Introduction
The anterior cruciate ligament (ACL) and meniscus
are intra-articular structures that have an inadequate healing
capacity (1-4).
Due to the high involvement of these structures in the knee
mobility and stability (3,4), injuries located at this site have
always posed a challenge to the physician, this leading to various
treatment protocols being developed and modified throughout the
years (5,6).
Biological products, such as platelet-rich plasma
(PRP), mesenchymal stem cells (MSC), collagen products or
hyaluronic acid, as a primary option or as an enhancement
treatment, have gained increasing popularity in the previous years.
New procedures have been designed and perfected to enhance the cell
receptivity and, therefore, the tissue repair in various areas of
the knee, such as ligaments or meniscal tissue (7-9).
Recently, teenagers and children involved in
professional or recreational athletics have increased considerably,
therefore generating a magnified incidence of sports-related knee
trauma. Additionally, the awareness of these injuries has expanded,
which results in an increased prevalence of knee lesions in the
pediatric population.
PRP is an autologous blood product containing
various growth factors, which have exhibited beneficial effects on
cell proliferation and differentiation, extracellular matrix
production, cell migration, chemotaxis and angiogenesis (10) [Table
I (11,12)]. In addition to the multiple growth
factors, desirable features of PRP include the absence of
immunogenicity phenomena, emphasizing its safety levels when
administered to patients (2,8).
 | Table IGrowth factors and their cellular
effects. |
Table I
Growth factors and their cellular
effects.
| Growth factor | Cellular effect |
|---|
| TGF-β1 | Enhances the
proliferative activity of fibroblasts |
| FGF-2 | Promotes
angiogenesis, cell migration, cell differentiation and
extracellular matrix production |
| IGF-1 | Has a chemotactic
effect on myoblasts and fibroblasts |
| PDGF | Stimulates the
proliferation and chemotaxis of mesenchymal cells |
| VEGF | Has a chemotactic
effect on macrophages and granulocytes; promotes angiogenesis |
Recently, PRP has become a popular choice for soft
tissue repair, for example, in aesthetic medicine (13). In terms of orthopedics and sports
medicine, it has shown promising results on pain relief in both
in vivo and in vitro models (8,14-16).
The menisci are crescent-shaped, fibrocartilaginous
structures that have a key-role in static weight-bearing,
knee-stabilization, compressive force distribution, joint
lubrication and proprioception (2-4).
Degenerative or osteoarthritic changes emerging after total or
subtotal meniscectomy emphasize the importance of preserving or
repairing these structures, whenever possible, as an endeavor to
maintain the normal functioning of the knee joint (4,17).
When discussing the clinical outcome of meniscal
injuries, the vascularization of this tissue is a significant
factor. From intrauterine development and shortly after birth, the
menisci are fully vascularized structures. With time, the
vascularization subsides, thus by the age of 10, only 10-30% of the
meniscus is vascularized, and at maturity, only 10-25% of the
peripheral tissue contains blood vessels (18,19) In
young patients, the meniscus has an adequate healing capacity,
while in teenagers and children aged over 10, two models related to
the location of the injury can be observed: One located in the
vascular area, where the blood supply delivers nutritive factors
which promote the differentiation of mesenchymal stem cells
(19) and one located in the
avascular area, where the healing process depends only on the
intrinsic self-repair capacity of the meniscus (18-20).
According to Lotysch et al (21), on MRI, meniscal lesions can be
classified into four different types: Grade 1 reveals a small focal
area of hyperintensity, with no extension to the articular surface.
In this case, non-operative care and physical therapy are
recommended. Grade 3 abnormal meniscal signals are referred to as
definite meniscal tears, and in this case, the meniscal repair is
an adequate treatment (especially in athletes and active patients).
Grade 2 includes linear areas of hyperintensity and no extension to
the articular surface (because some grade 2 signals were found to
be associated with a meniscal tear on arthroscopy, they were
further subdivided into 2a, 2b and 2c).
