1. Introduction
Despite the research data currently provided,
cutaneous and mucosal manifestations of SARS-CoV-2 infection remain
poorly known, particularly their prevalence, their morphological
characteristics, the pathogenic substrate, as well as their
diagnostic and prognostic significance. The ongoing pandemic, as
well as the emergence of new viral strains, possibly more
contagious and responsible for more severe disease (1), even with the development of several
promising vaccines, that, however, have lower efficacy in a large
group of individuals suffering from metabolic disorders, autoimmune
diseases or with iatrogenic immune suppression (2), make it imperative to understand the
complex clinical manifestations of COVID-19, including the signs
and symptoms on the skin and mucous membranes. Case reports refer
to a variety of morphological aspects that are either virus-induced
or associated with antiviral therapy or secondary to the
circumstances of the pandemic such as stress (herpes simplex,
herpes zoster and alopecia areata) and environmental factors
related to the use of antiseptics and disinfectants (contact
dermatitis or urticaria) (3-8).
According to a French study (conducted by Raymond-Poincaré
University Hospital, Garches, France), which involved ~40 patients
confirmed positive for COVID-19, the most common mucocutaneous
manifestations were: macular exanthema (32 patients; trunk and head
and neck were the areas preferentially involved, hand and feet were
spared), face edema (13 patients), oral lichenoid reaction (13
patients), enanthema (11 patients), macroglossia (10 patients),
cheilitis (5 patients), livedo reticularis (5 patients), urticarial
rashes (3 patients), maculopapular exanthema (3 patients), purpura
(2 patients), atopic dermatitis (1 patient), herpes (1 patient).
All the patients presented extremely itchy lesions (9).
The positive diagnosis of skin and mucosal lesions
in patients with COVID-19 is difficult and primarily requires the
exclusion of drug-induced dermatoses (10,11)
and of other eruptions with similar clinical expression,
particularly other viral infections. Cutaneous lesions in patients
with SARS-CoV-2 infections are extremely variable in morphological
patterns and their importance as a marker for the viral infection
and for disease prognosis is still debated (6,12-15).
Mucosal lesions are markedly less studied, but there are reports of
oral mucous membrane changes and ocular conjunctival or corneal
lesions in patients diagnosed with COVID-19, either as solitary
findings or in association with cutaneous manifestations, with
unclear pathogenic mechanisms, to date (9,16,17).
A classification of the cutaneous lesions associated
with SARS-CoV-2 infection based on the clinical aspect, pathogenic
hypotheses, histopathological findings, associated disease
severity, and prognostic importance, as well as a description of
the most commonly encountered oral and ocular mucosal lesions
during COVID-19 disease were reported in the present review.
2. Research methods
A literature search was conducted, using electronic
databases Key Elsevier, Medscape, PubMed, Google Scholar, for the
term ‘COVID-19’ in combination with ‘skin’, ‘cutaneous
manifestations’, ‘mucosal manifestations’, ‘rash’, ‘exanthem’,
‘enanthem’, ‘urticarial’, ‘chilblain’, ‘livedo’, ‘ocular mucosa’,
and ‘purpura’ to collect reports of skin and mucosal manifestations
described in patients with COVID-19. Case reports, case series, and
literature review-type articles were included in our research. A
brief review was created, based on 63 articles identified in the
literature.
3. Prevalence
Literature studies estimate a variable prevalence of
the cutaneous and mucosal manifestations related to SARS-CoV-2
infection, between 0.2% (18),
among Chinese patients, 15 out of 78 for Russian patients (3) and 20.4% in a study of 148 Italian
patients (5).
4. Pathogenesis
The pathogenic mechanism is unclear. It may include
the hyperactive immune response, the complement activation, and the
microvascular injury. However, there are currently two proposed
hypotheses, which classify the cutaneous manifestations of COVID-19
into two groups: i) manifestations linked to a direct
cytopathogenic effect on cells such as keratinocytes, which are
involved in numerous other viral infections (urticarial rashes,
reactions similar to drug eruptions, varicella-like lesions)
(12,19); and ii) manifestations linked to an
uncontrolled release of cytokines due to alterations involving
specific white blood cells, such as T cells and macrophages. This
second group could be divided into two other groups: a)
manifestations similar to those in macrophage activation syndrome
(acral ischemia, gangrene, retiform purpura, livedo racemosa) and
b) cutaneous manifestations observed in young patients and linked
to the activation of an early type I interferon (IFN) response
(chilblain-like lesions) (20).
This hypothesis may provide a possible explanation
of the pathophysiological mechanisms of the skin manifestations of
COVID-19 disease.
5. Classification and description of
cutaneous, oral, and ocular mucosal lesions
Several classifications have been proposed according
to the morphological pattern and histological changes. The clinical
patterns described include urticarial rash, confluent
erythematous/maculopapular/morbilliform rash, papulosquamous rash,
papulovesicular exanthem/varicella-like lesions, chilblains, livedo
reticularis/racemosa-like pattern, purpuric ‘vasculitic’ pattern,
vasculitides, livedo, and necrotic lesions (3,13,21-23).
Based on the morphological pattern, pathogenic
hypotheses, and histological changes, the classification of skin
manifestations was adapted into two main groups: i) inflammatory
and exanthematous rashes (urticarial rash, maculopapular rashes,
and papulovesicular rashes); and ii) vasculitic/vasculopathic
lesions: acral ischemic lesions such as chilblain-like and acral
ulcers, reticular purpuric lesions such as retiform purpura, livedo
reticularis/livedo racemosa, and purpuric vasculitis, purpuric
non-vasculitic lesions such as petechial rash and flexural and
periflexural purpuric dermatitis. The lesions on the oral and
ocular mucosa associated with SARS-CoV-2 infection are also
presented.
i) Inflammatory and exanthematous
rashes a) Urticarial rash
The urticarial rash associated with COVID-19 has
been reported in numerous publications. It was first mentioned by
Recalcati in 16.7% of the total skin manifestations related to
SARS-CoV-2 infection (5). Galván
et al came to the conclusion that it occurs in 19% of cases,
appears simultaneously with the systemic symptoms, lasts ~1 week,
and is associated with medium-high severity of the infection.
