Introduction
Peripherally inserted central catheters (PICCs) have
been widely applied for the administration of chemotherapy drugs
(1-3).
PICCs have the advantages of convenient operation, high safety,
long indwelling time and low maintenance difficulty (4), and can provide patients with
intravenous treatment for 7 days to 1 year, which is beneficial to
reduce damage to blood vessels and avoid patient suffering
associated with repeated punctures (5). Furthermore, PICC provides good venous
access for nutrition and chemotherapy in patients with cancer
(6). However, previous studies
have indicated that PICC application has a significant association
with the risk of adverse reactions, such as infection, phlebitis
and deep venous thrombosis, for patients with solid malignancies
receiving chemotherapy drugs (7-9).
Nevertheless, the application of PICC for chemotherapy drugs is
still relatively safe and effective (6). To ensure patient safety and increase
the detection rate of venous complications, clinical observation
alone is not sufficient. Therefore, conducting in-depth research on
the microscopic level of blood vessels is important.
Vinorelbine, a semi-synthetic vinca alkaloid, may
bind to tubulin and suppress mitotic microtubule polymerization
(10). It is a potent
chemotherapeutic drug for treating breast and non-small cell lung
cancer (11-13).
Although oral administration was approved for the clinical
application of vinorelbine in 2006 due to convenience and the low
risk of venous thrombosis, intravenous infusion exhibits higher
efficacy (14). For example, the
results of a phase II study demonstrated that patients with
non-small cell lung cancer treated with oral vinorelbine exhibited
good tolerance, but displayed limited overall survival time
compared with those treated with intravenous vinorelbine (15). Although PICCs exhibit various
advantages, the catheter is left in the vein for a long time, thus
phlebitis often occurs due to the stimulation of the blood vessel
wall by the catheter, the chemical stimulation of blood vessels via
the drug infusion and the low obstruction in patients with cancer
(1-3).
Therefore, it is important to strengthen clinical observation and
actively prevent, reduce or eliminate the occurrence of
complications as much as possible. Certain patients with an
invisible thrombus do not exhibit clinical symptoms, but present
with abnormal pathological and laboratory indicators, despite
thrombus formation. Therefore, to ensure patient safety and
increase the detection rate of venous complications, clinical
observation alone is not adequate. Thus, it is important to conduct
in-depth research at the microscopic level of blood vessels.
However, there is a lack of models with vinorelbine administration
via PICC and a lack of evidence regarding pathological changes of
PICC vein complications at different stages. The present study
aimed to construct a rabbit model with vinorelbine administration
via PICC to dynamically monitor phlebitis and thrombosis changes,
which may provide a reference for early prediction, timely
prevention and treatment of PICC-related chemotherapy
complications.
Materials and methods
Animals
The present study was approved by the Ethics
Committee of the School of Medicine, Jinhua Polytechnic (Jinhua,
China; approval no. 2019017). In total, 48 healthy New Zealand
rabbits (weight, 2.5-3.0 kg; age, 3-month-old) were provided by
Jinhua Center of Laboratory Animals, Jinhua Food and Drug
Inspection and Testing Institute (Jinhua, China), including 24
non-pregnant female rabbits and 24 male rabbits. The rabbits were
adaptively fed for 1 week in the animal experimental center in
single cages and randomly numbered. The housing conditions were as
follows: 18˚C; humidity, 65%; light/dark cycles, 12/12 h; and with
sufficient food and clean water. The animal experiments were
performed in accordance with the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health (ncbi.nlm.nih.gov/books/NBK54050/).
Construction of the PICC rabbit
model
The 1.9 Fr x50 cm single-lumen PICCs (Fr, unit of
the circumference of the catheter) were purchased from Unijet. The
lumen and outer wall of the catheter were rinsed with 15 ml normal
saline. Before use, the catheter was further rinsed with 5 ml
normal saline to remove bubbles. The rabbits were anesthetized via
an intraperitoneal injection of 30 mg/kg sodium pentobarbital (3%;
Sigma-Aldrich; Merck KGaA). After successful anesthesia, the length
of the PICC insertion was measured for each rabbit. To perform the
measurement procedures, the rabbit was placed in a supine position,
and keeping the rabbit's ears upright and parallel to the body at
180˚, the length of the PICC insertion was determined (21-26 cm) by
measuring the length from the puncture point (posterior auricular
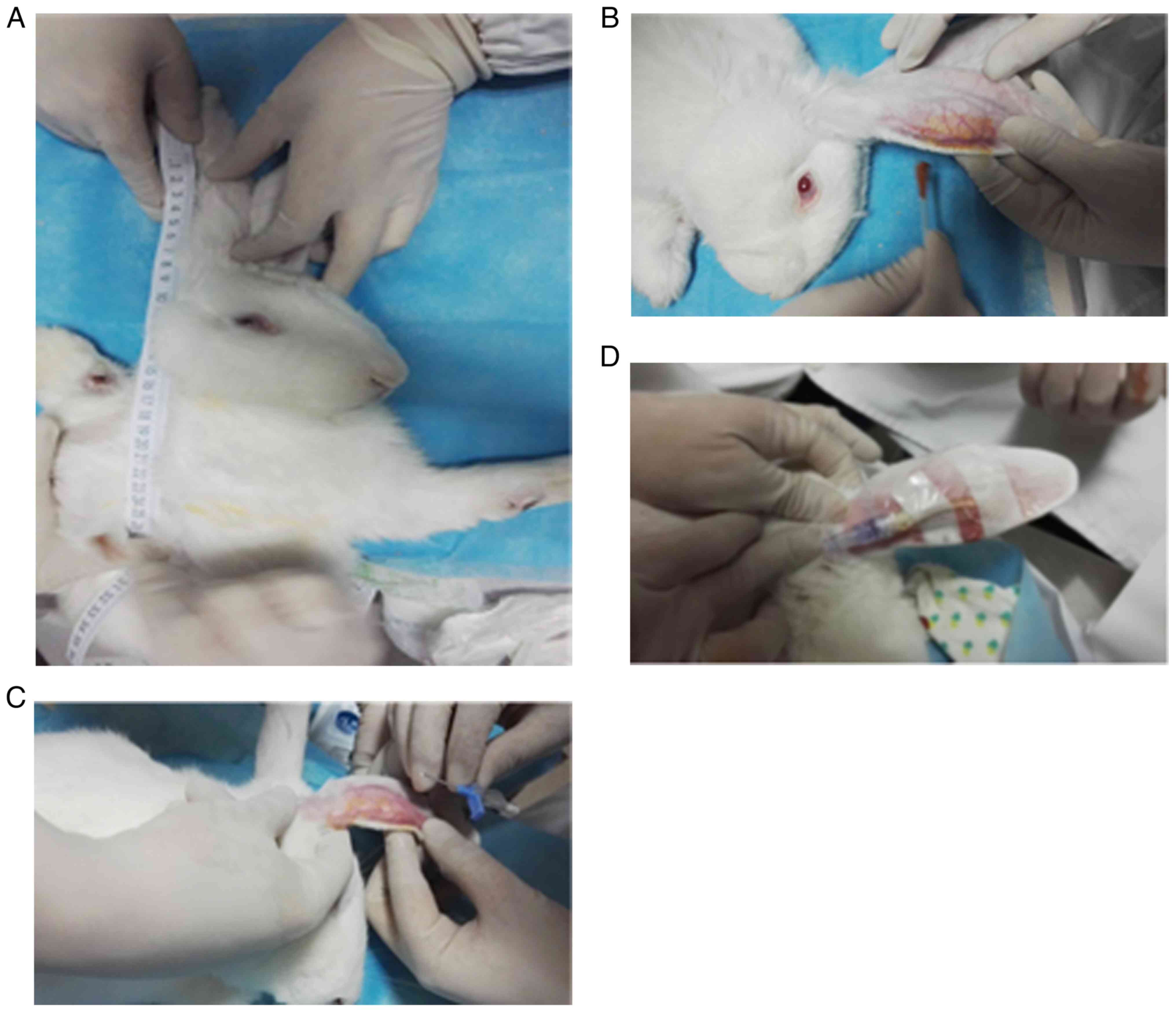
vein) to the most evident stop of the cardiac apex (Fig. 1A). Following skin preparation
(Fig. 1B), disinfection (Fig. 1B) and puncture (Fig. 1C), the PICC tube was inserted into
posterior auricular vein to the predetermined length (Fig. 1D). After checking patency and
initial fixation, 0.5 ml Lipiodol® (Jiangsu Hengrui
Pharmaceutical Co., Ltd.) was intrathecally injected. Subsequently,
whether the PICC tube reached the anterior vena cava was observed
under X-ray fluoroscopy. Blood samples (1.8 ml) were collected from
the ear vein. After flushing the PICC tube with 10 ml saline, the
catheter was closed and fixed with a 3M applicator. To prevent
accidental extubation from the rabbit ear, a recovery collar was
placed on each animal so as not to affect the blood circulation or
food intake.
