Introduction
Sepsis is an immune disease induced by microbial
invasion (1) and has a higher
incidence in the elderly (2). It
not only exhibits a high prevalence and fatality, but also may lead
to subsequent kidney injury (3),
cardiac autophagy (4), stroke
(5), and lung injury (6-13).
At present, due to the lack of a gold standard for early diagnosis
(14), it is difficult to
distinguish sepsis from other immune diseases, hindering its
prevention and treatment. Understanding sepsis from the perspective
of molecular biology is conducive to the discovery of reliable and
accurate biomarkers for the early diagnosis and treatment.
Long non-coding H19 (lncRNA H19) which is located on
human chromosome 11 and approximately 6,291-bp long, does not
directly encode genes but regulates gene expression at the
post-transcriptional level by pairing and binding to nucleic acid
sequences, thereby participating in various diseases (15). According to Wan et al, lncRNA
H19 induced inflammation and neuronal dysfunction in rats with
ischemia-reperfusion injury via the microRNA (miR)-21/PDCD4 pathway
(16). It also inhibited
epithelial-mesenchymal transition of endothelial cells through
tumor growth factor-β1 (TGF-β1) and Smad pathway in diabetic
retinopathy (17). Bitarafan et
al reported that upregulated lncRNA H19 may be involved in
coronary artery diseases (18). In
addition, lncRNA H19 was revealed to activate the signal transducer
and activator of transcription/enhancer of zeste homolog 2
(STAT3/EZH2) pathway by downregulating let-7, thus accelerating
malignant proliferation of esophageal cancer (19). Moreover, lncRNA H19 was capable of
regulating pulmonary fibrosis through the TGF-β1 pathway via
miR-140(20). It is worth
mentioning that the downregulated lncRNA H19 may be associated with
sepsis (21).
In the present study, lncRNA H19 was revealed to be
downregulated in the serum of septic patients, and thus it was
theorized that downregulated lncRNA H19 may be involved in sepsis
development and is valuable for early diagnosis. Cecum ligation and
puncture (CLP) was performed to establish rat models of
sepsis-induced acute lung injury (ALI). Moreover, starBase2.0
demonstrated that lncRNA H19 carried sequence fragments that bound
to miR-152-3p. Therefore, it was theorized that lncRNA H19 was
likely to be involved in sepsis by regulating miR-152-3p. The
lncRNA H19/miR-152-3p axis has been reported to play a role in
myeloma (22), but there is no
evidence that lncRNA H19 participates in sepsis through miR-152.
Therefore, the present study investigated the association between
the lncRNA H19/miR-152 axis and sepsis.
Materials and methods
Septic patients
A total of 85 patients with sepsis (57 males and 28
females, aged 72.74±6.68 years) admitted to the Second Affiliated
Hospital of Nanjing Medical University (Nanjing, China) from
January 2016 to March 2017 were enrolled. The inclusion criteria
were as follows: patients diagnosed with sepsis. The exclusion
criteria were as follows: Pregnant or lactating women; individuals
≥85 years old; patients with chronic lung-related diseases,
advanced adenocarcinoma, mental illness, or those unwilling to
cooperate with the treatment; patients with a history of sepsis or
blood transfusion. Another 76 healthy individuals undergoing
physical examinations were selected as a control group. The Ethics
Committee of The Second Affiliated Hospital of Nanjing Medical
University (Nanjing, China) approved the present study and patients
who participated in this research, signed the informed consent and
had complete clinical data.
Venous blood (5 ml) was sampled after 8 h fasting
and stored in Eppendorf (EP) tubes without anticoagulant, and then
centrifuged at 3x103 x g for 15 min at room temperature.
Subsequently, the supernatant was placed in an EP tube without
RNase, and centrifuged again at 1.2x104 x g for 5 min at
4˚C. Supernatant was collected and stored at -40˚C for testing.
Sepsis diagnostic criteria followed the guidelines
for emergency treatment of sepsis/septic shock in China (2018)
(23): Patients with infection or
suspected infection were diagnosed with sepsis when sepsis-related
sequential organ failure assessment (SOFA) score increased more
than 2 points from the baseline (24).
Rat model of sepsis
A total of 27 healthy Sprague-Dawley (SD) male rats
(aged 2-3 months, weighing 190-270 g; Hunan SJA Laboratory Animal
Co., Ltd.) were randomly allocated into a control group, model
group, and lncRNA H19 adenovirus (lncRNA H19-ad) group. The rats in
the lncRNA H19-ad group were injected with lncH19 expression
vectors (lentiviral packaging plasmids; cat. no. V79020; Shanghai
Sangon Biotech Co., Ltd.) to upregulate lncRNA H19. The model group
and lncRNA H19-ad group were anesthetized with 1% pentobarbital
sodium (40 mg/kg body weight) to be modeled via CLP. CLP was
performed at the middle of the abdomen, then the wound was sutured.
The rats were raised in cages under constant temperature (23˚C) and
humidity conditions (60%), and were free to move and eat under an
alternating 12-h light/dark cycle. The collection of serum samples
was as follows: Venous blood (1 ml) was sampled from the tail of
the rats, placed in an anticoagulant tube, then centrifuged at
3x103 x g at 4˚C for 30 min to collect the supernatant.
