Introduction
Tuberculosis (TB) caused by Mycobacterium
tuberculosis (M.tb) remains the principal killer among global
infectious diseases (1). According
to a WHO report 2018, it is estimated that 10 million persons had
incident TB, and 1.5 million TB-related mortalities occurred
(2). M.tb is unique among
bacterial pathogens as it can survive in the body for several
years. Therefore, finding effective therapeutic strategies to
manage this infectious disease is important (3). M.tb weakens host immune
function and therefore enables its long-term coexistence with the
host (4). M.tb virulence
factors exhibit numerous modes of action, including preventing the
formation and acidification of phagolysosomes, inhibiting the
formation of autophagosomes, resisting oxidative stress and
inhibiting cell autophagy (5).
Moreover, Ca2+ is a crucial secondary messenger in cells
that regulates cell cycle progression, proliferation and autophagy
(6). Its role in cell
proliferation and autophagy implies that increased or weakened
Ca2+ signals may result in cell death (7).
A previous study demonstrated that vitamin D3
induces autophagy in numerous types of cancer cells, such as lung
cancer and prostate cancer (8),
which suggests that the induction of autophagy may be a mechanism
whereby vitamin D3 exerts an anti-cancer effect (9). Another study reported that vitamin D3
can regulate the immune response to M.tb, which indicated
that vitamin D3 serves a vital role in TB treatment (10). Furthermore, vitamin D3 serves an
extensive role in Ca2+ homeostasis (11). Intracellular Ca2+ is
also an essential secondary messengers in the regulation of
autophagy (12). However, the role
of vitamin D3 in regulating cell autophagy and immunity is still
poorly understood.
Macrophages are a vital component of the immune
system and serve numerous roles in the immune response, such as
phagocytosis, bactericidal and in the secretion of inflammatory
factors (interleukin IL-1β, tumor necrosis factor α and IL-6)
(13). The THP-1 human leukemia
monocyte cell line has been widely used as a model to study the
immune response of monocytes (14). In the present study, the effect of
vitamin D3 on THP-1 cells infected by M.tb was explored,
including its function in the modulation of intracellular
Ca2+ and the induction of autophagy. THP-1 cells were
incubated with M.tb and the autophagic process and Ca2
+ concentration of the host cells following vitamin D3
treatment were investigated. Animal experiments were used to
further verify the role of vitamin D3 in promoting autophagy after
M.tb infection.
Materials and methods
Animals
A total of 16 male Balb/c mice (age, 6-8 weeks;
weight, 20±5 g) were obtained from the Jackson Laboratory. All
animal experiments and experimental protocols were approved by the
Ethics Committee of Ningxia University (Yinchuan, China; approval
no. 2020-013). The mice were maintained in a pathogen-free facility
at a constant temperature (24±1˚C) and 50-55% humidity with a 12-h
light/dark cycle and were allowed to eat and drink ad
libitum. M.tb purchased from Chinese Center for Disease
Control and Prevention was grown in Middlebrook 7H9 broth (BD
Diagnostic; Becton, Dickinson and Company) supplemented with 10%
OADC and 0.05% Tween-80 at 37˚C in a tissue incubator with an
atmosphere of 5% CO2. After one week of acclimatization,
the mice were randomly divided into the following four treatment
groups (n=4 mice/group): i) Control group (50 µl 0.9% normal saline
administered intranasally); ii) M.tb group [50 µl
M.tb (50 µg/ml) administered intranasally]; iii) vitamin D3
group [50 µl vitamin D3 (Beyotime Institute of Biotechnology; 5
µmol/ml) administered intranasally]; and iv) vitamin D3 +
M.tb group [50 µl vitamin D3 (5 µmol/ml) and 50 µl
M.tb (50 µg/ml) administered intranasally]. Intranasal
administration was performed under 1.5-2% isoflurane anesthesia
with 50 µl of the saline, M.tb and vitamin D3 (Beyotime,
China) instilled into one nostril. Treatment was repeated once a
day for seven days in each treatment group. The animals were placed
into the induction box (1.5-2% isoflurane). Once the animals were
completely anesthetized (3-5 min; animals did not attempt resume
prone position), anesthesia was maintained using 0.5-1.0%
isoflurane.
Histological examination
Mice were sacrificed by cervical dislocation at
indicated time points (7 days after M.tb and vitamin D3
treatment). Subsequently, the lungs were extracted from the mice,
washed once with PBS and fixed in 4% paraformaldehyde for 24 h at
room temperature. the fixed tissue was embedded in paraffin and
sliced into 4-µm-thick sections using a microtome. Samples were
then stained with H&E for 10 min at room temperature and images
captured with a light microscope (magnification, x100).
Cell culture
The monocytic THP-1 cell line (ATCC) was cultured in
RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Thermo Fisher Scientific, Inc.) in a 5%
CO2 incubator at 37˚C. Before incubation, the cells were
seeded into a 6-well plate at a cell density of 2x106
cell/well and treated with 100 ng/ml phorbol 12-myristate
13-acetate (PMA) at 37˚C for 24 h to transform into adherent
macrophages. A vision-based automatic cell counter was used to
determine the total number of cells.
Assessment of cell viability
The MTT assay (Thermo Fisher Scientific, Inc.) was
used to assess cell viability. THP-1 cells were seeded into a
96-well plate at a density of 5x103 cells/well
and treated with 100 ng/ml phorbol 12-myristate 13-acetate (PMA) at
37˚C for 24 h to transform into adherent macrophages. Subsequently,
the resulting THP-1 cells was treated with different concentrations
of vitamin D3 (0, 5, 20, 50 and 100 µmol/ml) at 37˚C for 24 h or
treated with vitamin D3 (5 µmol/ml) at 37˚C for 0, 6, 12, 24 and 36
h. Following incubation, 20 µl MTT solution was added to each well,
according to the manufacturer's instructions. The plate was then
transferred to an incubator for another 4 h at 37˚C; DMSO solution
(100 µl) was subsequently added to each well to dissolve the purple
formazan. Finally, the absorbance (560 nm) was measured using a
microplate reader (Bio-Rad Laboratories, Inc.).