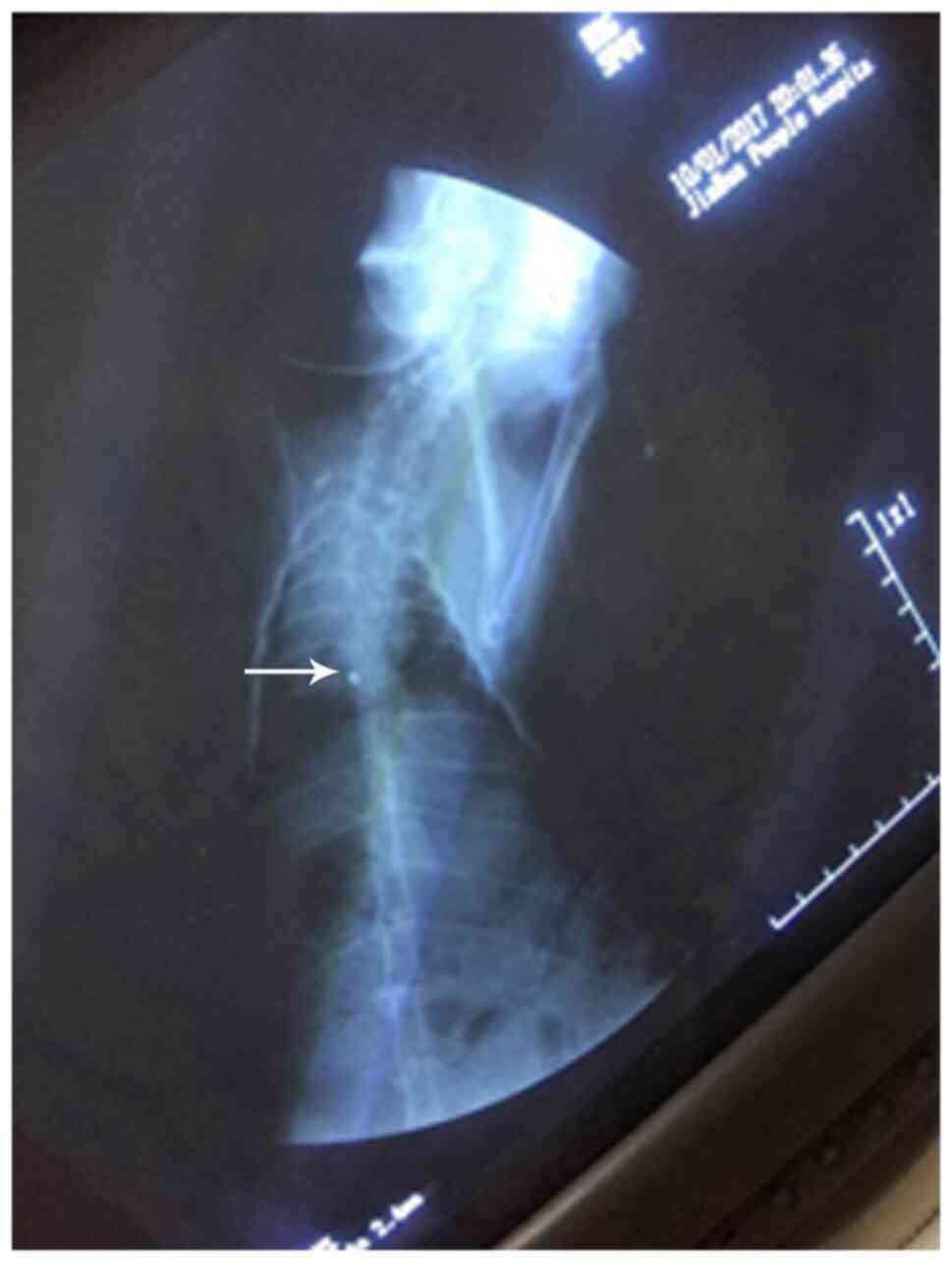
Evaluation of PICC models
The criteria for evaluating the successful
establishment of PICC models were as follows: i) The established
animal model of PICC was successful with one puncture; ii) there
was no local damage or bleeding in the puncture; iii) the end of
the catheter was not twisted or folded under X-ray fluoroscopy; and
iv) the PICC tube was placed in the anterior vena cava. As
presented in Fig. 2, the
transparent dot corresponded to the end of the catheter.
Animal grouping and modeling
The PICC model rabbits were randomly divided into
the following eight groups (n=6 per group): i) Control group (PICC
in place for 1 day); ii) 2nd day of PICC placement (received the
first cycle of vinorelbine administration); iii) 3rd day of PICC
placement; iv) 7th day of PICC placement; v) 14th day of PICC
placement; vi) 21st day of PICC placement; vii) 23rd day of PICC
placement (received the second cycle of vinorelbine
administration); and viii) 24th day of PICC placement. Vinorelbine
(10 mg/ml) was purchased from Jiangsu Haosoh Pharmaceutical Group
Co., Ltd. After being dissolved in 10 ml normal saline, vinorelbine
was slowly injected into the rabbit using an infusion pump for 15
min. The drug was administered once on the 2nd day and once on the
23rd day after intubation. After each administration, the tube was
sealed with heparin saline according to the pulse positive pressure
method (16). Based on the
Meeh-Rubner formula (17), the
body surface area of each rabbit was calculated, and 25
mg/m2 was used as the optimal dose of vinorelbine.
During the intermittent period of chemotherapeutics, PICC
intubation maintenance was completed according to regular
operations: The dressing was changed once a week; and when the
dressing was wet or curled, it was disinfected and replaced. After
the experiment, considering humane endpoints and animal welfare
(when the rabbit experienced severe pain, suffering or dying or
when the experiment was complete), all rabbits were euthanized via
an intravenous injection of 100 mg/kg sodium pentobarbital to
minimize suffering and distress. Death was verified by the complete
cessation of the heartbeat and breathing and disappearance of
reflexes.