Management for grade 2 meniscal lesions continue to
be controversial, as numerous patients have persisting pain after
conservative treatment. Blanke et al considered that this
category of patients could benefit from using biological products
as an enhancement of primary therapy (22).
ACL plays an important role in knee stability and
normal biomechanics (6). Injuries
at this site, mainly developed in sports participation, have been
associated with instability, decreased physical activity, low
quality of life overall and are considered to be some of the most
devastating knee injuries (19).
Long-term, patients with ACL lesions have the risk of developing
degenerative changes in the knee, meniscal tears or chondral
lesions (23,24). Although reconstruction is currently
the gold standard for ACL lesions, this procedure is associated
with multiple complications and incomplete return to sports
(25).
When addressing young patients, sports medicine and
orthopedics must take into consideration the importance of ensuring
that long-term complications of ACL and meniscal injuries are
diminished. The clinical outcome of this age category has the
singularity of a high percentage of patients involved in physical
activity (recreational/professional).
Due to young patients requiring a different
approach, there is a constant tendency of designing new protocols,
with literature suggesting that teenagers and children who have
suffered partial ACL tears, or grade 2 meniscal injuries (21), could benefit from the use of
biologics, such as PRP, in terms of pain relief, joint stability
improvement and resuming of physical activity (22,26).
In order to comprehensively assess knee function
impairment, as well as treatment efficiency, patient-reported
outcome measures (PROM) are necessary.
The revised Lysholm knee scoring scale (27) consists of eight domains which
evaluate: Pain (25 points), instability (25 points), locking (15
points), swelling (10 points), limp (5 points), stair climbing (10
points), squatting (5 points), and need for support (5 points).
Every question response has been appointed a score on an increasing
scale. The final score is represented by the sum of all the
answers, and may vary from 0 to 100. Higher scores have been
associated with an improved outcome.
The Lysholm knee scoring scale has an acceptable
test-retest reliability, criteria validity, construct validity,
responsiveness to change (28), and
it is currently used to assess various knee conditions.
Materials and methods
A retrospective observational study on 72 patients
(48 females and 24 males), aged between 11 and 17 years old, was
conducted on children and teenagers, defined as recreational
athletes, who are involved in sports activities at school or at
home, but who do not qualify as professionals. These patients were
admitted to the Pediatric Orthopedics Clinic of ‘Grigore
Alexandrescu’ Children's Emergency Hospital, (Bucharest, Romania)
between January 2018 and December 2019. Inclusion criteria were as
follows: patients who suffered a knee sprain and underwent X-ray
and MRI imaging, and were diagnosed with grade 2 meniscal injury
and grade 2 ACL lesion. Through the use of X-rays, the patients who
had a fracture, a subchondral fracture or any other bony avulsion
which would require other treatment solutions were excluded from
the present study. A total of 55 patients were diagnosed with a
grade 2 meniscal injury [MRI grading (21)] and 17 patients with grade 2 ACL
lesion (MRI grading). They underwent PRP as an enhancement of
primary treatment (after cast immobilization). The study was
approved (approval no. 23223/05.12.2017) by the Ethics Committee of
‘Grigore Alexandrescu’ Children's Emergency Hospital (Bucharest,
Romania). Preceding the study, all legal guardians of the patients
signed written informed consent after receiving a detailed
explanation of all the procedures the patients would undergo.
Upon admission, trauma history was recorded, and a
clinical exam and X-ray were performed. After fracture cases were
excluded using X-rays, the cases diagnosed as knee sprains
underwent cast immobilization for three weeks. Following removal of
cast immobilization, all patients followed a one-month recovery
protocol, which included physiotherapy, anti-inflammatory drugs,
and physical activity restraint. During this time, if there was no
improvement in pain levels, joint mobility, or knee swelling, an
MRI was performed. Following the MRI diagnosis, the patients
underwent PRP therapy. Since it meets the criteria of a PROM, the
Lysholm knee scoring scale was conducted before the first PRP
injection. The patients were also requested to rate their pain
intensity using the Numeric Pain Rating Scale (NPRS) (29). The same injection technique was used
for all patients, and no cast immobilization was applied. All
patients followed the same recovery protocol: Avoiding full
weight-bearing of the injured knee for one week and gradual
resumption of physical activities. For pain management, the
patients had a contraindication of taking non-steroidal
anti-inflammatory drugs (NSAIDs) (for six months).