Pruritus may be identified in 92% of cases (21). The International League of
Dermotological Societies (ILDS)/American Academy of Dermotology
(AAD) registry, including over 1,000 patients reported a median
duration of 4 days for urticarial rash (24). Freeman et al reported that
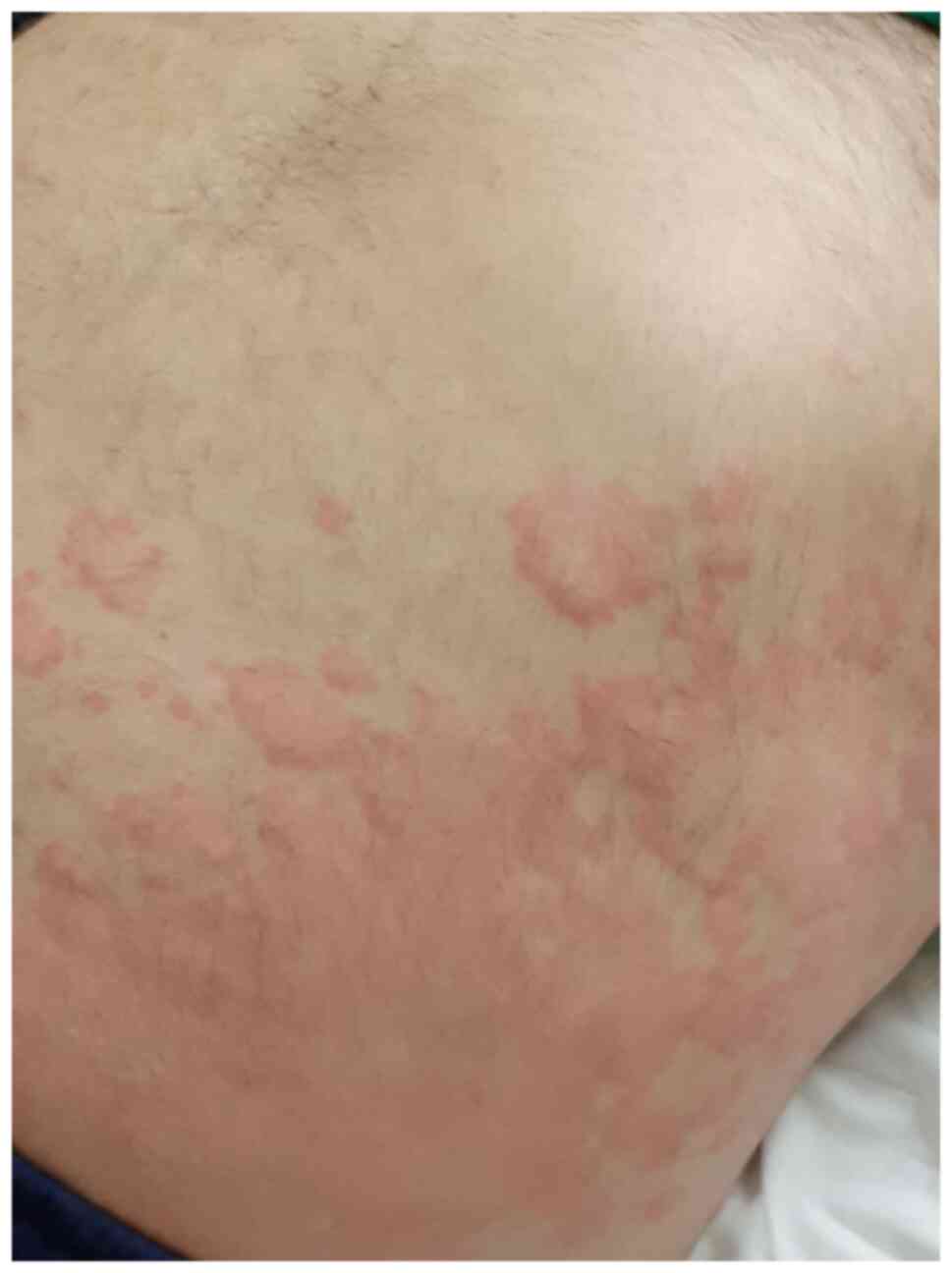
urticarial lesions could be identified in 16% of the total
cutaneous lesions, predominantly involve the trunk and the limbs,
sparing the acral sites in most cases (Fig. 1) (24). The proposed pathogenic hypotheses
include the unspecific activation of mast cells, direct endothelial
damage [angiotensin-converting enzyme 2 (ACE2+)],
antigen-antibody deposits, complement activation, and kinin pathway
activation. Whether the wheals are directly correlated with the
novel coronavirus remains unclear, the etiopathogenic substrate
being difficult to demonstrate, as urticaria may be drug-induced,
particularly by antibiotics (12,24,25).
With the use of histopathology, Rodríguez-Jiménez et al
identified a mild case of vacuolar interface dermatitis accompanied
by few necrotic keratinocytes compatible with an erythema
multiforme-like pattern (26).
Amatore et al also reported the presence of lichenoid and
vacuolar interface dermatitis, associated with mild spongiosis,
dyskeratotic basal keratinocytes and superficial perivascular
lymphocytic infiltrate in a patient diagnosed with COVID-19 who
presented with erythemato-edematous non-pruritic annular plaques
and fever (27).
b) Maculo-papular rashes
According to Galván et al, maculo-papular
rashes are the most common skin manifestations in patients with
COVID-19 (47% of the cases) (21).
They tend to occur concurrently or immediately following the other
symptoms of the disease and are suggested to be associated with
severe clinical forms, where the mortality may reach 2%. Pruritus
may be present in 56% of cases. The evolution is on average 8.6
days (21). Several subtypes have
been described: morbilliform rash, erythema elevatum diutinum-like