Ca2+ concentration
assay
THP-1 cells were seeded at a density of
5x103 cells/well in 96-well plates and treated with 100
ng/ml PMA at 37˚C for 24 h to transform into adherent macrophages.
Then the cells of different treatment groups were incubated with
M.tb (MOI:35) and vitamin D3 (5, 10 and 20 µmol/ml) at 37˚C
for 24 h. Cytosolic Ca2+ concentration was quantified
using Fluo-4 acetoxymethyl (AM; Thermo Fisher Scientific, Inc.). In
brief, Fluo-4 AM was diluted in PBS and the cells were incubated at
a final concentration of 1 µM for 30 min at 37˚C to ensure that
Fluo-4 AM was fully converted into Fluo-4 intracellularly. Cells
were then washed three times with PBS solution, and the
fluorescence intensity was assessed by acquiring emissions at 494
nm using flow cytometry. (Thermo Fisher Scientific, Inc.). FlowJo
version 10 software (FlowJo, LLC) was used for flow analysis. The
result was compared with the control group.
Western blotting
M-PER Mammalian Protein extraction reagent (Thermo
Fisher Scientific, Inc.) was used to extract total protein from
cells in the different treatment groups. A BCA kit was used to
determine protein concentration. Total protein (10 µg per lane) was
mixed with 6X loading buffer and separated using SDS-PAGE on a 10%
gel; separated proteins were subsequently transferred to a PVDF
membrane. After blocking with Superblock Blocking Buffer (Thermo
Fisher Scientific, Inc.) at 37˚C for 1 h, the membrane was
incubated with the following primary antibodies: anti-LC3 (cat. no.
14600-1-AP; 1:1,000; ProteinTech Group, Inc.), anti-sequestosome-1
(p62, cat. no. 18420-1-AP; 1:1,000; ProteinTech Group, Inc.),
anti-Beclin-1 (cat. no. 11306-1-AP; 1:1,000; ProteinTech Group,
Inc.), anti-autophagy-related 5 (ATG-5, cat. no. 66744-1-Ig;
1:1,000; ProteinTech Group, Inc.) and anti-AKT (cat. no.
60203-2-Ig; 1:1,000; ProteinTech Group, Inc.) overnight at 4˚C. The
PVDF membranes were washed five times with TBS containing 0.02%
Tween-20 (1,000 ml TBS and 2 ml Tween-20). Following the primary
incubation membranes were incubated with secondary antibodies
[HRP-conjugated Affinipure Goat Anti-Mouse IgG(H+L), cat. no.
SA00001-1; 1:10,000; ProteinTech Group, Inc. and HRP-conjugated
Affinipure Goat Anti-Rabbit IgG(H+L), cat. no. SA00001-2; 1:10,000;
ProteinTech Group, Inc.] at room temperature for 1 h. ECL
chemiluminescence kit (Thermo Fisher Scientific, Inc.) was used to
visualize protein bands. β-actin was used as an internal control.
The intensity of protein bands was analyzed using ImageJ (version
146; National Institutes of Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using the RNA Extraction Kit
(Takara Biotechnology Co., Ltd.), and cDNA was synthesized using a
High-Capacity cDNA Archive kit (Takara Biotechnology Co., Ltd.).
The following temperature protocol was used for reverse
transcription: 37˚C for 15 min and 85˚C for 5 sec. Subsequently,
the acquired cDNA was subjected to qPCR using an ABI 7500 Fast
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) and a SYBR-Green PCR Kit (Takara Biotechnology Co. Ltd.). The
following thermocycling conditions were used for qPCR: 95˚C for 30
sec, followed by 40 cycles of 95˚C for 5 sec and 65˚C for 30 sec.
The primers were synthesized by the Shanghai Sango Biotechnology
Co., Ltd., and primer sequences are presented in Table I. Relative expression levels was
analyzed using the 2-∆∆Cq method and normalized to
β-actin as an internal control (15).
 | Table ISequences of primers used for reverse
transcription-quantitative PCR. |
Table I
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5'→3') |
|---|
| AMPK | F:
TTGAAACCTGAAAATGTCCTGCT |
| | R:
GGTGAGCCACAACTTGTTCTT |
| LC3 | F:
AACATGAGCGAGTTGGTCAAG |
| | R:
GCTCGTAGATGTCCGCGAT |
| β-actin | F:
GCCAACCGCGAGAAGATGA |
| | R:
CCATCACGATGCCAGTGGTA |
Immunofluorescence staining
THP-1 cells were seeded at a density of
1x105 cells/well into 6-well plates and treated with 100
ng/ml PMA at 37˚C for 24 h to transform into adherent macrophages.
Then the cells of different treatment groups were incubated with
M.tb (MOI:35) and vitamin D3 (5 µmol/ml) at 37˚C for 24 h.
After being processed according to different treatment groups, the
cells were washed three times at 37˚C with PBS or Hanks' balanced
salt solution to remove the culture medium. Subsequently, 1 ml
Fluo-4 AM (1 µM) fluorescent probe (Thermo Fisher Scientific, Inc.)
was added to the cells and incubated for 40 min in the dark at
37˚C. The NucBlue™ Fixed Cell ReadyProbes™
Reagent (DAPI; 5 µM; Invitrogen; Thermo Fisher Scientific) was used
to stain the nucleus. Finally, a confocal laser scanning microscope
was used to detect the fluorescence of Fluo-4 to verify changes in
intracellular Ca2+ concentration.