Subsequently, rats were sacrificed with cervical dislocation and
received intraperitoneal injection of pentobarbital sodium (150
mg/kg body weight) until respiratory and cardiac arrest. Part of
the pulmonary tissue and blood were obtained for pathological
section examination and bacterial culture and identification,
respectively. The modeling was determined by pathological sections
and colony identification. In short, sepsis models were constructed
in model and lncRNA H19-ad groups, and the lncRNA H19-ad group
received an injection of pcDNA3-lncH19 expression vectors
(lentiviral packaging plasmids), while the control group was not
subjected to any treatment. Humane endpoints: Rats were dying or
suffering from severe organ failure that could not be treated
effectively. No deaths were reported before the end point of the
study. The cell line used to amplify lncRNA H19 was 293 cells
(ATCC). For plasmid transfection, 2 µg plasmid was used for cell
transfection, and the lentivirus was packaged with
pMDL/pRSV-Rev/VSVG-mixed plasmid and lentivirus plasmid. The
transfection kit used was Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). After transfection for 48 h, lentivirus
supernatant was collected, and then centrifuged at
1.5x103 x g at 4˚C for 5 min and stored at -80˚C for
subsequent experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
After serum sampling, rats were sacrificed and part
of the pulmonary tissue was ground into homogenate. A MiRNeasy
Serum/Plasma Kit (cat. no. 217184; Qiagen GmBH) was applied to
extract RNAs from serum, and TRIzol (Solarbio Life Sciences) was
used to isolate total RNAs from pulmonary tissues. The optical
density (OD) value of total RNAs at 260-280 nm was obtained by an
ultraviolet spectrophotometer, and those with a value of
OD260/OD280 >1.8 were used for subsequent
qPCR. FastKing one-step reverse transcription-fluorescence
quantitative kit (Beijing Tiangen Biotech Co., Ltd.) and ABI PRISM
7000 (Applied Biosystems; Thermo Fisher Scientific, Inc.) were
employed for RNA quantification. The fluorescence quantification
was performed using the TaqMan probe method (TaqMan; Applied
Biosystems; Thermo Fisher Scientific, Inc.). lncRNA H19 and
miR-152-3p primers were designed and synthesized by Sangon
Bioengineering (Shanghai) Co., Ltd. The primers were as follows:
lncRNA H19 forward, 5'-GTCCGGCCTTCCTGAACACCTT-3' and reverse,
5'-GCTTCACCTTCCAGAGCCGAT-3'; miR-152-3p forward,
5'-GCCTATAAACATCCGACTG-3' and reverse, 5'-GATCGCTGTCGTGGAAGTCG-3'.
The qPCR reaction system (50 µl) contained 1.25 µl forward primer,
1.25 µl reverse primer, 1.0 µl probe, 10 pg/g RNA template, 5 µl
50X ROX Reference Dye, and RNase-Free ddH2O was added to
a final volume of 50 µl. The reaction process was as follows:
reverse transcription at 50˚C for 30 min for 1 cycle;
pre-denaturing at 95˚C for 3 min for 1 cycle; denaturation at 95˚C
for 15 sec, annealing at 60˚C for 30 sec, for a total of 40 cycles.
ABI PRISM 7000 was used for analyses. U6 and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as internal
reference genes, and the data were normalized using the
2-ΔΔCq method (25). The
forward primer of GAPDH was 5'-CGGAATTCGTGAAGCTCGGAGTCAACGG-3'; and
the reverse primer was 5'-CGGGATCCCAGGAGCGCAGGGTTAGTCA-3'. The
upstream primer of U6 was 5'-ATTGGAACGATACAGAGAAGATT-3'; and the
downstream primer was 5'-GGAACGCTTCACGAATTTG-3'.
Western blotting
The protein extract consisted of the following
reagents: protein inhibitor +20 mmol Tris-HCl solution (pH 7.5;
both from Solarbio Life Science). After serum sampling, rats were
sacrificed and part of the pulmonary tissue was ground into
homogenate. The homogenate was mixed with protein extract (1 ml),
and the mixture was pipetted repeatedly until the complete lysis of
cells. The extract was centrifuged in a pre-cooled centrifuge
(1.6x104 x g) for 20 min at 4˚C. Protein concentration
in the supernatant was determined by bicinchoninic acid (BCA), and
the proteins (20 µg protein loaded per lane) were separated by
sodium dodecylsulphate-polyacrylamide gel electrophoresis
(SDS-PAGE) (5% spacer gel and 10% separation gel). Then, the
separated proteins were transferred to a nitrocellulose (NC)
membrane and maintained for 1 h at room temperature [sealed with 5%
skim milk/phosphate-buffered saline (PBS) solution]. Subsequently,
the primary antibody against β-actin was added, and maintained
overnight at 4˚C. The NC membrane was washed three times with PBS
solution, and then goat anti-rabbit secondary antibody [horseradish
peroxidase (HRP) conjugate] was added and maintained for 1 h at
room temperature. Finally, after washing with PBS solution, the NC
membrane was visualized with enhanced chemiluminescence (ECL)
solution. ImageJ software (version 1.60; National Institutes of
Health) was used to analyze the relative protein expression levels.
β-Actin was used as the internal reference protein, and the
relative expression level of the protein was calculated as follows:
Relative protein expression level=gray value of the test band/gray
value of the β-actin band. Apoptosis-related proteins [caspase-3
(product code ab32351; 1:5,000), caspase-9 (product code ab32539;
1:1,0000), B-cell lymphoma-2 (Bcl-2; product code ab196495;
1:2,000), and BCL2-associated X (Bax; product code ab32503;
1:10,000)], inflammatory factors [tumor necrosis factor-α (TNF-α;
product code ab205587; 1:1,000), interleukin (IL)-6 (product code
ab208113; 1:1,000) and IL-17 (product ab218013; 1:1,000)], β-actin
antibody, goat anti-rabbit secondary antibody H&L
(DyLight® 488; product code ab96899; 1:20,000), cleaved
caspase-3 (product code ab214430; 1:5,000) and cleaved caspase-9
(product code ab2324; 1:5,000) were all purchased from Abcam.
Enzyme-linked immunosorbent assay
(ELISA)
Venous blood (1 ml) sampled from the tail of
laboratory rats was placed in an anticoagulant tube, then
centrifuged (3x103 x g) at 4˚C for 30 min to collect the
supernatant. The ELISA kits (Abcam) detected the levels of IL-17
(product code ab214028), TNF-α (product code ab208348), and IL-6
(product code ab222503).
Wet to dry (W/D)weight ratio,
myeloperoxidase (MPO), superoxide dismutase (SOD), malondialdehyde
(MDA)
After obtaining serum samples part of the pulmonary
tissue was ground into homogenate. MPO was determined by
colorimetry using an MPO colorimetric activity assay kit (cat. no.
K744; BioVision, Inc.) according to the instructions of the kit.
Trichloroacetic acid (10%) was added into ground tissue samples,
and centrifuged at 4x103 x g at 4˚C for 10 min.
Subsequently, 0.6% thiobarbituric acid was added to the
supernatant, followed by heating in a boiling water bath for 15
min. Subsequently the absorbance at 532, 600 and 450 nm was
detected. Additionally, the SOD concentration was determined by a
Super Oxide Disruption Activity Assay Kit (product no. ab65354;
Abcam) according to the instructions of the kit.