rash, erythema multiforme-like rash (typical target-like lesions
mainly on the extremities, but also on the trunk, possibly induced
by the virus), and digitiform papulosquamous rash (9,21,23,28).
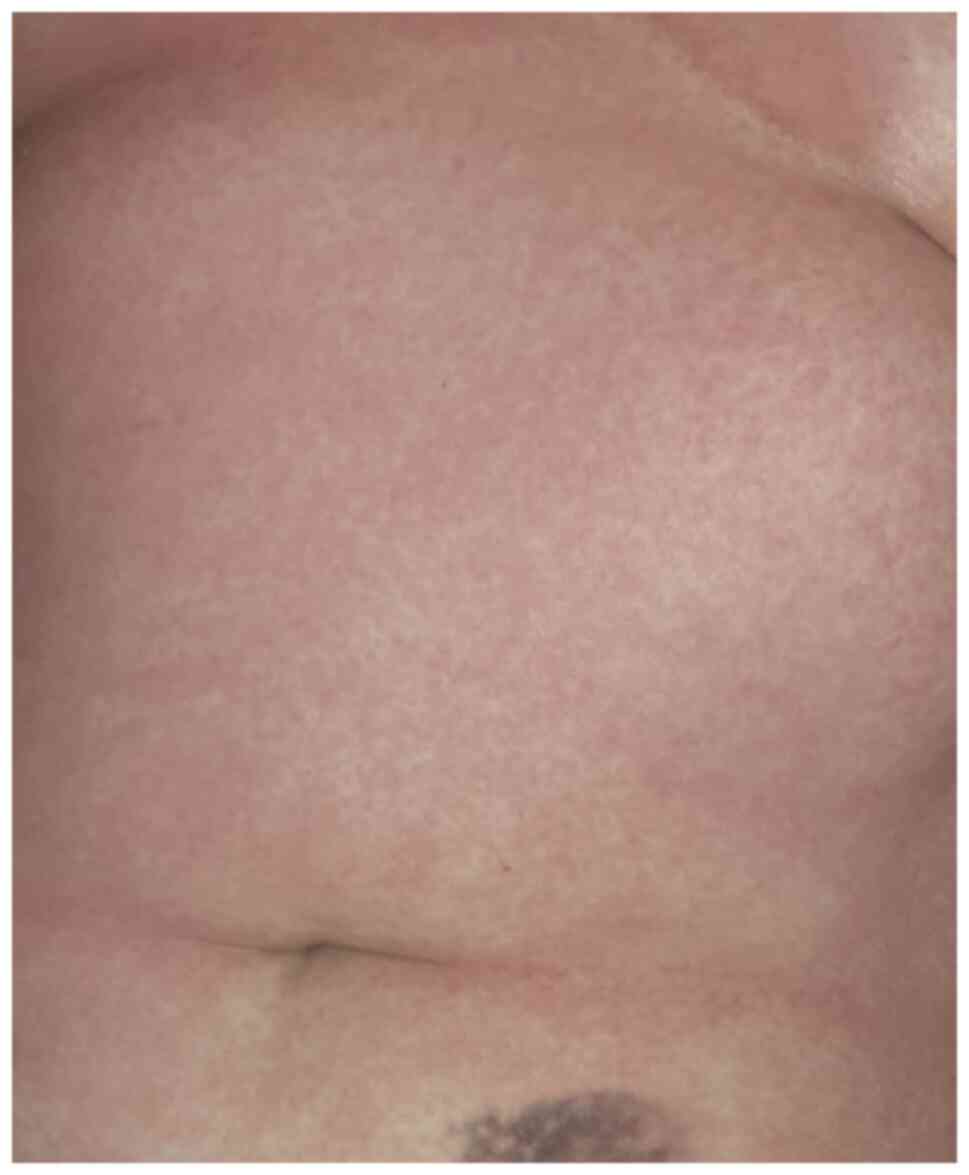
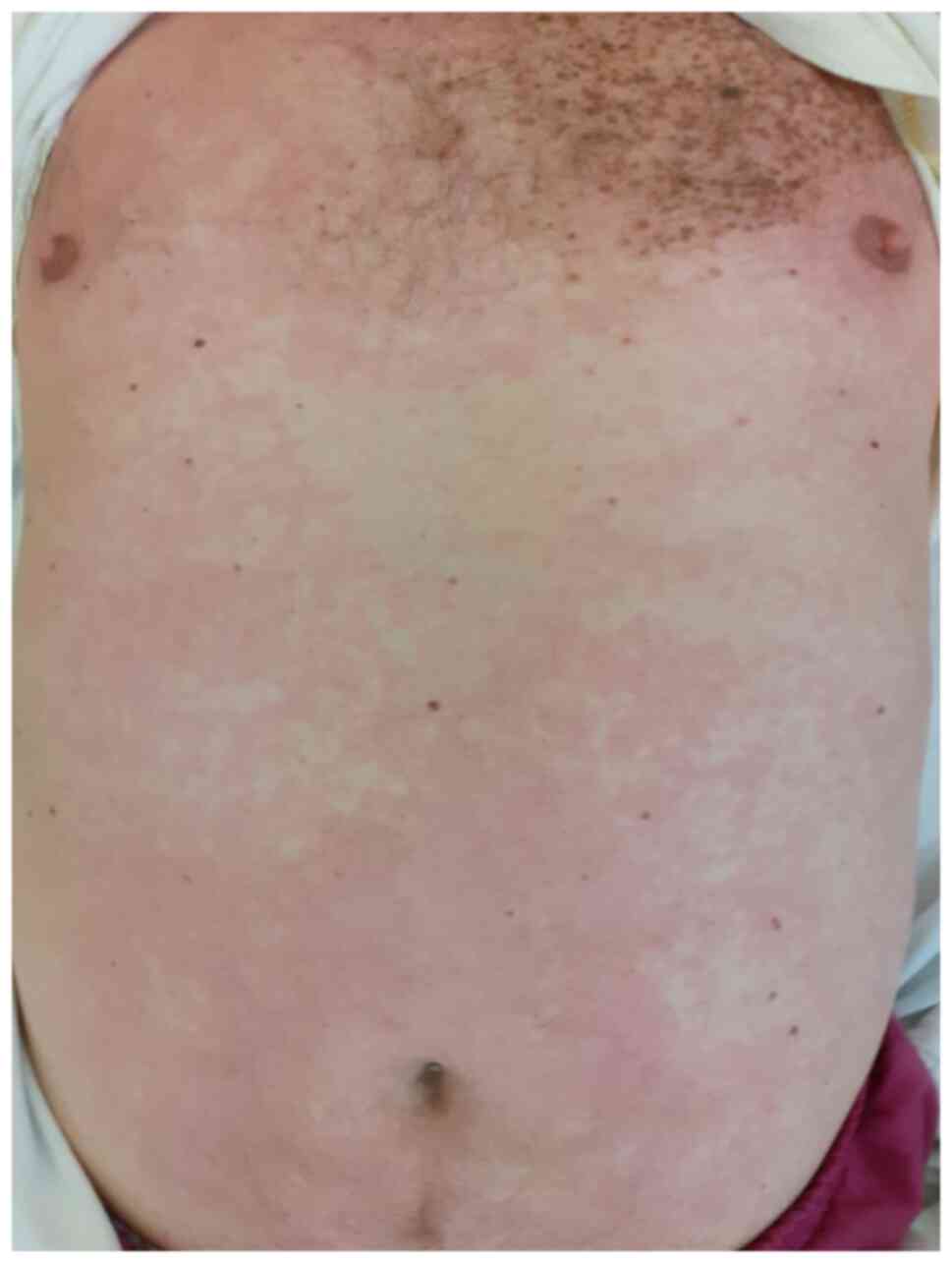
Morbilliform rash presents with maculo-papules or non-pruritic
erythematous plaques, predominantly distributed on the trunk and
extremities of the limbs excluding the face and the mucous
membranes (Figs. 2 and 3). Onset is frequently following the onset
of COVID-19 systemic symptoms. Differential diagnosis includes
other viral exanthems and drug-induced cutaneous reactions
(21-23,28).
Pityriasis rosea, including typical and atypical digitiform
papulosquamous rash, has been described in association with
SARS-CoV-2 infection, presumably as an expression of the immune
response of the body to high levels of proinflammatory cytokines
(21,28,29).
Histopathology of these erythematous eruptions, as described by
Gianotti et al, revealed vascular damage in all the 3 cases
examined (30). Reymundo et
al observed a mild superficial perivascular lymphocytic
infiltrate on the histology of 4 patients (31). By contrast, Herrero-Moyano et
al observed neutrophilic infiltrate in 8 patients with late
maculopapular eruptions. Collectively, the histopathology reveals
changes similar to other viral rashes (32).
c) Papulovesicular
exanthem/varicella-like lesions
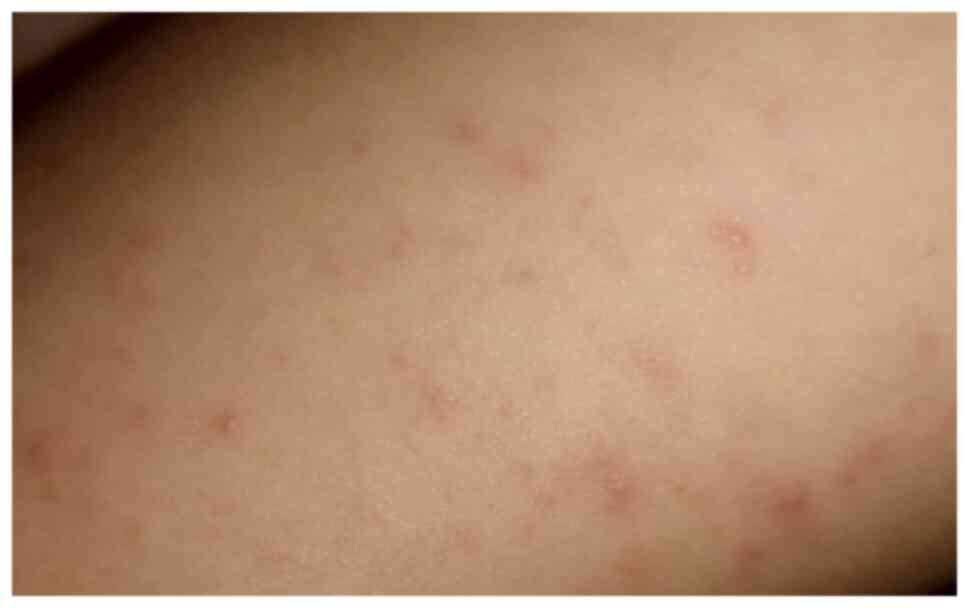
Marzano et al revealed that the
papulovesicular exanthems have a 9% prevalence, the median age is
60 years, but children may also be affected (33). In an unpublished study conducted in
eight Italian dermatology units, skin lesions were reported to
appear in most cases 3 days following the systemic symptoms and to
disappear after 8 days, without scarring (13). The clinical aspect consists of a
vesicular eruption similar to varicella (Fig. 4), or miliaria rubra-like
disseminated lesions, located mainly on the torso (13,33).
