Introduction
Hand, foot and mouth disease (HFMD) broke out in
Fuyang, Anhui in 2008, resulting in many children's deaths and
since then, the prevalence of HFMD has been rising rapidly in China
(1). HFMD primarily infects
children under the age of 6 years and is caused by Coxsackie virus
A16 (Cox A16) and enterovirus 71 (EV71) (2). The rapid progression of HFMD may lead
to serious nervous system complications involving cerebritis,
meningitis and high mortality (3,4).
These severe cases are primarily caused by EV71 infection. However,
the pathogenic mechanism of EV71 infection is not yet completely
understood and there is no specific drug against EV71
infection.
Viral infection induces several types of programmed
cell death (PCD), such as apoptosis (5) and pyroptosis (6). Certain types of PCD are part of the
stress response used by the body to eliminate the virus; however,
other types promote viral infection and spread in the body. For
example, hepatitis B virus induces apoptosis of liver cancer cells
(7) and EV71 infection induces
pyroptosis (8). In the present
study, it was hypothesized that the pathogenesis of EV71 infection
may be associated with apoptosis and pyroptosis of infected
cells.
Apoptosis is an autonomous form of PCD that is
controlled by genes, such as Bcl-2, p53, c-myc and caspases
(9,10). The morphological manifestations of
apoptosis include decreased cell volume, rupture of the nuclear
membrane and nucleolus, intact cell membrane structure and
separation of apoptotic bodies from apoptotic cells (11). Apoptosis does not lead to
inflammation in the surrounding cells (12). However, inhibiting EV71-mediated
apoptosis can protect cells (13).
Pyroptosis is a newly identified type of PCD that
was discovered by Brennan and Cookson in salmonella-infected
macrophages (14). Pyroptosis is
identified by the presence of swollen cells, which rupture and
cause the release of IL-1β and IL-18(15), thereby inducing an inflammatory
response in the body (16). The
inflammatory response triggered by EV71 is the primary cause of
high levels of inflammation observed in severe HFMD (17,18).
Considering the association between pyroptosis and inflammation, it
was hypothesized that suppressing EV71-mediated pyroptosis may lead
to identification of novel anti-inflammatory agents for the
treatment of HFMD.
Current anti-inflammatory therapies include
steroidal and non-steroidal treatments, both of which have side
effects (19,20). The aim of the present study was to
identify anti-inflammatory ingredients in natural plants, such as
those used in Traditional Chinese medicine. Throughout years of
clinical practice, the effect of Astragalus membranaceus
(AM) on inflammation has gradually been recognized (21). Total astragalosides (ASTs), the
primary active ingredients of AM, are used in the treatment of
nephrotic syndrome (22) and can
inhibit the proliferation of gastric cancer cells (23), prevent oxidative damage (24) and protect against inflammation
(25). Therefore, the present
study examined whether ASTs attenuate EV71-induced cell injury by
investigating the effect of ASTs on cell viability, viral
replication and release and cell apoptosis and pyroptosis. The
present study aimed to enhance understanding of the pathogenic
mechanism of EV71 and develop novel strategies for EV71
infection.
Materials and methods
Cell lines and culture
Human normal gastric epithelial cell (GES-1 cells)
and human rhabdomyosarcoma cells (RD) were obtained from Fenyang
College of Shanxi Medical University (Fenyang, China) and cultured
in DMEM (Boster Biological Technology) supplemented with 10% FBS
(Biological Industries Technology), 1% penicillin and 1%
streptomycin (Boster Biological Technology). The cells were
maintained in an incubator (Thermo Fisher Scientific, Inc.) at 37˚C
with 5% CO2.
Tissue culture infectious dose 50
(TCID50) assay
EV71 was generously supplied by Professor Zhendong
Zhao from the Chinese Academy of Medical Sciences & Peking
Union Medical College, Institute of Pathogen Biology (Beijing,
China). The EV71 titer was measured using TCID50 assay. Steps are
as follows: RD cells were seeded into 96-well plates
(1x104/well) and incubated overnight at 37˚C in an
incubator. EV71 was serially diluted
1x101-1x1011 times in centrifuge tubes and
then added to the 96-well plates (8 repeated/dilution). The 96-well
plates were observed under the light inverted microscope
(magnification, x40) once every day for 5 days and ~50% of the
cytopathic effect in the wells was recorded. TCID50 results were
calculated according to the Reed-Münch method (26) using the following formula:
Plaque-forming units=0.7 x TCID50.
Viral infection
GES-1 cells were seeded into 6-well plates
(1x106/well) and infected with EV71 at a specified
multiplicity of infection (MOI; 0, 1, 3 and 5) at 37˚C for 24 h,
and infected cells and supernatant were collected at 2,000 x g for
3 min at room temperature. Proteins were extracted from cells and
analyzed using western blotting. The aforementioned supernatant was
collected to measure the virus titer.
Antibodies and reagents
BCA protein detection kit (cat. no. AR0146) and ECL
western blot detection kit (cat. no. AR1171) were obtained from
Boster Biological Technology. Total astragalosides (cat. no.
SA8600) were purchased from Beijing Solarbio Science &
Technology Co, Ltd. Horseradish peroxidase-conjugated goat
anti-rabbit IgG (H+L) antibody (cat. no. CW0103) was purchased from
CoWin Biosciences. Anti-caspase-3 (cat. no. WL02117), anti-NLR
family, pyrin domain containing 3 (NLRP3; cat. no. WL02635),
anti-cleaved (c)-caspase-3 (cat. no. WL01992) and anti-pro-IL-1β
(cat. no. WL02257) antibodies were purchased from Wanleibio Co.,
Ltd. Anti-gasdermin D protein (GSDMD; cat. no. A17308) and
anti-pro-caspase-1 (cat. no. A0964) antibodies were obtained from
ABclonal Biotech Co., Ltd. Anti-PARP (cat. no. AP102), anti-c-PARP
(cat. no. AF1567) antibodies, Lactate dehydrogenase (LDH) detection
kit (cat. no. C0017), DAPI (cat. no. C1002) and BeyoRT II First
Strand cDNA Synthesis kit (cat. no. D7168) were obtained from
Beyotime Institute of Biotechnology. Anti-c-caspase-1 (cat. no.
BS65650) antibody was obtained from Bioworld Technology, Inc.
Anti-VP1 (cat. no. GTX132313) antibody was purchased from GeneTex,
Inc. Cell Counting Kit-8 (CCK-8; cat. no. 40203ES60) assay and
Hieff Universal Blue qPCR SYBR Green Master mix (cat. no. 11141ES)
were purchased from Yeasen Biotech Co., Ltd.