A total of 1 month after the first PRP injection (3
months after the initial trauma), the clinical outcome evaluation
was conducted using the Lysholm knee scoring scale and the NRS of
pain.
After the first follow-up (at one month after PRP
treatment), the patients included in our study had a check-up every
six months.
Demographic data, pain intensity (NRS) and Lysholm
score were analyzed using the unpaired t-test. The statistical
significance was determined using a conventional P-value of
<0.05 and a confidence interval (CI) of 95%. Analyses were
performed using Medcalc 19.4.0 software (MedCalc Software Ltd.) and
Excel (Microsoft Office Pack; Microsoft Corporation).
Results
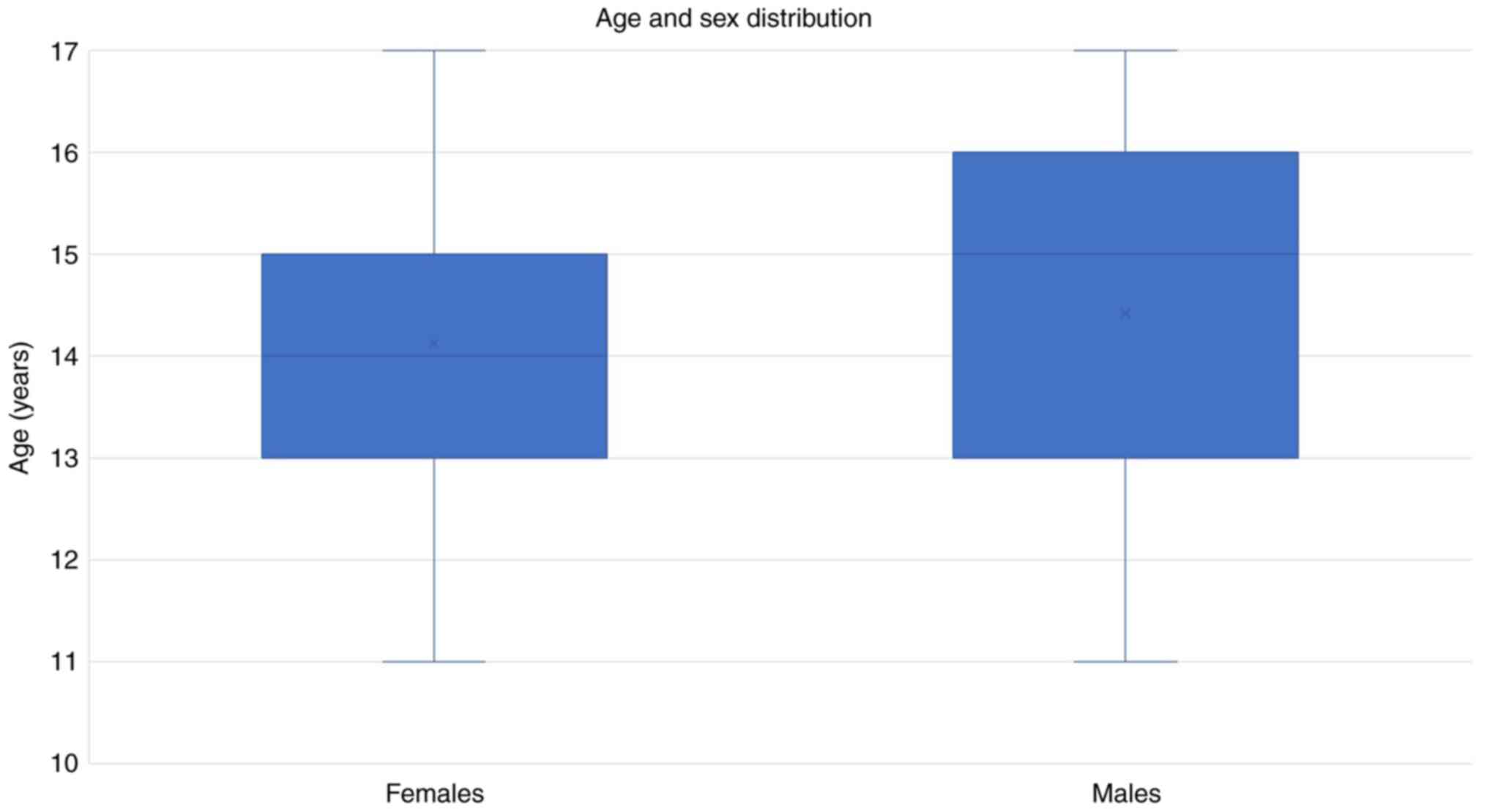
Our study included 72 subjects (48 females and 24
males), with a mean age of 14.15 vs. 14.42 years old (P=0.67), as
demonstrated in Fig. 1. The
patients were diagnosed with a grade 2 meniscal injury [MRI grading
(21)] or grade 2 ACL lesion.
Pain on the NRS had a mean value of 7.81 before PRP,
while at the one-month follow-up, it had a mean value of 2.22 (as
revealed in Table II). The
unpaired sample t-test was performed. The difference between pain
before and after PRP was 5.59, with a 95% CI and an associated
P-value of 0.0065.
 | Table IIComparison of pain and Lysholm score
before and after PRP. |
Table II
Comparison of pain and Lysholm score
before and after PRP.
| Variable | Before PRP N=72 | One month after PRP
N=72 | Unpaired sample
t-test | Before PRP N=72 |
|---|
| Pain (NRS), mean
value ± SEM, N | 7.819±0.1599 | 2.22±0.2714 | 5.59 P=0.0065 | 7.819±0.1599 |
| Lysholm score, mean
value ± SEM, N | 43.90±2.244 | 85.64±2.152 | 41.73±3.109
P=0.0004 | 43.90±2.244 |
The Lysholm score before PRP had a mean value of