The dry and wet weight of the right middle and lower
lobes were measured to calculate the W/D weight ratio.
Dual-luciferase reporter gene
assay
Lung epithelial cells (BEAS-2B; ATCC) were cultured
at 37˚C in a 5% CO2 incubator with a culture medium
system containing Dulbecco's modified Eagle's Medium (DMEM)
(Hyclone; Cytiva), 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Sceintific, Inc.), and 1% penicillin/streptomycin solution
(100X; Solarbio Life Science). starBase2.0(26) predicted the targeting binding loci
between lncRNA H19 and miR-152-3p. GLO-H19-wild type (wt) and
GLO-H19-mutant (mut) vectors were constructed and co-transfected
with miR-152-3p mimics (5'-UCAGUGCAUGACAGAACUUGG-3') and NC-mimics
(5'-ACUACUGAGUGACAGUAGA-3') (designed and synthesized by Sangon
Biotech Co., Ltd.) respectively. The transfection kit used was
Lipofectamine 2000. The transfected cells were cultured on 96-well
plates for 48 h. Then, a Dual-luciferase reporter gene assay system
(Promega Corporation) was employed to determine the luciferase
activity. In this study, pGLO plasmid vectors (Bio-Rad
Laboratories, Inc.) were used. The relative luciferase activity was
measured as follows: Relative luciferase activity=luciferase
activity of glowworm/internal reference luciferase activity of sea
pansy.
Statistical analysis
The data were statistically analyzed with SPSS 20.0
(Asia Analytics formerly SPSS, China) and visualized with GraphPad
Prism8.0 (GraphPad Software, Inc.). Measurement data were expressed
by the mean ± standard deviation (X±SD). Between-group comparisons
were performed by independent sample's t-test and multi-group
comparisons by one way ANOVA, followed by pairwise post hoc
comparisons (Dunnett's test). The counting data were expressed by
cases/percentage [n(%)], and the inter-group comparisons adopted
the χ2 test. Pearson correlation analysis was used to
determine the correlations between lncRNA H19 and patient data.
Receiver operating characteristic (ROC) curve was employed to
assess the diagnostic value of lncRNA H19 for septic patients. All
data were analyzed with two-tailed tests. The confidence interval
(CI) was set at 95%. P<0.05 indicated a statistically
significant difference.
Results
lncRNA H19 is expressed at a low level
in septic patients and rats
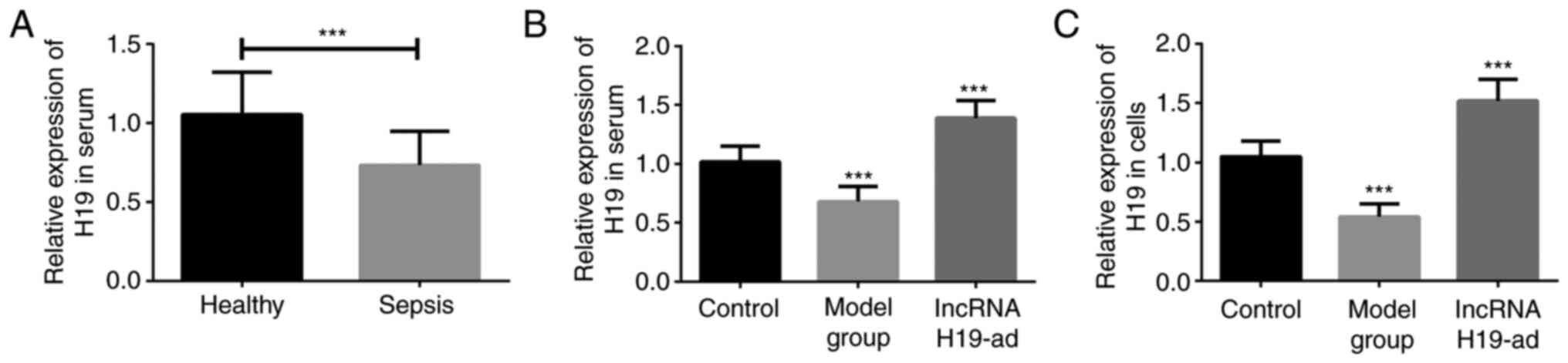
Firstly, the serum samples of 85 septic patients and
76 healthy individuals were collected for lncRNA H19 quantification
with qPCR. Serum lncRNA H19 in septic patients was significantly
lower than that in healthy individuals (Fig. 1A). Subsequently, CLP was performed
on rats to construct septic models. qPCR quantified the expression
of lncRNA H19, and the results revealed that it was downregulated
in the serum and pulmonary tissue of septic rats (model group)
compared with the control group (Fig.
1B and C). The aforementioned
results suggested a close association between lncRNA H19 and
sepsis.
Clinical value of lncRNA H19
Correlations between lncRNA H19 and general data of
septic patients were statistically analyzed. lncRNA H19 was not
revealed to be correlated to sex and body mass index (BMI), but was
correlated to age, history of alcoholism, smoking, white blood cell
(WBC), procalcitonin (PCT), sequential organ failure assessment
(SOFA) and acute physiology and chronic health evaluation II
(APACHEII) scores, as well as sepsis-induced ALI (Table I). In addition, lncRNA H19 was
negatively correlated to miR-152-3p. Therefore, lncRNA H19 may be a
biomarker of sepsis.
 | Table ICorrelation between general data and
H19 (n=85). |
Table I
Correlation between general data and
H19 (n=85).