Cell treatment and morphological
observation
GES-1 cells were plated into 6-well plates
(1x106/well) and divided into four treatment groups:
Control (treated with DMEM), EV71 (MOI=5), AST (10 µg/ml) and EV71
+ ASTs (ASTs were added 2 h before EV71 infection). The cells were
cultured for 24 h in an incubator at 37˚C with 5% CO2.
The morphology of cells was observed and photographed using a light
inverted microscope (magnification, x100; Nikon Corporation).
CCK-8 assay
Cells in the logarithmic growth phase were selected
for the experiment. GES-1 cells were treated as aforementioned,
then co-cultured with CCK-8 reagent for 2 h. Absorbance was
determined using a microplate reader (BioTek Instruments, Inc.) at
450 nm to calculate cell viability as follows: Cell viability
(%)=(Absorbancetreatment-Absorbancecontrol)/(Absorbanceblack-Absorbancecontrol)
x100. The blank well only contained medium and CCK-8 reagent and
the experiment was repeated three times.
Measurement of LDH release
LDH release was used to assess cell damage. GES-1
cells supernatant was collected using centrifugation (Eppendorf
tubes; Eppendorf) at 400 x g for 5 min at room temperature. LDH was
measured using an LDH detection kit, according to the
manufacturer's instructions. LDH activity (%) was calculated as
follows: (LDHtreatment-LDH
control)/(LDHmax-LDHcontrol)
x100.
DAPI nuclear staining
After GES-1 cells were treated for 24 h as
aforementioned, the culture medium was discarded. Cells were rinsed
three times with PBS for 3 min each and then fixed with 4%
paraformaldehyde for 20 min at 4˚C. After discarding
paraformaldehyde, the cells were rinsed with PBS five times and
then stained with DAPI (1:10,000) at room temperature for 5 min.
The cells were observed using a fluorescence microscope
(magnification, x200).
Western blotting
Cells from the control and experimental groups were
placed in 1.5-ml centrifuge tubes, then lysed with 60 µl RIPA lysis
buffer (Beyotime Institute of Biotechnology) and the total protein
concentration was determined using a BCA kit. Subsequently, ~30 µg
total extracted protein (per well) from each group was separated
using 12% SDS-PAGE and then transferred to a nitrocellulose (NC)
membrane. The NC membrane was blocked with 5% skimmed milk for 2 h
at room temperature and incubated with poly (ADP-ribose) polymerase
(PARP; 1:1,000), c-PARP (1:1,000), caspase-3 (1:1,000), c-caspase-3
(1:1,000), NLRP3 (1:1,000), GSDMD (1:1,000), pro-caspase-1
(1:1,000), c-caspase-1 (1:1,000), pro-IL-1β (1:1,000) and virus
structural protein VP1 (1:2,000) antibodies overnight at 4˚C.
Following primary antibody incubation, the membrane was incubated
with goat anti-rabbit IgG secondary antibody (1:5,000). The target
proteins were observed using an ECL chromogenic kit by ChemiDoc™ MP
Imaging System (Bio-Rad Laboratories, Inc.). ImageJ software
(version 6.0; National Institutes of Health) was used for
densitometry.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and
reverse-transcribed into cDNA using a BeyoRT II First Strand cDNA