Tammaro et al presented data from their combined experience
in Rome (Italy) and Barcelona (Spain), and described similar
lesions to ones identified in infections caused by members of the
Herpesviridae family (34).
Differential diagnosis must include herpetic infections and
Grover's Disease (32). As a
pathogenic hypothesis, direct viral damage to basal keratinocytes
may be considered (28,29,33,34).
According to Mahé et al, histologically, these exanthems
reveal acantholysis, intraepidermal vesicles with suprabasal
clefts, prominent, ‘pomegranate-like’ dyskeratosis and suspected
viral inclusions in multinucleated cells (35). Fernandez-Nieto et al
identified another case of papulovesicular eruption which revealed
extensive epidermal necrosis with acantholysis and swelling of
keratinocytes, balloon degeneration of keratinocytes, and signs of
endothelitis in the dermal vessels (36).
ii) Vasculitic/vasculopathic lesions
associated with SARS-CoV-2
The current hypotheses consider that the
vasculitic/vasculopathic manifestations could be the result of
small vessel occlusion, or it could be a neurogenic,
microthrombotic, immune complex-mediated mechanism. A direct
correlation with the SARS-CoV-2 virus has yet to be demonstrated
(37,38).
a) Ischemic acral lesions
Ischemic acral lesions were described in patients
with COVID-19, presenting two types of clinical manifestations:
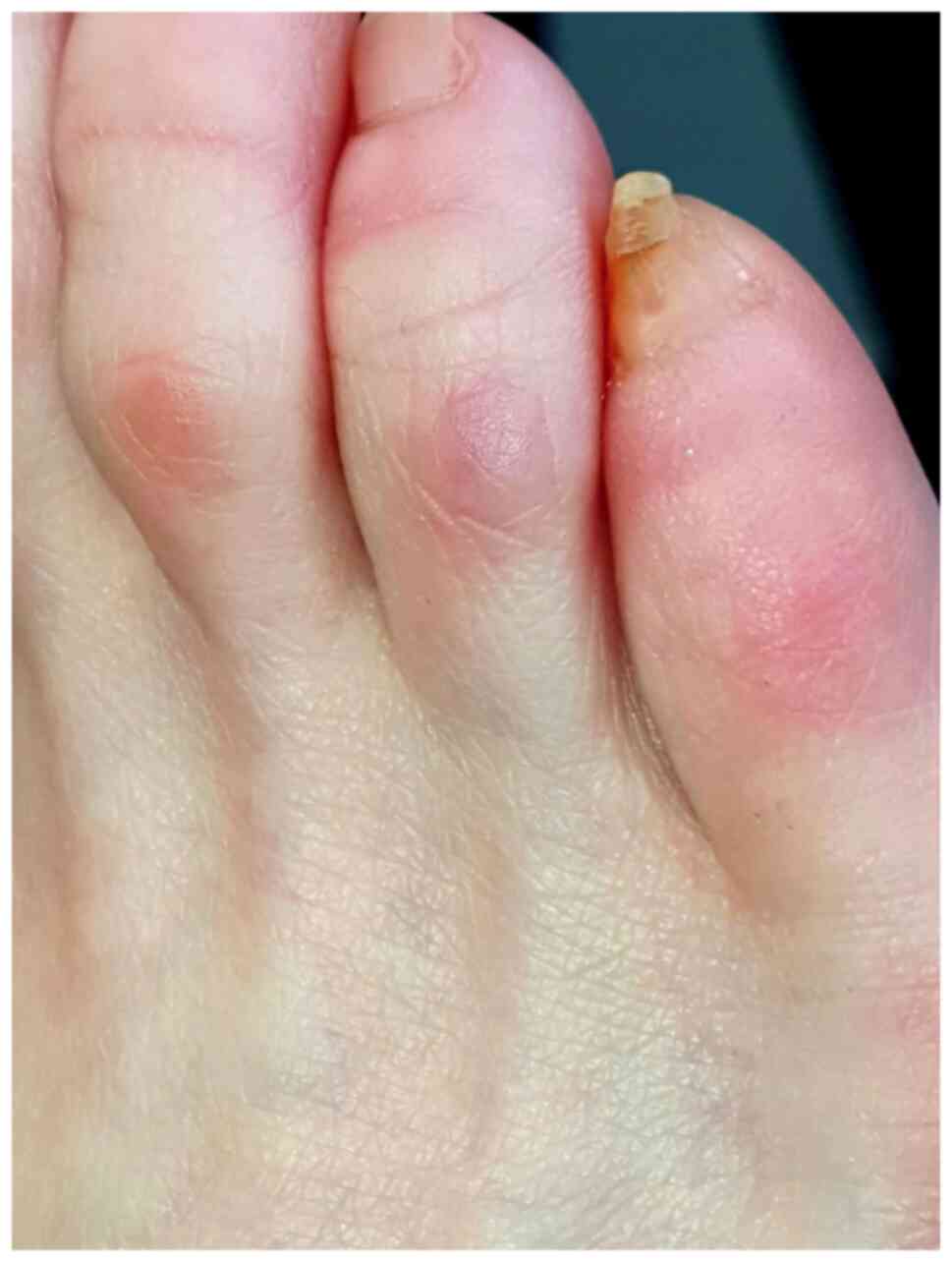
chilblain-like/perniosis and acral ulcers. Chilblain-like lesions
are painful cyanotic, red-purple macular or papular lesions, with
acral disposition, particularly in the lower limbs (toes, but also
plantar and calcaneal), accompanied by edema (Fig. 5) (21). These lesions occur mainly in
children with asymptomatic clinical forms, in 19-40% of adults with
less severe disease (16% hospitalized) and in females (68% of
cases), at younger ages (31.7 years on average). They appear later
in the evolution of the COVID-19 disease, without cold exposure or
other predisposing substrates, on average after the 9th day, but in
certain cases, even following the recovery period (3,7,11,19,20).
The evolution is towards resolution in 2-8 weeks, with a duration
of symptoms between 10 and 133 days, according to the ILDS/AAD
registry (24). In patients with
critical COVID-19 disease and disseminated intravascular
coagulation severe manifestations with cyanosis of the toes,
bullous lesions, and dry gangrene have been described (22,23).