Statistical analysis
All experiments were repeated at least three times.
All data were analyzed using the SPSS 13.0 software (SPSS, Inc.).
Unpaired Student's t-tests were used to compare differences between
two groups, whereas one-way ANOVA followed by Tukey's post hoc test
was used to compare differences between more than two groups. Data
are presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
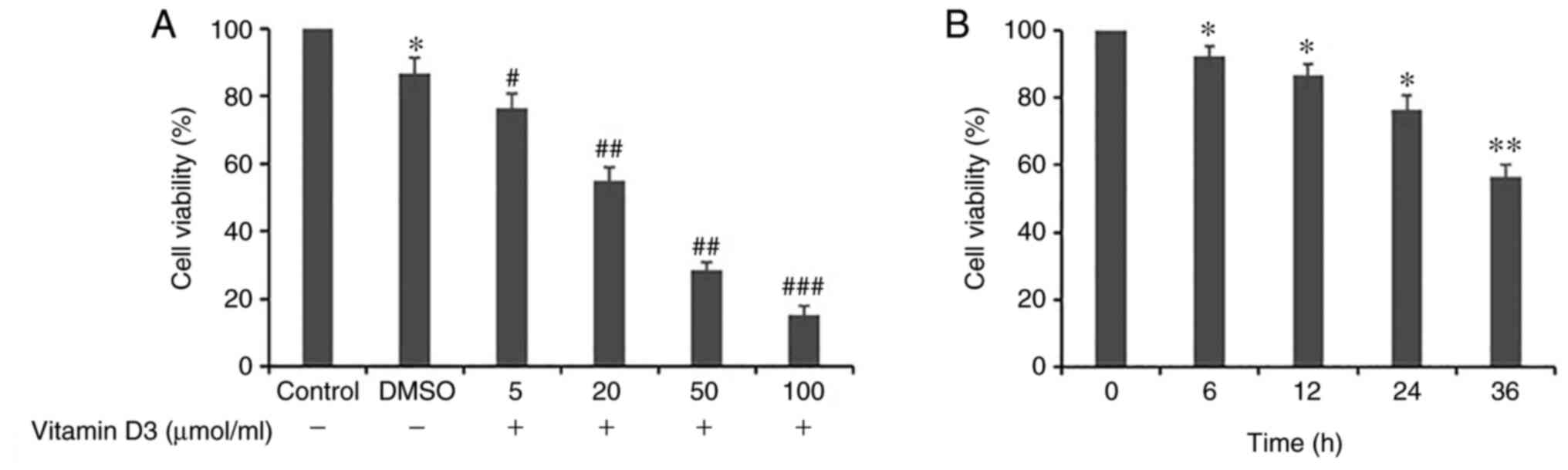
Vitamin D3 decreases the viability of
THP-1 cells in a dose- and time-dependent manner
To investigate the effects of vitamin D3 on THP-1
cell viability, the THP-1 cells were either treated with different
concentrations of vitamin D3 (0, 5, 20, 50 or 100 µmol/ml) for 24 h
(Fig. 1A) or treated with vitamin
D3 (5 µmol/ml) for 0, 6, 12, 24 and 36 h (Fig. 1B). The results demonstrated that
the viability of THP-1 cells was significantly decreased by vitamin
D3 in a dose- and time-dependent manner compared with the
respective control group; as vitamin D3 was dissolved in DMSO,
cells treated with a culture medium of 0.03% DMSO were used as the
control. It was demonstrated that when the vitamin D3 concentration
reached 5 µmol/ml, cellular viability was statistically different
from the control group; therefore, this concentration was chosen
for subsequent experiments.
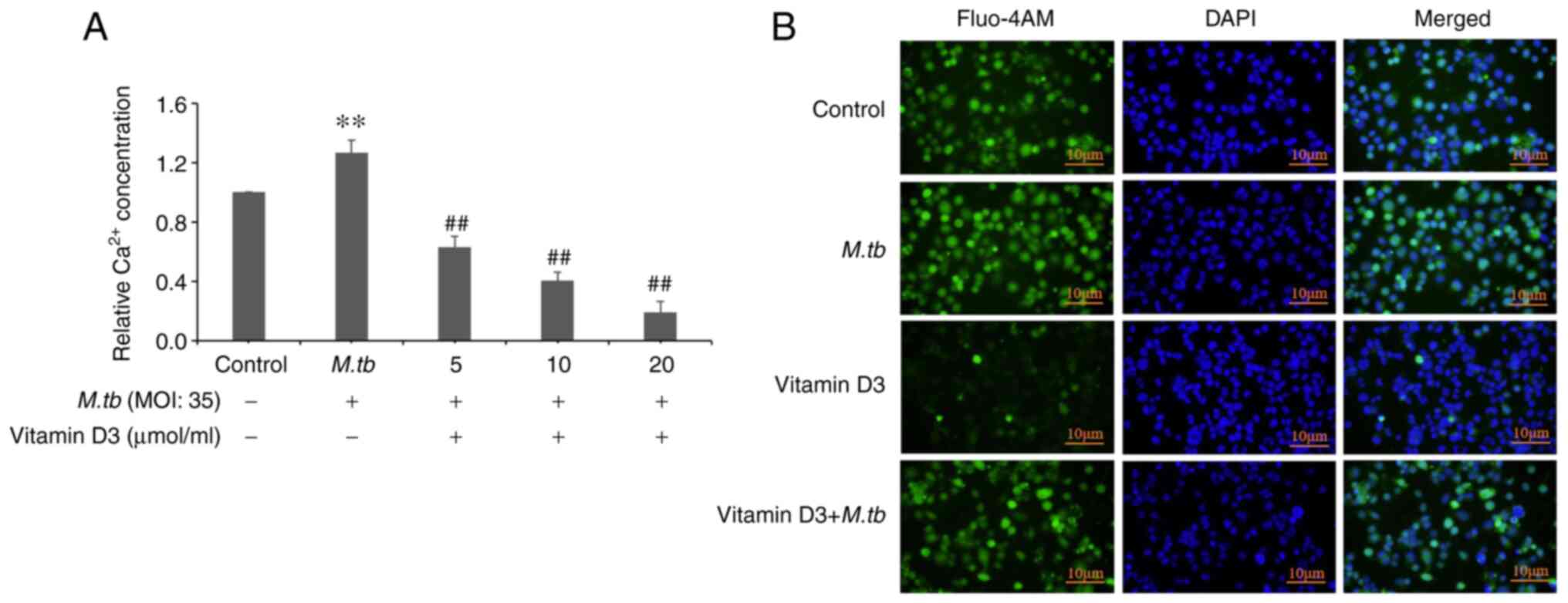
Effect of vitamin D3 on
Ca2+ concentration in THP-1 cells infected with
M.tb
Impaired Ca2+ homeostasis can lead to
cellular dysfunction and autophagy (16). Whether Ca2+ homeostasis
of THP-1 cells infected by M.tb is altered under vitamin D3
treatment, and whether vitamin D3 could induce autophagy by
inhibiting Ca2+ concentration was therefore
investigated. To examine the Ca2+ homeostasis of THP-1
cells infected with M.tb following vitamin D3 treatment, the
intracellular Ca2+ concentration was quantified using
Fluo-4 AM-treated THP-1 cells. Compared with the control group,
M.tb infection for 24 h induced an ~26% increase in
Ca2+ concentration (Fig.
2A). Compared with the M.tb group, it was demonstrated
that the vitamin D3 + M.tb (5, 10 and 20 µmol/ml) groups had
a significant inhibitory effect on Ca2+ concentration.
Immunofluorescence assay results also demonstrated that vitamin D3
treatment markedly attenuated Ca2+ concentration
compared with the control and M.tb groups (Fig. 2B). Compared with the vitamin D3
group, the Ca2+ concentration in the vitamin D3 +