43.90 points and of 85.64 points at the one month follow up
(Table II). The difference was of
41.73 points, with a 95% CI and an associated P-value of
0.0004.
In addition, the single assessment numeric
evaluation of pain and the Lysholm score between the patients who
had been diagnosed with ACL injuries vs. those who had been
diagnosed with meniscal lesions were compared.
As demonstrated in Table III, pain levels (NRS) were similar
in patients admitted with ACL lesions vs. patients admitted with
meniscal lesions (7.58 vs. 7.89). The Lysholm score before the
first PRP injection had a mean value of 46.11 points in patients
who had sustained ACL lesions vs. 43.21 in patients who had
sustained meniscus lesions (the difference was not statistically
significant, P=0.63).
 | Table IIIPain (NRS), Lysholm score variations:
ACL lesions vs. meniscal lesions. |
Table III
Pain (NRS), Lysholm score variations:
ACL lesions vs. meniscal lesions.
| Variable | ACL lesions,
N=17 | Meniscal lesions,
N=55 | Unpaired sample
t-test difference | P-value |
|---|
| Pain (NRS) before
PRP, mean ± SEM | 7.58±0.32 | 7.89±0.18 | 0.31±0.37 | 0.63 |
| Pain (NRS) after PRP,
mean ± SEM | 1.58±0.30 | 2.41±0.36 | 0.82±0.45 | 0.07 |
| Pain (NRS) level
decrease (before/after PRP) ± SEM | 6±0.41 N=17 | 5.57±0.36 | -0.52±0.54 | 0.08 |
| Lysholm score before
PRP, mean ± SEM | 46.11±4.47 | 43.21±2.60 | -2.89±5.31 | 0.09 |
| Lysholm score after
PRP, mean ± SEM | 90±1.76 | 84.29±2.74 | -5.73±3.22 | 0.08 |
| Lysholm score
increase (before/after PRP), mean ± SEM | 43.88±4.76 | 41.07±3.32 | -2.80±6.55 | 0.60 |
One month after the PRP treatment, pain on the NRS
decreased with a mean of 6 points in patients with ACL lesions vs.