General indicator observations
The local skin temperature, redness and extent of
swelling at the puncture point were observed for the rabbits in
each group. The criteria for evaluating the degree of phlebitis
according to the phlebitis grading standard of the American Society
of Intravenous Nursing (18) were
as follows: i) Grade 0, no clinical symptoms and signs; ii) grade
I, local pain, redness or edema, no cord-like changes in the vein
and no induration on touch; iii) grade II, local pain, redness,
swelling or edema, string-like changes in veins, no induration,
mild swelling, burning sensation and moderate pain; and iv) grade
III, local pain, redness or edema, string-like changes in veins and
palpable induration. Furthermore, venous thrombosis was evaluated
based on the following indicators: i) Whether the puncture blood
vessel infusion was unobstructed or not; ii) whether the local skin
was swollen and displayed edema or not; and iii) the thrombus
shape, color and composition according to pathological
analysis.
Hematoxylin and eosin (H&E)
staining
Following removal of the PICC tube, two sections (5
cm) were removed at the front and back of the catheter;
furthermore, the ear vein and the anterior vena cava (3 cm) were
removed. Ear vein, anterior vena cava and catheter samples were
fixed with 10% formalin for 24 h at 4˚C. Following dehydration
using an ethanol series, the samples were cleared using xylene
I/II. Paraffin-embedded samples were sliced into 20, 50, 100 or 200
µm-thick sections. Subsequently, H&E staining was performed.
Following dewaxing and rehydrating, the sections were stained with
hematoxylin dye solution for 20 min at room temperature, followed
by eosin staining for 1 min at room temperature. The sections were
then dehydrated and cleared. After sealing with a neutral balsam,
stained sections were visualized using a light microscope (Olympus
Corporation; magnification, x15 or x20).
Prothrombin time
A total of 2 ml ear vein blood samples were
collected for measuring prothrombin time. Prothrombin time was
determined using ACL-TOP700 automatic coagulation analyzer
[ACL-TOP700; Wofen Medical Equipment Trading (Beijing) Co.,
Ltd.].
ELISA
Blood samples obtained from the ear vein were used
for ELISA. Samples were maintained at room temperature for 2 h.
Subsequently, the samples were centrifuged at 1,000 x g for 20 min
at 4˚C and then stored at -20˚C. The serum levels of E-selectin
(cat. no. SEA029Rb; Cloud-Clone Corp.), P-selectin (cat. no.
SEA569Ra), interleukin (IL)-2 (cat. no. SEA073Rb), IL-6 (cat. no.
SEA079Rb), C-reactive protein (CRP; cat. no. SEA821Rb) and D-dimer
(D2D; cat. no. CEA506Rb; all Cloud-Clone Corp.) were detected using
the corresponding ELISA detection kits according to manufacturer's
instructions. Optical density values were determined using a
microplate reader (Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism software (version 7.0; GraphPad Software, Inc.). Data are
presented as the mean ± standard deviation. Each experiment was
repeated three times independently. Comparisons among multiple
groups were performed using one-way ANOVA followed by Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Construction of a PICC chemotherapy
rabbit model
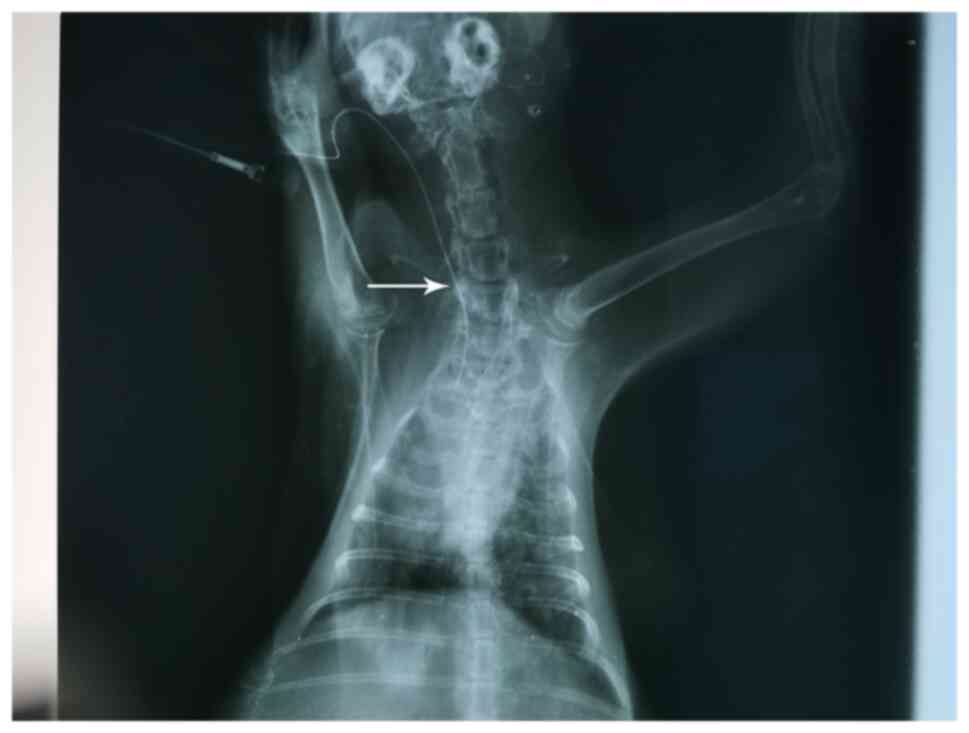
In the present study, a PICC chemotherapy model was
successfully established in 48 rabbits. To observe the pathological
changes of the puncture point during PICC catheterization, the
rabbits were randomly separated into eight experimental groups. A
course of chemotherapy includes 2-3 cycles (19); the present study was based on two
cycles. X-rays were captured to confirm that the end of the
catheter was not twisted or folded, and was placed in the anterior
vena cava (Fig. 3). In each group,
the local puncture site displayed no injury or bleeding. Thus, it
was determined that the PICC models were successfully
constructed.