M.tb group was markedly enhanced. These results suggested
that vitamin D3 treatment inhibited Ca2+ concentration
in THP-1 cells infected by the M.tb.
Effect of vitamin D3 on
autophagy-related factors in THP-1 cells
Vitamin D3 exhibited a significant inhibitory effect
on the cellular viability of THP-1, aforementioned. However,
whether vitamin D3 induced autophagy in THP-1 had not been
explored. To determine whether vitamin D3 regulated autophagy in
THP-1 cells, the protein expression levels of autophagy-associated
proteins, p62, LC3, Beclin-1, ATG-5 and AKT, were analyzed by
western blotting (Fig. 3).
Compared to the M.tb group, treatment with vitamin D3
significantly increased the protein expression levels of p62,
LC3Ⅱ/LC3Ⅰ, Beclin-1 and ATG-5 under M.tb infection (Fig. 3B-E). However, treatment with
vitamin D3 significantly suppressed the protein expression levels
of AKT under M.tb infection compared with the control
(Fig. 3F). Moreover, the increase
in LC3 and AMP activated protein kinase (AMPK) mRNA expression
levels in the vitamin D3 and vitamin D3 + M.tb groups was
higher compared with the M.tb group (Fig. 3G and H). Western blotting and RT-qPCR analyses
indicated that vitamin D3 may stimulate autophagy in THP-1 cells.
Taken together, these results suggested that vitamin D3 may inhibit
M.tb infection by increasing autophagy in THP-1 cells.
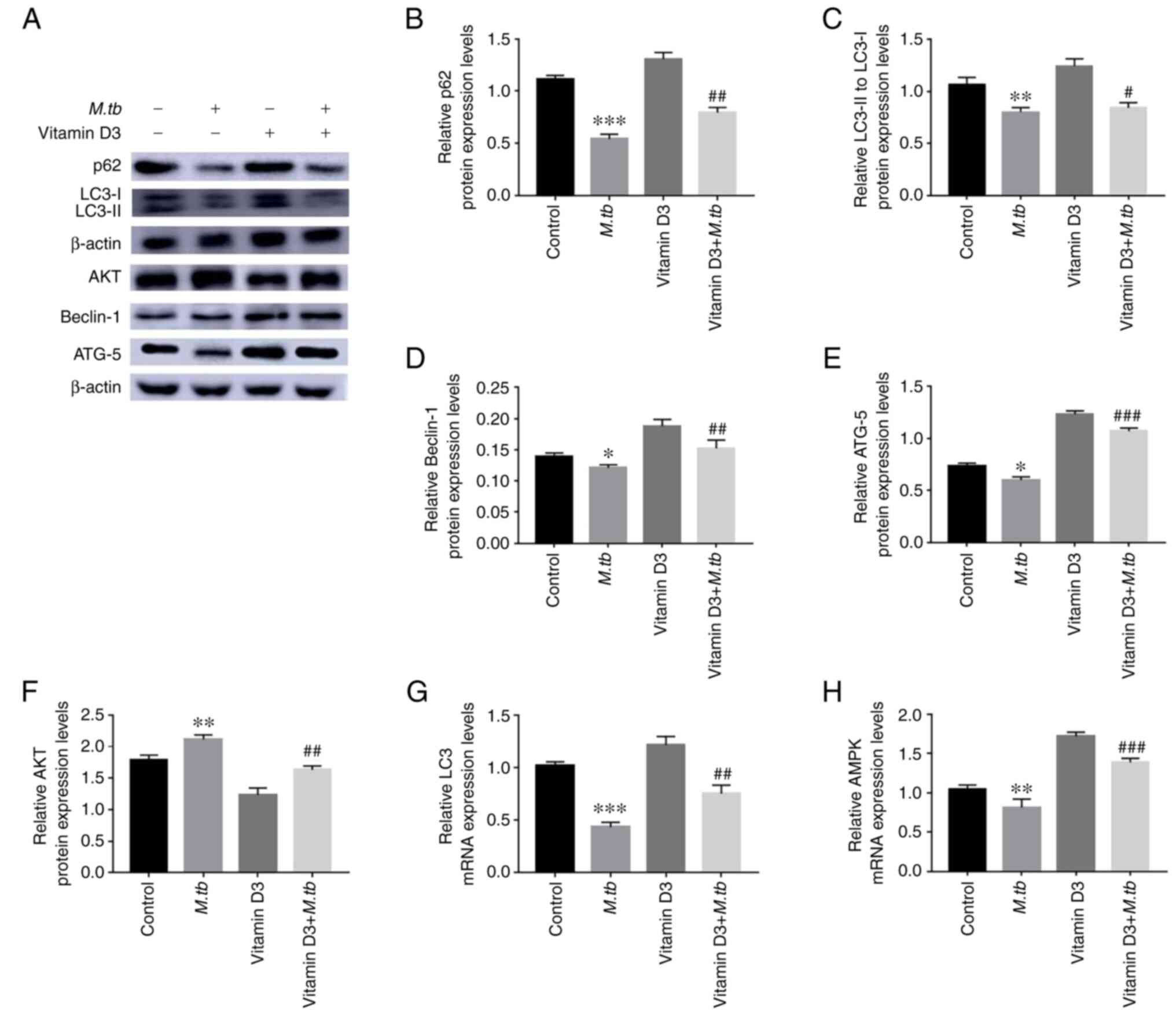 | Figure 3Effect of M.tb and vitamin D3
on THP-1 cell autophagy. (A) Following vitamin D3 and/or
M.tb treatment for 24 h, the protein expression levels of
autophagy markers, including p62, LC3Ⅱ/LC3Ⅰ, Beclin-1, ATG-5 and
AKT in THP-1 cells were analyzed by western blotting. (B) p62, (C)
LC3Ⅱ/LC3Ⅰ, (D) Beclin-1, (E) ATG-5 and (F) AKT protein
semi-quantitative expression levels. β-actin was used as a loading
control. The mRNA expression levels of (G) LC3 and (H) AMPK were
standardized using the 2-ΔΔCq method. Data are presented
as the mean ± standard deviation of three independent experiments.
*P<0.05, **P<0.01,
***P<0.001 vs. control; and #P<0.05,
##P<0.01, ###P<0.001 vs. M.tb.
ATG-5, autophagy-related 5; M.tb, Mycobacterium
tuberculosis; p62, sequestosome-1. |
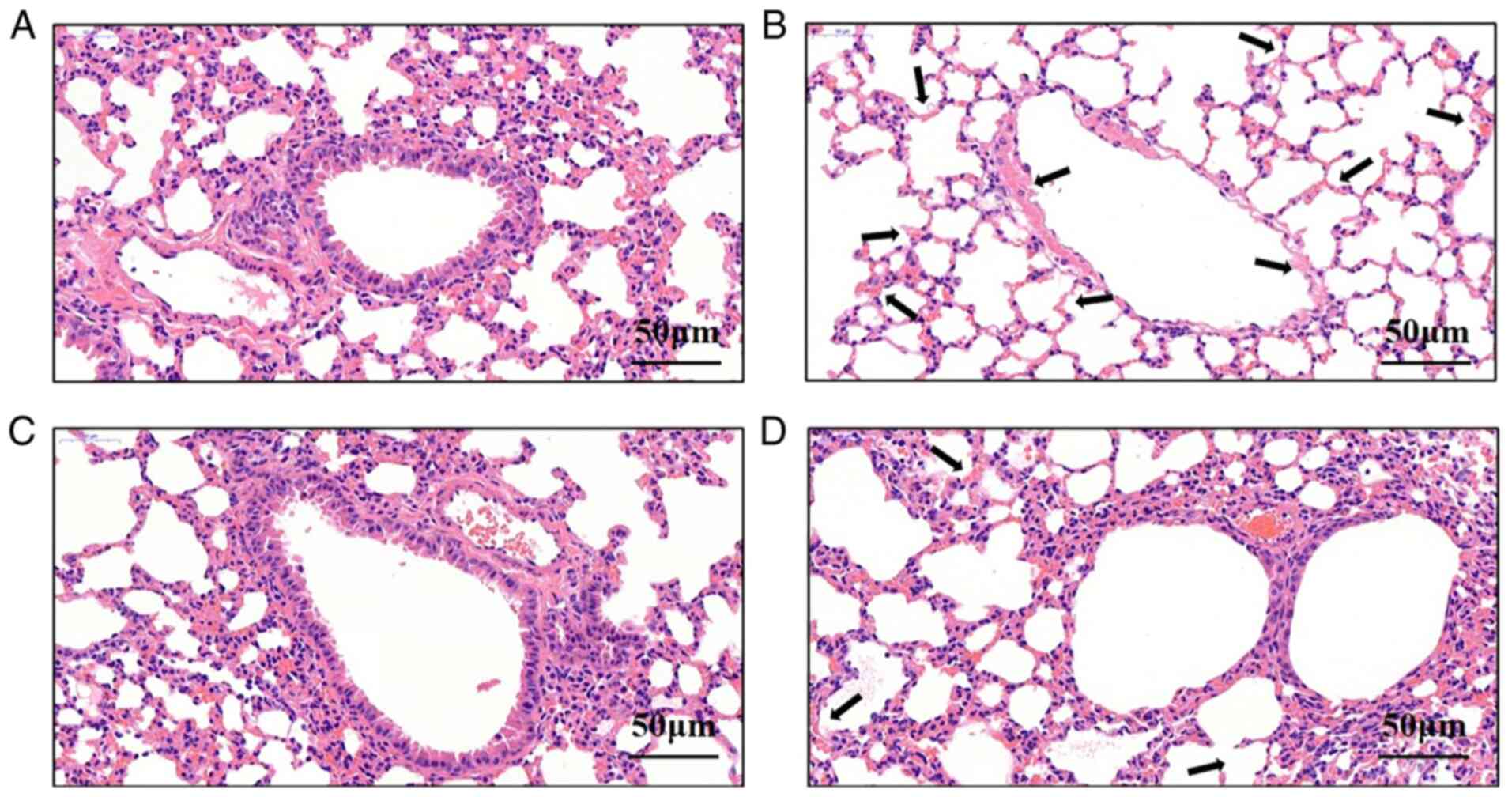
Vitamin D3 attenuates lung damage
following M.tb infection
After the Balb/c mice were treated intranasally with
vitamin D3 and/or M.tb, pathological sections were produced
and H&E staining was performed. Pulmonary tissue sections from
