Introduction
Ophthalmic herpes zoster (OHZ) is a frequent
localization of zoster (1/5 of cases) that is characterized by
typical vesicular lesions arranged on an erythematous-edematous
background along the path of the trigeminal nerve ophthalmic branch
(1). OHZ is five times more
frequent than the mandibular and maxillary branches' damage
(2). The impairment of the
nasociliary ramification (ophthalmic branch) is clinically
expressed by the Hutchinson sign (specific lesions on the lateral
edge of the nasal pyramid up to the tip of the nose), and predicts
ocular damage (3).
The increasing incidence for herpes zoster,
including OHZ, may be due to immunosenescence (increased risk after
60 years) and acquired immunosuppression [the increasing number of
neoplasms (particularly haematologic)], transplanted and patients
infected with human immunodeficiency virus (HIV), the use of
immunosuppressive, chemotherapeutic, biological therapies, systemic
corticosteroids for long periods, virulence of the varicella-zoster
virus (VZV) and the general immune status of the patient (4).
In OHZ, acquired immunosuppression is frequently
induced by organ transplants and immunosuppressive therapies, HIV
infection (15-25 times higher) and neoplasms (5). OHZ may present with severe forms in
immunosuppressed patients, with long, exhaustive evolutions, which
require early systemic therapies with permanent monitoring of
potential complications (6).
Delayed referrals to specialist doctors and cataract surgery favour
the severe forms of OHZ (7,8). OHZ has a higher risk of developing
ocular complications; before antiviral therapy the risk was 50% and
now the risk has decreased to under 29% (1).
The most common complications associated with OHZ
are cutaneous, including ulcerations, cellulitis, vicious scars in
necrotic forms, ectropion, entropion and risk of bacterial
superinfections with multi-resistant germs, such as
methicillin-resistant Staphylococcus aureus (MRSA)
and Pseudomonas aeruginosa (9).
Ocular complications can be diverse, from mild,
reversible to severe complications resulting in irreversible
blindness. The following may occur at the oculo-palpebral level:
Episcleritis, scleritis, keratitis, conjunctivitis, iridocyclitis,
anterior and posterior uveitis, ciliary body ischemia, secondary
glaucoma, vitritis, cataracts, pupillary lesions (Horner syndrome),
paralysis of cranial nerves (III, IV-VI), orbital apex syndrome,
orbital abscess, lesions of the optic nerve (optic nerve atrophy,
retrobulbar optic neuropathy) or retinal lesions (hemorrhagic
retinitis, acute retinal necrosis, choroiditis, branchial or
central retinal artery obstruction and retinal detachment)
(6,10). Acute retinal necrosis is often
caused by VZV and presents as acute iridocyclitis, inflammation of
the vitreous body, necrotizing retinitis, occlusive retinal
vasculitis, rapid loss of vision and retinal detachment (11).
Among the neurological complications in OHZ, the
most common is postherpetic neuralgia, which increases with age
(36.6% of patients with OHZ are >60 years; 47.5% of patients
with ophthalmic herpes are >70 years) (12). Following the remission of acute
infection, chronic inflammation persists in the trigeminal area,
with perineural lymphocyte infiltrates (trigeminal tract and
mesencephalic nucleus) lasting months or years after the eruption
of OHZ (12). Manifestation of
postherpetic neuralgia decreases with time, reaching 30% at 6 weeks
and 9% 1 year after onset (13).
Other neurological complications include cranial nerves (III, IV
and VI) palsies, Ramsay-Hunt syndrome, palpebral ptosis,
sympathetic ophthalmia, contralateral hemiplegia, encephalitis and
myelitis (12,14,15).
In severe forms of zoster, mortality can be high, particularly in
disseminated types (5-15%) (16).
The antiviral agents (for example, acyclovir)
inhibits viral DNA polymerase, thus decreasing viral replication
(17). Therefore, the oral
antiviral therapies or intravenous antiviral agents (for severe
forms or for patients who are immunosuppressed) decrese
complications in OHZ.