Synthesis kit (Beyotime Institute of Biotechnology) at 42˚C for 60
min, then left to stand at 80˚C for 10 min to terminate the
reaction. qPCR was performed using Hieff Universal Blue qPCR SYBR
Green Master mix. The amplification reaction mixture consisted of
10 µl 2X SYBR Green mix, a specific primer set (0.4 µl each), cDNA
(1 µg) and diethylpyrocarbonate (DEPC)-treated water to make the
total volume of 20 µl. The following thermocycling conditions were
used: Initial denaturation at 95˚C for 2 min; followed by 40 cycles
of denaturation at 95˚C for 10 sec and extension at 55˚C for 30
sec. GAPDH was used as an internal control and mRNA expression
levels were analyzed using the 2-ΔΔCq method (27). The following primer pairs were
used: EV71 forward, 5'-CGCACAGGGTCACTCAGAAC-3' and reverse,
5'-GCCCATTGCCACCAGTAGAC-3' and GAPDH forward,
5'-ACAACTTTGGCATTGTGGAA-3' and reverse,
5'-GATGCAGGGATGATGTTCTG-3'.
Statistical analysis
All experiments were independently repeated three
times and data are presented as the mean ± SD. One-way ANOVA
followed by Tukey's post hoc was used to compare multiple groups,
and Welch's test followed by Games-Howell post hoc test was applied
to data with unequal variances; unpaired Student's t-test was used
to compare statistical differences between two groups using SPSS
20.0 (SPSS, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
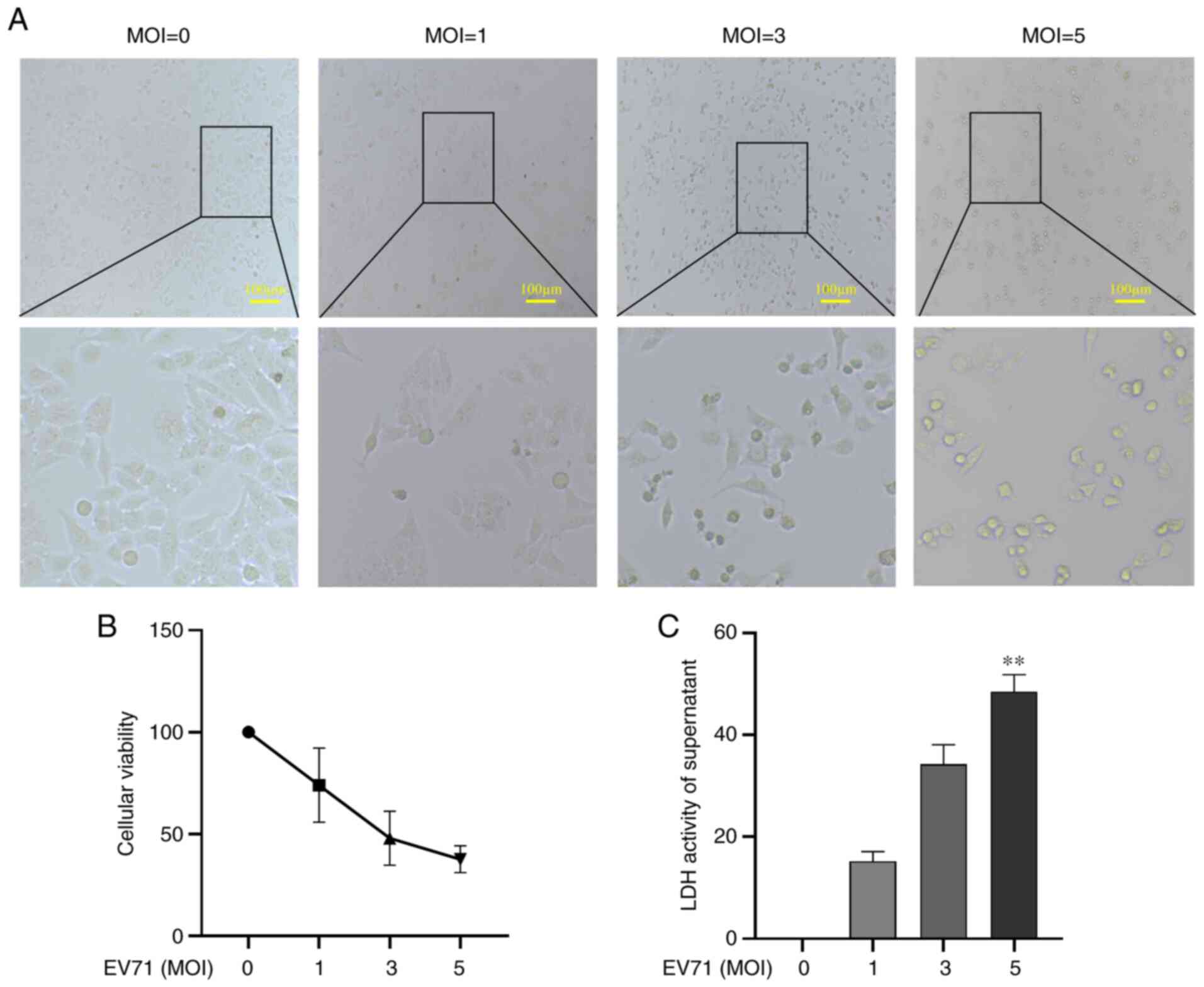
EV71 infection causes direct damage to
cells
To assess whether EV71 infection damages GES-1
cells, GES-1 cells were infected with EV71 at various MOIs (0, 1, 3
and 5) for 24 h. Cell morphology was then observed under an
inverted microscope. The results showed that the cells became
round, the cytoplasm shrank and the overall number of cells
decreased (Fig. 1A). Cell
viability was detected using a CCK-8 assay; EV71 infection caused
GES-1 cell death in a dose-dependent manner (P<0.01; Fig. 1B). LDH release was used to detect
the damage to GES-1 cells; the results revealed that GES-1 cells
were markedly damaged with the increase of MOIs, with MOI of 5
causing a significant increase in LDH release (P<0.01; Fig. 1C). It was concluded that EV71
infection caused direct damage to GES-1 cells.
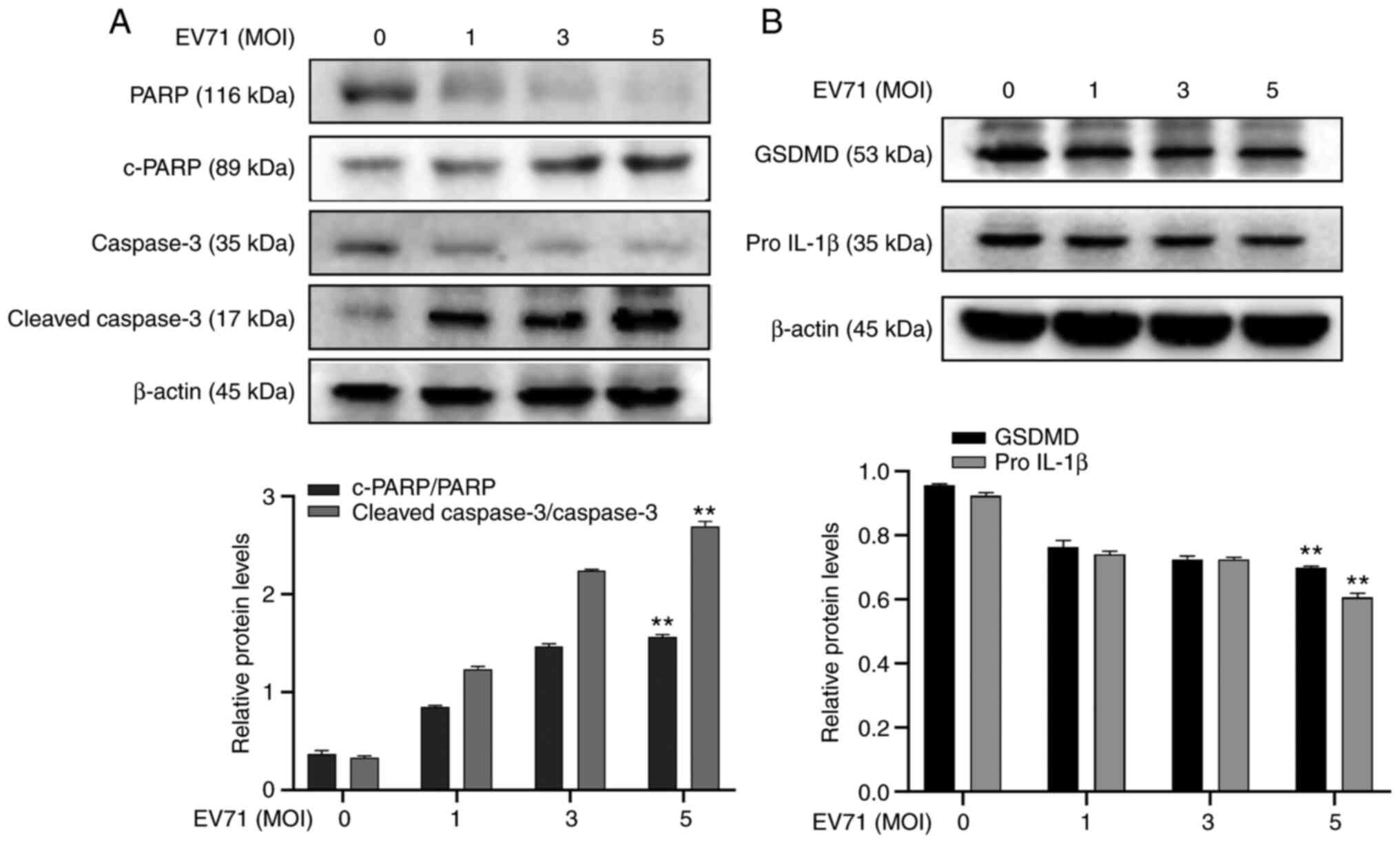
EV71 induces apoptosis and
pyroptosis
Considering the association between EV71 and
induction of PCD (8,28), EV71-induced cell apoptosis and
pyroptosis were investigated. GES-1 cells were infected with EV71
at different MOIs for 24 h. The expression of apoptosis-associated
proteins PARP, c-PARP, Caspase-3 and c-caspase-3 were analyzed
using western blotting. Levels of PARP and caspase-3 protein
decreased following EV71 infection (P<0.01; Fig. 2A). The pyroptosis-associated
proteins pro-GSDMD and pro-IL-1β were also detected using western
blotting. The results showed that pro-GSDMD and pro-IL-1β in GES-1
cells were decreased following EV71 infection (P<0.01; Fig. 2B). It was concluded that EV71
induces apoptosis and pyroptosis in GES-1 cells.