the control group (Fig. 4A)
exhibited clear alveolar structure without edema, congestion or
inflammatory cell infiltrates in the interstitial fluid. However,
pulmonary tissues from the M.tb group (Fig. 4B) were damaged and inflammatory
cell infiltrates and pathological changes were present. In the
vitamin D3 (Fig. 4C) and vitamin
D3 + M.tb groups (Fig. 4D),
nasal administration was repeated for 7 days. Compared with the
M.tb group, the degree of alveolar wall damage and
infiltrating inflammatory cells in the vitamin D3 + M.tb
group markedly decreased. These results suggested that vitamin D3
could reduce lung damage following M.tb infection by
promoting cell autophagy.
Discussion
TB, caused by the bacterial pathogen M.tb,
remains one of the deadliest infectious diseases, and the number of
deaths and infections has continued to surge in the past 200 years
(17). The latest WHO report
estimates about 1.6 million global deaths annually from TB
(18). Vitamin D3 induces
anti-mycobacterial activity in mononuclear phagocytes (19) and an early study demonstrated that
vitamin D3 is a potent antiproliferative agent in different tissues
and cells, including the epithelial tissue, the vascular wall, the
kidney, cancer cells and immune cells (20). Therefore, studying the autophagic
mechanism of THP-1 cells infected with M.tb and induced by
vitamin D3 will not only help understand the pathological mechanism
of TB but also aid in discovering novel therapeutics for TB.
Previous study have indicated that THP-1 cells can
be differentiated into mature macrophage-like cells using PMA
(21). THP-1 cells resemble
primary monocytes and macrophages in their morphological and
functional properties and are a suitable, safe and reliable model
to study macrophage functions and responses (22). THP-1 is an immortalized cell line
with a high growth rate. Compared with PBMC-derived monocytes,
THP-1 cells better facilitate reproducibility of findings.
Furthermore, it has been reported that the THP-1 cell line is more
sensitive to cellular Ca2+ signals. Therefore, human
monocyte THP-1 cells were used in the present study to simulate
vitamin D3-induced macrophage autophagy and to investigate the
effective clearance of cells infected with M.tb by
autophagy. In the present study, THP-1 cells were incubated with
M.tb for 24 h and the other treatment groups were treated
with vitamin D3 or vitamin D3 + M.tb for 24 h. The results