Case report
A male, aged 54 years old, diagnosed with chronic
lymphocytic leukemia (CLL), failed to attend follow-up
consultations for 1 year. The patient was admitted to the Emergency
Hospital of Sibiu County's Dermatology Clinic in August 2016 due to
an extensive, extremely painful, erythematous, vesicular-bullous
rash, marked edema of the right fronto-orbital level and half of
the scalp, impossibility of opening the palpebral cleft and
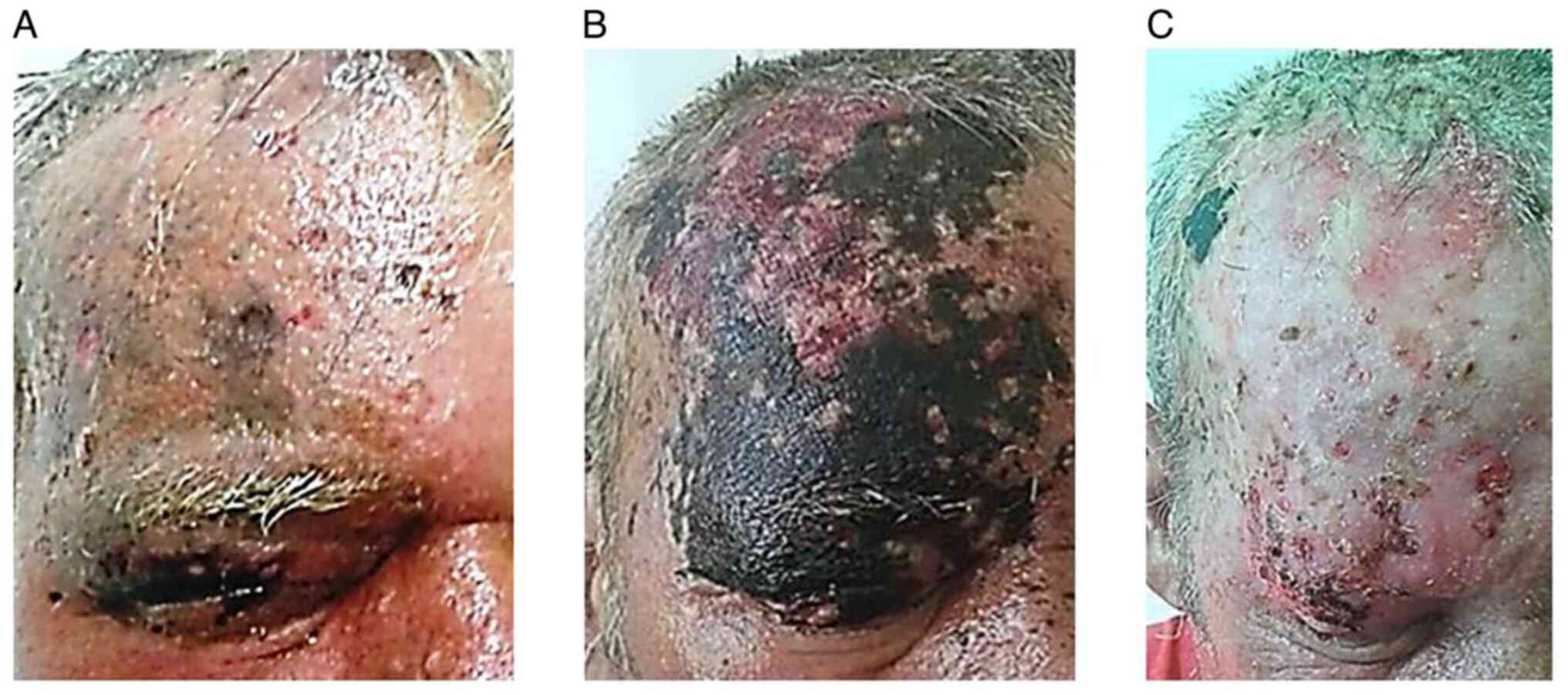
high-intensity headaches (Fig. 1A),
which debuted 1 week prior. Following admission, the patient had
subfebrile temperature (37.5˚C), with large, painful, hard lymph
nodes, adherent to the superficial and deep planes on the right
side of the submandibular area.
Ophthalmologic examination of the right eye (RE)
revealed conjunctival changes (chemosis, mixed conjunctival
hyperemia and purulent conjunctival secretion), at the level of the
anterior pole corneal changes (epithelial and stromal edema, and
hypotransparency of the cornea), palpebral changes (hard edema and
erythematous-vesicular-bullous rash) and protrusion of the eyeball.
