Introduction
Spinal cord injury (SCI) is a serious disabling
disease of the central nervous system, principally caused by
physical trauma, degenerative diseases and infections, including
HIV and bacteria (1,2). The pathology of SCI is divided into
primary and secondary injury. Primary injury refers to the injury
caused by direct or indirect external force on the spinal cord,
which is irreversible. Secondary injury involves a series of
complex reactions that occur on the basis of the primary injury,
including local edema, ischemia, focal hemorrhage, oxidative stress
and inflammatory reactions, which are reversible and preventable
(3). Nerve repair is the major
approach to self-repair after SCI; however, the lack of
self-regenerative ability of neurons generally leads to poor
recovery (4). Therefore,
researchers worldwide have conducted multiple studies on the
mechanism of SCI treatment (5,6). The
mechanism of the proposed treatment can be summarized as inhibiting
glial scar formation and inflammation, improving microcirculation,
inhibiting neuronal cell apoptosis and promoting cell repair and
regeneration (5). Inhibiting cell
apoptosis is an important part of the repair and treatment
mechanism of SCI and serves a central role in regulating SCI
(6).
As a synthetic opioid receptor agonist-antagonist,
butorphanol (Fig. 1A) is widely
used in perioperative analgesia and compound anesthesia. Its main
mechanism is to stimulate κ-opioid receptor (KOR) to exert a spinal
analgesic effect. Butorphanol affects KOR and can partially
activate the KOR in the G protein activation pathway and fully
activate the KOR in the β-arrestin recruitment pathway (7). Previous studies have suggested that
butorphanol can reduce myocardial ischemia-reperfusion injury by
inhibiting inflammation, oxidative stress and apoptosis (8-10).
Butorphanol can also suppress the inflammation in
lipopolysaccharide (LPS) induced H9C2 cells in an in vitro
sepsis model (11).
Carrageenan-induced inflammation in rat paws can be alleviated by
treatment with butorphanol (12).
In addition, butorphanol can reduce inflammatory infiltration
injury to alleviate brain damage as a consequence of sepsis
(13). Furthermore, butorphanol is
considered to relieve neuronal inflammation and apoptosis arising
from ischemia-hypoxia-reperfusion (14). It was hypothesized that butorphanol
may protect against inflammation and apoptosis in neuronal
injury.
A previous study indicated that butorphanol can
inhibit the activation of p38 and JNK phosphorylation during
myocardial ischemia-reperfusion (9) and the activation of p38 and JNK
signaling is involved in SCI-induced neuronal activity damage,
inflammatory factor release and apoptosis (15,16).
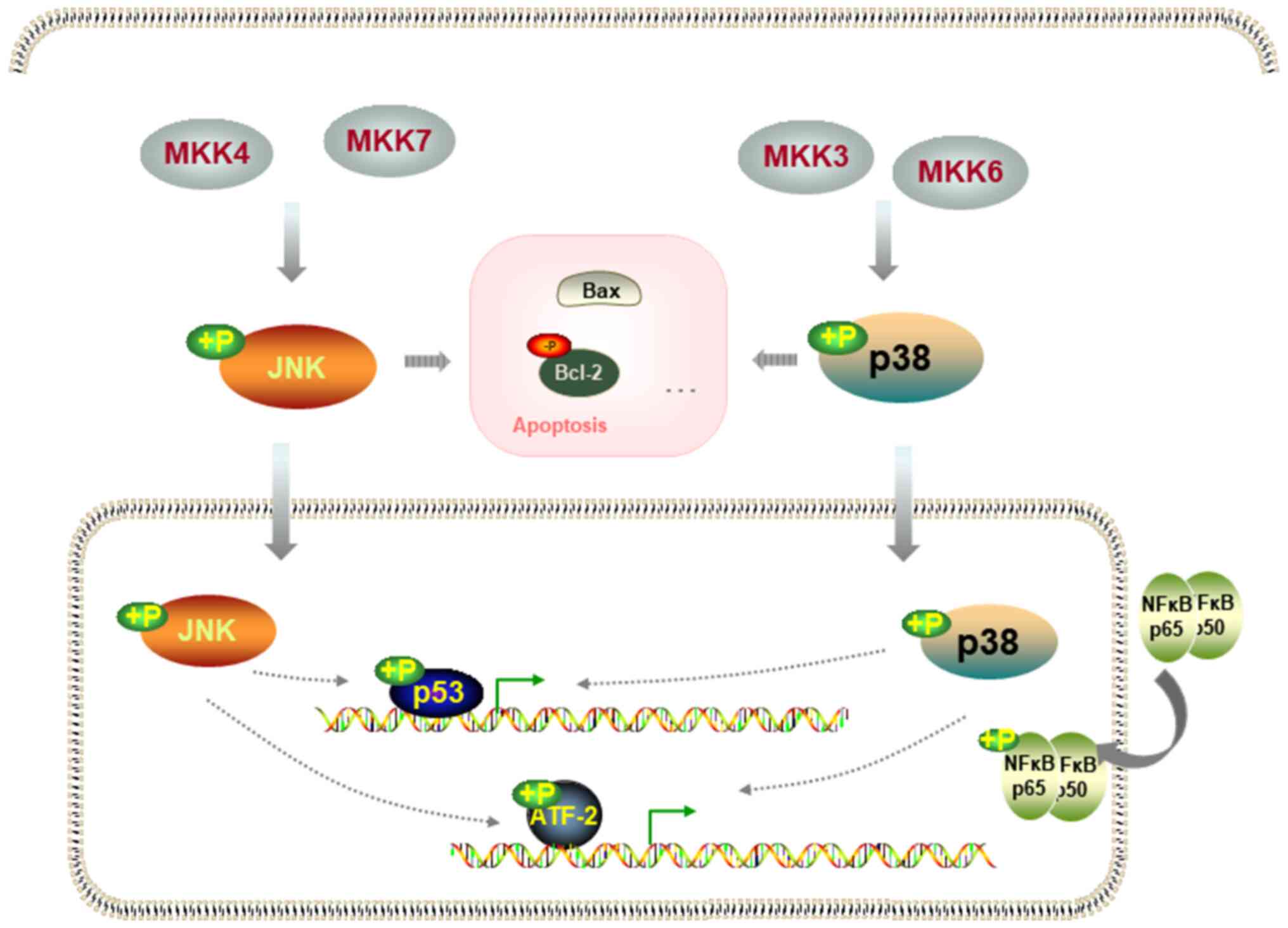
According to the Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway database (https://www.genome.jp/kegg/pathway.html), the
activation of JNK and p38 signaling can stimulate downstream
activating transcription factor 2 (ATF2) and p53 to induce
apoptosis signaling pathways. ATF2 and P53 participate in the
inflammatory and apoptotic effects of SCI (17,18).