demonstrated that incubation with M.tb significantly
decreased the protein expression levels of the autophagic proteins,
p62, LC3Ⅱ/LC3Ⅰ, Beclin-1 and ATG-5. Compared with the M.tb
group, the expression levels of p62, LC3Ⅱ/LC3Ⅰ, Beclin-1 and ATG-5
in the vitamin D3 + M.tb groups were significantly
increased. Furthermore, the mRNA expression levels of LC3 and AMPK
were also significantly increased in the vitamin D3 + M.tb
group. Compared with the M.tb group, the autophagy-related
protein, AKT, which is considered to be a negative regulator of
autophagy, was reduced in the vitamin D3 + M.tb groups.
These results are consistent with another result of the present
study, which demonstrated that vitamin D3 had a significant
inhibitory effect on the Ca2+ concentration (23), which may have induced autophagy and
explains the high efficiency of vitamin D3 in inhibiting the growth
of cancer cells (24).
Autophagy serves a vital role in cell survival under
certain stress conditions by scavenging proteins and damaged
organelles to maintain cellular homeostasis and integrity (25). For example, the p62 protein is
located at the autophagosome formation site and can bind to the
autophagosomal localization protein LC3 and the family of
ubiquitin-like proteins (26).
ATG5, as autophagy proteins, is critical for autophagy at the stage
of autophagosome formation (27).
Moreover, Beclin-1 is a mammalian autophagic protein involved in
diverse biological processes, including tumor suppression and cell
death (28). AKT is also known as
protein kinase B, and it is an oncogenic protein that regulates
cell survival, proliferation, growth and autophagy. As a negative
regulator of autophagy and apoptosis, the AKT/mTOR signaling
pathway serves an important role in regulating autophagy (29). AKT inhibitors directly inhibit the
activity of AKT and therefore can attenuate cancer proliferation
(30). LC3 and AMPK are essential
cytokines involved in the regulation of autophagy (31). The data obtained in the present
study demonstrated that vitamin D3 treatment resulted in a
significant upregulation of p62, LC3Ⅱ/LC3Ⅰ, Beclin-1 and ATG-5 and
a significant decrease in the AKT protein expression level. These
results supported the hypothesis that vitamin D3 increases
autophagy of THP-1 cells infected by M.tb. Therefore,
vitamin D3 may stimulate innate immune antibacterial activity of
THP-1 cells by enhancing mechanisms associated with autophagy.