a mean decrease of 5.57 in patients with meniscal injuries. It was
observed that the Lysholm score improved with a mean of 43.88
points in patients with ACL injuries, while in those who had
suffered meniscal lesions, the increase had a standard of 41.07
points.
These differences between ACL and meniscal lesions,
although important, were not significant statistically. It is
considered that these discrepancies between the two groups could be
clinically meaningful.
Regarding sports and daily physical activities, all
72 patients, before sustaining the injuries, were classified as
recreational athletes. A total of 1 month after PRP treatment, 60
patients (83.3%) were able to return to sports.
After the first one-month follow up, the patients
included in our study had periodical check-ups every six months,
with a mean follow-up time of 2 years. No local side effects
(swelling, locking, and erythema) or worsening of symptoms were
reported.
Discussion
As a primary or secondary treatment, biological
products remain controversial when it comes to the field of
orthopedics. Despite promising, the current literature has not yet
clearly elucidated the benefits of PRP treatment for meniscal or
ACL repairs.
A study conducted by Dallo et al (30) concluded that, compared with standard
reconstruction procedures, biological augmentation approaches for
ACL tears are associated with a faster return to sports, improved
healing, and improved proprioception. This theory is in agreement
with our results: An improvement in the Lysholm knee scoring scale,
an adequate pain level control (NRS) and the resumption of sports
from recreational athletes who have undergone PRP treatment.
In terms of meniscal healing, numerous studies
(14-16)
have acknowledged that PRP injections could provide an improved
clinical outcome in cases where the conservative approach was
unsuccessful. Although encouraging, the existing results encounter
the limitations of small study groups, the biased, subjective
perception of pain from patients, or lack of post-procedure MRI
studies, which could support the clinical findings. Moreover,
animal-based models, which depict molecular mechanisms of PRP, map
a discrepancy between results (16), extending even further the
controversy of the benefits of biologics for meniscal and ACL
tears.
The results emerged from our study highlight the
theory that there is a clear correlation between the variations in
the Lysholm knee scoring scale and pain perception on the NRS
[correspondingly to the conclusion reached by Sueyoshi et al
(31), that although weak, this
interrelationship is statistically significant], and also between
the resumption of sports and daily living activities. Our findings
compel us to consider that, in cases where the conservative
treatment and physical therapy for partial meniscal and ACL tears
fail to succeed, PRP could establish a satisfactory outcome and a
significant decline of long-term complications and physical
impairments.
At the one-month follow-up, none of the patients
included in our study reported any local side effects or
deterioration of the affected knee condition.
The main limitation of our study was the lack of MRI
follow-up and the small number of cases included. Further
investigation on larger groups, accompanied by post-procedure MRI,
could produce an improved understanding of the role that biologics
actually play in modern medicine.
In conclusion, PRP is a minimally invasive
treatment, efficient for young recreational athletes, contributing
to the resuming of sports at three months after the initial
trauma.
Meniscal or ACL tears in adolescents can benefit
from PRP in pain relief and resuming of sports.
Grade 2 ACL lesions have an improved response with
PRP therapy than partial meniscal tears in terms of pain
relief.
PRP is a safe, minimally invasive treatment method,
without side effects, which does not require sedation or follow-up
in post-intervention.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All the data are registered at ‘Grigore
Alexandrescu’ Children's Emergency Hospital, Bucharest, Romania and
are available from AU upon reasonable request.