Neuronal cell apoptosis is a complex
pathophysiological change that occurs following SCI and the
increase in apoptosis affects self-repair (19). Therefore, preventing neuronal cell
apoptosis can promote the recovery of nerve function. The PC12 cell
line is a differentiated cell line of rat adrenal medulla
phochromocytoma and has the general characteristics of
neuroendocrine cells. Due to its passage characteristics, this cell
line is widely used in neurophysiological and neuropharmacological
research (20,21). The present study aimed to explore
the effects of butorphanol on the neuronal inflammatory response
and apoptosis in PC12 cells.
Materials and methods
Cell culture
PC12 cells were purchased from The Cell Bank of Type
Culture Collection of The Chinese Academy of Sciences and cultured
in DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS (Invitrogen; Thermo Fisher Scientific, Inc.). Cells were
maintained at 37˚C in a 5% CO2 atmosphere.
Cells were divided into the following groups:
Untreated (control), butorphanol, lipopolysaccharide (LPS), LPS +
butorphanol and LPS + butorphanol + anisomycin. Cells were
pretreated with butorphanol (1, 2 and 4 µM; GlpBio Technology)
(14) and/or anisomycin (1 µM;
GlpBio Technology Inc.) (22) for
6 h and then induced with LPS (5 µg/ml; MilliporeSigma) (23) for 12 h for the establishment of the
model.
Cell Counting Kit 8 (CCK8) assay
PC12 cells were seeded in a 96-well plate at a
density of 6x103 cells/well. To determine the effect of
different doses of butorphanol on cell viability, butorphanol (1, 2
and 4 µM) was added to the plates and cells were incubated for 24 h
as in a previous study (14). To
determine the effect of LPS and butorphanol on cell viability,
cells were pretreated with butorphanol for 6 h and then incubated
with LPS for 12 h. Following incubation with 10 µl CCK8 solution
(Beyotime Institute of Biotechnology) for another 2 h at 37˚C, the
optical density was measured at a wavelength of 450 nm using a
microplate reader (Thermo Fisher Scientific, Inc.).
Western blotting
PC12 cells were plated into 6-well plates at a
density of 3x105 cells/well. Protein lysates were
prepared using RIPA lysis buffer (Beyotime Institute of
Biotechnology). Following quantification of the protein
concentration using a BCA protein assay kit (cat. no. P0012;
Beyotime Institute of Biotechnology), protein samples (30 µg per
lane) were subjected to 12% SDS-PAGE and transferred to PVDF
membranes. Following blocking with 5% non-fat milk, for 2 h at room
temperature, blots were incubated overnight at 4˚C with the
indicated primary antibodies. Subsequently, the membranes were
further incubated for 2 h at room temperature with the
corresponding secondary antibodies. Next, membranes were developed
with SuperSignal west femto maximum sensitivity substrate (Pierce;
Thermo Fisher Scientific, Inc.) and ImageJ software (version 1.6,
National Institutes of Health) was used for analysis.
Phosphorylated (p-)p38 (cat. no. ab4822; dilution, 1:1,000), p38
(cat. no. ab170099; dilution, 1:2,000), p-JNK (cat. no. ab124956;
dilution, 1:5,000), JNK (cat. no. ab208035; dilution, 1:500), ATF2
(cat. no. ab239361; dilution, 1:1,000) and Histone H3 (cat. no.
ab1791; dilution, 1:2,000) primary antibodies were obtained from
Abcam. p-ATF2 (cat. no. MA5-33115; dilution, 1:1,000), p53 (cat.
no. 21891-1-AP; dilution, 1:1,000), p65 (cat. no. 14-6731-81;
dilution, 1:1,000), Bcl2 (cat. no. PA5-27094; dilution, 1:1,000),
Bax (cat. no. PA5-11378; dilution, 1:2,000), cleaved caspase3 (cat.
no. PA5-114687; dilution, 1:1,000), GAPDH (cat. no. MA1-16757;
dilution, 1:2,000) and anti-rabbit secondary antibodies (cat. no.
G-21234; dilution, 1:100,000) were purchased from Thermo Fisher
Scientific. Protein bands were visualized using an enhanced
chemiluminescence kit (Beyotime Institute of Biotechnology), which
were quantified by imageJ 1.8 software (National institutes of
Health).
Activity of lactate dehydrogenase
(LDH)
The activity of LDH in PC12 cells was determined
using an LDH Assay kit (cat. no. C0016; Beyotime Institute of
Biotechnology) according to the manufacturer's instructions. The
optical density was measured at a wavelength of 490 nm using a
microplate reader (Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
PC12 cells were seeded in a 6-well plate at a
density of 6x105 cells/well at 37˚C. Total RNA was
extracted from the cultured cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions and reverse transcribed into cDNA using
the FSQ-101 reverse transcription system kit (Toyobo Life Science)
according to the manufacturer's protocols. The qPCR reactions were
performed using SYBR Green qPCR Master Mix (Roche Applied Science)
on a 7500 Real-time system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The thermocycling conditions were: 95˚C for 2
min, followed by 40 cycles at 95˚C for 10 sec, 60˚C for 35 sec and
72˚C for 10 sec. The primer sequences used were as follows: TNF-α
forward, 5'-TTCCCAAATGGGCTCCCTCT-3' and reverse,
5'-GTGGGCTACGGGCTTGTCAC-3'; IL-1β forward,
5'-TCCAGGATGAGGACCCAAGC-3' and reverse,
5'-TCGTCATCATCCCACGAGTCA-3'; IL-6 forward,
5'-TCTGGGAAATCGTGGAAATGAG-3' and reverse,
5'-TCTCTGAAGGACTCTGGCTTTGTC-3'; and GAPDH forward,
5'-TCTCTGCTCCTCCCTGTTCT-3' and reverse, 5'-TACGGCCAAATCCGTTCACA-3'.