The posterior pole was difficult to examine due to severity of the
swollen eyelids and hypotransparency of the cornea. In both eyes,
fine crystalline densifications were present; however, motility was
preserved in all quadrants. Examination of the left eye (LE) was
within normal limits. Laboratory investigations confirmed the
leukemic status (leukocytes, 120,000/mm3), inflammatory
syndrome and superinfection of ulcerations with MRSA.
After 24 h, eruption of the zoster disseminated.
Within 3 days, the lesions rapidly evolved towards deep, necrotic
ulcerations, extending in the periphery and depth, at the right
fronto-parietal level (Fig. 1B),
including the palpebral level, where it caused palpebral retraction
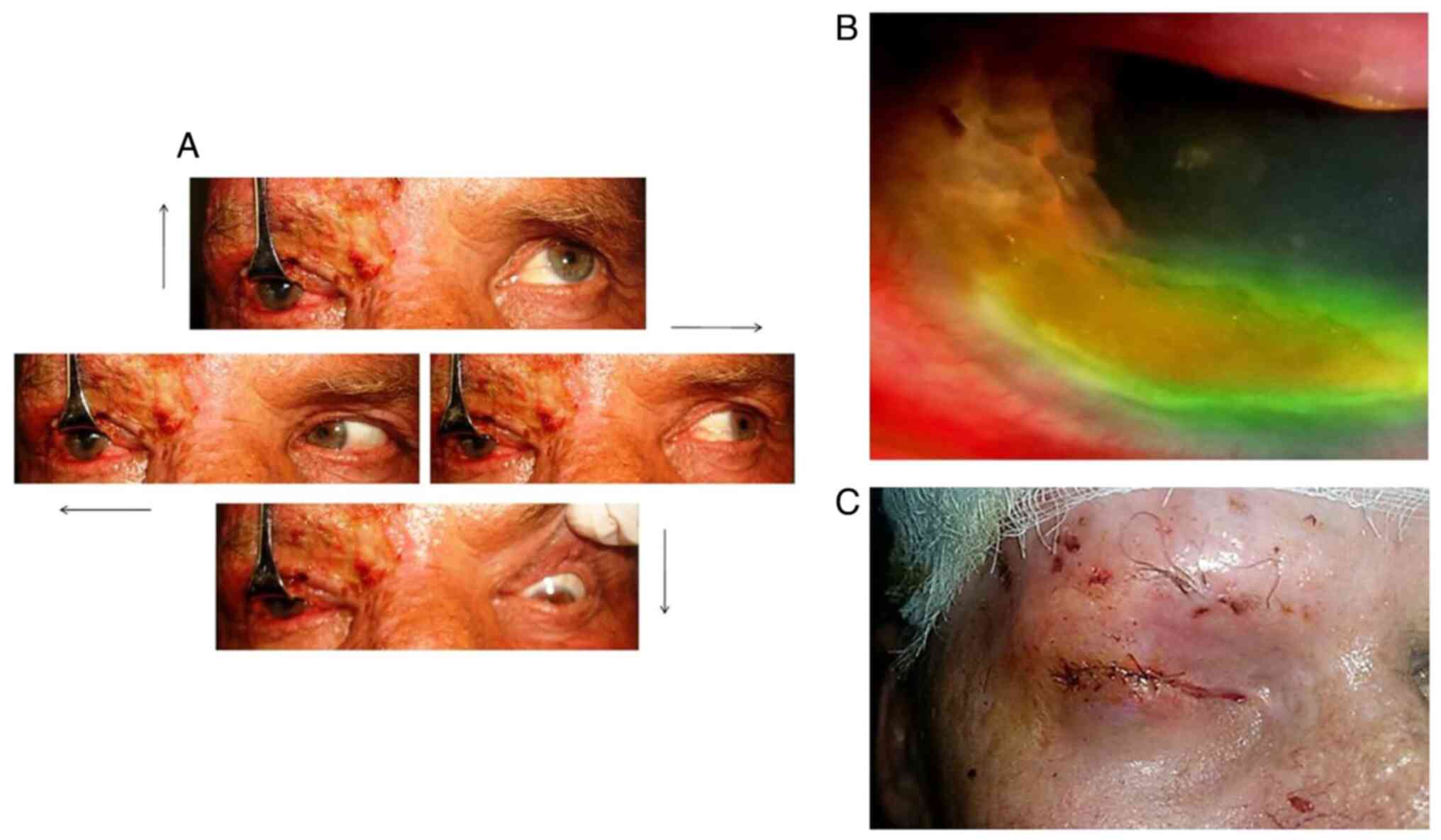
and lagophthalmia. Ophthalmological examination revealed decreased
visual acuity ofthe RE (at 50 cm), RE hypotransparency in 2/3
inferior cornea, chemosis, immobile eyeball, semimydriasis,
fixed/non-reflexive pupils, exophthalmia (RE, 23 mm; LE, 16 mm) and
RE complete ophthalmoplegia (Fig.