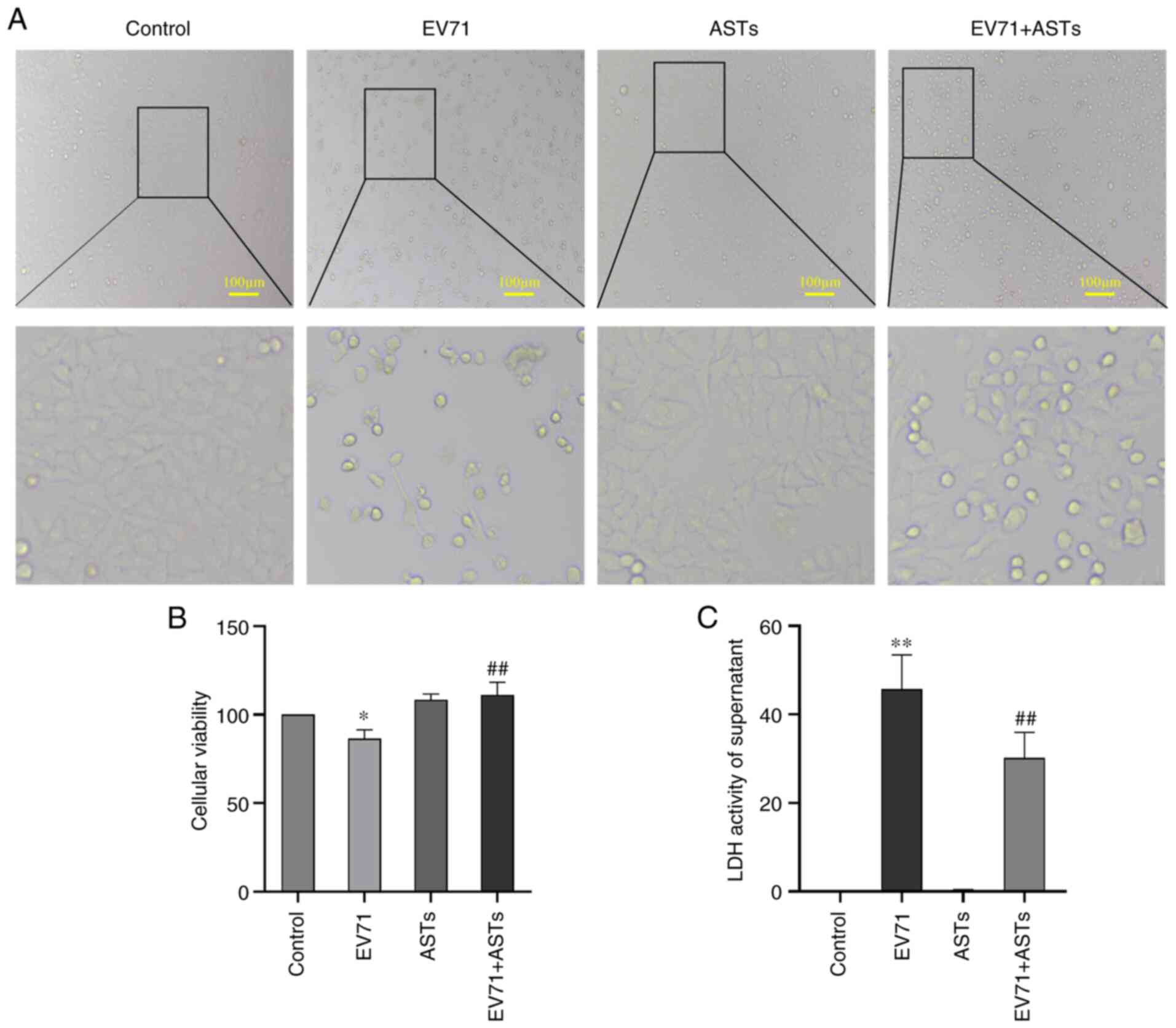
ASTs alleviate EV71 infection-induced
cell damage
To assess the protective effect of ASTs on EV71
infection-induced cell damage, GES-1 cells were treated with ASTs
(10 µg/ml) 2 h before infection with EV71 (MOI=5, as >50% of
GES-1 cells were damaged) for 24 h. GES-1 cell morphology was
observed under a microscope. The number of ASTs-treated cells was
higher than that of cells infected with EV71 (Fig. 3A). The viability of AST-treated
cells infected with EV71 was detected using CCK-8 assay; the
results showed that ASTs improved the viability of EV71-infected
GES-1 cells (P<0.01; Fig. 3B).
EV71 infection-induced damage in AST-treated cells was measured
using a LDH assay. The results showed that ASTs alleviated EV71
infection-induced cell damage (P<0.01; Fig. 3C).