| Characteristics | n (%) or mean ±
SD | H19 | t/r | P-value |
|---|
| Sex | | | 1.851 | 0.0677 |
|
Male | 57 (67.06) | 0.75±0.13 | | |
|
Female | 28 (32.94) | 0.69±0.16 | | |
| Age | 72.74±6.68 | 0.73±0.21 | 0.784 | 0.0026 |
| BMI | 20.26±1.01 | 0.73±0.21 | 0.117 | 0.2871 |
| History of
alcoholism | | | 2.660 | 0.0094 |
|
Yes | 61 (71.76) | 0.76±0.19 | | |
|
No | 24 (28.24) | 0.65±0.11 | | |
| Smoking
history | | | 3.115 | 0.0025 |
|
Yes | 54 (63.53) | 0.77±0.17 | | |
|
No | 31 (36.47) | 0.66±0.13 | | |
| WBC
(109/l) | 13.96±4.56 | 0.73±0.21 | -0.739 | <0.0001 |
| MDA (nmol/ml) | 16.95±4.66 | 0.73±0.21 | -0.759 | <0.0001 |
| SOD (kU/l) | 41.86±9.26 | 0.73±0.21 | -0.794 | <0.0001 |
| PCT (ng/ml) | 9.12±2.53 | 0.73±0.21 | -0.647 | <0.0001 |
| SOFA | 8.57±2.34 | 0.73±0.21 | -0.713 | 0.0146 |
| APACHEII | 16.98±4.42 | 0.73±0.21 | -0.687 | 0.0069 |
| Sepsis type | | | 2.510 | 0.0140 |
| Sepsis without
ALI | 36 | 0.71±0.18 | | |
| Sepsis with
ALI | 49 | 0.69±0.15 | | |
| miR-152 | 1.36±0.16 | 0.73±0.21 | -0.718 | 0.0125 |
ROC curve that was employed to assess the diagnostic
value of lncRNA H19 demonstrated that the area under the curve
(AUC) of lncRNA H19 for early diagnosis of sepsis was 0.8197 (95%
CI, 0.77 to 0.91), while that for diagnosis of sepsis patients with
ALI and without ALI was 0.9141 (95% CI, 0.87 to 0.96) and 0.8399
(95% CI, 0.77 to 0.91), respectively (Fig. 2; Table
II). The outcomes indicated the significant value of lncRNA H19
for early diagnosis of sepsis.
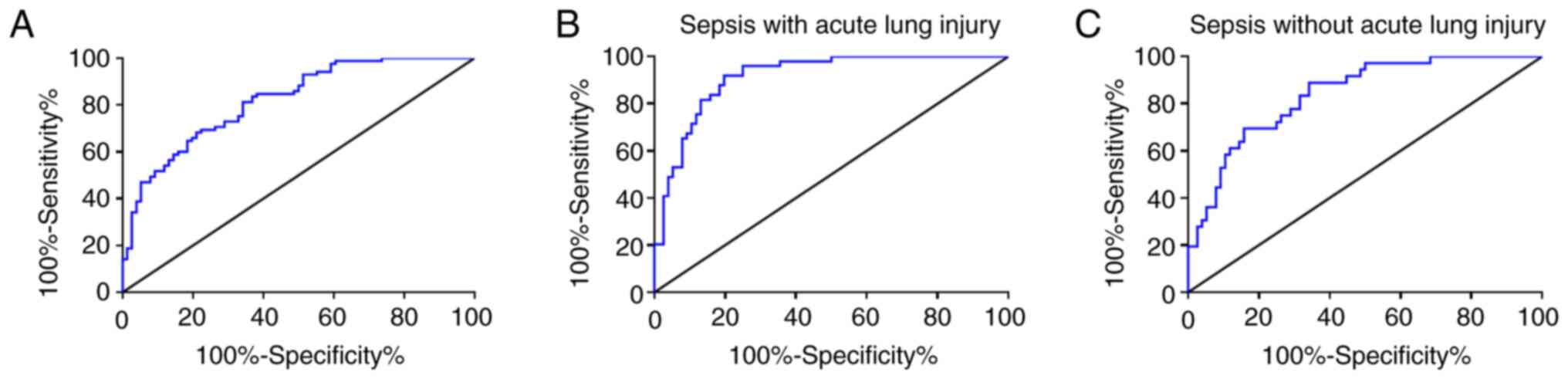 | Figure 2ROC analysis of lncRNA H19 for early
diagnosis of sepsis. (A) lncRNA H19 for early diagnosis of sepsis,
n=85; AUC=0.8197; 95% CI, 0.77 to 0.91. (B) lncRNA H19 for
diagnosis of septic patients with ALI, n=49; AUC=0.9141; 95% CI,
0.87 to 0.96. (C) lncRNA H19 for diagnosis of septic patients
without ALI, n=36; AUC=0.8399; 95% CI, 0.77 to 0.91. ROC, receiver
operating characteristic; lncRNA, long non-coding RNA; ALI, acute
lung injury; AUC, area under the curve; CI, confidence
interval. |
 | Table IIROC curve of lncRNA H19. |
Table II
ROC curve of lncRNA H19.
| Patients | AUC | SE | 95% CI | P-value |
|---|
| Sepsis without
ALI | 0.8399 | 0.0377 | 0.77-0.91 | <0.0001 |
| Sepsis with
ALI | 0.9141 | 0.0247 | 0.87-0.96 | <0.0001 |
| Sepsis | 0.8197 | 0.0322 | 0.76-0.88 | <0.0001 |
Upregulation of lncRNA H19 reduces
inflammation and pulmonary apoptosis in septic rats
Caspase-3, caspase-9, Bax, and Bcl-2 in pulmonary
tissue were detected to investigate the effects of lncRNA H19 on
sepsis. Lung injury is characterized by pulmonary apoptosis, and
the increase of pro-apoptotic proteins (caspase-3 and caspase-9)
and the decrease of anti-apoptotic protein (Bcl-2) are capable of
inducing apoptosis directly (27).
Therefore, the aforementioned four proteins were indicators in the
evaluation of pulmonary apoptosis. Caspase-3, caspase-9, and Bax
were increased while Bcl-2 was decreased in septic rats compared to
the control group. In addition, upregulation of lncRNA H19
suppressedcaspase-3, caspase-9 and Bax and increased Bcl-2
expression compared to the model group (Fig. 3A and D). As a systemic inflammatory response
syndrome, sepsis inevitably causes changes in inflammatory factors
(TNF-α, IL-6 and IL-17) (28-30),
thus the levels of these factors were determined as well to
evaluate the inflammatory response. TNF-α, IL-6, and IL-17 were
increased in the pulmonary tissue and serum of septic rats compared
to the control group, while upregulation of lncRNA H19 reduced
their levels compared with the model group (Fig. 3B and C). These results indicated that
upregulation of lncRNA H19 reduced pulmonary inflammation and
apoptosis in septic rats.