The expression of target genes was quantified using the
2-∆∆cq method (24).
TUNEL assay
A TUNEL assay kit (cat. no. C1086; Beyotime
Institute of Biotechnology) was used. PC12 cells were fixed with 4%
paraformaldehyde for 30 min at room temperature and permeated with
PBS containing 0.3% Triton X-100 for another 5 min at room
temperature. The detection solution was prepared and the procedure
was performed according to the manufacturer's instructions. The
coverglass was sealed and cells were observed under a fluorescence
microscope (magnification, x200; Olympus Corporation).
Bioinformatics and statistical
analysis
The KEGG pathway database (www.kegg.jp/kegg/pathway) is a collection of pathway
maps representing the molecular interaction, reaction and relation
networks. All experimental data are presented as the mean ±
standard deviation and experiments were performed in triplicate.
Statistical analyses were conducted using GraphPad Prism 8.0
software (GraphPad Software, Inc.). One-way analysis of variance
followed by a Tukey's post hoc test was applied to compare
differences among multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
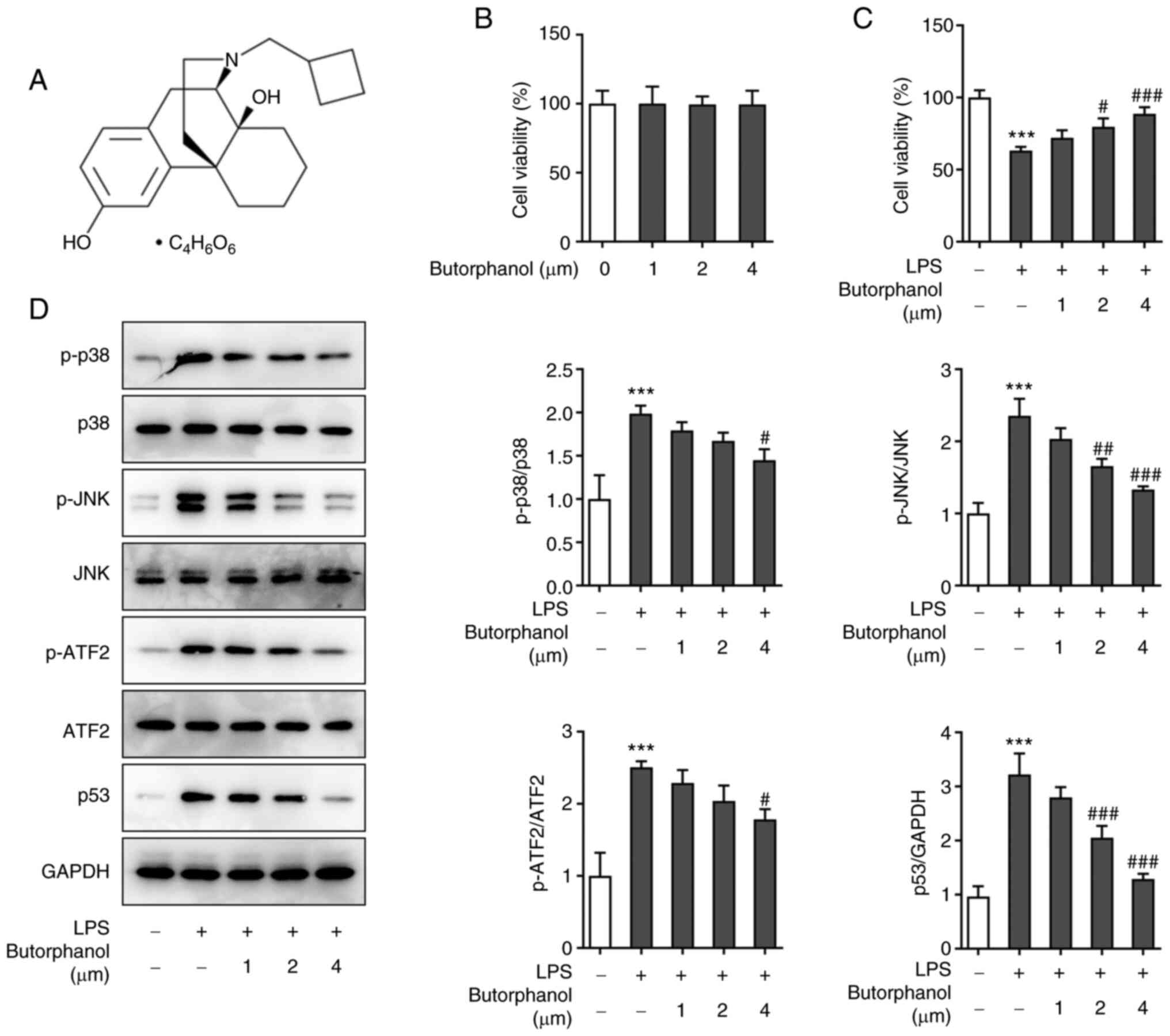
Effects of different doses of
butorphanol on LPS-induced reduction of PC12 cell viability and
p38/JNK/ATF2/p53 signaling
The effect of different doses of butorphanol on PC12
cell viability was assessed using a CCK8 assay. Cell viability was
not significantly altered following treatment with doses between
0-4 µM (Fig. 1B). Subsequently,
the viability of cells treated with butorphanol and LPS was
determined. Cells were divided into five groups: Control, LPS and
LPS + butorphanol (1, 2 and 4 µM). The viability of cells in the
LPS group was markedly decreased, whereas the viability of cells
pretreated with butorphanol increased in a concentration-dependent
manner, which suggested that butorphanol protected the cells
against the inhibitory effect of LPS on cell viability (Fig. 1C). Subsequently, the levels of
p-p38, p-JNK, p-ATF2, p53, p38, JNK and ATF2 were examined using
western blot analysis. The levels of p-p38, p-JNK, p-ATF2 and p-p53
were upregulated following treatment with LPS and downregulated
following pretreatment with butorphanol compared with the LPS
group. Additionally, there were no significant changes in the
expression levels of p38, JNK and ATF2 (Fig. 1D). The aforementioned results
indicated that the maximum dose of butorphanol in the experiment
could not only improve cell viability, but also effectively
inhibited signal protein expression. Therefore, butorphanol at a
concentration of 4 µM was used in subsequent experiments.