2A).
Given the severity of the disease and
immunosuppressed status induced by CLL, acyclovir was injected at
3x750 mg/day for 7 days, followed by oral therapy with 4 g/day of
acyclovir for an additional 10 days. This therapy was associated
with systemic anti-inflammatory, antialgic treatment, opioids,
antiepileptics, antibiotics (gentamicin 160 mg/day for 7 days +
cotrimoxazole 4x480 mg/day for 14 days), antifungals and group B
vitamin therapy. Necrectomy and disinfection with antiseptic
solutions were performed locally, as well as application of
epithelializing ointments with Kanamycin sulfate. Simultaneously,
the patient was evaluated and treated by the ophthalmologist with
topical ocular antivirals (acyclovir), antibiotics
(chloramphenicol), epithelializing (vitamin A and guma xantan) and
mydriatic agents (Tropicamide). Resumption of chemotherapy was
unable to be performed for CLL due to the risk of viral
multiplication and the possibility of other complications.
After 1 month, the patient was discharged with
fronto-parietal granular ulcerations, palpebral scar-like
retractile ulcerations, alopecia of RE eyelashes and eyebrows and
significant reduction of RE visual acuity (Fig. 1C). The patient was admitted in
September 2016 to the Ophthalmology Clinic for ocular pain, marked
photophobia and almost complete loss of visual acuity in the RE
(the patient perceived hand movement) 1 week after discharge. RE
examination revealed abundant conjunctival secretion, corneal and
conjunctival hypoesthesia, corneal ulcer in the half lower part of
the cornea, with high permeability to fluorescein and peripheral
corneal neovascularisation (Fig.
2B). Furthermore, the patient presented low RE visual acuity,
perceived hand movements and hyphema (4-5 mm blood in the inferior
part of anterior chamber). Intraocular tension was within normal
limits (RE, 14 mm Hg; LE, 18 mm Hg), which excluded the diagnosis
of secondary glaucoma. MRSA was isolated from the
conjunctival secretion, with the same spectrum of sensitivity as
during the previous admission. In this case, the corneal ulcer was
complicated by the anterior hemorrhagic uveitis and the disciform
keratitis that evolved unfavorably towards the neurotrophic
keratitis superinfected with MRSA. At the fronto-parietal
level, there were ulcerations covered by yellow, adherent and
painful crusts.
The systemic therapy included 3 weeks of antivirals,
antibiotics (cotrimoxazole for 2 weeks), antifungals and group B
vitamins. Topical antibiotics and epithelizing agents were applied
on the ulcerations. Corneal epithelialization agents, heparinized
blood sample of the patient, glucose solution and other nutrients
were administered on the ocular surface. After 3 weeks of
treatment, the patient presented with a persistent corneal
epithelial defect in 2/3 of the lower part, hyphema of smaller
dimensions (~0.5 mm), lagophthalmia due to persistent upper eyelid
necrosis, ophthalmoplegia and fronto-parietal granular ulcerations.
Thus, blepharoraphy (a type of surgery, which is performed to
maintain the anatomical integrity of the eyeball) was performed to
prevent corneal ulcerations that may have resulted in perforation
and loss of the eyeball. Blepharoraphy was performed to restore the
necrotic upper eyelid and inferior eyelid (Fig. 2C).