The causal correlation between chilblain-like lesions and
SARS-CoV-2 infection is still debated. French researchers did not
identify chilblains predictive for COVID-19, as they investigated
40 patients suffering from chilblains, with the nasopharyngeal test
(PCR) negative for all of these patients and positive serology in
only one-third of them (39,40).
However, the increased occurrence of chilblain-like lesions during
the COVID-19 pandemic, particularly in young patients with no
history of predisposing factors, such as exposure to cold, as well
as pathological reports of positive immunohistochemistry for
SARS-CoV-2 from skin biopsies are arguments in favor of a
COVID-19-associated type of chilblain (10,41).
The pathogenesis of SARS-CoV-2-associated chilblain involves
vasospasm and microthrombosis through an increase in the
vasoconstricting, prothrombotic and inflammatory pathway of
angiotensin 2 induced by the viral cell infection, as well as
acquired coagulopathy or endothelial cytotoxicity mediated by
CD8+ T lymphocytes and robust interferon I (IFN-I)
response (20,22,37,41).
Histological examination revealed lymphoid-lymphoplasmacytic
infiltrate in the dermis possibly extending to the hypodermis and
signs of endothelial activation or plump endothelial cells in the
venules surrounded by infiltrate (12,42).
An acral ulcer occurs in critically ill patients
with COVID-19 with multiorgan involvement and manifests with
purplish induration, livedoid plaques, bedsores. The pathogenic
mechanism hypothesis focuses on the systemic coagulopathy leading
to cutaneous ischemia (22).
b) Reticular purpuric lesions
Reticular purpuric lesions include retiform
purpura, livedo reticularis and livedo racemosa. Retiform
purpura is a severe manifestation that occurred in 82% of the
hospitalized patients with acute respiratory distress syndrome. Its
manifestations may include widespread purpura, hemorrhagic bullae,
microthrombosis, progressive thrombocytopenia, with or without
livedo racemosa (22).
Livedo reticularis/racemosa
pattern
Livedo describes a condition of slow blood flow and
blue cutaneous discoloration, which has been divided into 2 groups:
livedo reticularis, generally associated with cold-induced
cutaneous vasoconstriction and livedo racemosa, more frequently
associated with focal impairment of blood flow such as Sneddon's
syndrome. These lesions are described to appear any time during
SARS-CoV-2 infection, mostly in older patients, localized on the
limbs (13,21,22).
Livedo reticularis-like patterns are frequently mild, transient,
unilateral, or bilateral and not associated with thromboembolic
complications (43). On the
contrary, livedo racemosa-like patterns have often been described
in patients with severe coagulopathy (13,21).
Regarding the histopathology, Magro et al described
pauci-inflammatory microthrombotic vasculopathy observed in three
patients. They also demonstrated that in the racemosa-like pattern,
in patients with a severe infection of COVID-19, the vascular
thrombosis was associated with a minimal interferon response which
increased viral replication and complement activation, probably
involved in the pathophysiology of its clinical complications
(41). Genovese et al
distinguished the group of livedo reticularis/racemosa-like from
the purpuric ‘vasculitic’ pattern since only the last one is
considered the expression of a true vasculitis process (13).
Purpuric ‘vasculitic’ pattern
An Italian multicentric study revealed that this
pattern is likely to be very rare representing 8.2% of skin
manifestations (9). Joob and
Wiwanitkit described the first purpuric lesion during the COVID-19
pandemic, as a petechial rash. Vasculitic lesions were described to
appear more frequently in elderly patients with severe COVID-19,
representing the cutaneous manifestations associated with the
highest risk of death (44). The
clinical appearance is that of palpable purpura, petechiae,
hemorrhagic blisters, ulcers, with distribution on the lower limbs,
or with purple and necrotic lesions similar to leukocytoclastic
vasculitis. It occurs late during SARS-CoV-2 infection (21,22).
The pathogenic mechanism involves a complement-mediated
inflammation caused by the immune complexes deposited in small
vessels, with tissue destruction, associated with pro-coagulant
status. Differential diagnosis includes drug-induced vasculitis
(20,22). A severe clinical form of vasculitis
has been described, involving multisystem inflammatory syndrome in
children who develop an exaggerated immune response to SARS-CoV-2
virus, with clinical manifestations similar to Kawasaki disease
[acute vasculitis affecting children under 5 years of age and may
lead to coronary aneurysms in 25% of untreated cases, triggered by
an external factor, infectious agent, in individuals with genetic
susceptibility (CASP3, HLA II, BLK, CD40)] (45-47).
The mechanism of association of Kawasaki disease with viral
infection is incompletely elucidated, but it is assumed that
cytokines (IL-1, IL-6, IL-18) released by infected cells induce
endothelial injury with vasculitic manifestations (47).
c) Purpuric non-vasculitic
pattern
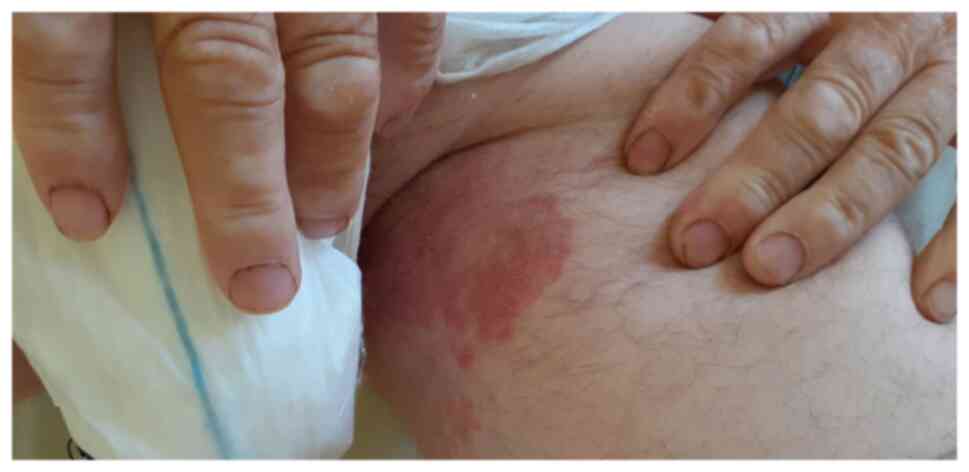
A petechial rash, probably secondary to
thrombocytopenia, has been described, accompanied by
macular/maculopapular lesions as a result of a non-vasculitic
inflammatory process or as secondary lesions during the evolution
of a maculopapular exanthem. Purpuric periflexural and flexural
lesions (Fig. 6) have also been
reported, with incompletely elucidated pathogenesis. These lesions
were considered purpuric non-vasculitic since there was no
histological evidence of a vasculitic inflammatory process
(21,22). Another dermatologic manifestation
described in two patients in association with COVID-19 was the ‘red
half-moon nail sign’, with no associated cutaneous lesions, that
appeared between 2 and 14 days since the disease onset and
persisted following the remission of respiratory symptoms (48,49).
Oral mucosal lesions in patients with
SARS-CoV-2 infection
Changes in oral mucous membranes in the context of
SARS-CoV-2 disease have also been reported (16,17).
Petechial, macular and maculo-petechial enanthems were described in
patients with COVID-19 disease, accompanied by a papulovesicular
rash, periflexural purpura, and erythema-multiforme-like rash.