Dynamic monitoring of catheter-related
thrombosis in rabbits with administration of vinorelbine via
PICC
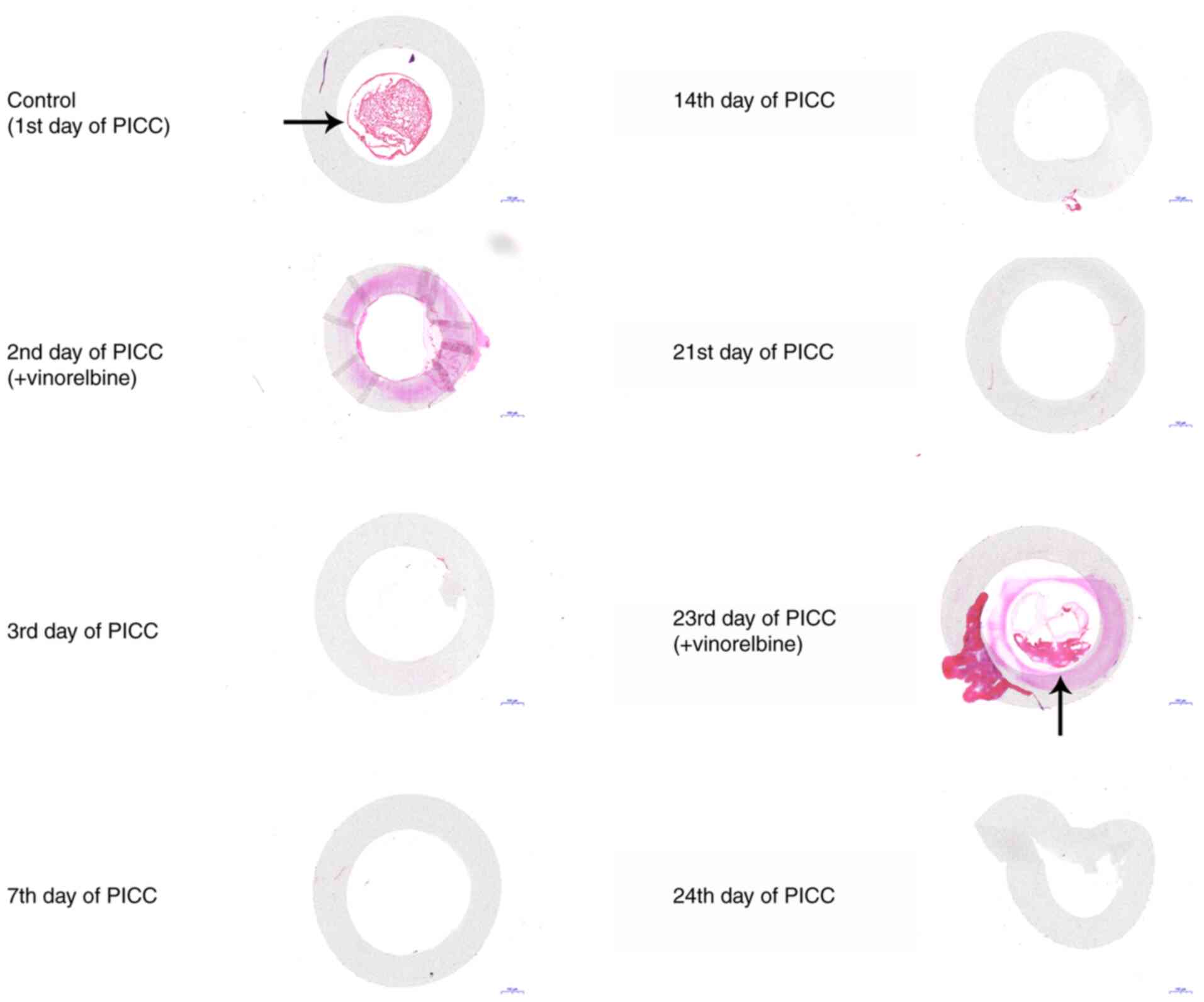
At different time periods, the PICC tube was removed
and the two segments at the front and back of the catheter were
surgically dissected. H&E staining of the middle of the
catheter was performed to observe catheter-related thrombosis. On
the 1st day of PICC implantation, the H&E staining results
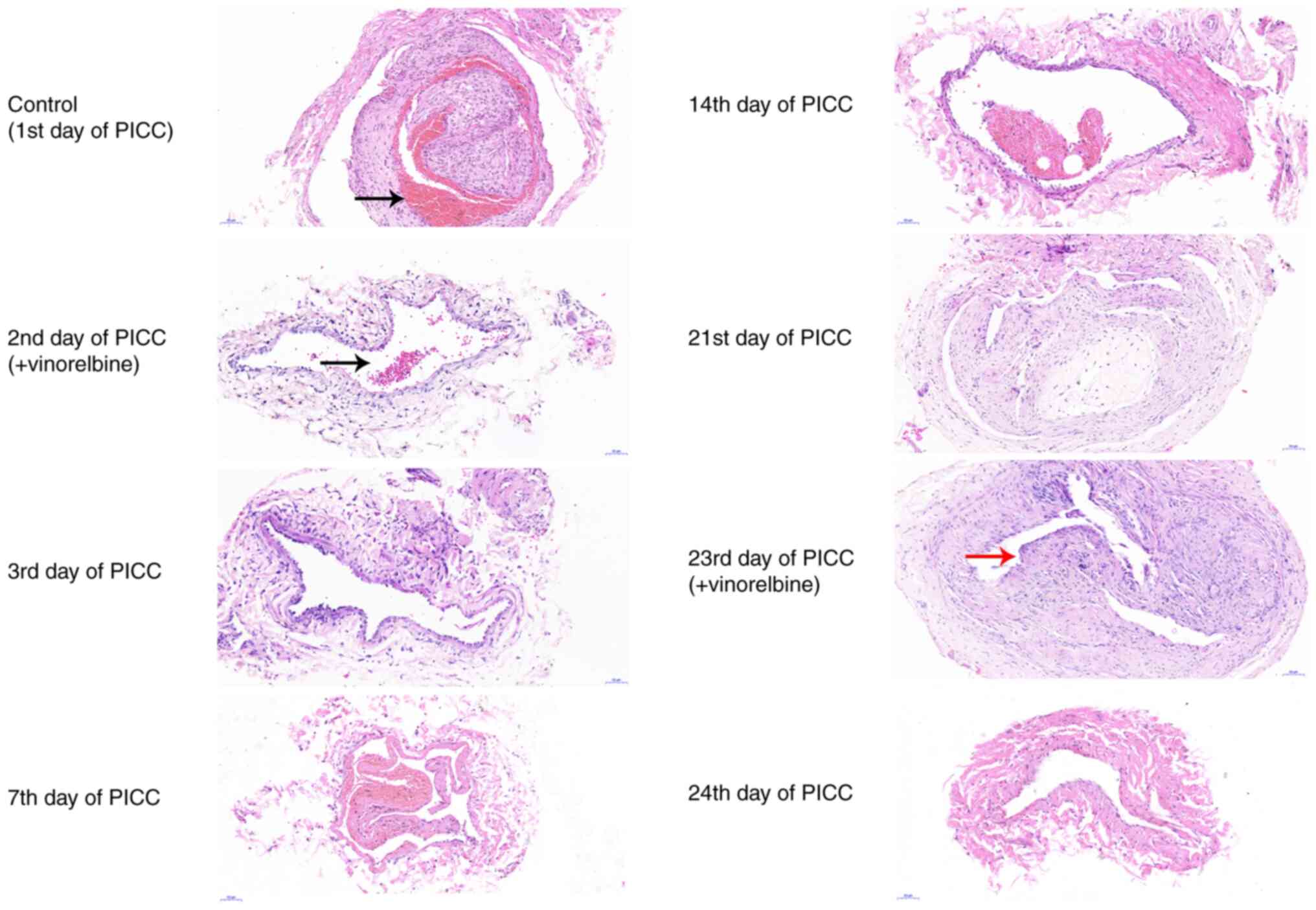
revealed that a thrombus filled the catheter (Fig. 4). On the 2nd day of PICC
implantation, no thrombus was observed in the catheter after
administration of vinorelbine. The catheter-related thrombus was
continuously monitored on the 3rd, 7th, 14th and 21st days of PICC
implantation. As indicated by the H&E staining results, there
was no thrombus in the catheter. On the 23rd day of PICC
implantation, a small thrombus was observed in the catheter.
However, the thrombus was no longer visible on the 24th day.
Collectively, the results indicated that catheter-related
thrombosis primarily occurred on the 1st day of PICC implantation
and the 23rd day of chemotherapy administration.