After 2 months, the patient returned with partially
epithelialized ulcerations of the scalp, with blepharoraphy and was
referred to the Department of Hematology from County Emergency
Hospital of Sibiu (Romania), for CLL therapy. Table I lists the complications associated
with OHZ.
 | Table IComplications associated with
ophthalmic herpes zoster (OHZ) in different types of cancer. |
Table I
Complications associated with
ophthalmic herpes zoster (OHZ) in different types of cancer.
| Authors, year | Case report no. | Age, years | Sex | Cancer | Complications | (Refs.) |
|---|
| Cheema et al,
2019 | 1 | 63 | M | B-cell lymphoma | Necrotic ulcers on
tri-segmental CN V distribution, trismus, right CN III and IV
palsies, endotheliitis, iridocyclitis and
blepharoconjunctivitis | (18) |
| Srinivasan et
al, 2009 | 2 | 66 | F | Breast cancer |
Meningoencephalitis | (19) |
| Harthan and Borgman,
2013 | 3 | 84 | F | Breast cancer | CN III palsy with
ophthalmoplegia | (20) |
| Khalafallah et
al, 2013 | 4 | 72 | F | Multiple myeloma | Herpetic neuralgia,
conjunctivitis and corneal pseudodendrites | (21) |
| | 5 | 50 | F | Multiple myeloma | Optic neuritis | (21) |
| | 6 | 68 | M | Multiple myeloma | Postherpetic
neuralgia | (21) |
| Rajkumar and Baum,
2016 | 7 | 71 | F | Uterine and thyroid
cancers | Cerebral venous sinus
thrombosis | (22) |
| Letchuman and
Donohoe, 2019 | 8 | 62 | M | Laryngeal
cancer | Ramsey-Hunt
syndrome, left CN VI and VII palsies, diplopia and conductive
hearing loss | (23) |
| Mercier et
al, 2019 | 9 | 68 | M | Lung
adenocarcinoma | Ramsey-Hunt
syndrome | (24) |
| Chen et al,
2016 | 10 | 63 | M | Chronic lymphocytic
leukemia | Ramsey-Hunt
syndrome and exposure keratopathy | (25) |
| Pointdujour et
al, 2014 | 11 | 70 | F | Chronic lymphocytic
leukemia | Acute orbital
syndrome | (26) |
| Sanghvi et
al, 2006 | 12 | 83 | F | Chronic lymphocytic
leukemia | Cicatricial
ectropion | (27) |
Discussion
OHZ appears in 1/5 of all cases with herpes zoster,
accompanied by potential skin, ocular, neurological or visceral
complications (1). Reactivation,
multiplication and retrograde migration of VZV along the trigeminal
nerve pathway (ophthalmic branch) is clinically expressed by a
specific eruption on the corresponding dermatome, with variable
ocular damage that is mostly limited at the cornea. Under
immunosuppression, particularly that acquired through neoplasms or
immunosuppressive therapies, the risk of developing severe forms
that evolve towards disabling complications, such as retractable
scars, blindness and cranial nerve palsies, is higher compared with
immunocompetent patients (1,6).
Currently, there are only a few published cases of
OHZ with multiple complications in patients with cancer (18-27).
These published cases and additional articles were searched on
PubMed using the following terms: ‘Ophthalmic herpes zoster’,
‘cancer patients’, ‘multiple complications’ and ‘case report’. A
total of 12 cases of complicated OHZ in patients with
hematological, breast, uterine, thyroid, laryngeal and lung cancers
were identified (Table I).
In the present study, considering the
immunosuppression acquired by CLL as a major risk factor, the
patient developed a severe form of OHZ with multiple complications
(Table II). The rapid evolution of
ulcers in a necrotic form suggests that OHZ is associated with
ecthyma gangrenosum, induced by Pseudomonas aeruginosa, due
to immunosuppression (28). The
bacteriological exam infirms this suspicion but confirms that
ulcers were superinfected with MRSA.
 | Table IISevere and multiple complications
developed by the patient in the present study. |
Table II
Severe and multiple complications
developed by the patient in the present study.