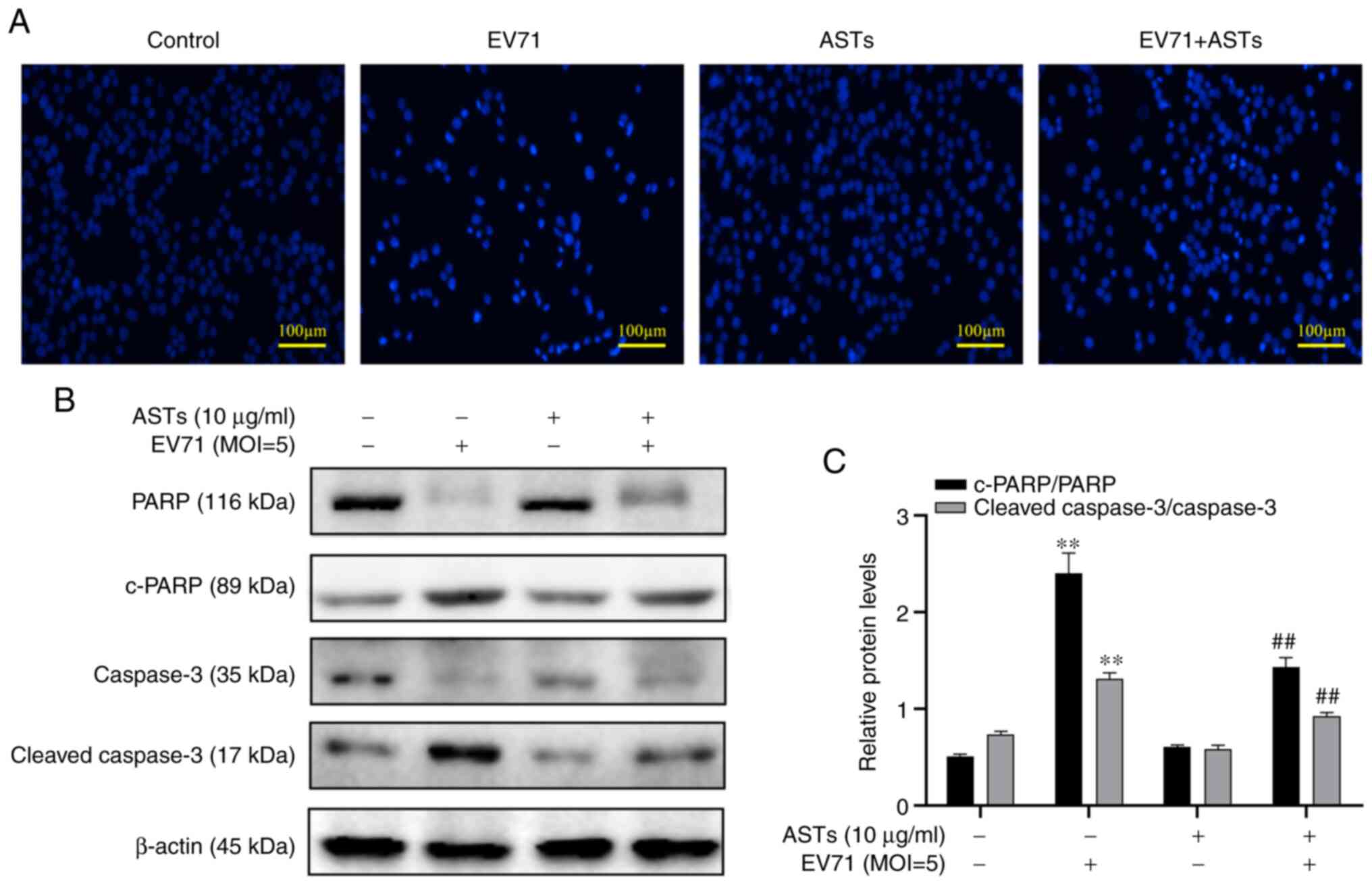
ASTs inhibit GES-1 cell apoptosis
To assess whether ASTs inhibit GES-1 cell apoptosis,
DAPI was used to stain the nucleus of GES-1 cells. The number of
cells was found to be decreased and the nucleus shrank following
EV71 infection. However, these pathological changes in the nucleus
were reversed following treatment with ASTs (Fig. 4A). In addition, changes in the
expression levels of apoptotic proteins were measured using western
blotting. The results revealed lower c-PARP and c-caspase-3 levels
in EV71-infected and AST-treated GES-1 cells compared with the
control group (P<0.01; Fig. 4B
and C).
ASTs inhibit pyroptosis in GES-1
cells
To determine whether ASTs inhibit GES-1 cell
pyroptosis, western blotting was used to detect marker proteins of
pyroptosis. Compared with the control group, the expression levels
of pyroptosis-associated proteins NLRP3 and c-caspase-1 were lower,
and those of pro-GSDMD, pro-caspase-1 and pro-IL-1β was
significantly higher in EV71-infected and AST-treated GES-1 cells
(P<0.05 or P<0.01; Fig. 5A
and B).
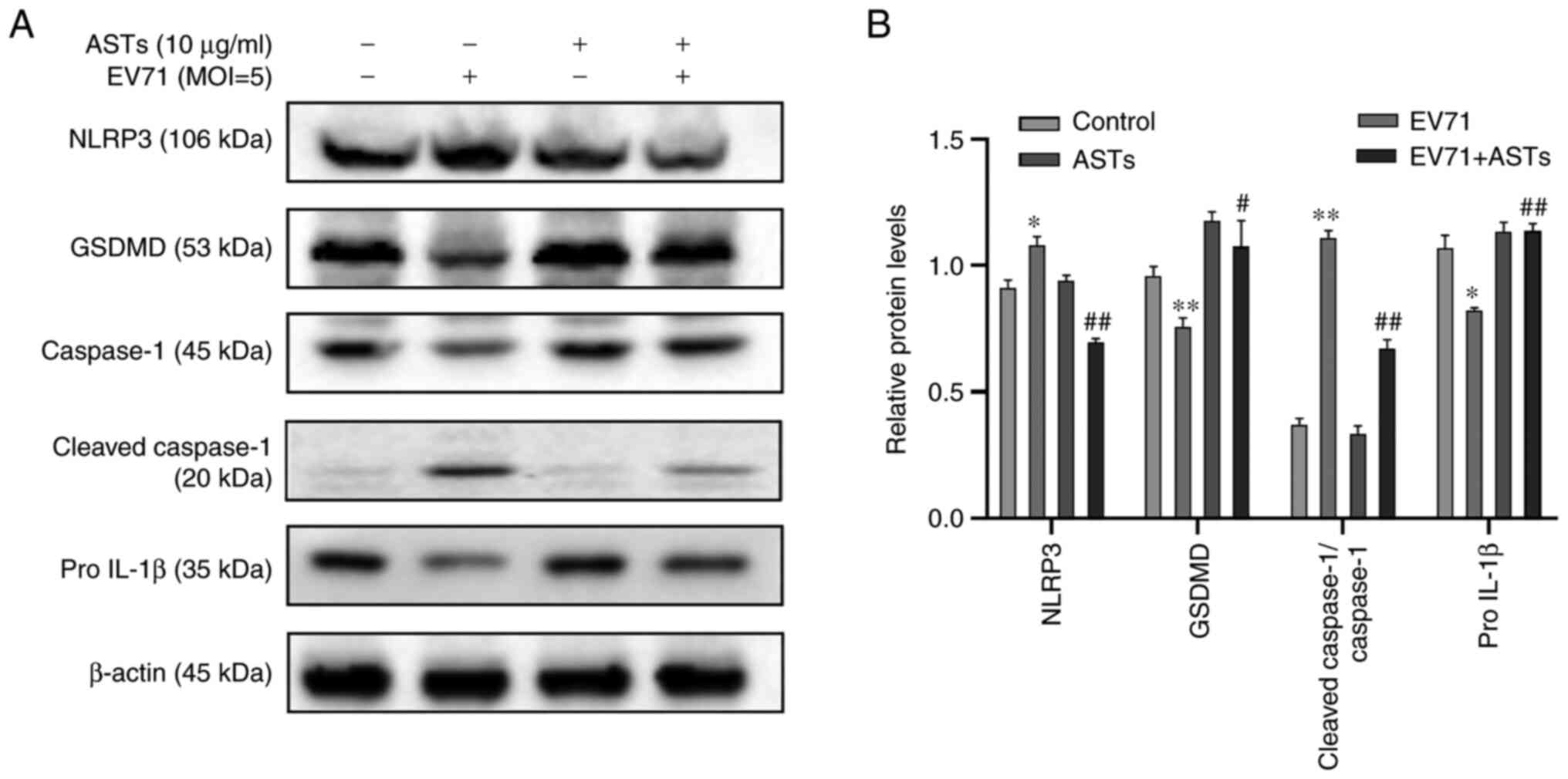 | Figure 5ASTs inhibit pyroptosis in GES-1
cells. (A) To determine the effect of ASTs on pyroptosis, GES-1
cells were seeded in culture plates and infected with EV71 at a MOI
of 5 in AST medium. The cells were collected and total protein was
measured using western blotting. Anti-NLRP3, anti-GSDMD,
anti-pro-caspase-1, anti-c-caspase-1 and anti-IL-1β antibodies were
used to analyze the levels of pyroptosis in cells. (B) Relative
protein expression levels are also presented. Data are presented as
the mean ± SD (n=3). *P<0.05 and
**P<0.01 vs. control; #P<0.05 and
##P<0.01 vs. EV71. ASTs, total astragalosides; EV71,
enterovirus 71; NLRP3, NLR family, pyrin domain containing 3;
GSDMD, gasdermin D protein; MOI, multiplicity of infection; c-,
cleaved. |
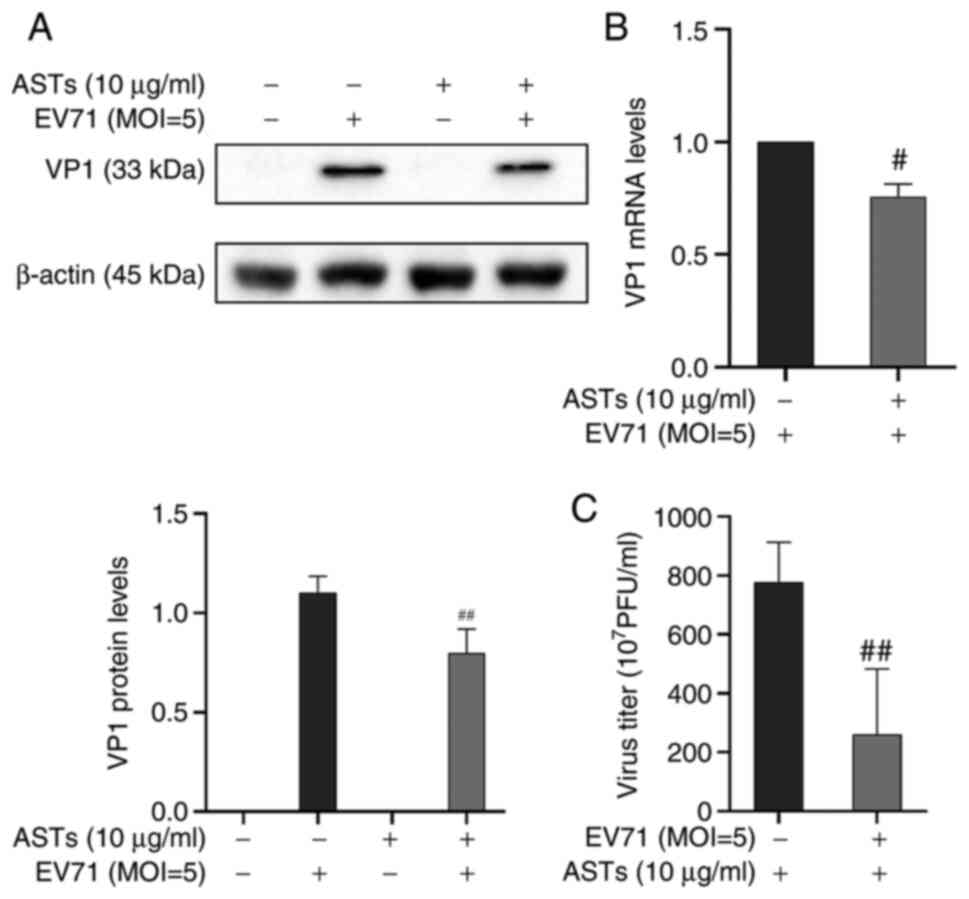
ASTs inhibit EV71 replication
ASTs inhibited apoptosis and pyroptosis to alleviate
EV71-induced cell damage. Therefore, it was investigated whether
the effect of ASTs on cell pyroptosis and apoptosis was mediated by