Dynamic monitoring of ear vein
thrombosis after administration of vinorelbine via PICC
H&E staining was performed to examine the
pathological changes in ear vein tissues at numerous time points
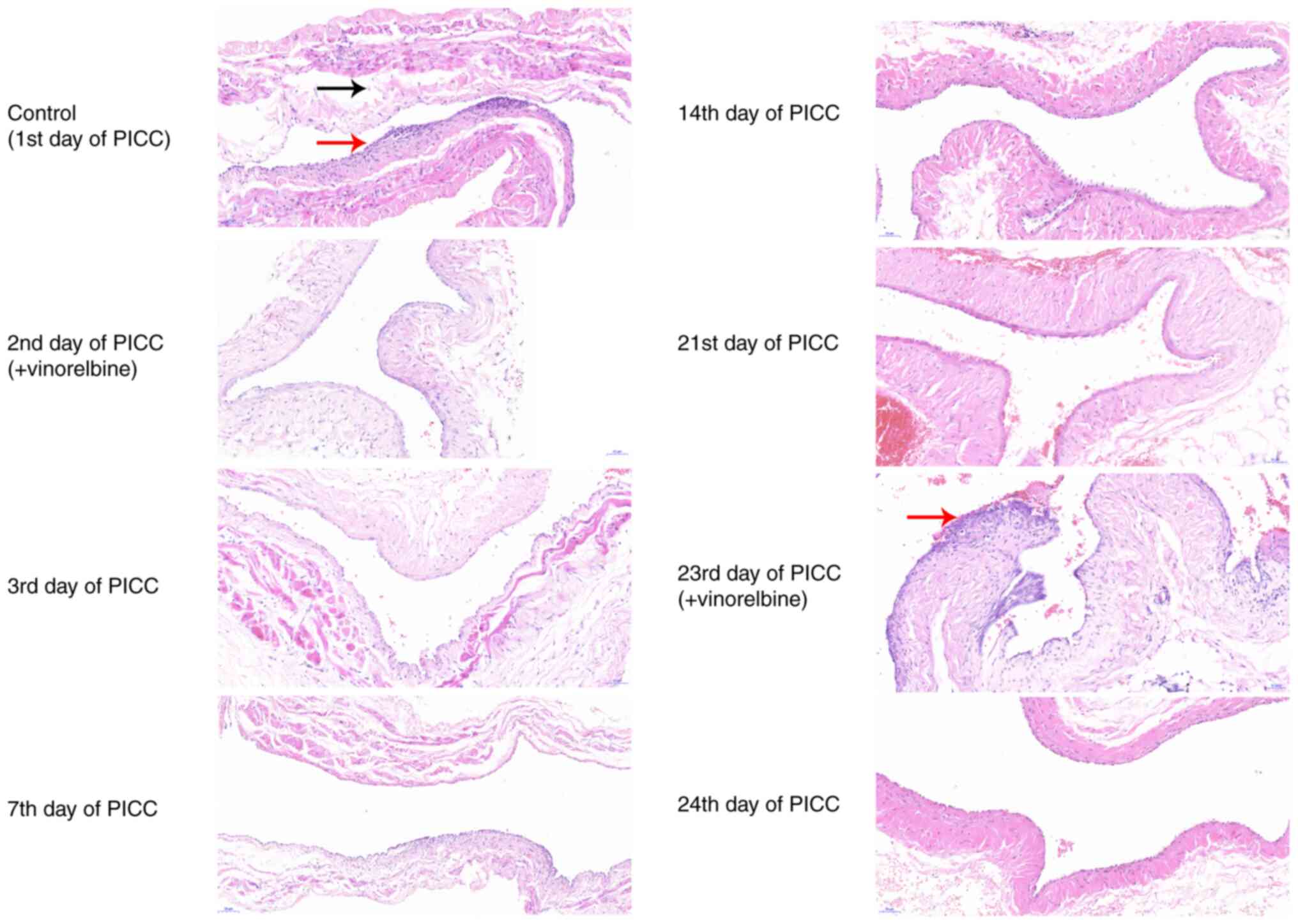
(Fig. 5). On the 1st day of PICC
implantation, the intima of the ear veins was irregularly ruptured,
and the lumen was characterized by thrombosis. On the 2nd day of
catheterization, there was slight ear vein thrombosis, the intima
was irregularly ruptured, and inflammatory cell infiltration was
observed following vinorelbine administration. On the 3rd, 7th and
14th days of PICC implantation, ear vein thrombosis and
inflammatory cell infiltration were notably improved, the intima
was hyperplastic, and scars were formed in the lumen of the ear
veins. Furthermore, the media and adventitia had no obvious
lesions, and the lumen was not completely occluded. On the 21st and
23rd (after vinorelbine administration) days of PICC implantation,
the results revealed that there were evident scars and inflammatory
cell infiltration in the lumen. On the 24th day, the blood vessels
of the ear veins were not damaged, there was no obvious scar in the
lumen and thrombosis had disappeared. Therefore, the results
indicated that ear vein thrombosis was primarily caused by PICC
puncture, and administration of vinorelbine may induce inflammatory
cell infiltration.
Dynamic monitoring of pathological
injury in the anterior vena cava after administration of
vinorelbine via PICC
The end of the anterior vena cava was obtained at
different time periods to investigate pathological injury via
H&E staining. On the 1st day of PICC implantation, the vascular
intima of the anterior vena cava was irregularly ruptured (Fig. 6). Furthermore, the vessel wall was
thickened, which was accompanied by immune cell infiltration. On
the 2nd (after vinorelbine administration), 3rd and 7th days of
PICC implantation, the vascular intima of the anterior vena cava
was relatively intact, with a slightly thickened wall. Moreover,
inflammatory cell infiltration was distinctly ameliorated. On the
14th and 21st days, the vascular intima of the anterior vena cava
was intact and the wall was normal. However, low level immune cell
infiltration was observed. On the 23rd day of PICC implantation
after administration of vinorelbine, there was distinct
inflammatory cell infiltration in the anterior vena cava. On the
24th day, the vascular intima of the anterior vena cava was
relatively intact, the wall was normal and there was almost no
infiltration of inflammatory cells. These results indicated that
vinorelbine administered via PICC could induce phlebitis, and over
time phlebitis was gradually ameliorated within the first cycle of
vinorelbine and phlebitis was significantly alleviated within 1 day
of the second cycle of vinorelbine
Evaluation of prothrombin time after
administration of vinorelbine via PICC
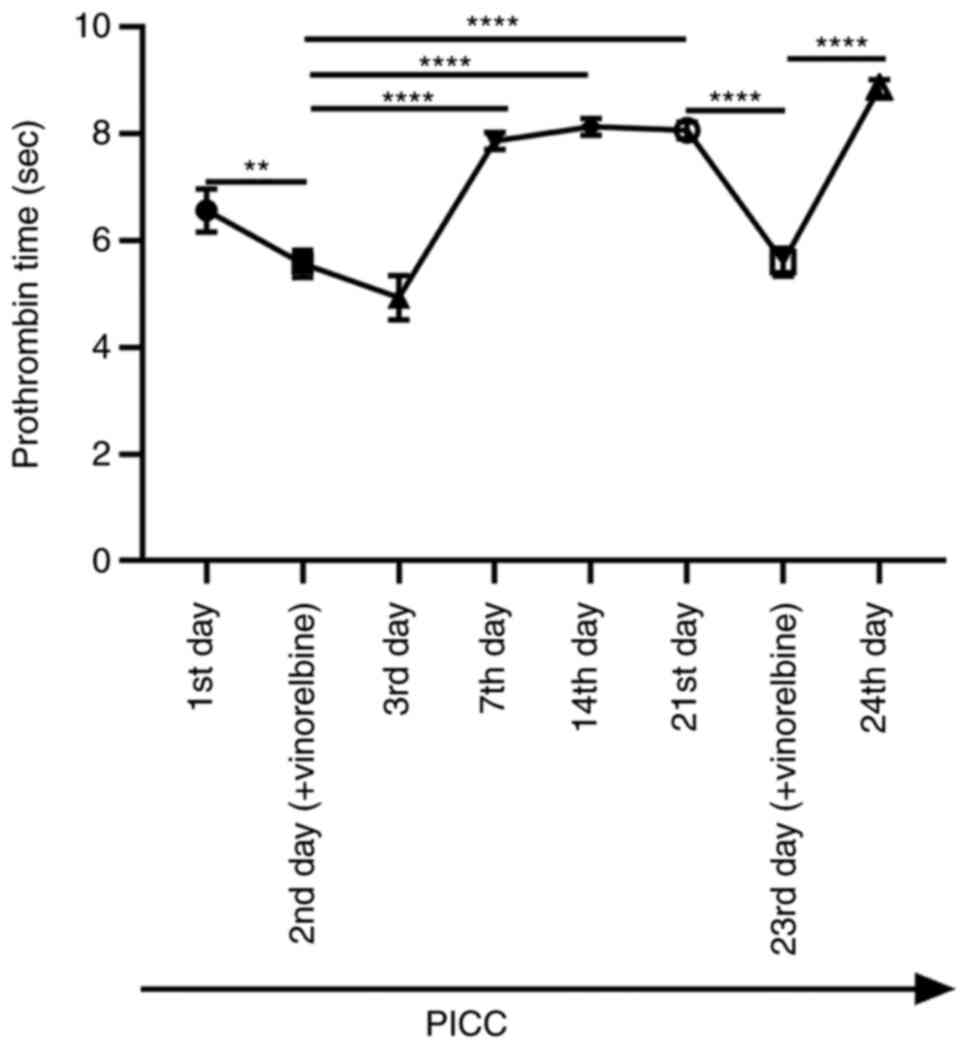
To assess the function of the extrinsic coagulation
system, the prothrombin time of ear vein blood samples that were
collected at different time points was assessed. The results
demonstrated that the prothrombin time was significantly shortened
after administration of vinorelbine on the 2nd day of PICC
implantation compared with that on the 1st day (Fig. 7). Compared with that on the 2nd day
of PICC implantation with vinorelbine administration, the
prothrombin time was significantly prolonged on the 7th, 14th and
21st days. On the 23rd day, after administration of vinorelbine,
the prothrombin time was significantly reduced compared with that
on the 21st day. By contrast, the prothrombin time was
significantly increased on the 24th day compared with that on the
23rd day. Therefore, the results indicated that administration of
vinorelbine via PICC reduced prothrombin time on the day of
administration.