Authors' contributions
AH, AS, ACh and AU analyzed and interpreted the
patients' data regarding the efficiency of PRP therapy in meniscal
and ACL lesions, graded by MRI. AT revised the literature data and
analyzed other studies where the treatment with PRP injections
provided favorable clinical outcomes. RPD and ACo researched the
studies that were included as references. FF designed the study,
and critically revised the manuscript and approved the current form
of the article in order to be submitted to the journal. All the
authors read and approved the final manuscript and agree to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The study was approved (approval number
23223/05.12.2017) by the Ethics Committee of ‘Grigore Alexandrescu’
Children's Emergency Hospital (Bucharest, Romania) which follows
the World Medical Association Code of Ethics (Declaration of
Helsinki, 1967). Preceding the study, all legal guardians signed a
written informed consent after receiving a detailed explanation of
all the procedures the patients would undergo.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vavken P and Murray MM: The potential for
primary repair of the ACL. Sports Med Arthrosc Rev. 19:44–49.
2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Seedhom BB, Dowson D and Wright V:
Proceedings: Functions of the menisci. A preliminary study. Ann
Rheum Dis. 33(111)1974.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Renström P and Johnson RJ: Anatomy and
biomechanics of the menisci. Clin Sports Med. 9:523–538.
1990.PubMed/NCBI
|
|
4
|
Krause WR, Pope MH, Johnson RJ and Wilder
DG: Mechanical changes in the knee after meniscectomy. J Bone Joint
Surg Am. 58:599–604. 1976.PubMed/NCBI
|
|
5
|
Arnoczky SP and Warren RF:
Microvasculature of the human meniscus. Am J Sports Med. 10:90–95.
1982.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Amis AA: Anterior cruciate ligament
replacement. Knee stability and the effects of implants. J Bone
Joint Surg Br. 71:819–824. 1989.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Murray MM, Spindler KP, Ballard P, Welch
TP, Zurakowski D and Nanney LB: Enhanced histologic repair in a
central wound in the anterior cruciate ligament with a
collagen-platelet-rich plasma scaffold. J Orthop Res. 25:1007–1017.
2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gobbi A, Karnatzikos G, Mahajan V and
Malchira S: Platelet-rich plasma treatment in symptomatic patients
with knee osteoarthritis: Preliminary results in a group of active
patients. Sports Health. 4:162–172. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cheng M, Wang H, Yoshida R and Murray MM:
Platelets and plasma proteins are both required to stimulate
collagen gene expression by anterior cruciate ligament cells in
three-dimensional culture. Tissue Eng Part A. 16:1479–1489.
2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ionescu LC, Lee GC, Huang KL and Mauck RL:
Growth factor supplementation improves native and engineered
meniscus repair in vitro. Acta Biomater. 8:3687–3694.
2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Clendenen TV, Arslan AA, Lokshin AE, Idahl
A, Hallmans G, Koenig KL, Marrangoni AM, Nolen BM, Ohlson N,
Zeleniuch-Jacquotte A and Lundin E: Temporal reliability of
cytokines and growth factors in EDTA plasma. BMC Res Notes.
3(302)2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kim ES, Kim JJ and Park EJ: Angiogenic
factor-enriched platelet-rich plasma enhances in vivo bone
formation around alloplastic graft material. J Adv Prosthodont.
2:7–13. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Samadi P, Sheykhhasan M and Khoshinani HM:
The use of platelet-rich plasma in aesthetic and regenerative
medicine: A comprehensive review. Aesthetic Plast Surg. 43:803–814.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Popescu MB, Carp M, Tevanov I, Nahoi CA,
Stratila MA, Haram OM and Ulici A: Isolated meniscus tears in
adolescent patients treated with platelet-rich plasma
intra-articular injections: 3-Month clinical outcome. Biomed Res
Int. 2020(8282460)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pujol N, Salle De Chou E, Boisrenoult P
and Beaufils P: Platelet-rich plasma for open meniscal repair in
young patients: Any benefit? Knee Surg Sports Traumatol Arthrosc.
