholar
|