Dynamic monitoring of inflammation-
and thrombosis-related factors in rabbits administered with
vinorelbine via PICC
Inflammation- and thrombosis-related indexes were
examined in blood samples obtained from ear veins via ELISA at
different time points. The levels of CRP after administration of
vinorelbine on the 2nd day of PICC implantation were significantly
increased compared with those on the 1st day of catheterization
(Fig. 8A). However, on the 7th,
14th and 21st days of PICC implantation, CRP levels were
significantly lowered compared with those on the 2nd day. On the
23rd day of PICC implantation after vinorelbine administration, CRP
levels were significantly elevated compared with those on the 21st
day, but this effect was significantly reversed on the 24th day.
The levels of P-selectin, E-selectin and IL-6 were also determined
in ear vein blood samples. P-selectin, E-selectin and IL-6 levels
were significantly higher on the 2nd day of PICC after vinorelbine
administration compared with those on the 1st day of
catheterization (Fig. 8B-D).
Nevertheless, the levels of these markers were significantly
decreased on the 7th, 14th and 21st days of PICC implantation
compared with those on the 2nd day. Following administration of
vinorelbine on the 23rd day of catheterization, P-selectin,
E-selectin, and IL-6 levels were significantly elevated compared
with those on the 21st day, but this effect was reversed on the
24th day. As demonstrated in Fig.
8E, IL-2 levels were significantly reduced after vinorelbine
administration on the 2nd day of PICC catheterization compared with
those on the 1st day of catheterization. On the 7th, 14th and 21st
days, IL-2 levels were significantly increased in a time-dependent
manner compared with those on the 2nd day. After administration of
vinorelbine on the 23rd day of catheterization, IL-2 levels were
significantly reduced compared with those on the 21st day, but then
significantly increased on the 24th day. D2D levels were
significantly higher on the 2nd day of PICC implantation after
vinorelbine administration compared with those on the 1st day of
PICC (Fig. 8F). On the 7th, 14th
and 21st days, D2D levels were significantly decreased compared
with those on the 2nd day. However, significantly increased D2D
levels were detected following administration of vinorelbine on the
23rd day compared with those on the 21st day. The levels of D2D
displayed a significant decline on the 24th day compared with those
on the 23rd day. These results revealed that vinorelbine
administration via PICC could induce an inflammatory response and
thrombosis formation.
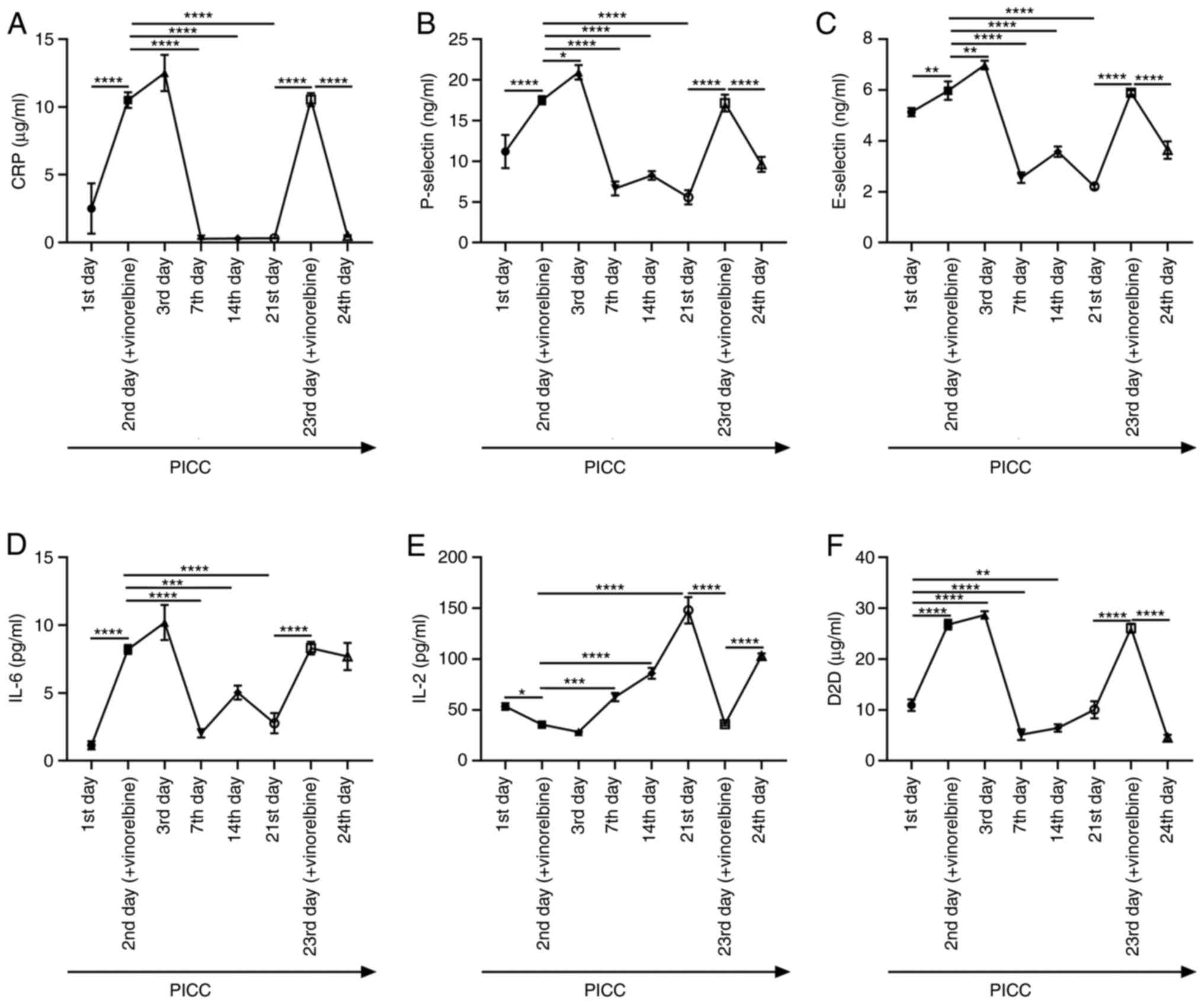 | Figure 8Inflammation- and thrombosis-related
factors in rabbits with PICC administration of vinorelbine. Levels
of (A) CRP, (B) P-selectin, (C) E-selectin, (D) IL-6, (E) IL-2 and
(F) D2D were determined in ear vein blood samples from rabbits with
PICC placement at eight different time points by performing ELISA.