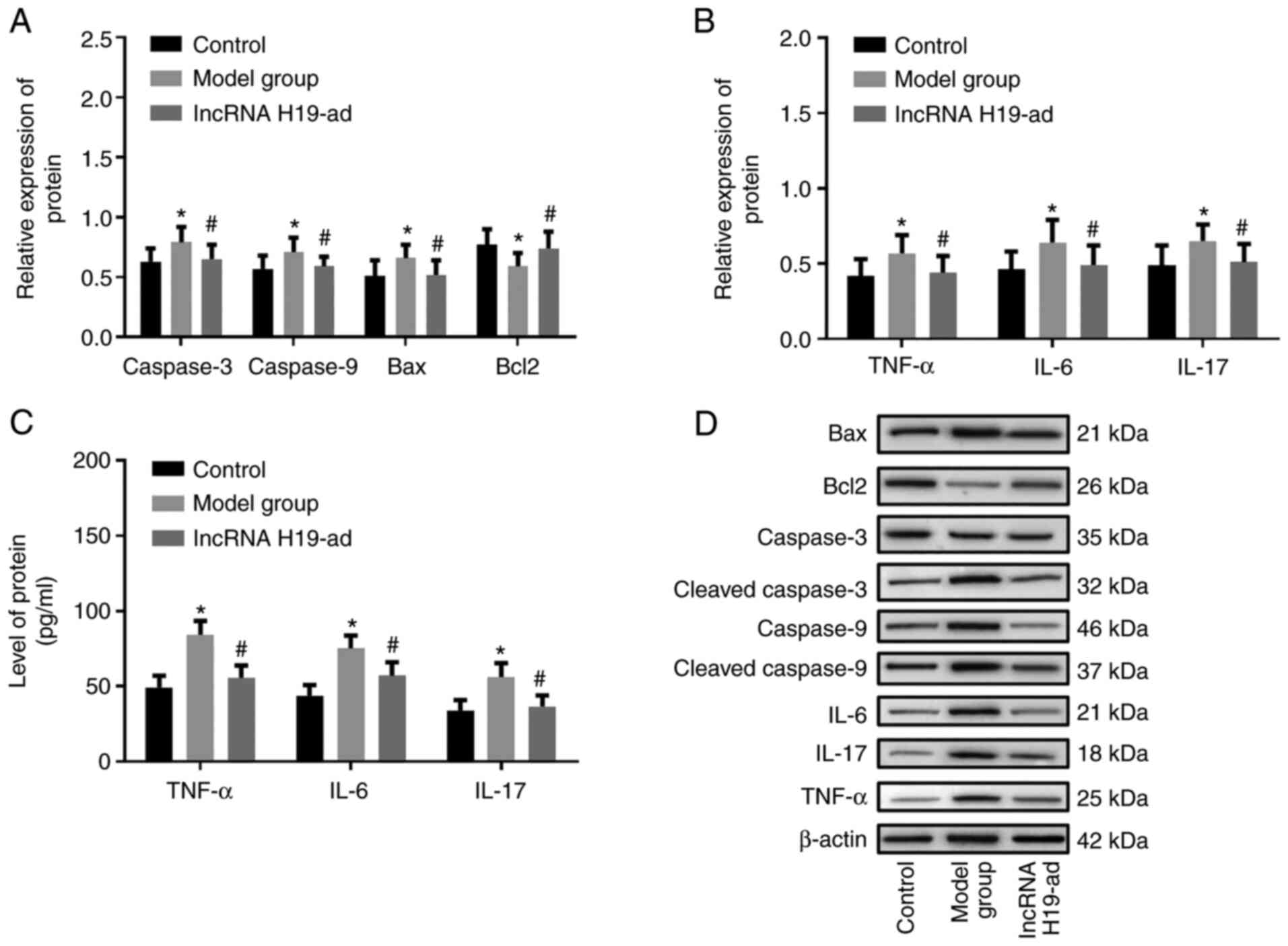 | Figure 3Upregulation of lncRNA H19 reduces
pulmonary inflammation and apoptosis in septic rats. (A) lncRNA H19
downregulated caspase-3, caspase-9, Bax and upregulated Bcl-2. (B)
Upregulation of lncRNA H19 reduced TNF-α, IL-6 and IL-17 in
pulmonary tissue. (C) Upregulation of lncRNA H19 reduced TNF-α,
IL-6 and IL-17 in serum. (D) Western blotting of caspase-3,
caspase-9, Bax, Bcl-2, TNF-α, IL-6 and IL-17. *P<0.05
vs. the control group; #P<0.05 vs. the model group.