23:51–58. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ishida K, Kuroda R, Miwa M, Tabata Y,
Hokugo A, Kawamoto T, Sasaki K, Doita M and Kurosaka M: The
regenerative effects of platelet-rich plasma on meniscal cells in
vitro and its in vivo application with biodegradable gelatin
hydrogel. Tissue Eng. 13:1103–1112. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ding C, Martel-Pelletier J, Pelletier JP,
Abram F, Raynauld JP, Cicuttini F and Jones G: Meniscal tear as an
osteoarthritis risk factor in a largely non-osteoarthritic cohort:
A cross-sectional study. J Rheumatolo. 34:776–784. 2007.PubMed/NCBI
|
|
18
|
Clark CR and Ogden JA: Development of the
menisci of the human knee joint. Morphological changes and their
potential role in childhood meniscal injury. J Bone Joint Surg Am.
65:538–547. 1983.PubMed/NCBI
|
|
19
|
Makris EA, Hadidi P and Athanasiou KA: The
knee meniscus: Structure-function, pathophysiology, current repair
techniques, and prospects for regeneration. Biomaterials.
32:7411–7431. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kobayashi K, Fujimoto E, Deie M, Sumen Y,
Ikuta Y and Ochi M: Regional differences in the healing potential
of the meniscus-an organ culture model to eliminate the influence
of microvasculature and the synovium. Knee. 11:271–278.
2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lotysch M, Mink J, Crues JV and Schwartz
SA: Magnetic resonance imaging in the detection of meniscal
injuries. Magn Reson Imaging. 4(185)1986.
|
|
22
|
Blanke F, Vavken P, Haenle M, von Wehren
L, Pagenstert G and Majewski M: Percutaneous injections of platelet
rich plasma for treatment of intrasubstance meniscal lesions.
Muscles Ligaments Tendons J. 5:162–166. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lohmander LS, Englund PM, Dahl LL and Roos
EM: The long-term consequence of anterior cruciate ligament and
meniscus injuries: Osteoarthritis. Am J Sports Med. 35:1756–1769.
2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lohmander LS, Ostenberg A, Englund M and
Roos H: High prevalence of knee osteoarthritis, pain, and
functional limitations in female soccer players twelve years after
anterior cruciate ligament injury. Arthritis Rheum. 50:3145–3152.
2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nelson IR, Chen J, Love R, Davis BR,
Maletis GB and Funahashi TT: A comparison of revision and rerupture
rates of ACL reconstruction between autografts and allografts in
the skeletally immature. Knee Surg Sports Traumatol Arthrosc.
24:773–779. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Braun HJ, Wasterlain AS and Dragoo JL: The
use of PRP in ligament and meniscal healing. Sports Med Arthrosc
Rev. 21:206–212. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tegner Y and Lysholm J: Rating systems in
the evaluation of knee ligament injuries. Clin Orthop Relat Res.
198:43–48. 1985.PubMed/NCBI
|
|
28
|
Kocher MS, Steadman JR, Briggs KK, Sterett
WI and Hawkins RJ: Reliability, validity, and responsiveness of the
Lysholm knee scale for various chondral disorders of the knee. J
Bone Joint Surg Am. 86:1139–1145. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Iohom G: Chapter 11-Clinical assessment of
postoperative pain. In: Postoperative Pain Management: An
Evidence-Based Guide to Practice. Shorten G, Carr D, Harmon D, Puig
M and Browne J (eds). Saunders Elsevier, Philadelphia, PA,
pp102-108, 2006.
|
|
30
|
Dallo I, Chahla J, Mitchell JJ,
Pascual-Garrido C, Feagin JA and LaPrade RF: Biologic approaches
for the treatment of partial tears of the anterior cruciate
ligament: A current concepts review. Orthop J Sports Med.
5(2325967116681724)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sueyoshi T, Emoto G and Yato T:
Correlation between single assessment numerical evaluation score
and lysholm score in primary total knee arthroplasty patients.
Arthroplast Today. 4:99–102. 2017.PubMed/NCBI View Article : Google Scholar
|