regulation of EV71. The expression of the EV71 structural protein,
VP1, was detected using western blotting and VP1 mRNA expression
levels were measured using RT-qPCR. Results showed that the protein
and mRNA expression of VP1 was decreased following AST treatment
(P<0.01 or P<0.05, respectively; Fig. 6A and B). A TCID50 assay was used to detect EV71
release in the cell culture supernatant. The results showed that
ASTs inhibited EV71 release in GES-1 cells (P<0.01; Fig. 6C). These results showed that ASTs
inhibited EV71 replication and release.
Discussion
HFMD is a pediatric disease caused by EV71 infection
that is prevalent worldwide (29).
The incidence of HFMD in China has been rising since 2008, but
there are no specific antiviral drugs for the control of EV71
infection (30). Therefore,
identifying effective antiviral agents against EV71 viral infection
is urgent.
Previous studies have shown that the active
components of certain plants exert antiviral effects. For example,
Kalanchoe gracilis leaf extract inhibits both Cox A16 and
EV71 infection in vivo and in vitro by inhibiting
viral non-structural protein 2A protease activity (31). Apigenin directly binds to
heterogeneous nuclear ribonucleoprotein A2 protein to prevent EV71
infection (32). Oblongifolin
M extracted from Garcinia oblongifolia suppresses EV71
replication by downregulating endoplasmic reticulum resident
protein 57(33). Baicalin inhibits
replication of EV71 by inhibiting expression of EV1 3D polymerase
(34). As an active component of
AM, ASTs exert obvious protective effects in cell injury. Li et
al (24) found that ASTs
protect the kidney from shock wave-induced oxidation injury. In a
previous study, the EV71 receptors scavenger receptor class B
member 2 and P-selectin glycoprotein ligand-1 were found to be
distributed in the human gastrointestinal tract, and the stomach
was the primary site of EV71 infection (35). Our previous experiment showed that
GES-1 cells are sensitive to EV71(36). However, the present study
discovered that EV71 infection led to shrinkage of GES-1 cell
cytoplasm and decrease in cell number, while ASTs alleviated GES-1
cell damage.