lncRNA, long non-coding RNA; Bax, BCL2-associated X; Bcl-2, B-cell
lymphoma-2; TNF-α, tumor necrosis factor-α; IL, interleukin. |
Upregulation of lncRNA H19 improves
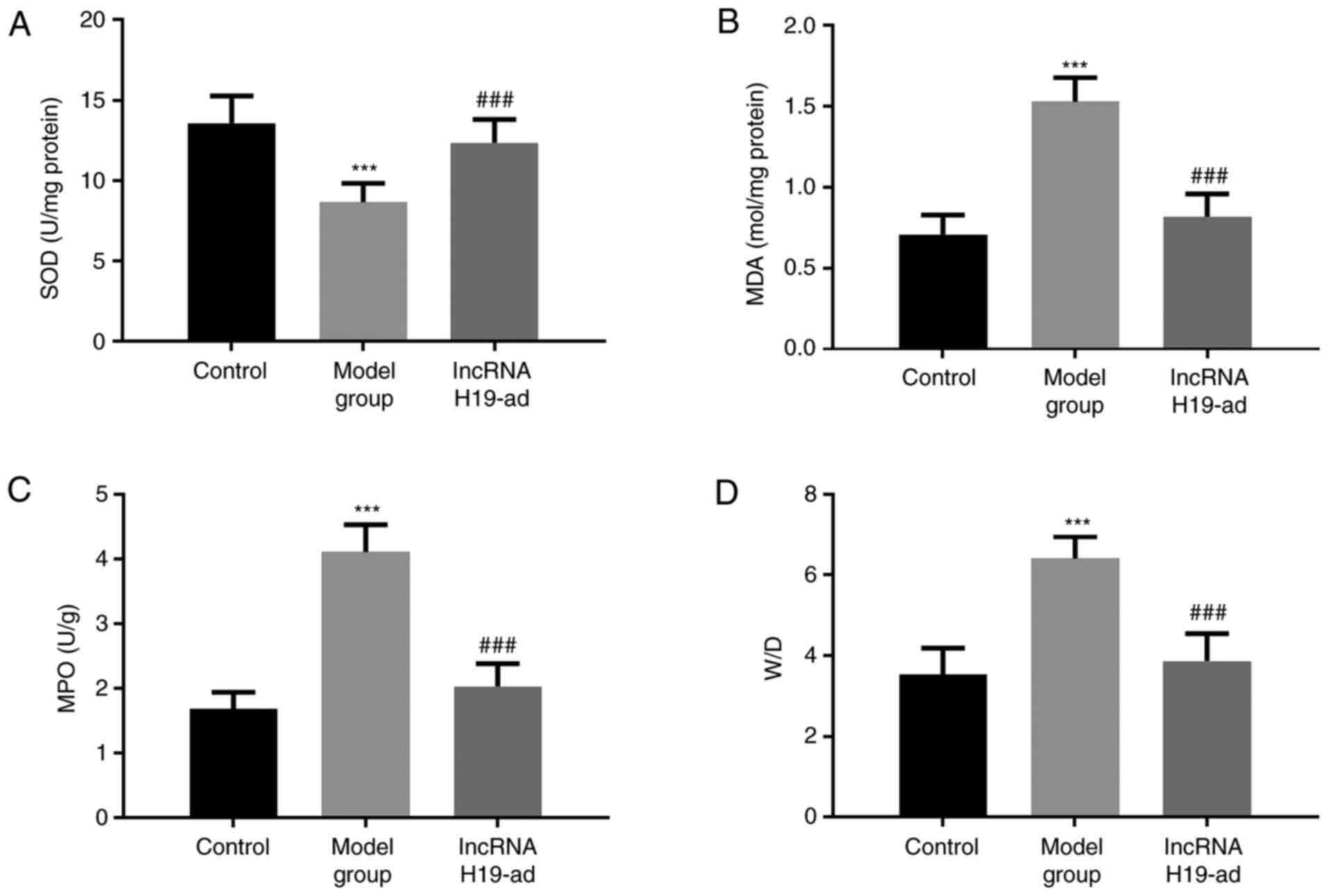
pulmonary function in septic rats
The effects of lncRNA H19 on pulmonary function were
evaluated by W/D, MPO, SOD, and MDA (Fig. 4). Compared with the control group,
SOD was decreased while MDA, MPO and W/D were increased in septic
rats. Compared with model group, upregulation of lncRNA H19
increased SOD and decreased MDA, MPO and W/D. Therefore,
upregulation of lncRNA H19 improved pulmonary function in septic
rats.
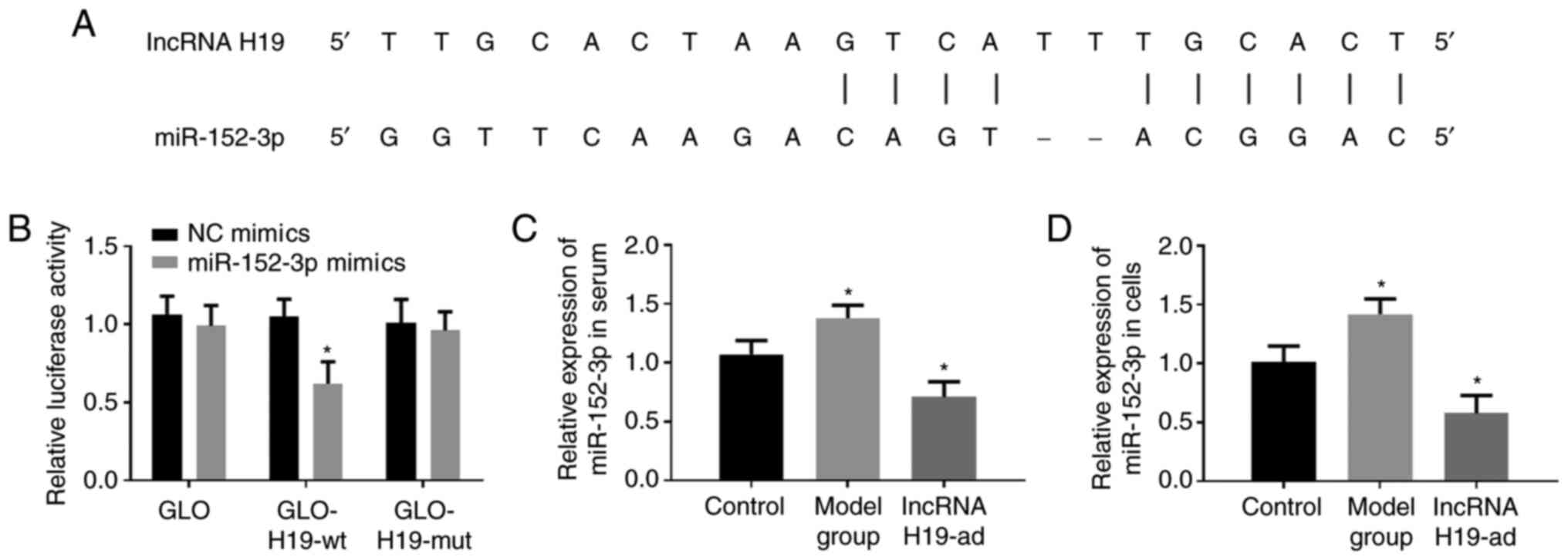
miR-152-3p is the target gene of
lncRNA H19
starBase2.0 predicted that lncRNA H19 was capable of
binding to miR-152-3p (Fig. 5A).
Moreover, upregulation of lncRNA H19 suppressed the expression of
miR-152-3p in serum and tissue (Fig.
5C and D). Therefore, it was
theorized that miR-152-3p could bind to and negatively regulate
miR-152. Dual-luciferase reporter gene assay was adopted to verify
the authenticity of the binding sites. The results revealed that
the co-transfection of lncRNA H19-wt and miR-152-3p-mimics
decreased the luciferase activity, while no significant changes
were observed by other co-transfection combinations (Fig. 5B). The aforementioned results
indicated that lncRNA H19 downregulated miR-152-3p by binding to
it.