| A, Skin |
|---|
| Complication | Clinical
manifestation |
|---|
| RE palpebral and
right fronto-parietal necrotic ulcerations superinfected with
MRSA | Extensive necrotic
ulcerations in the right hemicranium |
| RE vicious
palpebral scars | In evolution, by
detaching the necrosis, the ulcerations healed with retractable
scars and necrosis of the RE upper eyelid |
| B, Ocular |
| Complication | Clinical
manifestation |
| Herpetic
keratoconjunctivitis | RE visual acuity to
hand motion. |
| | Chemosis, purulent
conjunctival secretion. |
| | Round oval areas of
epithelial and stromal edema, descemet folds (signs specific to
disciform keratitis, complication that usually appears late) |
| Complete
ophthalmoplegia | Right, immobile
eyeball, semimydriasis, fixed and no reflexive pupil |
| Lagophthalmia | Lack of substance
in the RE upper eyelid with defective confrontation of the eyelids,
lagophthalmia, exposure keratopathy, corneal ulceration and require
blepharorhaphy |
| Anterior
hemorrhagic uveitis with hyphema | Marked photophobia,
semimydriasic pupil, fixed, non-reflexive, unevenly dilated, oval,
anterior chamber with inflammatory reaction and 4-5 mm hyphema
arranged inferiorly and nasally |
| RE blindness | Loss of visual
acuity in RE |
| C,
Neurological |
| Complication | Clinical
manifestation |
| Post-herpetic
neuralgia | Painful headaches
of increased intensity on the right hemicranium (unilaterally at
the level of the scalp, forehead, upper eyelid and the middle third
of the lower eyelid of RE, nose wing and right eyeball) |
Complete ophthalmoplegia (simultaneous paralysis of
the three cranial nerves, III, IV and VI) is a rare complication
(11-29% of cases with OHZ) (20).
Shin et al (29) performed a
study at Moorfields Eye Hospital on 146 patients with OHZ who were
>50 years and found that 6.82% of patients had ophthalmoplegia
(2.73% complete form; 2.05% unilateral form; 1.36% unilateral form
associated with proptosis and 0.68% bilateral form with proptosis)
(29).
Multiple cranial nerve paralysis in OHZ can be
explained by the direct cytopathic effect of the virus, acting on
surrounding neuronal tissues and inflammation of the trigeminal
nerve due to VZV reactivation that can spread through the cavernous
sinus, thus affecting other nerves (30). At the vascular level, occlusive
vasculitis may occur induced by the chronic inflammatory cellular
changes of the virus (31).
In the present study, the cytopathic effect of VZV
was aggravated by the immunosuppressed status and delayed treatment
due to late presentation. Regarding the evolution of the corneal
ulcer to anterior hemorrhagic uveitis, there are only a few cases
of uveitis with hyphema reported (32-35).
Hyphema, a complication of OHZ, has been reported in even fewer
cases (36). The cause of bleeding
is assumed to be a process of occlusive vasculitis (32).
In the analysis of the immediate prognosis, it is
important to consider the risk of the corneal perforation of the
ulcer, evolution towards corneal leukoma/loss of the eyeball,
chronic ocular inflammation, acute retinal necrosis with the
consecutive loss of vision and the orbital apex syndrome (37). In assessing the late prognosis, it
is important to consider that postherpetic neuralgia can last for
months/years, with an increased risk of the patient's
immunosuppression (38). In
addition, immunocompromised patients have a higher risk (40%) of
visceral dissemination, and the recovery of ophthalmoplegia is
extremely difficult (may take 2-18 months) (4).
The prognosis was reserved due to the general
oncological pathology, and the severe form of OHZ with multiple and
severe ocular, cutaneous and neurological complications that
developed.
In conclusion, in the present study, the severe form
of OHZ was favored by non-timely referral of the patient to a
medical service, the immunosuppression acquired through CLL and
patient non-compliance with therapy. Thus severe OHZ with multiple
complications, including cutaneous (ulceronecrotic, superinfected
ulcers and scars), ophthalmologic (palpebral retraction and
necrosis, lagophthalmia, kerato-conjunctivitis, total
ophthalmoplegia, anterior hemorrhagic uveitis with hyphema and RE
blindness) and neurologic (postherpetic neuralgia) occurred.
The peculiarity of this case consists of the
extremely rare occurrence of complete ophthalmoplegia within the
OHZ and the hyphema in the herpetic uveitis. This case is among the
few of OHZ with multiple and severe complications in
immunosuppressed cancer patients reported in the literature.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GMI was involved in research-creation and study
design, analysis and interpretation of patient data, manuscript
drafting and critical revision of the manuscript for important
intellectual content. DMS was involved in data acquisition,
analysis and interpretation of patient data, manuscript drafting,
and study design. RCC was involved in data acquisition, analysis
and interpretation of patient data, manuscript drafting and study
design. MR was involved in research-creation, design, analysis and
interpretation of patient data, manuscript drafting and critical
revision of the manuscript for important intellectual content. All
authors have read and approved the final manuscript. All authors
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The study has been approved by the Ethics Committee
of the County Emergency Hospital of Sibiu, Romania (approval no.