Apoptosis is an autonomous type of PCD (9). EV71, hepatitis B and C and other
viruses induce apoptosis (7,37,38).
Viruses tend to use host cells to induce apoptosis of tissue or
immune cells to infect the body (39). The present study found that EV71
infection decreased expression levels of apoptosis marker proteins
caspase-3 and PARP in GES-1 cells, and ASTs ameliorated this
effect. Thus, the present study showed that EV71 induced apoptosis
of GES-1 cells and ASTs suppressed this process. These results
suggested that ASTs inhibited EV71 replication and virus-induced
cytopathic effects, such as apoptosis and pyroptosis. However, the
exact mechanism requires further in vivo studies.
A variety of cytokines, such as TNF-α, IL-1, IL-6,
IL-12, IFN-α, IFN-β, monocyte chemoattractant protein-1 and IL-8
are rapidly produced in the body following infection with
microorganisms, which is a significant cause of acute respiratory
distress syndrome and multiple organ failure (40). EV71 infection induces IL-4, IL-6,
IL-12, TNF and IFN production and pyroptosis (36,41).
Cells undergoing pyroptosis have been shown to produce IL-1β and
IL-18 cytokines, which induce inflammation in the body (42). The present study revealed that EV71
infection induced an increase in the protein level of NLRP3, while
it decreased the protein expression levels of GSDMD, pro-caspase-1
and pro-IL-1β in GES-1 cells. Conversely, ASTs inhibited pyroptosis
and IL-1β production to suppress EV71-induced inflammation. Thus,
ASTs exerted both antiviral and anti-inflammatory effects, which
may support the further clinical application of ASTs in the
treatment of EV71-induced HFMD. Future studies should aim to
provide insight into the mechanism of AST-induced antiviral effects
in vitro, as well as searching for potential antiviral
targets. The present results will be further demonstrated in animal
models. However, an animal model of virus infection requires a
special biosafety laboratory and there are few laboratories in the
world with a license. The present cell experiments were performed
in a Biosafety Level II laboratory (43).
In conclusion, the findings of the present study
suggested that EV71 infection may induce GES-1 cell apoptosis and
pyroptosis, while ASTs may suppress these processes and alleviate
cytotoxicity. This protective effect may be due to the ability of
ASTs to suppress the replication and release of EV71 in GES-1
cells.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 81301426), the Provincial
Natural Science Foundation of Shanxi (grant no. 201901D111329), the
Mega Research and Development Projects of Lüliang (grant no.
2020SHFZ38), The Program of Fenyang College, Shanxi Medical
University (grant no. 2020B01) and The Key Laboratory Platform
Construction Projects of Lüliang (grant no. 2020ZDSYS17).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL, XZ and JH conceived and designed the
experiments. XZ, JH, CS, JD and QH performed the experiments. JH
analyzed the data. XZ and JH wrote the manuscript. QL and XZ
revised the manuscript. All authors have read and approved the
final manuscript. XZ and JH confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gao W, Hou M, Liu X, Li Z, Yang Y and
Zhang W: Induction of SOCS expression by EV71 infection promotes
EV71 replication. Biomed Res Int. 2020(2430640)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fu M, Bai J, Gao S, Chang Z, Zhou X and
Long JE: Construction and characterization of an infectious cDNA
clone of enterovirus 71: A rapid method for rescuing infectious
virus based on stable cells expressing T7 polymerase. Arch Virol.
166:627–632. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hu Y, Xu Y, Huang Z, Deng Z, Fan J, Yang
R, Ma H, Song J and Zhang Y: Transcriptome sequencing analysis of
SH-SY5Y cells infected with EV71 reveals the potential neuropathic
mechanisms. Virus Res. 282(197945)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fu Y, Zhang L, Zhang F, Tang T, Zhou Q,
Feng C, Jin Y and Wu Z: Exosome-mediated miR-146a transfer
suppresses type I interferon response and facilitates EV71
infection. PLoS Pathog. 13(e1006611)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ampomah PB and Lim LHK: Influenza A
virus-induced apoptosis and virus propagation. Apoptosis. 25:1–11.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xie WH, Ding J, Xie XX, Yang XH, Wu XF,
Chen ZX, Guo QL, Gao WY, Wang XZ and Li D: Hepatitis B virus X
protein promotes liver cell pyroptosis under oxidative stress
through NLRP3 inflammasome activation. Inflamm Res. 69:683–696.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hu X, Jiang J, Ni C, Xu Q, Ye S, Wu J, Ge
F, Han Y, Mo Y, Huang D and Yang L: HBV integration-mediated cell
apoptosis in HepG2.2.15. J Cancer. 10:4142–4150. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang Y, Qin Y, Wang T, Chen Y, Lang X,
Zheng J, Gao S, Chen S, Zhong X, Mu Y, et al: Pyroptosis induced by
enterovirus 71 and coxsackievirus B3 infection affects viral
replication and host response. Sci Rep. 8(2887)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ding Q, Zhang W, Cheng C, Mo F, Chen L,
Peng G, Cai X, Wang J, Yang S and Liu X: Dioscin inhibits the
growth of human osteosarcoma by inducing G2/M-phase arrest,
apoptosis, and GSDME-dependent cell death in vitro and in vivo. J
Cell Physiol. 235:2911–2924. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Karam JA, Lotan Y, Karakiewicz PI, Ashfaq
R, Sagalowsky AI, Roehrborn CG and Shariat SF: Use of combined
apoptosis biomarkers for prediction of bladder cancer recurrence
and mortality after radical cystectomy. Lancet Oncol. 8:128–136.
2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Häcker G: The morphology of apoptosis.
Cell Tissue Res. 301:5–17. 2000.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wyllie AH, Kerr JF and Currie AR: Cell
death: The significance of apoptosis. Int Rev Cytol. 68:251–306.
1980.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Song F, Yu X, Zhong T, Wang Z, Meng X, Li
Z, Zhang S, Huo W, Liu X, Zhang Y, et al: Caspase-3 inhibition
attenuates the cytopathic effects of EV71 infection. Front
Microbiol. 9(817)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cookson BT and Brennan MA:
Pro-inflammatory programmed cell death. Trends Microbiol.
9:113–114. 2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Brennan MA and Cookson BT: Salmonella
induces macrophage death by caspase-1-dependent necrosis. Mol
Microbiol. 38:31–40. 2000.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gaul S, Leszczynska A, Alegre F, Kaufmann
B, Johnson CD, Adams LA, Wree A, Damm G, Seehofer D, Calvente CJ,
et al: Hepatocyte pyroptosis and release of inflammasome particles
induce stellate cell activation and liver fibrosis. J Hepatol.