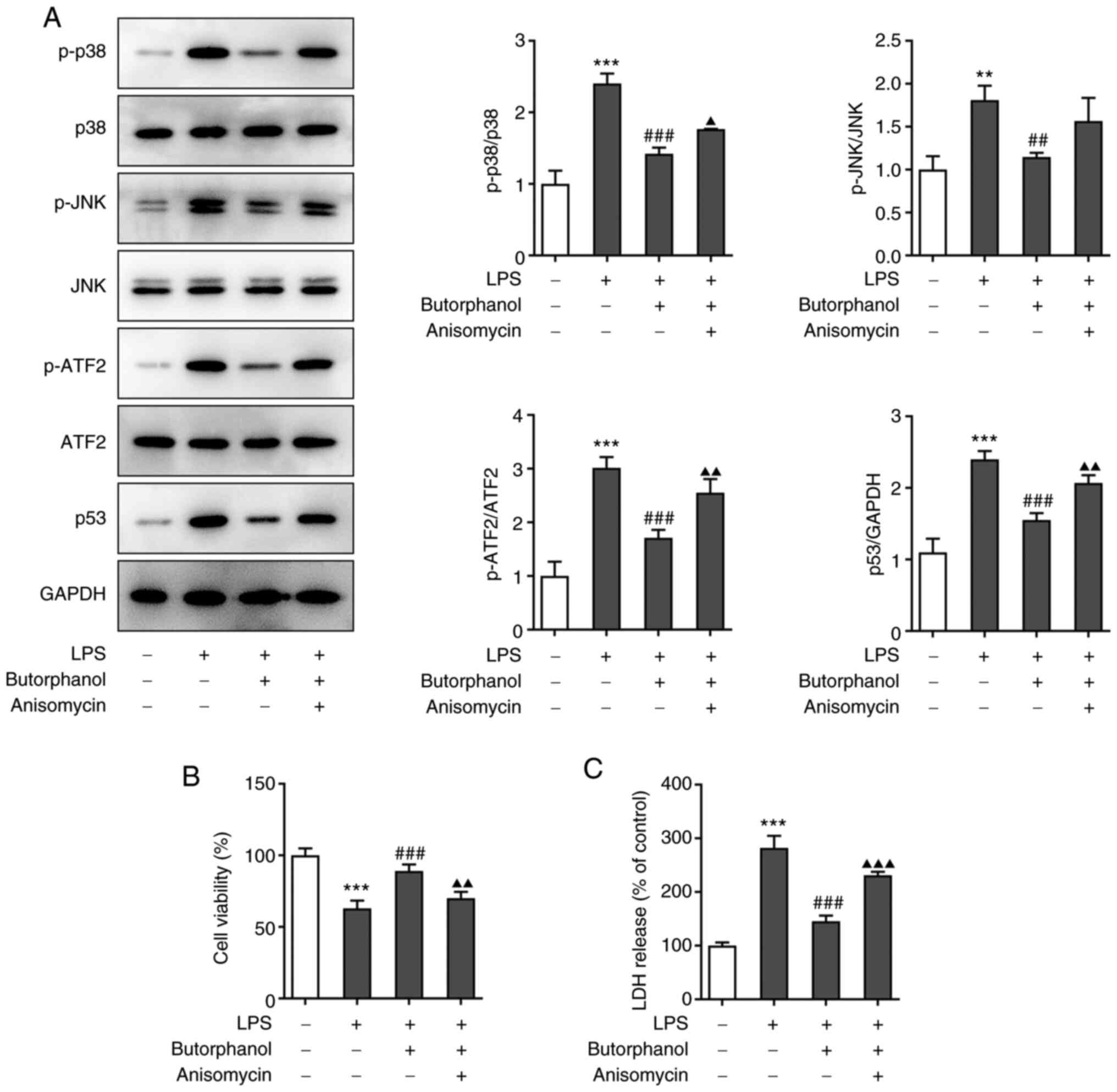
Butorphanol protects cells against the
inhibitory effects of LPS on cell viability via p38/JNK/ATF2/p53
signaling
In order to explore the mechanism of the MAPK
signaling pathway in the regulation of cells by butorphanol,
anisomycin was used to pretreat cells. Cells were divided into four
groups: Control, LPS, LPS + 4 µM butorphanol and LPS + 4 µM
butorphanol + anisomycin. The protein expression levels of pathway
signaling molecules in each group were determined using western
blot analysis. The levels of p-p38, p-JNK, p-ATF2 and p-p53 were
all upregulated in cells subjected to additional anisomycin
treatment compared with the LPS + 4 µM butorphanol group, whereas
there was no effect on the expression levels of p38, JNK and ATF2
(Fig. 2A). In addition, cell
viability in these four groups was assessed and the results
indicated that cell viability was decreased following treatment
with anisomycin compared with the LPS + 4 µM butorphanol group
(Fig. 2B). Furthermore, the
activity of LDH in each group was measured using an assay kit. The
levels of LDH were increased in the LPS group compared with the
control group and decreased in the LPS + 4 µM butorphanol group
compared with the LPS group. Interestingly, the LDH levels were
elevated again following the addition of anisomycin compared with
the LPS + 4 µM butorphanol group (Fig.
2C).