Discussion
How to perform accurate early diagnosis of sepsis, a
thorny public health issue, is the focus of present sepsis
research. Due to their highly active participation in the
progression of sepsis, non-coding RNAs have become a research
hotspot. Thus, seeking biomarkers for early diagnosis of sepsis
from non-coding RNAs has also been considered a new approach to
challenge sepsis. Fang et al observed that lncRNA H19 was
downregulated in the peripheral blood of septic patients (21), and the aberrant downregulation was
also reported in our study. Therefore, it is theorized that the
downregulated lncRNA H19 may have potential value for early
diagnosis of sepsis and participate in its process.
By analyzing the relationship between the general
data and lncRNA H19, the close association between downregulated
lncRNA H19 and sepsis was revealed. In addition, ROC curve
demonstrated that the AUC of lncRNA H19 for the diagnosis of sepsis
was 0.8197 (95% CI, 0.76 to 0.88, P<0.0001). Therefore, lncRNA
H19 was revealed as a biomarker of sepsis and achieved high early
diagnostic value.
In addition, the association between lncRNA H19 and
sepsis was also explored by upregulating lncRNA H19. Pulmonary
apoptosis and inflammatory responses are pathological
manifestations of sepsis-induced ALI (27), therefore, studying the effect of
lncRNA H19 on both could assess its effects on this disease. It was
determined that upregulated lncRNA H19 not only alleviated
inflammatory responses in septic rats by downregulating TNF-α, IL-6
and IL-17, but also inhibited pulmonary apoptosis by downregulating
caspase-3, caspase-9, and Bax. In addition, upregulated lncRNA H19
also improved pulmonary function (W/D, MPO, SOD, and MDA). It is
worth mentioning that upregulation of lncRNA H19 triggered
downregulation of miR-152-3p in pulmonary tissue. starBase
predicted that lncRNA H19 was capable of binding to miR-152-3p.
Dual-luciferase reporter gene assay demonstrated that
co-transfection of lncRNA H19-wt and miR-152-3p significantly
decreased luciferase activity, indicating that lncRNA H19
alleviated sepsis-induced ALI by downregulating miR152.
miR-152-3p is located on human chromosome 11 with a
length of approximately 87bp. The most common biological function
of miR-152-3p is to reduce mRNA stability at a post-transcriptional
level (22). Numerous studies have
revealed that it participates in the pathological pathways of a
number of diseases. In liver-related diseases, miR-152-3p has been
revealed to affect liver cell viability and liver fibrosis through
cyclin-dependent kinase 8 (CDK8) and GLI family zinc finger 3
(GLI3) (31,32). Downregulated miR-152-3p promoted the
growth of liver cancer cells (33).
In addition, aberrantly expressed miR-152-3p has also been revealed
to be associated with prostate cancer, keloids, and sepsis
(34-36).
According to our findings, as a downstream target gene of lncRNA
H19, miR-152-3p may mediate the regulation of lncRNA H19 on
pulmonary apoptosis and inflammation. Thus, the lncRNA
H19/miR-152-3p axis exhibits potential therapeutic value for
sepsis, and upregulating lncRNA H19 may alleviate sepsis-induced
ALI.
Our study explored the relationship between lncRNA
H19 and sepsis in terms of diagnostic value and molecular biology,
and reported that lncRNA H19 was valuable for early diagnosis of
sepsis and was capable of inhibiting its progression via
miR-152-3p. In the future, the target genes and signaling pathways
located downstream of miR-152-3p will be investigated and the
molecular network of lncRNA H19 in sepsis will be improved, in
order to provide more systematic and reliable references for sepsis
treatment. There are several limitations in the present study, such
as failure to further detect more indicators and lack of survival
analyses due to limited time and equipment support. We will address
these limitations in our future work.
In conclusion, lncRNA H19 was revealed as a
potential early diagnostic biomarker for sepsis. Moreover, it
alleviated sepsis-induced ALI and reduced inflammation in rats by
downregulating miR-152-3p, which revealed that upregulation of
lncRNA H19 may hinder the progression of sepsis.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Natural Science Research
Projects of Jiangsu Higher Education (grant no. 19KJB320001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ designed the study and drafted the manuscript.
LS, MZ and LH were responsible for the collection and analysis of
the experimental data. HC revised the manuscript critically for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Second Affiliated Hospital of Nanjing Medical University
(Nanjing, China). Patients who participated in this research,
signed the informed consent and had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lever A and Mackenzie I: Sepsis:
Definition, epidemiology, and diagnosis. BMJ. 335:879–883.
2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Martin-Loeches I, Guia MC, Vallecoccia MS,
Suarez D, Ibarz M, Irazabal M, Ferrer R and Artigas A: Risk factors
for mortality in elderly and very elderly critically ill patients
with sepsis: A prospective, observational, multicenter cohort
study. Ann Intensive Care. 9(26)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gómez H and Kellum JA: Sepsis-induced
acute kidney injury. Curr Opin Crit Care. 22:546–553.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sun Y, Cai Y and Zang QS: Cardiac
autophagy in sepsis. Cells. 8(141)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shao IY, Elkind MSV and Boehme AK: Risk
factors for stroke in patients with sepsis and bloodstream
infections. Stroke. 50:1046–1051. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen X, Wang T, Song L and Liu X:
Activation of multiple toll-like receptors serves different roles
in sepsis-induced acute lung injury. Exp Ther Med. 18:443–450.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wu J, Yan X and Jin G: Ulinastatin
protects rats from sepsis-induced acute lung injury by suppressing
the JAK-STAT3 pathway. J Cell Biochem. 120:2554–2559.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zheng H, Liang W, He W, Huang C, Chen Q,
Yi H, Long L, Deng Y and Zeng M: Ghrelin attenuates sepsis-induced
acute lung injury by inhibiting the NF-κB, iNOS, and Akt signaling
in alveolar macrophages. Am J Physiol Lung Cell Mol Physiol.