*P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001. PICC,
peripherally inserted central catheter; CRP, C-reactive protein;
IL, interleukin; D2D, D-dimer. |
Discussion
In the present study, a rabbit model with
vinorelbine administration via PICC was successfully established.
During each time point of PICC intubation, the puncture points,
vascular pathological changes and serum observation indexes were
assessed. Eight time points of PICC intubation were used to observe
phlebitis and venous thrombosis. Administration of vinorelbine via
PICC distinctly induced catheter-related thrombosis, ear vein
thrombosis and pathological damage in the anterior vena cava.
Furthermore, prothrombin time was significantly decreased, and the
inflammatory response was significantly enhanced after vinorelbine
administration via PICC. With increasing time after PICC
administration of vinorelbine, the aforementioned pathological
changes were notably improved.
X-rays confirmed that the PICC rabbit model was
successfully constructed in the present study. For patients with
cancer receiving chemotherapy or nutrition, PICC has become the
main method of venous access (20-22).
However, PICC-related venous thrombosis is the most common
complication (23). For example,
it has been reported that PICC is associated with a high risk of
catheter-related deep venous thrombosis in a randomized multicenter
trial (3). Consistently, the
results of the present study revealed that the catheter was filled
with a thrombus on the 1st day of PICC implantation. However, after
administration of 25 mg/m2 vinorelbine on the 2nd and
23rd day of PICC, the vein thrombus was observed. Furthermore,
pathological damage in the anterior vena cava was relatively
minimal on the day of vinorelbine administration. However, with
increasing time the pathological damage was gradually ameliorated.
In a recent monocentric and randomized trial, PICC displayed higher
safety and effectiveness compared with a centrally inserted central
catheter. Moreover, the use of PICC could effectively reduce the
risk of infection and thrombosis (24). However, whether vinorelbine
injection via PICC is effective and safe requires long-term
investigation in a larger cohort.
Prothrombin time refers to the time required to add
excessive tissue thromboplastin and calcium ions to plasma lacking
platelets to convert prothrombin to thrombin, resulting in plasma
coagulation (25-27).
Prothrombin time is an indicator reflecting the activity of
coagulation factors Ⅰ, Ⅱ, Ⅴ, Ⅶ and Ⅹ in the plasma (28). The prothrombin time measurement is
a barrier-free screening test to check the function of the
extrinsic coagulation system, and it is also an important
monitoring index for clinical anticoagulation therapy (29). In the present study, compared with
that on the 1st day of PICC, the prothrombin time was significantly
shortened following vinorelbine administration, but significantly
increased over time. In a previous study, a positive correlation
between serum vinorelbine levels and the number of platelets was
reported (30).
Serum inflammation- and thrombosis-related factors
were examined by performing ELISA. The results demonstrated that
CRP, P-selectin, E-selectin, IL-6 and D2D levels were distinctly
elevated following vinorelbine administration. CRP, which is a
non-specific diagnostic inflammatory biomarker, is involved in
mediating innate immune responses (31). The levels of CRP in the plasma rise
sharply when the body is infected or tissues are damaged (32). In the early stage of acute
inflammation, P-selectin participates in the process of recruiting
leukocytes to the injured site (33). Moreover, P-selectin is closely
related to deep vein thrombosis (34). D2D is a fibrin degradation product,
and its elevated levels indicate a hypercoagulable state and
secondary fibrinolysis in the body (35). It has become a diagnostic marker
for deep vein thrombosis (36). In
the present study, administration of vinorelbine via PICC
significantly elevated serum CRP, P-selectin, E-selectin, IL-6 and
D2D levels but decreased serum IL-2 levels, indicating that
inflammation and thrombosis could be induced by vinorelbine
administration via PICC. A previous study demonstrated that
vinorelbine reduces serum IL-2 levels in a Lewis lung cancer mouse
model (37).
However, the present study had certain limitations.
Firstly, to dynamically observe phlebitis and venous thrombosis,
additional time points should be assessed. Moreover, the potential
underlying mechanisms should be explored in future studies.
The present study successfully constructed a rabbit
model with vinorelbine administration via PICC, and dynamically
observed phlebitis and venous thrombosis. On the day of vinorelbine
administration via PICC, there was a high risk of phlebitis and
thrombosis, which suggested that anticoagulation therapy and
patient care should be provided in that time period. Therefore, the
present study provided a reference for early prediction, timely
prevention and treatment of PICC-related chemotherapy venous
complications, and also provided a theoretical basis for timely
vascular protection, anticoagulation therapy and effective patient
care.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Zhejiang
Province Experimental Animal Science and Technology Planning
Project in 2018 (grant no. 2018C37095).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LH conceived and designed the study. GC and QH
performed the majority of experiments and data analysis, and wrote
the manuscript. BH, LZ and LF performed a small number of
experiments and data analysis, and wrote and revised the
manuscript. LH and GC confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the School of Medicine, Jinhua Polytechnic (Jinhua,
China; approval no. 2019017).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Keren R, Shah SS, Srivastava R, Rangel S,
Bendel-Stenzel M, Harik N, Hartley J, Lopez M, Seguias L, Tieder J,
et al: Comparative effectiveness of intravenous vs oral antibiotics
for postdischarge treatment of acute osteomyelitis in children.
JAMA Pediatr. 169:120–128. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Campagna S, Gonella S, Berchialla P,
Morano G, Rigo C, Zerla PA, Fuzzi R, Corona G, Storto S, Dimonte V
and Mussa B: Can peripherally inserted central catheters be safely
placed in patients with cancer receiving chemotherapy? A
retrospective study of almost 400,000 catheter-days. Oncologist.
24:e953–e959. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Taxbro K, Hammarskjöld F, Thelin B, Lewin
F, Hagman H, Hanberger H and Berg S: Clinical impact of
peripherally inserted central catheters vs implanted port catheters
in patients with cancer: An open-label, randomised, two-centre
trial. Br J Anaesth. 122:734–741. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yu D, Zhang K, Huang L, Zhao B, Zhang X,
Guo X, Li M, Gu Z, Fu G, Hu M, et al: Detection of peripherally
inserted central catheter (PICC) in chest X-ray images: A
multi-task deep learning model. Comput Methods Programs Biomed.
197(105674)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jumani K, Advani S, Reich NG, Gosey L and
Milstone AM: Risk factors for peripherally inserted central venous
catheter complications in children. JAMA Pediatr. 167:429–435.
2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Robinson A, Souied O, Bota AB, Levasseur
N, Stober C, Hilton J, Kamel D, Hutton B, Vandermeer L, Mazzarello
S, et al: Optimal vascular access strategies for patients receiving
chemotherapy for early-stage breast cancer: A systematic review.