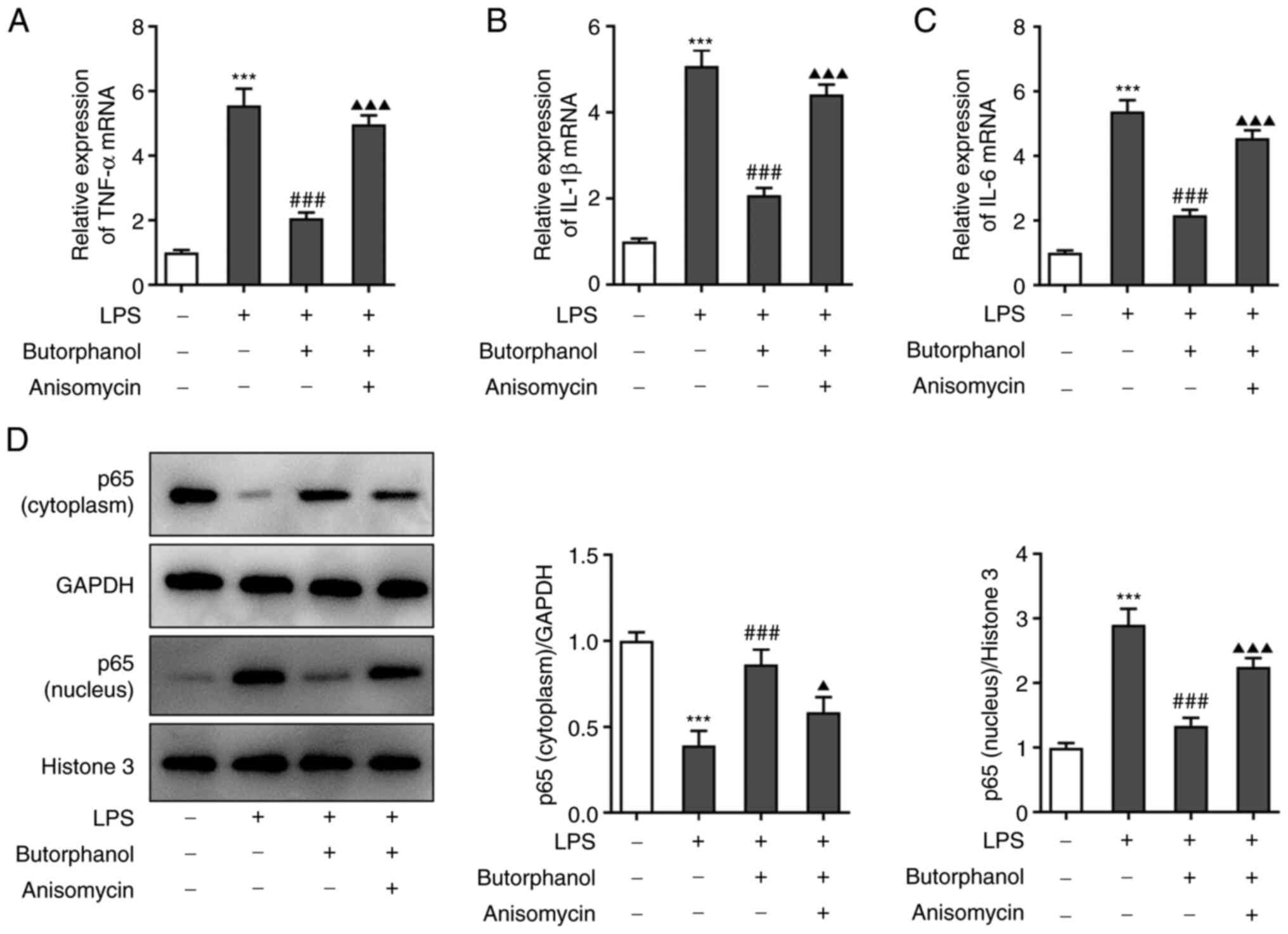
Butorphanol reduces LPS-induced
inflammatory factor release and apoptosis via p38/JNK/ATF2/p53
signaling
Based on the findings on cell viability, the effects
of butorphanol on inflammatory factors and cell apoptosis were
studied. The expression levels of TNF-α, IL-1β and IL-6 in the
aforementioned four groups were measured using RT-qPCR. The results
revealed that butorphanol reduced the increase in the levels of
inflammatory factors caused by LPS and anisomycin suppressed the
effect of butorphanol to some extent (Fig. 3A-C). Next, the expression levels of
p65 (cytoplasm) and p65 (nucleus) were determined using western
blot analysis. The results revealed that LPS promoted the transfer
of p65 into the nucleus and butorphanol could restrain the entry of
p65 into the nucleus. As expected, anisomycin reversed the
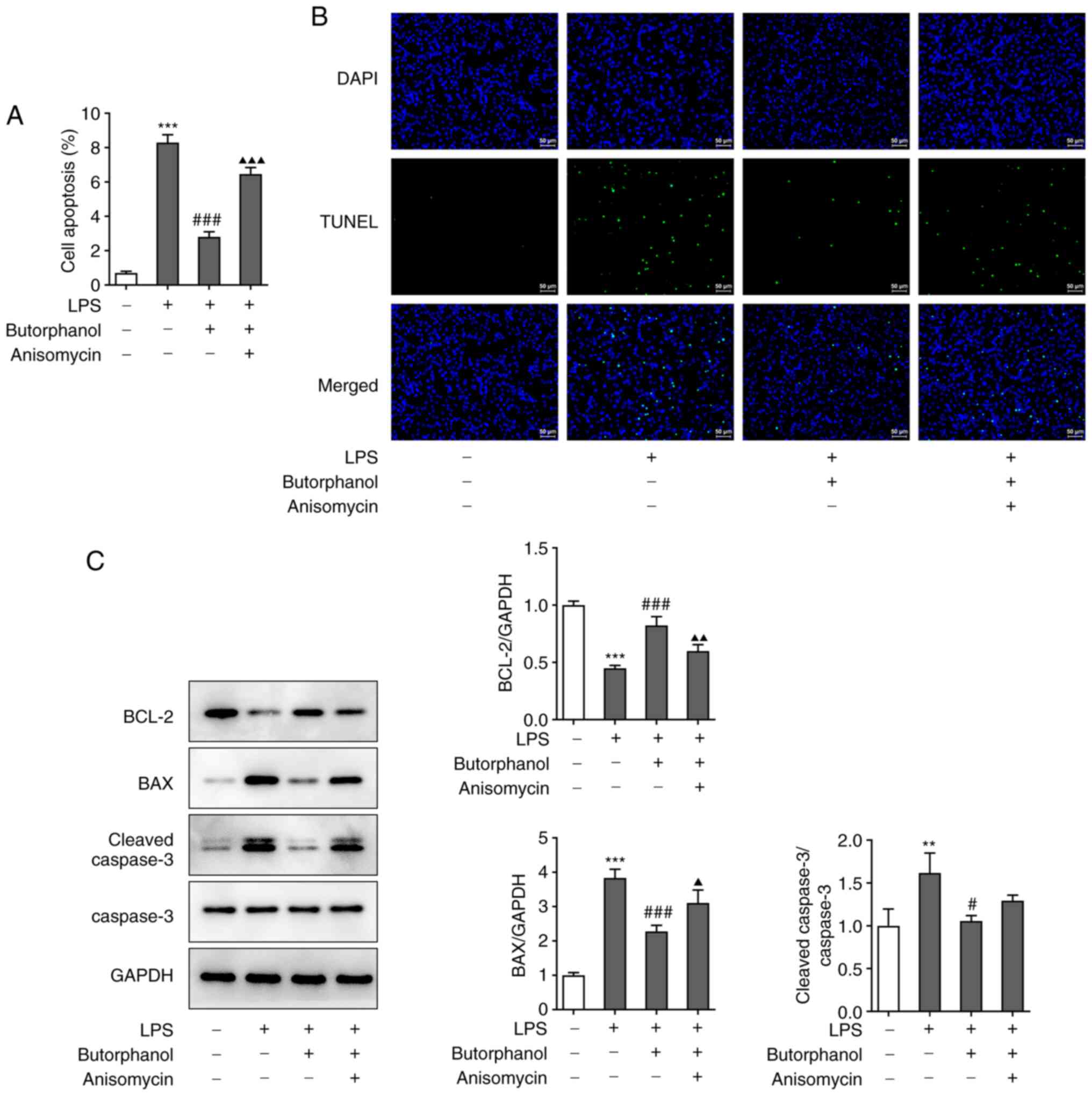
inhibitory effect of butorphanol on the transfer of p65 (Fig. 3D). Furthermore, a TUNEL assay and
western blot analysis were used to evaluate cell apoptosis.