74:156–167. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Duan G, Yang H, Shi L, Sun W, Sui M, Zhang
R, Wang X, Wang F, Zhang W, Xi Y and Fan Q: Serum inflammatory
cytokine levels correlate with hand-foot-mouth disease severity: A
nested serial case-control study. PLoS One.
9(e112676)2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Han J, Wang Y, Gan X, Song J, Sun P and
Dong XP: Serum cytokine profiles of children with human enterovirus
71-associated hand, foot, and mouth disease. J Med Virol.
86:1377–1385. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
James DS: The multisystem adverse effects
of NSAID therapy. J Am Osteopath Assoc. 99 (Suppl 11):S1–S7.
1999.PubMed/NCBI
|
|
20
|
Polderman JA, Farhang-Razi V, Van Dieren
S, Kranke P, DeVries JH, Hollmann MW, Preckel B and Hermanides J:
Adverse side effects of dexamethasone in surgical patients.
Cochrane Database Syst Rev. 8(CD011940)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang X, Liang T, Yang W, Zhang L, Wu S,
Yan C and Li Q: Astragalus membranaceus injection suppresses
production of interleukin-6 by activating autophagy through the
AMPK-mTOR pathway in lipopolysaccharide-stimulated macrophages.
Oxid Med Cell Longev. 2020(1364147)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sai YP, Song YC, Chen XX, Luo X, Liu J and
Cui WJ: Protective effect of astragalosides from radix astragali on
adriamycin-induced podocyte injury. Exp Ther Med. 15:4485–4490.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
OuYang Y, Huang J, OuYang Z and Kang J:
Enrichment and purification process of astragalosides and their
anti-human gastric cancer MKN-74 cell proliferation effect. Afr
Health Sci. 14:22–27. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li X, He D, Zhang L, Cheng X, Sheng B and
Luo Y: A novel antioxidant agent, astragalosides, prevents shock
wave-induced renal oxidative injury in rabbits. Urol Res.
34:277–282. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li C, Yang F, Liu F, Li D and Yang T:
NRF2/HO-1 activation via ERK pathway involved in the
anti-neuroinflammatory effect of astragaloside IV in LPS induced
microglial cells. Neurosci Lett. 666:104–110. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cheng JH, Sun YJ, Zhang FQ, Zhang XR, Qiu
XS, Yu LP, Wu YT and Ding C: Newcastle disease virus NP and P
proteins induce autophagy via the endoplasmic reticulum
stress-related unfolded protein response. Sci Rep.
6(24721)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li Q, Cheng F, Zhou K, Fang L, Wu J, Xia
Q, Cen Y, Chen J and Qing Y: Increased sensitivity to TNF-α
promotes keloid fibroblast hyperproliferation by activating the
NF-κB, JNK and p38 MAPK pathways. Exp Ther Med.
21(502)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Xu T, Li Y, Wu HL, Chen H, Wu H, Guo M,
Zhao M, Wang C, Lin T, Lin Z, et al: The inhibition of enterovirus
71 induced apoptosis by durvillaea antarctica through P53 and STAT1
signaling pathway. J Med Virol. 93:3532–3538. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
You L, Chen J, Liu W, Xiang Q, Luo Z, Wang
W, Xu W, Wu K, Zhang Q, Liu Y and Wu J: Enterovirus 71 induces
neural cell apoptosis and autophagy through promoting ACOX1
downregulation and ROS generation. Virulence. 11:537–553.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ma JQ, Sun YZ, Ming QL, Tian ZK, Yang HX
and Liu CM: Ampelopsin attenuates carbon tetrachloride-induced
mouse liver fibrosis and hepatic stellate cell activation
associated with the SIRT1/TGF-β1/Smad3 and autophagy pathway. Int
Immunopharmacol. 77(105984)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang CY, Huang SC, Zhang Y, Lai ZR, Kung
SH, Chang YS and Lin CW: Antiviral ability of Kalanchoe
gracilis leaf extract against enterovirus 71 and coxsackievirus
A16. Evid Based Complement Alternat Med.
2012(503165)2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang W, Qiao H, Lv Y, Wang J, Chen X, Hou
Y, Tan R and Li E: Apigenin inhibits enterovirus-71 infection by
disrupting viral RNA association with trans-acting factors. PLoS
One. 9(e110429)2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang M, Dong Q, Wang H, He Y, Chen Y,
Zhang H, Wu R, Chen X, Zhou B, He J, et al: Oblongifolin M, an
active compound isolated from a Chinese medical herb Garcinia
oblongifolia, potently inhibits Enterovirus 71 reproduction
through downregulation of ERp57. Oncotarget. 7:8797–8808.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li X, Liu Y, Wu T, Jin Y, Cheng J, Wan C,
Qian W, Xing F and Shi W: The antiviral effect of baicalin on
enterovirus 71 in vitro. Viruses. 7:4756–4771. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jiao XY, Guo L, Huang DY, Chang XL and Qiu
QC: Distribution of EV71 receptors SCARB2 and PSGL-1 in human
tissues. Virus Res. 190:40–52. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cao L and Zhang X, Yuan S, Cheng K and
Zhang X: Autophagy induced by enterovirus 71 regulates the
production of IL-6 through the p38MAPK and ERK signaling pathways.
Microb Pathog. 131:120–127. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Li H, Bai Z, Li C, Sheng C and Zhao X:
EV71 infection induces cell apoptosis through ROS generation and
SIRT1 activation. J Cell Biochem. 121:4321–4331. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Guo X, Liu WL, Yang D, Shen ZQ, Qiu ZG,
Jin M and Li JW: Hepatitis C virus infection induces endoplasmic
reticulum stress and apoptosis in human fetal liver stem cells. J
Pathol. 248:155–163. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Iannello A, Debbeche O, Martin E, Attalah
LH, Samarani S and Ahmad A: Viral strategies for evading antiviral
cellular immune responses of the host. J Leukoc Biol. 79:16–35.
2006.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Guo XJ and Thomas PG: New fronts emerge in
the influenza cytokine storm. Semin Immunopathol. 39:541–550.
2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Shao P, Wu X, Li H, Wu Z, Yang Z and Yao
H: Clinical significance of inflammatory cytokine and chemokine
expression in hand, foot and mouth disease. Mol Med Rep.
15:2859–2866. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhuo L, Chen X, Sun Y, Wang Y, Shi Y, Bu
L, Xia W, Han J, Chen D and Li X: Rapamycin inhibited pyroptosis
and reduced the release of IL-1β and IL-18 in the septic response.
Biomed Res Int. 2020(5960375)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kruse RH, Puckett WH and Richardson JH:
Biological safety cabinetry. Clin Microbiol Rev. 4:207–241.
1991.PubMed/NCBI View Article : Google Scholar
|