24505)
Patient consent for publication
Written informed consent for publication was
provided by the patient prior to the study start.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tran KD, Falcone MM, Choi DS, Goldhardt R,
Karp CL, Davis JL and Galor A: Epidemiology of herpes zoster
ophtalmicus: Recurrence and chronicity. Ophtalmology.
123:1469–1475. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Galil K, Choo PW, Donahue JG and Platt R:
The sequelae of herpes zoster. Arch Intern Med. 157:1209–1213.
1997.PubMed/NCBI
|
|
3
|
Shaikh S and Ta CN: Evaluation and
management of herpes zoster ophthalmicus. Am Fam Physician.
66:1723–1730. 2002.PubMed/NCBI
|
|
4
|
Dworkin RH, Johnson RW, Breuer J, Gnann
JW, Levin MJ, Backonja M, Betts RF, Gershon AA, Haanpaa ML,
McKendrick MW, et al: Recommendations for the management of herpes
zoster. Clin Infect Dis. 44 (Suppl 1):S1–S26. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Wiafe B: Herpes zoster ophthalmicus in
HIV/AIDS. Community Eye Health. 16:35–36. 2003.PubMed/NCBI
|
|
6
|
Opstelten W and Zaal MJ: Managing
ophthalmic herpes zoster in primary care. BMJ. 331:147–151.
2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Long MD, Martin C, Sandler RS and
Kappelman MD: Increased risk of herpes zoster among 108 604
patients with inflammatory bowel disease. Aliment Pharmacol Ther.
37:420–429. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Korber A, Franckson T, Grabbe S and
Dissemond J: Ambilateral reactivation of herpes zoster V2 following
cataract operation of both eyes. J Eur Acad Dermatol Venereol.
21:712–713. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Atzori L, Ferreli C, Zucca M, Fanni D and
Aste N: Facial cellulitis associated with complicating ophthalmic
herpes zoster. Dermatol Online J. 10:2004.PubMed/NCBI
|
|
10
|
Lavaju P, Badhu BP and Shah S: Herpes
zoster ophthalmicus presenting as orbital abscess along with
superior orbital fissure syndrome: A case report. Indian J
Ophthalmol. 63:733–735. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Anthony CL, Bavinger JC and Yeh S:
Advances in the diagnosis and management of acute retinal necrosis.
Ann Eye Sci. 5(28)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zaal MJ, Völker-Dieben HJ and D'Amarao J:
Prognostic value of Hutchinson's sign in acute herpes zoster
ophthalmicus. Graefes Arch Clin Exp Ophthalmol. 241:187–191.
2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Scott FT, Leedham-Green ME, Barrett-Muir
WY, Hawrami K, Gallagher WJ, Johnson R and Breuer J: A study of
shingles and the development of postherpetic neuralgia in East
London. J Med Virol. 70 (Suppl 1):S24–S30. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rousseau A, Bourcier T, Colin J and
Labetoulle M: Herpes zoster ophthalmicus-diagnosis and management.
US Ophthalm Rev. 6:119–124. 2013.
|
|
15
|
Liesegang TJ: Herpes zoster ophthalmicus
natural history, risk factors, clinical presentation, and
morbidity. Ophthalmology. 115 (Suppl 2):S3–S12. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gnann JW Jr and Whitley RJ: Clinical
practice. Herpes zoster. N Engl J Med. 347:340–346. 2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kausar S, Said Khan F, Ishaq Mujeeb Ur
Rehman M, Akram M, Riaz M, Rassol G, Hamid Khan A, Saleem I, Shamim
S and Malik A: A review: Mechanism of action antiviral drugs. Int J
Immunopathol Pharmacol. 35(20587384211002621)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cheema HS, Diedrich AM, Kyne BM and Toeque
M: A case of tri-segmental cranial nerve V herpes zoster. IDCases.
18(e00642)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Srinivasan S, Ahn G and Anderson A:
Meningoencephalitis-complicating herpes zoster ophthalmicus
infection. J Hosp Med. 4:E19–E22. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Harthan JS and Borgman CJ: Herpes zoster
ophthalmicus-induced oculomotor nerve palsy. J Optom. 6:60–65.