Anisomycin increased the number of apoptotic cells, which suggested
that anisomycin blocked the inhibitory effect of butorphanol on
cell apoptosis (Fig. 4A). The
results of the TUNEL assay demonstrated that the apoptotic cells in
the LPS group emitted more fluorescence and that butorphanol
markedly reduced the number of apoptotic cells (Fig. 4B). Additionally, the expression
levels of apoptosis-related proteins were determined using western
blot analysis. The expression levels of Bax and cleaved caspase 3
were upregulated in the LPS group. Furthermore, both their
expression levels were decreased in the LPS + 4 µM butorphanol
group and upregulated in the LPS + 4 µM butorphanol + anisomycin
group, whereas the expression levels of Bcl2 exhibited the opposite
trend (Fig. 4C).
Discussion
SCI is a destructive neurological and pathological
state that can severely affect the quality of life of patients
(25). Its pathophysiology
includes ischemia, oxidative stress, inflammation, apoptosis and
motor dysfunction (26). In recent
years, with the continuing in-depth study of pathological
mechanisms, novel treatment strategies have been proposed to
overcome neurodegenerative events. While these strategies restore
the stability of the spine, reducing secondary SCI and improving
the survival of patients remains unresolved and requires more
research (27). Common clinical
treatment methods focus on surgery, drug treatment and cell therapy
(28). The choice of surgical
methods and timing should be considered based on the overall
condition of the patient, so that surgical treatment tends to be
more individualized (29).
High-dose hormone shock therapy is still questioned for side
effects; however, in the early stages of SCI, the dose can be
appropriately reduced to reduce the occurrence of complications
(30). In addition, despite being
the most promising treatment for SCI, cell therapy still faces
numerous problems, such as whether the transplanted cells can
survive, whether the axon regeneration direction is accurate and
whether it can establish effective synaptic connections with the
neurons of the host (31).
Furthermore, hyperbaric oxygen, pulsed electricity, local cold
therapy, acupuncture and other uncommon therapies are also under
continuous development (32,33).
The inflammatory response has been demonstrated to
be involved in the occurrence and development of SCI (34). Following SCI, the pro-inflammatory
factor TNF-α is the first to increase and it acts synergistically
with other pro-inflammatory factors to produce lipid peroxides and
oxygen free radicals (34). TNF-α
can promote the activity of cells such as microglia and astrocytes,
stimulate cells and promote matrix proliferation (34). On the other hand, TNF-α can also
cause apoptosis and affect neuronal function (35). Furthermore, IL-1β induces cell
apoptosis, stimulates the expression of adhesion factors and, when
its expression increases, promotes the inflammatory response
(36). IL-6 can act on macrophages
to promote their differentiation and infiltration, upregulate the
expression of other cytokines and actively participate in the
secondary injury of spinal cord nerve tissue (37,38).
In the present study, the expression levels of TNF-α, IL-1β and
IL-6 were determined and butorphanol was found to reduce the
increase in TNF-α, IL-1β and IL-6 levels caused by LPS via the MAPK
signaling pathway. This indicated that butorphanol could slow down
the inflammatory response in nerve cells. Additionally, LDH is one
of the important enzyme systems for anaerobic glycolysis and
gluconeogenesis. It can catalyze the reduction and oxidation
reaction between propionic acid and L-lactic acid and is widely
present in human tissues (39).
Hypoxia in spinal cord tissues will accelerate, or even cause,
excessive glycolysis and increase LDH levels (40). The present study revealed that
butorphanol can reduce the increase in LDH levels.
Neuronal cell apoptosis usually refers to the
programmed death of neuronal cells and is the primary cause of
delayed spinal cord cell death after SCI (41). Neuronal cell apoptosis is a complex
pathophysiological process, which mainly involves the protease
cascade mediated by members of the caspase family, in which
caspase-3 serves a pivotal role (42). A previous study suggested that the
activity of caspase-3 contributes to neuronal cell apoptosis
following traumatic brain injury and experimental transient
cerebral ischemia in mice (43).
Furthermore, the anti-apoptotic genes Bcl-2 and Bcl-xL, the
pro-apoptotic genes Bax, Bad and Bcl-2 interacting killer and p65
transportation to the nucleus are also involved in the process of
cell apoptosis (44,45). In the present study, butorphanol
was found to reduce the elevation of pro-apoptotic genes and the
expression of p65 in the nucleus, thereby inhibiting cell
apoptosis.