These mucosal lesions occurring concurrently with a skin rash are
indicative of a viral etiology, rendering the examination of the
oral mucosa an important step in differentiating between
drug-induced exanthems and viral-induced skin rashes in the context
of the SARS-CoV-2 pandemic (16).
Lingual pain was described in patients with COVID-19, possibly due
to the higher expression of ACE2 receptor in the epithelial cells
of the tongue (17). Oral ulcers,
similar to recurrent herpes simplex or recurrent aphthous
stomatitis have been reported by several authors. Pathogenic
hypotheses focus on vascular and arterial thrombosis in small and
medium-sized vessels (16,17). Lichen-planus-like lesions have been
reported in patients that had been diagnosed with COVID-19 in the
previous 12 months (16,17). In a Spanish study, 45.7% of 666
patients presented mucocutaneous lesions. On the oral mucosa,
transient lingual papillitis was identified in 11.5% of cases,
recurrent aphthous stomatitis in 6.9% of cases, glossitis with
lateral indentations in 6.6% of cases, and depapilating glossitis
in 3.9% of cases (16). The
pathogenic mechanism for these manifestations is not yet fully
understood.
Ocular mucosa lesions in patients with
SARS-CoV-2 infection
The conjunctiva is considered to be an important
part of the eye mucosa. It has a consistent barrier role against
environmental and infectious agents due to numerous immunologic
features common to other mucosal tissues (CD4+,
CD8+ T cells, B cells, mast cells, dendritic cells, and
Langerhans cells) (50,51). The confirmation of both ACE2 (as the
key entry receptor) and cellular serine protease TMPRSS2 expression
on the ocular surface cells (52)
makes the conjunctiva vulnerable for SARS-CoV-2 infection. In
addition, the wide immunohistochemical detection of CD147 (promoter
of viral invasion into the host cells), in different eye structures
explains the viral propagation and enables ocular surface cells for
further person-to-person transmission. The incidence of
conjunctivitis in COVID-19 patients largely varies from 0.8% to
4.8% (18,53). A higher incidence (~3%) has been
noted in severe COVID-19 cases as compared with only 0.7% in mild
to moderate disease (54). The
conjunctival signs, mostly bilateral, usually include mild to
moderate hyperemia, follicular changes, chemosis, and discharge. A
limited number of severe cases develop conjunctival pseudomembranes
or corneal lesions (epithelial defects or subepithelial
infiltrates) (55-57).
The timing of conjunctivitis largely varies, certain patients
reporting conjunctivitis-related symptoms (foreign body sensation,
itching, and occasionally photophobia) before respiratory symptoms
or fever. The possibility of contracting the SARS-CoV-2 infection
via the eye is, at least in theory, plausible as the nasolacrimal
duct may transport viral particles from the ocular surface to
highly susceptible nasal epithelial cells from the inferior meatus
(58). Other intriguing
observations are that the detection of SARS-CoV-2 RNA in tears is
not always associated with ocular manifestations as not every
COVID-19 patient with conjunctivitis has a positive tear sample
(59,60).
6. Discussion and conclusions
Although millions of cases have been registered, no
pathognomonic dermatological signs and symptoms for the disease
have been identified yet. The polymorphic skin and mucosal lesions
associated with SARS-CoV-2 infection are not an argument for the
viral etiology, as usually, a certain virus is responsible for a
single type of dermatologic manifestation. However, the increased
incidence of the aforementioned clinical patterns of dermatologic
conditions during the pandemic, suggests the association with the
SARS-CoV-2 virus. The diverse clinical aspects may be explained by
pathogenic differences between distinct strains of the virus,
differences related to the host reactivity, and the possibility of
co-infections. In contrast, skin and mucosal manifestations during
COVID-19 may not only be related to the virus itself, but also to
the viral-induced vasculitis and thrombotic vasculopathy, or they
may be due to adverse reactions to the prescribed drugs (6-8,61).
The most common side effects associated with several of the
often-prescribed drugs for COVID-19 infection (antimalarials) were
maculopapular exanthematous reactions, urticaria, and psoriasis
exacerbation. Oral antiretroviral combination lopinavir/ritonavir
may be responsible for Stevens-Johnson syndrome (8). Temporal association between urticarial
lesions and maculopapular eruptions with SARS-CoV-2 infection, when
they appear concurrently as the systemic symptoms may be indicative
of a viral etiology, rather than a drug-induced one (8). It is currently considered that two
types of skin manifestations may be characteristic of the COVID-19
disease chilblain-like lesions and papulovesicular lesions.
Therefore RT-PCR for SARS-CoV-2 (if the onset is less than 4 weeks
previous) or serological testing (IgM, IgG) for a potential
SARS-CoV-2 infection should be added to the investigation protocol
in patients without known risk factors who develop pernio-like
lesions or in patients with papulovesicular rashes. Cases of
COVID-19 with a clinical picture consisting of an infectious rash
alone have been reported, making it imperative to investigate a
febrile rash for the novel coronavirus as a possible cause
(30,61).
The description of the mucocutaneous manifestations
associated with COVID-19 reviewed in this article may be helpful in
the early recognition of cutaneous signs that are associated with
severe complications (such as livedoid, necrotic or maculopapular
lesions) and to establish prompt management essential in improving
patients prognosis. Patients with autoimmune and chronic
inflammatory disorders, such as psoriasis, atopic dermatitis,
lupus, scleroderma, and hidradenitis suppurativa may require
special care and adjustment of their immune-suppressive therapy
protocol in order to maximize the chances for an effective response
to anti-Covid-19 vaccines (2).
Acknowledgements
Not applicable.
Funding
Funding: Publishing fees were supported by the Association of
Dermatologists of Moldova.
Availability of data and materials
Not applicable.
Authors' contributions
MPT and DEB contributed to the study design,
participated in the entire review process, and prepared the
manuscript. IME and CIB contributed to collecting the relevant
literature, data analysis, and critical interpretation. MSC, MG,
and DCB conceived the review and modified the manuscript. ACP, AD
and ACN ensured that all questions related to the accuracy or
integrity of the work are appropriately investigated and resolved.
All authors read and approved the final version of the manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Docea AO, Tsatsakis A, Albulescu D,
Cristea O, Zlatian O, Vinceti M, Moschos SA, Tsoukalas D, Goumenou
M, Drakoulis N, et al: A new threat from an old enemy: Re-emergence
of coronavirus (Review). Int J Mol Med. 45:1631–1643.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Calina D, Docea AO, Petrakis D, Egorov AM,
Ishmukhametov AA, Gabibov AG, Shtilman MI, Kostoff R, Karvalho F,
Vinceti M, et al: Towards effective COVID-19 vaccines: Updates,
perspectives and challenges (Review). Int J Mol Med. 46:3–16.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Potekaev NN, Zhukova OV, Protsenko DN,
Demina OM, Khlystova EA and Bogin V: Clinical characteristics of
dermatologic manifestations of COVID-19 infection: Case series of
15 patients, review of literature, and proposed etiological
classification. Int J Dermatol. 59:1000–1009. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gisondi P, Piaserico S, Conti A and Naldi
L: Dermatologists and SARS-CoV-2: The impact of the pandemic on
daily practice. J Eur Acad Dermatol Venereol. 34:1196–1201.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Recalcati S: Cutaneous manifestations in
COVID-19: A first perspective. J Eur Acad Dermatol Venereol.