2013.
|
|
21
|
Khalafallah AA, Woodgate M, Koshy K and
Patrick A: Ophthalmic manifestations of herpes zoster virus in
patients with multiple myeloma following bone marrow
transplantation. BMJ Case Rep. 2013(bcr2012007625)2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rajkumar A and Baum E: Cerebral venous
sinus thrombosis resulting from herpes zoster infection in an older
adult. Ann Longterm Care Clin Care Aging. 24:27–30. 2016.
|
|
23
|
Letchuman V and Donohoe CD: Brainstem and
cerebellar involvement in Ramsay Hunt syndrome. Case Rep
Otolaryngol. 2019(7605056)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mercier T, Deslypere G and Nackaerts K:
Ramsay Hunt syndrome: A rare complication of herpes zoster
infection in a lung cancer patient. Acta Clin Belg. 74:355–358.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen I, Fohtung RB, Oughli HA, Bauer R,
Mattar C, Powderly WG and Thoelke MS: Concurrent Ramsay Hunt
syndrome and disseminated herpes zoster in a patient with relapsed
chronic lymphocytic leukemia. IDCases. 8:79–82. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pointdujour R, Temnogorod J, Mancini R,
Chang SH, Esmaeli B and Shinder R: Acute orbital syndrome in herpes
zoster ophthalmicus. Invest Ophthalmol Vis Sci. 55(4082)2014.
|
|
27
|
Sanghvi CA, Leatherbarrow B and Ataullah
S: Cicatricial ectropion due to herpes zoster ophthalmicus. J
Postgrad Med. 52:153–154. 2006.PubMed/NCBI
|
|
28
|
Birlutiu V, Birlutiu RM, Baicu M and Iancu
GM: A case report of double etiology of ecthyma gangrenosum:
Pseudomonas aeruginosa and Enterococcus faecalis in
an immunocompromised child occurred during influenza evolution.
Medicine (Baltimore). 98(e15651)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shin HM, Lew H and Yun YS: A case of
complete ophthalmoplegia in herpes zoster ophthalmicus. Korean J
Ophthalmol. 19:302–304. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lee JH, Heo HJ, Kim KM, Lee HG, Baek SM
and Jung DW: Herpes zoster in the ophthalmic branch of the
trigeminal ganglia obscuring cavernous sinus thrombosis due to
streptococcus constellatus subsp. Constellatus-a case report.
Anesth Pain Med (Seoul). 15:205–208. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Trottini M and DelGiofice M: When herpes
causes 3rd nerve palsy, patients and practitioners suddenly have to
deal with multiple issues. Rev Optom. 153:24–25. 2016.
|
|
32
|
Okuniki Y, Sakai J, Kezuka T and Goto H: A
case of herpes zoster uveitis with severe hyphema. BMC Ophthalmol.
14(74)2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Akpek EK and Gottsch JD: Herpes zoster
sine herpete presenting with hyphema. Ocul Immunol Inflamm.
8:115–118. 2000.PubMed/NCBI
|
|
34
|
Arvinth R, Zahari M and Reddy SC: Herpes
zoster anterior uveitis and hyphema. Eur J Medical Health Sci.
3:1–3. 2021.
|
|
35
|
Hayasaka S, Watanabe M, Yamamoto Y, Noda
S, Sekimoto M and Setogawa T: Herpes zoster ophthalmicus
complicated by hyphema and hemorrhagic glaucoma. Ophtalmologica.
196:185–187. 1988.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Katherine SBH, Ngim YS, Juliana J and
Ramli N: Herpes zoster keratouveitis with hypopyon and hyphema.
Taiwan J Ophthalmol. 10:54–57. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lee CY, Tsai HC, Jung Lee SS and Chen YS:
Orbital apex syndrome: An unusual complication of herpes zoster
ophthalmicus. BMC Infect Dis. 15(33)2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Forbes HJ, Bhaskaran K, Thomas SL, Smeeth
L, Clayton T, Mansfield K, Minassian C and Langan SM:
Quantification of risk factors for postherpetic neuralgia in herpes
zoster patients: A cohort study. Neurology. 87:94–102.
2016.PubMed/NCBI View Article : Google Scholar
|