In conclusion, butorphanol may suppress the neuronal
inflammatory response and apoptosis in PC12 cells via inhibition of
p38/JNK/ATF2/p53 signaling (Fig.
5). A previous study has indicated that butorphanol can relieve
neuronal inflammation and apoptosis arising from
ischemia-hypoxia-reperfusion (14)
and the present study is consistent with the previous studies,
which all indicated that neuronal inflammation and apoptosis are
alleviated following butorphanol treatment. The novelty of the
present study was that it explored the protective effects on
LPS-induced PC12 cells through p38/JNK/ATF2/p53 signaling. However,
the present study was limited to experiments based on PC12 cells,
which provided the basis for the use of butorphanol in other cell
lines. Furthermore, the potential toxicity of butorphanol, the
suitable dose of butorphanol and its protective effects in animal
models and later in humans with pheochromocytoma should be explored
in the future to strengthen the credibility of the present study.
Whether the effects mediated by butorphanol through activation of
the KOR on PC12 cells and whether PC12 cells express KOR or other
opioid receptors are all worth considering and exploring in future
studied. With the gradual deepening of the understanding of
inhibition of neuronal cell apoptosis and the continuous
advancement of medical technology, novel treatment approaches to
clinical SCI may emerge in the near future.
Acknowledgements
Not applicable.
Funding
Funding: This study was funded by the Key R&D Project of
Hainan Science and Technology Department (grant no.
2019YFC0840705).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TH, YH and SL designed and conceived the study. TH
and SL conducted the experiments and analyzed the data with the
help of HC, LF and WY. TH and SL drafted the manuscript which was
revised by TH. All authors have read and approved the final
manuscript. TH and SL confirmed the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Levin SN and Lyons JL: HIV and spinal cord
disease. Handb Clin Neurol. 152:213–227. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ahuja CS, Wilson JR, Nori S, Kotter MRN,
Druschel C, Curt A and Fehlings MG: Traumatic spinal cord injury.
Nat Rev Dis Primers. 3(17018)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Orr MB and Gensel JC: Interactions of
primary insult biomechanics and secondary cascades in spinal cord
injury: Implications for therapy. Neural Regen Res. 12:1618–1619.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shao A, Tu S, Lu J and Zhang J: Crosstalk
between stem cell and spinal cord injury: Pathophysiology and
treatment strategies. Stem Cell Res Ther. 10(238)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sandrow-Feinberg HR and Houlé JD: Exercise
after spinal cord injury as an agent for neuroprotection,
regeneration and rehabilitation. Brain Res. 1619:12–21.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhaohui C and Shuihua W: Protective
effects of SIRT6 against inflammation, oxidative stress, and cell
apoptosis in spinal cord injury. Inflammation. 43:1751–1758.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ji J, Lin W, Vrudhula A, Xi J, Yeliseev A,
Grothusen JR, Bu W and Liu R: Molecular interaction between
butorphanol and κ-opioid receptor. Anesth Analg. 131:935–942.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wu Y, Wan J, Zhen WZ, Chen LF, Zhan J, Ke
JJ, Zhang ZZ and Wang YL: The effect of butorphanol
postconditioning on myocardial ischaemia reperfusion injury in
rats. Interact Cardiovasc Thorac Surg. 18:308–312. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Huang LH, Li J, Gu JP, Qu MX, Yu J and
Wang ZY: Butorphanol attenuates myocardial ischemia reperfusion
injury through inhibiting mitochondria-mediated apoptosis in mice.
Eur Rev Med Pharmacol Sci. 22:1819–1824. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang H, Wang JL, Ren HW, He WF and Sun M:
Butorphanol protects on myocardial ischemia/reperfusion injury in
rats through MAPK signaling pathway. Eur Rev Med Pharmacol Sci.
23:10541–10548. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tang W, Luo L, Hu B and Zheng M:
Butorphanol alleviates lipopolysaccharide-induced inflammation and
apoptosis of cardiomyocytes via activation of the κ-opioid
receptor. Exp Ther Med. 22(1248)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vachon P and Moreau JP: Butorphanol
decreases edema following carrageenan-induced paw inflammation in
rats. Contemp Top Lab Anim Sci. 41:15–17. 2002.PubMed/NCBI
|
|
13
|
Meng J, Jiang SJ, Jiang D and Zhao Y:
Butorphanol attenuates inflammation via targeting NF-κB in septic
rats with brain injury. Eur Rev Med Pharmacol Sci. 23 (3
Suppl):S161–S170. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang Z, Wang L, Hu Y and Wang F:
Butorphanol protects PC12 cells against OGD/R-induced inflammation
and apoptosis. Mol Med Rep. 22:1969–1975. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhao P, Chao W and Li W: FBXW5 reduction
alleviates spinal cord injury (SCI) by blocking microglia activity:
A mechanism involving p38 and JNK. Biochem Biophys Res Commun.
514:558–564. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Geng W and Liu L: MiR-494 alleviates
lipopolysaccharide (LPS)-induced autophagy and apoptosis in PC-12
cells by targeting IL-13. Adv Clin Exp Med. 28:85–94.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang HW, Ding JD, Zhang ZS, Zhao SS, Duan
KY, Zhu BQ, Zhao WF, Chai ZT and Liu XW: Critical Role of p38 in
spinal cord injury by regulating inflammation and apoptosis in a
rat model. Spine (Phila Pa 1976). 45:E355–E363. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gao L, Xu W, Fan S, Li T, Zhao T, Ying G,
Zheng J, Li J, Zhang Z, Yan F, et al: MANF attenuates neuronal
apoptosis and promotes behavioral recovery via Akt/MDM-2/p53
pathway after traumatic spinal cord injury in rats. Biofactors.
2018.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
19
|
Fan H, Zhang K, Shan L, Kuang F, Chen K,
Zhu K, Ma H, Ju G and Wang YZ: Reactive astrocytes undergo M1
microglia/macrohpages-induced necroptosis in spinal cord injury.