34:e212–e213. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ahouach B, Harent S, Ullmer A, Martres P,
Bégon E, Blum L, Tess O and Bachmeyer C: Cutaneous lesions in a
patient with COVID-19: Are they related? Br J Dermatol.
183(e31)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Iordache AM, Docea AO, Buga AM, Mitrut R,
Albulescu D, Zlatian O, Ianosi S, Ianosi G, Neagoe D, Sifaki M, et
al: The incidence of skin lesions in contrast media-induced
chemical hypersensitivity. Exp Ther Med. 17:1113–1124.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Martinez-Lopez A, Cuenca-Barrales C,
Montero-Vilchez T, Molina-Leyva A and Arias-Santiago S: Review of
adverse cutaneous reactions of pharmacologic interventions for
COVID-19: A guide for the dermatologist. J Am Acad Dermatol.
83:1738–1748. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mascitti H, Bonsang B, Dinh A, Assan F,
Perronne V, Leblanc T, Duran C, Bouchand F, Matt M, Le Gal A, et
al: Clinical cutaneous features of patients infected with
SARS-CoV-2 hospitalized for pneumonia: A cross-sectional study.
Open Forum Infect Dis. 7(ofaa394)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Le Cleach L, Dousset L, Assier H, Fourati
S, Barbarot S, Boulard C, Bourseau Quetier C, Cambon L, Cazanave C,
Colin A, et al: Most chilblains observed during the COVID-19
outbreak occur in patients who are negative for COVID-19 on
polymerase chain reaction and serology testing. Br J Dermatol.
183:866–874. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Brănișteanu DE, Ianoşi SL, Dimitriu A,
Stoleriu G, Oanţǎ A and Brănișteanu DC: Drug-induced rowell
syndrome, a rare and difficult to manage disease: A case report.
Exp Ther Med. 15:785–788. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kaya G, Kaya A and Saurat JH: Clinical and
histopathological features and potential pathological mechanisms of
skin lesions in COVID-19: Review of the literature.
Dermatopathology (Basel). 7:3–16. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Genovese G, Moltrasio C, Berti E and
Marzano AV: Skin manifestations associated with COVID-19: Current
knowledge and future perspectives. Dermatology. 237:1–12.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Darlenski R and Tsankov N: COVID-19
pandemic and the skin: What should dermatologists know? Clin
Dermatol. 38:785–787. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Suchonwanit P, Leerunyakul K and
Kositkuljorn C: Cutaneous manifestations in COVID-19: Lessons
learned from current evidence. J Am Acad Dermatol. 83:e57–e60.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jimenez-Cauhe J, Ortega-Quijano D, de
Perosanz-Lobo D, Burgos-Blasco P, Vañó-Galván S, Fernandez-Guarino
M and Fernandez-Nieto D: Enanthem in patients with COVID-19 and
skin rash. JAMA Dermatol. 156:1134–1136. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rochefort J and Chaux AG: Oral mucosal
lesions and Covid-19: Symptoms and/or complication? J Oral Med Oral
Surg. 27(23)2021.
|
|
18
|
Guan W, Ni Z, Hu Y, Liang W, Ou C, He J,
Liu L, Shan H, Lei C, Hui DSC, et al: Clinical characteristics of
coronavirus disease 2019 in China. N Engl J Med. 382:1708–1720.
2020.
|
|
19
|
Aida Maranduca M, Liliana Hurjui L,
Constantin Branisteanu D, Nicolae Serban D, Elena Branisteanu D,
Dima N and Lacramioara Serban I: Skin-a vast organ with
immunological function (Review). Exp Ther Med. 20:18–23.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hubiche T, Cardot-Leccia N, Le Duff F,
Seitz-Polski B, Giordana P, Chiaverini C, Giordanengo V, Gonfrier
G, Raimondi V, Bausset O, et al: Clinical, laboratory, and
interferon-alpha response characteristics of patients with
chilblain-like lesions during the COVID-19 pandemic. JAMA Dermatol.
157:202–206. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Galván Casas C, Català A, Carretero
Hernández G, Rodríguez-Jiménez P, Fernández-Nieto D,
Rodríguez-Villa Lario A, Navarro Fernández I, Ruiz-Villaverde R,
Falkenhain-López D, Llamas Velasco M, et al: Classification of the
cutaneous manifestations of COVID-19: A rapid prospective
nationwide consensus study in Spain with 375 cases. Br J Dermatol.
183:71–77. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Marzano AV, Cassano N, Genovese G,
Moltrasio C and Vena GA: Cutaneous manifestations in patients with
COVID-19: A preliminary review of an emerging issue. Br J Dermatol.
183:431–442. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gisondi P, PIaserico S, Bordin C, Alaibac
M, Girolomoni G and Naldi L: Cutaneous manifestations of SARS-CoV-2
infection: A clinical update. J Eur Acad Dermatol Venereol.
34:2499–2504. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Freeman EE, McMahon DE, Lipoff JB,
Rosenbach M, Kovarik C, Desai SR, Harp J, Takeshita J, French LE,
Lim HW, et al: The spectrum of COVID-19-associated dermatologic
manifestations: An international registry of 716 patients from 31
countries. J Am Acad Dermatol. 83:1118–1129. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Seirafianpour F, Sodagar S, Pour Mohammad
A, Panahi P, Mozafarpoor S, Almasi S and Goodarzi A: Cutaneous
manifestations and considerations in COVID-19 pandemic: A
systematic review. Dermatol Ther. 33(e13986)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rodríguez-Jiménez P, Chicharro P, De
Argila D, Muñoz-Hernández P and Llamas-Velasco M: Urticaria-like
lesions in COVID-19 patients are not really urticaria-a case with
clinicopathological correlation. J Eur Acad Dermatol Venereol.
34:e459–e460. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Amatore F, Macagno N, Mailhe M, Demarez B,
Gaudy-Marqueste C, Grob JJ, Raoult D, Brouqui P and Richard MA:
SARS-CoV-2 infection presenting as a febrile rash. J Eur Acad
Dermatol Venereol. 34:e304–e306. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tan SW, Tam YC and Oh CC: Skin
manifestations of COVID-19: A worldwide review. JAAD Int.
2:119–133. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Usher AD: WHO launches crowdfund for
COVID-19 response. Lancet. 395(1024)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gianotti R, Recalcati S, Fantini F, Riva
C, Milani M, Dainese E and Boggio F: Histopathological study of a
broad spectrum of skin dermatoses in patients affected or highly
suspected of infection by COVID-19 in the Northern Part of Italy:
Analysis of the many faces of the viral-induced skin diseases in
previous and new reported cases. Am J Dermatopathol. 42:564–570.
2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Reymundo A, Fernáldez-Bernáldez A, Reolid
A, Butrón B, Fernández-Rico P, Muñoz-Hernández P, De Argila D,
Wiesner T and Llamas-Velasco M: Clinical and histological
characterization of late appearance maculopapular eruptions in
association with the coronavirus disease 2019. A case series of
seven patients. J Eur Acad Dermatol Venereol. 34:e755–e757.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Herrero-Moyano M, Capusan TM,
Andreu-Barasoain M, Alcántara-González Ruano-Del Salado M,
Sánchez-Largo Uceda ME, Calzado-Villarreal L and Pérez-González Y:
A clinicopathological study of eight patients with COVID-19
pneumonia and a late-onset exanthema. J Eur Acad Dermatol Venereol.