Breast Cancer Res Treat. 171:607–620. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fallouh N, McGuirk HM, Flanders SA and
Chopra V: Peripherally inserted central catheter-associated deep
vein thrombosis: A narrative review. Am J Med. 128:722–738.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lv S, Liu Y, Wei G, Shi X, Chen S and
Zhang X: The anticoagulants rivaroxaban and low molecular weight
heparin prevent PICC-related upper extremity venous thrombosis in
cancer patients. Medicine (Baltimore). 98(e17894)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pu YL, Li ZS, Zhi XX, Shi YA, Meng AF,
Cheng F, Ali A, Li C, Fang H and Wang C: Complications and costs of
peripherally inserted central venous catheters compared with
implantable port catheters for cancer patients: A meta-analysis.
Cancer Nurs. 43:455–467. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Perri F, Lazzari G, Scarpati GD and
Silvano G: Oral vinorelbine: A feasible and safe partner for
radiotherapy in the treatment of locally advanced non-small cell
lung cancer. Onco Targets Ther. 9:2359–2364. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Diaby V, Tawk R, Sanogo V, Xiao H and
Montero AJ: A review of systematic reviews of the
cost-effectiveness of hormone therapy, chemotherapy, and targeted
therapy for breast cancer. Breast Cancer Res Treat. 151:27–40.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Harbeck N, Huang CS, Hurvitz S, Yeh DC,
Shao Z, Im SA, Jung KH, Shen K, Ro J, Jassem J, et al: Afatinib
plus vinorelbine versus trastuzumab plus vinorelbine in patients
with HER2-overexpressing metastatic breast cancer who had
progressed on one previous trastuzumab treatment (LUX-Breast 1): An
open-label, randomised, phase 3 trial. Lancet Oncol. 17:357–366.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mondal A, Gandhi A, Fimognari C, Atanasov
AG and Bishayee A: Alkaloids for cancer prevention and therapy:
Current progress and future perspectives. Eur J Pharmacol.
858(172472)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jonna S, Reuss JE, Kim C and Liu SV: Oral
chemotherapy for treatment of lung cancer. Front Oncol.
10(793)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rossi D, Catalano V, Alessandroni P,
Fedeli A, Fedeli SL, Giordani P, Baldelli AM, Casadei V, Ceccolini
M, Ugolini M, et al: A phase II study of single-agent oral
vinorelbine in patients with pretreated advanced non-small-cell
lung cancer. Clin Lung Cancer. 8:382–385. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Markert JE, Jasien JV, Turner DC, Huisingh
C, Girkin CA and Downs JC: IOP, IOP transient impulse, ocular
perfusion pressure, and mean arterial pressure relationships in
nonhuman primates instrumented with telemetry. Invest Ophthalmol
Vis Sci. 59:4496–4505. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ohwada K: Body surface area of the golden
Syrian hamster. Jikken Dobutsu. 41:221–224. 1992.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Oragano CA, Patton D and Moore Z:
Phlebitis in intravenous amiodarone administration: Incidence and
contributing factors. Crit Care Nurse. 39:e1–e12. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wilson BE, Jacob S, Yap ML, Ferlay J, Bray
F and Barton MB: Estimates of global chemotherapy demands and
corresponding physician workforce requirements for 2018 and 2040: A
population-based study. Lancet Oncol. 20:769–780. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bertoglio S, Cafiero F, Meszaros P,
Varaldo E, Blondeaux E, Molinelli C and Minuto M: PICC-PORT totally
implantable vascular access device in breast cancer patients
undergoing chemotherapy. J Vasc Access. 21:460–466. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bertoglio S, Faccini B, Lalli L, Cafiero F
and Bruzzi P: Peripherally inserted central catheters (PICCs) in
cancer patients under chemotherapy: A prospective study on the
incidence of complications and overall failures. J Surg Oncol.
113:708–714. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Qi F, Cheng H, Yuan X and Zhang L:
Comparison of PICC and TIVAP in chemotherapy for patients with
thyroid cancer. Oncol Lett. 20:1657–1662. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang G, Li Y, Wu C, Guo L, Hao L, Liao H,
Xiao X, Liu S and Luo L: The clinical features and related factors
of PICC-related upper extremity asymptomatic venous thrombosis in
cancer patients: A prospective study. Medicine (Baltimore).
99(e19409)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Picardi M, Della Pepa R, Cerchione C,
Pugliese N, Mortaruolo C, Trastulli F, Giordano C, Grimaldi F,
Zacheo I, Raimondo M, et al: A frontline approach with peripherally
inserted versus centrally inserted central venous catheters for
remission induction chemotherapy phase of acute myeloid leukemia: A
randomized comparison. Clin Lymphoma Myeloma Leuk. 19:e184–e194.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ng VL: Prothrombin time and partial
thromboplastin time assay considerations. Clin Lab Med. 29:253–263.
2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tripodi A: Prothrombin time international
normalized ratio monitoring by self-testing. Curr Opin Hematol.
11:141–145. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yang DT, Robetorye RS and Rodgers GM: Home
prothrombin time monitoring: A literature analysis. Am J Hematol.
77:177–186. 2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Naghadeh HT, Maghsudloo M and Tabatabaei
MR: Coagulation factors V, VIII, and X, prothrombin time and
activated partial thromboplastin time test results in thawed plasma
stored at 1-6˚C for 5 days. Blood Transfus. 9:95–98.
2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Onundarson PT, Francis CW, Indridason OS,
Arnar DO, Bjornsson ES, Magnusson MK, Juliusson SJ, Jensdottir HM,
Vidarsson B, Gunnarsson PS, et al: Fiix-prothrombin time versus
standard prothrombin time for monitoring of warfarin
anticoagulation: A single centre, double-blind, randomised,
non-inferiority trial. Lancet Haematol. 2:e231–240. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gusella M, Pasini F, Caruso D, Barile C,
Modena Y, Fraccon AP, Bertolaso L, Menon D, Crepaldi G, Bononi A,
et al: Clinical outcomes of oral metronomic vinorelbine in advanced
non-small cell lung cancer: Correlations with pharmacokinetics and
MDR1 polymorphisms. Cancer Chemother Pharmacol. 83:493–500.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yao Z, Zhang Y and Wu H: Regulation of
C-reactive protein conformation in inflammation. Inflamm Res.
68:815–823. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pathak A and Agrawal A: Evolution of
C-reactive protein. Front Immunol. 10(943)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Setiadi H, Yago T, Liu Z and McEver RP:
Endothelial signaling by neutrophil-released oncostatin M enhances
P-selectin-dependent inflammation and thrombosis. Blood Adv.
3:168–183. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wei Y, Chen X, Shen H, Wu W, Cao G, Chen
W, Wang Y, Shen H, Yu S and Zhang J: P-selectin level at first and
third day after portal hypertensive splenectomy for early
prediction of portal vein thrombosis in patients with cirrhosis.
Clin Appl Thromb Hemost. 24 (9_Suppl):76S–83S. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Riva N, Camporese G, Iotti M, Bucherini E,
Righini M, Kamphuisen PW, Verhamme P, Douketis JD, Tonello C,
Prandoni P, et al: Age-adjusted D-dimer to rule out deep vein
thrombosis: Findings from the PALLADIO algorithm. J Thromb Haemost.
16:271–278. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wells PS, Anderson DR, Rodger M, Forgie M,
Kearon C, Dreyer J, Kovacs G, Mitchell M, Lewandowski B, Kovacs MJ,
et al: Evaluation of D-dimer in the diagnosis of suspected
deep-vein thrombosis. N Engl J Med. 349:1227–1235. 2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Orlandi P, Banchi M, Alì G, Desidero TD,
Fini E, Fontanini G and Bocci G: Active metronomic vinorelbine
schedules decrease plasma interleukin-2 levels in mice with Lewis
lung carcinoma. J Chemother. 33:198–202. 2021.PubMed/NCBI View Article : Google Scholar
|