Mol Neurodegener. 11(14)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tian JS, Liu SB, He XY, Xiang H, Chen JL,
Gao Y, Zhou YZ and Qin XM: Metabolomics studies on
corticosterone-induced PC12 cells: A strategy for evaluating an in
vitro depression model and revealing the metabolic regulation
mechanism. Neurotoxicol Teratol. 69:27–38. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Katsetos CD, Herman MM, Balin BJ, Vinores
SA, Hessler RB, Arking EJ, Karkavelas G and Frankfurter A: Class
III beta-tubulin isotype (beta III) in the adrenal medulla: III.
Differential expression of neuronal and glial antigens identifies
two distinct populations of neuronal and glial-like (sustentacular)
cells in the PC12 rat pheochromocytoma cell line maintained in a
Gelfoam matrix system. Anat Rec. 250:351–365. 1998.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lu S, Luo Y, Zhou P, Yang K, Sun G and Sun
X: Ginsenoside compound K protects human umbilical vein endothelial
cells against oxidized low-density lipoprotein-induced injury via
inhibition of nuclear factor-κB, p38, and JNK MAPK pathways. J
Ginseng Res. 43:95–104. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ma Z, Lu Y, Yang F, Li S, He X, Gao Y,
Zhang G, Ren E, Wang Y and Kang X: Rosmarinic acid exerts a
neuroprotective effect on spinal cord injury by suppressing
oxidative stress and inflammation via modulating the Nrf2/HO-1 and
TLR4/NF-κB pathways. Toxicol Appl Pharmacol.
397(115014)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hearn JH and Cross A: Mindfulness for
pain, depression, anxiety, and quality of life in people with
spinal cord injury: A systematic review. BMC Neurol.
20(32)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ambrozaitis KV, Kontautas E, Spakauskas B
and Vaitkaitis D: Pathophysiology of acute spinal cord injury.
Medicina (Kaunas). 42:255–261. 2006.PubMed/NCBI(In Lithuania).
|
|
27
|
Mourelo Fariña M, Salvador de la Barrera
S, Montoto Marqués A, Ferreiro Velasco ME and Galeiras Vázquez R:
Update on traumatic acute spinal cord injury. Part 2. Med
Intensiva. 41:306–315. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Galeiras Vázquez R, Ferreiro Velasco ME,
Mourelo Fariña M, Montoto Marqués A and Salvador de la Barrera S:
Update on traumatic acute spinal cord injury. Part 1. Med
Intensiva. 41:237–247. 2017.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
29
|
Witiw CD and Fehlings MG: Acute spinal
cord injury. J Spinal Disord Tech. 28:202–210. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rouanet C, Reges D, Rocha E, Gagliardi V
and Silva GS: Traumatic spinal cord injury: Current concepts and
treatment update. Arq Neuropsiquiatr. 75:387–393. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cofano F, Boido M, Monticelli M, Zenga F,
Ducati A, Vercelli A and Garbossa D: Mesenchymal stem cells for
spinal cord injury: Current options, limitations, and future of
cell therapy. Int J Mol Sci. 20(2698)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ahuja CS, Nori S, Tetreault L, Wilson J,
Kwon B, Harrop J, Choi D and Fehlings MG: Traumatic Spinal Cord
Injury-Repair and Regeneration. Neurosurgery. 80:S9–S22.
2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tang H, Guo Y, Zhao Y, Wang S, Wang J, Li
W, Qin S, Gong Y, Fan W, Chen Z, et al: Effects and mechanisms of
acupuncture combined with mesenchymal stem cell transplantation on
neural recovery after spinal cord injury: Progress and prospects.
Neural Plast. 2020(8890655)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Orr MB and Gensel JC: Spinal cord injury
scarring and inflammation: Therapies targeting glial and
inflammatory responses. Neurotherapeutics. 15:541–553.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tyor WR, Avgeropoulos N, Ohlandt G and
Hogan EL: Treatment of spinal cord impact injury in the rat with
transforming growth factor-beta. J Neurol Sci. 200:33–41.
2002.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhou W, Yuan T, Gao Y, Yin P, Liu W, Pan
C, Liu Y and Yu X: IL-1β-induces NF-κB and upregulates microRNA-372
to inhibit spinal cord injury recovery. J Neurophysiol.
117:2282–2291. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Barros AGC, Cristante AF, Santos GBD,
Natalino RJM, Ferreira RJR and Barros-Filho TEP: Evaluation of the
effects of erythropoietin and interleukin-6 in rats submitted to
acute spinal cord injury. Clinics (Sao Paulo).
74(e674)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gensel JC and Zhang B: Macrophage
activation and its role in repair and pathology after spinal cord
injury. Brain Res. 1619:1–11. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ding J, Karp JE and Emadi A: Elevated
lactate dehydrogenase (LDH) can be a marker of immune suppression
in cancer: Interplay between hematologic and solid neoplastic
clones and their microenvironments. Cancer Biomark. 19:353–363.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lv R, Du L, Zhang L and Zhang Z: Polydatin
attenuates spinal cord injury in rats by inhibiting oxidative
stress and microglia apoptosis via Nrf2/HO-1 pathway. Life Sci.
217:119–127. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Crowe MJ, Bresnahan JC, Shuman SL, Masters
JN and Beattie MS: Apoptosis and delayed degeneration after spinal
cord injury in rats and monkeys. Nat Med. 3:73–76. 1997.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sekhon LH and Fehlings MG: Epidemiology,
demographics, and pathophysiology of acute spinal cord injury.
Spine (Phila Pa 1976). 26 (24 Suppl):S2–S12. 2001.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Gashmardi N, Hosseini SE, Mehrabani D,
Edalatmanesh MA and Khodabandeh Z: Impacts of bone marrow stem
cells on caspase-3 levels after spinal cord injury in mice. Iran J
Med Sci. 42:593–598. 2017.PubMed/NCBI
|
|
44
|
Thompson CD, Zurko JC, Hanna BF,
Hellenbrand DJ and Hanna A: The therapeutic role of interleukin-10
after spinal cord injury. J Neurotrauma. 30:1311–1324.
2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Takada Y, Singh S and Aggarwal BB:
Identification of a p65 peptide that selectively inhibits NF-kappa
B activation induced by various inflammatory stimuli and its role
in down-regulation of NF-kappaB-mediated gene expression and
up-regulation of apoptosis. J Biol Chem. 279:15096–15104.
2004.PubMed/NCBI View Article : Google Scholar
|