A previous study has reported that reductions in the
concentration of Ca2+ are associated with the triggering
of autophagy (32). Furthermore,
vitamin D3 alters Ca2+ homeostasis in cells (33). However, the effects of vitamin D3
in THP-1 cells following M.tb infection and its association
with intracellular Ca2+ homeostasis, is still unknown.
Therefore, in the present study the Ca2+ concentration
in the cells treated with vitamin D3 was tracked by the cytoplasmic
Ca2+ specific indicator Furo-4 AM. The results
demonstrated that vitamin D3 significantly reduced Ca2+
concentration and significantly enhanced the expression levels of
autophagy-associated proteins in THP-1 cells infected by
M.tb compared with the M.tb group. These results
therefore suggested that cytosolic Ca2+ may be crucial
for vitamin D3-induced autophagy.
M.tb is a typical parasitic bacterium that
can evade the antimicrobial effect of macrophages through various
mechanisms and survive in host immune cells, such as macrophages
(34). A previous study reported
that in M.tb-infected mice, the rupture of foam cells due to
exacerbated infection and/or inflammation and the release of their
contents likely sustains the disease pathology and generation of
caseum, which leads to progressive destruction of lung tissues
(35). Kimmey et al
(36) demonstrated that
M.tb can evade autophagic responses in the mouse model.
Induction of autophagy promotes maturation and acidification of
M.tb phagosomes and their conversion into mycobactericidal
organelles. The present study aimed to determine whether vitamin D3
could reduce lung damage following M.tb infection by
promoting cell autophagy. Furthermore, various autophagy-related
protein expression levels were analyzed to investigate this
potential autophagic mechanism. The results of the present study
suggested that incubation with M.tb significantly decreased
autophagy-related protein expression levels, whereas vitamin D3
significantly promoted the activation of p62, LC3Ⅱ/LC3Ⅰ, Beclin-1
and ATG-5, and inhibited AKT protein expression levels, when
induced by M.tb infection. Based on the above conclusions,
it was speculated that vitamin D3 may promote phagosomal formation
and autolysosomal maturation by significantly inhibiting the
concentration of Ca2+ and likely enhancing the signal
transduction in the host cell to promote the fusion of phagosomes
and lysosomes. It is therefore possible that the autophagy
signaling pathways of macrophages may have been activated to
promote autophagy.
In conclusion, the present study demonstrated that
the molecular mechanism of vitamin D3 promoted autophagy in THP-1
cells infected by M.tb. However, the precise mechanism by
which vitamin D3 promotes the expression of autophagy-related
proteins needs further research and it may also be necessary to
repeat the experiments in a different type of macrophage cell line
to further test the hypothesis. To the best of our knowledge, the
present study is the first to report that vitamin D3 significantly
reversed pulmonary injury of M.tb-infected mice, possibly
via the autophagic pathway, thereby improving autophagic
dysfunction. The results indicated that the mechanism of action of
vitamin D3 may involve the attenuation of autophagic flux
dysfunction through inhibition of the Ca2+ signaling
pathway.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the National Natural
Science Foundation of China (grant no. 31772710), Key Project of
Research and Development of Ningxia Hui Autonomous Region of China
(grant no. 2020BEG03019), Natural Science Foundation of Ningxia
(grant no. 2021AAC03109), Key Project of Research and Development
of Ningxia Hui Autonomous Region of China (grant no. 2019BBF02005)
and Natural Science Foundation of Ningxia (grant no.
2021AAC03299).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YuW designed the project, revised the article and
provided technical guidance. DX designed the project, revised the
article and coordinated all aspects of the present work. YiW
participated in all experiments, performed data analysis, created
the figures and wrote the article. XL and FS were responsible for
sample preparation and documentation. YiW and XL confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All animal experiments and experimental protocols
were approved by the Ethics Committee of Ningxia University
(Yinchuan, China; approval no. 2020-013).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sharma S and Meena LS: Potential of
Ca2+ in Mycobacterium tuberculosis
H37Rv pathogenesis and survival. Appl Biochem
Biotechnol. 181:762–771. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chakaya JM, Harries AD and Marks GB:
Ending tuberculosis by 2030-Pipe dream or reality? Int J Infect
Dis. 92S:S51–S54. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
de Martino M, Lodi L, Galli L and
Chiappini E: Immune response to Mycobacterium tuberculosis:
A narrative review. Front Pediatr. 7(350)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Xu G, Wang J, Gao GF and Liu CH: Insights
into battles between Mycobacterium tuberculosis and
macrophages. Protein Cell. 5:728–736. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Paik S and Jo EK: An interplay between
autophagy and immunometabolism for host defense against
mycobacterial infection. Front Immunol. 11(603951)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Monteith GR, Davis FM and Roberts-Thomson
SJ: Calcium channels and pumps in cancer: Changes and consequences.
J Biol Chem. 287:31666–31673. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Huang W, Lu C, Wu Y, Ouyang S and Chen Y:
T-type calcium channel antagonists, mibefradil and NNC-55-0396
inhibit cell proliferation and induce cell apoptosis in leukemia
cell lines. J Exp Clin Cancer Res. 34(54)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Abu El Maaty MA and Wölfl S: Vitamin D as
a novel regulator of tumor metabolism: Insights on potential
mechanisms and implications for anti-cancer therapy. Int J Mol Sci.