34:e460–e464. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Marzano AV, Genovese G, Fabbrocini G,
Pigatto P, Monfrecola G, Piraccini BM, Veraldi S, Rubegni P, Cusini
M, Caputo V, et al: Varicella-like exanthem as a specific
COVID-19-associated skin manifestation: Multicenter case series of
22 patients. J Am Acad Dermatol. 83:280–285. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tammaro A, Adebanjo GAR, Parisella FR,
Pezzuto A and Rello J: Cutaneous manifestations in COVID-19: The
experiences of Barcelona and Rome. J Eur Acad Dermatol Venereol.
34:e306–e307. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mahé A, Birckel E, Merklen C, Lefèbvre P,
Hannedouche C, Jost M and Droy-Dupré L: Histology of skin lesions
establishes that the vesicular rash associated with COVID-19 is not
‘varicella-like’. J Eur Acad Dermatol Venereol. 34:e559–e561.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fernandez-Nieto D, Ortega-Quijano D,
Jimenez-Cauhe J, Burgos-Blasco P, de Perosanz-Lobo D, Suarez-Valle
A, Cortes-Cuevas JL, Carretero I, Garcia-Del Real C and
Fernandez-Guarino M: Clinical and histological characterization of
vesicular COVID-19 rashes: A prospective study in a tertiary care
hospitall. Clin Exp Dermatol. 45:872–875. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cappel MA, Cappel JA and Wetter DA: Pernio
(Chilblains), SARS-CoV-2, and COVID toes unified through cutaneous
and systemic mechanisms. Mayo Clin Proc. 96:989–1005.
2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hoenig LJ: Update on the cutaneous
manifestations of COVID-19. Clin Dermatol. 38(507)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bouaziz JD, Duong TA, Jachiet M, Velter C,
Lestang P, Cassius C, Arsouze A, Domergue Than Trong E, Bagot M,
Begon E, et al: Vascular skin symptoms in COVID-19: A French
observational study. J Eur Acad Dermatol Venereol. 34:e451–e452.
2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Guelimi R, Salle R, Dousset L, Assier H,
Fourati S, Barbarot S, Boulard C, Bourseau Quetier C, Cambon L,
Cazenave C, et al: Cutaneous manifestations during COVID-19
epidemic: COVID-skin study of the French society of dermatology.
Ann Dermatol Venereol. 147(A135)2020.doi.org/10.1016/j.annder.2020.09.116.
|
|
41
|
Magro C, Mulvey JJ, Berlin D, Nuovo G,
Salvatore S, Harp J, Baxter-Stoltzfus A and Laurence J: Complement
associated microvascular injury and thrombosis in the pathogenesis
of severe COVID-19 infection: A report of five cases. Transl Res.
220:1–13. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Alramthan A and Aldaraji W: Two cases of
COVID-19 presenting with a clinical picture resembling chilblains:
First report from the Middle East. Clin Exp Dermatol. 45:746–748.
2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Manalo IF, Smith MK, Cheeley J and Jacobs
R: A dermatologic manifestation of COVID-19: Transient livedo
reticularis. J Am Acad Dermatol. 83(700)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Joob B and Wiwanitkit V: COVID-19 can
present with a rash and be mistaken for dengue. J Am Acad Dermatol.
82(e177)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
European Centre for Disease Prevention and
Control: Paediatric inflammatory multisystem syndrome and
SARS-CoV-2 infection in children - May 15, 2020. ECDC, Stockholm,
2020.
|
|
46
|
Verdoni L, Mazza A, Gervasoni A, Martelli
L, Ruggeri M, Ciuffreda M, Bonanomi E and D'Antiga L: An outbreak
of severe Kawasaki-like disease at the Italian epicentre of the
SARS-CoV-2 epidemic: An observational cohort study. Lancet.
395:1771–1778. 2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Whittaker E, Bamford A, Kenny J, Kaforou
M, Jones CE, Shah P, Ramnarayan P, Fraisse A, Miller O, Davis P, et
al: Clinical characteristics of 58 children with a pediatric
inflammatory multisystem syndrome temporally associated with
SARS-CoV-2. JAMA. 324:259–269. 2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Méndez-Flores S, Zaladonis A and
Valdes-Rodriguez R: COVID-19 and nail manifestation: Be on the
lookout for the red half-moon nail sign. Int J Dermatol.
59(1414)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Neri I, Guglielmo A, Virdi A, Gaspari V,
Starace M and Piraccini BM: The red half-moon nail sign: A novel
manifestation of coronavirus infection. J Eur Acad Dermatol
Venereol. 34:e663–e665. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Sacks EH, Wieczorek R, Jakobiec FA and
Knowles DM II: Lymphocytic subpopulations in the normal human
conjunctiva. A monoclonal antibody study. Ophthalmology.
93:1276–1283. 1986.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Rodrigues MM, Rowden G, Hackett J and
Bakos I: Langerhans cells in the normal conjunctiva and peripheral
cornea of selected species. Invest Ophthalmol Vis Sci. 21:759–765.
1981.PubMed/NCBI
|
|
52
|
Zhou L, Xu Z, Castiglione GM, Soiberman
US, Eberhart CG and Duh EJ: ACE2 and TMPRSS2 are expressed on the
human ocular surface, suggesting susceptibility to SARS-CoV-2
infection. Ocul Surf. 18:537–544. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zhang X, Chen X, Chen L, Deng C, Zou X,
Liu W, Yu H, Chen B and Sun X: The evidence of SARS-CoV-2 infection
on ocular surface. Ocul Surf. 18:360–362. 2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Loffredo L, Pacella F, Pacella E, Tiscione
G, Oliva A and Violi F: Conjunctivitis and COVID-19: A
meta-analysis. J Med Virol. 92:1413–1414. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Salducci M and La Torre G: COVID-19
emergency in the cruise's ship: A case report of conjunctivitis.
Clin Ter. 171:e189–e191. 2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Navel V, Chiambaretta F and Dutheil F:
Haemorrhagic conjunctivitis with pseudomembranous related to
SARS-CoV-2. Am J Ophthalmol Case Rep. 19(100735)2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Cheema M, Aghazadeh H, Nazarali S, Ting A,
Hodges J, McFarlane A, Kanji JN, Zelyas N, Damji KF and Solarte C:
Keratoconjunctivitis as the initial medical presentation of the
novel coronavirus disease 2019 (COVID-19). Can J Ophthalmol.
55:e125–e129. 2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Sungnak W, Huang N, Bécavin C, Berg M,
Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D,
Sampaziotis F, et al: SARS-CoV-2 entry factors are highly expressed
in nasal epithelial cells together with innate immune genes. Nat
Med. 26:681–687. 2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Xia J, Tong J, Liu M, Shen Y and Guo D:
Evaluation of coronavirus in tears and conjunctival secretions of
patients with SARS-CoV-2 infection. J Med Virol. 92:589–594.
2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L
and Wu K: Characteristics of ocular findings of patients with
coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA
Ophthalmol. 138:575–578. 2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Su CJ and Lee CH: Viral exanthem in
COVID-19, a clinical enigma with biological significance. J Eur
Acad Dermatol Venereol. 34:e251–e252. 2020.PubMed/NCBI View Article : Google Scholar
|