18(3184)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mondul AM, Weinstein SJ, Layne TM and
Albanes D: Vitamin D and cancer risk and mortality: State of the
science, gaps, and challenges. Epidemiol Rev. 39:28–48.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Martineau AR: Old wine in new bottles:
Vitamin D in the treatment and prevention of tuberculosis. Proc
Nutr Soc. 71:84–89. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang L, Jiang X, Pfau D, Ling Y and
Nathan CF: Type I interferon signaling mediates Mycobacterium
tuberculosis-induced macrophage death. J Exp Med.
218(e20200887)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rizzuto R, Pinton P, Ferrari D, Chami M,
Szabadkai G, Magalhães PJ, Di Virgilio F and Pozzan T: Calcium and
apoptosis: Facts and hypotheses. Oncogene. 22:8619–8627.
2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Komohara Y, Fujiwara Y, Ohnishi K and
Takeya M: Tumor-associated macrophages: Potential therapeutic
targets for anti-cancer therapy. Adv Drug Deliv Rev. 99:180–185.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chanput W, Mes J, Vreeburg RA, Savelkoul
HF and Wichers HJ: Transcription profiles of LPS-stimulated THP-1
monocytes and macrophages: A tool to study inflammation modulating
effects of food-derived compounds. Food Funct. 1:254–261.
2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kang P, Zhang W, Chen X, Yi X, Song P,
Chang Y, Zhang S, Gao T, Li C and Li S: TRPM2 mediates
mitochondria-dependent apoptosis of melanocytes under oxidative
stress. Free Radic Biol Med. 126:259–268. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang CH, Kao CH, Chen YF, Wei YH and Tsai
TF: Cisd2 mediates lifespan: Is there an interconnection among
Ca²+ homeostasis, autophagy, and lifespan? Free Radic
Res. 48:1109–1114. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bussi C and Gutierrez MG: Mycobacterium
tuberculosis infection of host cells in space and time. FEMS
Microbiol Rev. 43:341–361. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Adeniji AA, Knoll KE and Loots DT:
Potential anti-TB investigational compounds and drugs with
repurposing potential in TB therapy: A conspectus. Appl Microbiol
Biotechnol. 104:5633–5662. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gough ME, Graviss EA and May EE: The
dynamic immunomodulatory effects of vitamin D3 during Mycobacterium
infection. Innate Immun. 23:506–523. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Svensson D, Nebel D and Nilsson BO:
Vitamin D3 modulates the innate immune response through regulation
of the hCAP-18/LL-37 gene expression and cytokine production.
Inflamm Res. 65:25–32. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Riendeau CJ and Kornfeld H: THP-1 cell
apoptosis in response to Mycobacterial infection. Infect Immun.
71:254–259. 2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chanput W, Mes JJ and Wichers HJ: THP-1
cell line: An in vitro cell model for immune modulation approach.
Int Immunopharmacol. 23:37–45. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hashemipour S, Lalooha F, Zahir Mirdamadi
S, Ziaee A and Dabaghi Ghaleh T: Effect of vitamin D administration
in vitamin D-deficient pregnant women on maternal and neonatal
serum calcium and vitamin D concentrations: A randomised clinical
trial. Br J Nutr. 110:1611–1616. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Luong KV and Nguyen LT: The beneficial
role of vitamin D and its analogs in cancer treatment and
prevention. Crit Rev Oncol Hematol. 73:192–201. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Galati S, Boni C, Gerra MC, Lazzaretti M
and Buschini A: Autophagy: A player in response to oxidative stress
and DNA damage. Oxid Med Cell Longev. 2019(5692958)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lamark T, Svenning S and Johansen T:
Regulation of selective autophagy: The p62/SQSTM1 paradigm. Essays
Biochem. 61:609–624. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zheng W, Xie W, Yin D, Luo R, Liu M and
Guo F: ATG5 and ATG7 induced autophagy interplays with UPR via PERK
signaling. Cell Commun Signal. 17(42)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
He C and Levine B: The beclin 1
interactome. Curr Opin Cell Biol. 22:140–149. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Song M, Bode AM, Dong Z and Lee MH: AKT as
a therapeutic target for cancer. Cancer Res. 79:1019–1031.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Revathidevi S and Munirajan AK: Akt in
cancer: Mediator and more. Semin Cancer Biol. 59:80–91.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Revathidevi S and Munirajan AK: An
overview of autophagy: Morphology, mechanism, and regulation.
Antioxid Redox Signal. 20:460–473. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kania E, Pająk B and Orzechowski A:
Calcium homeostasis and ER stress in control of autophagy in cancer
cells. Biomed Res Int. 2015(352794)2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Pinton P, Giorgi C, Siviero R, Zecchini E
and Rizzuto R: Calcium and apoptosis: ER-mitochondria
Ca2+ transfer in the control of apoptosis. Oncogene.
27:6407–6418. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Padhi A, Pattnaik K, Biswas M, Jagadeb M,
Behera A and Sonawane A: Mycobacterium tuberculosis LprE
suppresses TLR2-dependent cathelicidin and autophagy expression to
enhance bacterial survival in macrophages. J Immunol.
203:2665–2678. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
El-Sharkawy A and Malki A: Vitamin D
signaling in inflammation and cancer: Molecular mechanisms and
therapeutic implications. Molecules. 25(3219)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kimmey JM, Huynh JP, Weiss LA, Park S,
Kambal A, Debnath J, Virgin HW and Stallings CL: Unique role for
ATG5 in neutrophil-mediated immunopathology during M.
tuberculosis infection. Nature. 528:565–569. 2015.PubMed/NCBI View Article : Google Scholar
|