317:L381–L391. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Park I, Kim M, Choe K, Song E, Seo H,
Hwang Y, Ahn J, Lee SH, Lee JH, Jo YH, et al: Neutrophils disturb
pulmonary microcirculation in sepsis-induced acute lung injury. Eur
Respir J. 53(1800786)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zou Y, Bao S, Wang F, Guo L, Zhu J, Wang
J, Deng X and Li J: FN14 blockade on pulmonary microvascular
endothelial cells improves the outcome of sepsis-induced acute lung
injury. Shock. 49:213–220. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Aziz M, Ode Y, Zhou M, Ochani M, Holodick
NE, Rothstein TL and Wang P: B-1a cells protect mice from
sepsis-induced acute lung injury. Mol Med. 24(26)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang J, Gong S, Wang F, Niu M, Wei G, He
Z, Gu T, Jiang Y, Liu A and Chen P: Granisetron protects
polymicrobial sepsis-induced acute lung injury in mice. Biochem
Biophys Res Commun. 508:1004–1010. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Englert JA, Bobba C and Baron RM:
Integrating molecular pathogenesis and clinical translation in
sepsis-induced acute respiratory distress syndrome. JCI Insight.
4(e124061)2019.PubMed/NCBI View Article : Google Scholar : (Online ahead of
print).
|
|
14
|
Delahanty RJ, Alvarez J, Flynn LM, Sherwin
RL and Jones SS: Development and evaluation of a machine learning
model for the early identification of patients at risk for sepsis.
Ann Emerg Med. 73:334–344. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rezaei M, Mokhtari MJ, Bayat M, Safari A,
Dianatpuor M, Tabrizi R, Asadabadi T and Borhani-Haghighi A: Long
non-coding RNA H19 expression and functional polymorphism rs217727
are linked to increased ischemic stroke risk. BMC Neurol.
21(54)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wan P, Su W, Zhang Y, Li Z, Deng C, Li J,
Jiang N, Huang S, Long E and Zhuo Y: lncRNA H19 initiates
microglial pyroptosis and neuronal death in retinal
ischemia/reperfusion injury. Cell Death Differ. 27:176–191.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Thomas AA, Biswas S, Feng B, Chen S,
Gonder J and Chakrabarti S: lncRNA H19 prevents
endothelial-mesenchymal transition in diabetic retinopathy.
Diabetologia. 62:517–530. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bitarafan S, Yari M, Broumand MA,
Ghaderian SM, Rahimi M, Mirfakhraie R, Azizi F and Omrani MD:
Association of increased levels of lncRNA H19 in PBMCs with risk of
coronary artery disease. Cell J. 20:564–568. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen MJ, Deng J, Chen C, Hu W, Yuan YC and
Xia ZK: lncRNA H19 promotes epithelial mesenchymal transition and
metastasis of esophageal cancer via STAT3/EZH2 axis. Int J Biochem
Cell Biol. 113:27–36. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang X, Cheng Z, Dai L, Jiang T, Jia L,
Jing X, An L, Wang H and Liu M: Knockdown of long noncoding RNA H19
represses the progress of pulmonary fibrosis through the
transforming growth factor β/Smad3 pathway by regulating miR-140.
Mol Cell Biol. 39:e00143–19. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fang Y, Hu J, Wang Z, Zong H, Zhang L,
Zhang R and Sun L: lncRNA H19 functions as an Aquaporin 1
competitive endogenous RNA to regulate microRNA-874 expression in
LPS sepsis. Biomed Pharmacother. 105:1183–1191. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zheng JF, Guo NH, Zi FM and Cheng J: Long
non-coding RNA H19 promotes tumorigenesis of multiple myeloma by
activating BRD4 signaling by targeting miR-152-3p. Mol Cell Biol.
40:e00382–19. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu XW, Ma T, Cai Q, Wang L, Song HW and
Liu Z: Elevation of serum PARK7 and IL-8 levels is associated with
acute lung injury in patients with severe sepsis/septic shock. J
Intensive Care Med. 34:662–668. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ferreira FL, Bota DP, Bross A, Mélot C and
Vincent JL: Serial evaluation of the SOFA score to predict outcome
in critically ill patients. JAMA. 286:1754–1758. 2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42:D92–D97. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xie W, Lu Q, Wang K, Lu J, Gu X, Zhu D,
Liu F and Guo Z: miR-34b-5p inhibition attenuates lung inflammation
and apoptosis in an LPS-induced acute lung injury mouse model by
targeting progranulin. J Cell Physiol. 233:6615–6631.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Murray A, Gow AJ, Venosa A, Andres J,
Malaviya R, Adler D, Yurkow E, Laskin JD and Laskin DL: Assessment
of mustard vesicant lung injury and anti-TNF-α efficacy in rodents
using live-animal imaging. Ann N Y Acad Sci. 1480:246–256.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chepurnova DA, Samoilova EV, Anisimov AA,
Verin AD and Korotaeva AA: Compounds of IL-6 receptor complex
during acute lung injury. Bull Exp Biol Med. 164:609–611.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gouda MM and Bhandary YP: Acute lung
injury: IL-17A-mediated inflammatory pathway and its regulation by
curcumin. Inflammation. 42:1160–1169. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yin T, Liu MM, Jin RT, Kong J, Wang SH and
Sun WB: miR-152-3p modulates hepatic carcinogenesis by targeting
cyclin-dependent kinase 8. Pathol Res Pract.
215(152406)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li L, Zhang L, Zhao X, Cao J, Li J and Chu
G: Downregulation of miR-152 contributes to the progression of
liver fibrosis via targeting Gli3 in vivo and in vitro. Exp Ther
Med. 18:425–434. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang WL, Yu DJ and Zhong M: lncRNA HAGLROS
accelerates the progression of lung carcinoma via sponging
microRNA-152. Eur Rev Med Pharmacol Sci. 23:6531–6538.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Moya L, Meijer J, Schubert S, Matin F and
Batra J: Assessment of miR-98-5p, miR-152-3p, miR-326 and miR-4289
expression as biomarker for prostate cancer diagnosis. Int J Mol
Sci. 20(1154)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang R, Bai Z, Wen X, Du H, Zhou L, Tang
Z, Yang Z and Ma W: miR-152-3p regulates cell proliferation,
invasion and extracellular matrix expression through by targeting
FOXF1 in keloid fibroblasts. Life Sci. 234(116779)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dong L, Li H, Zhang S and Yang G: miR-148
family members are putative biomarkers for sepsis. Mol Med Rep.
19:5133–5141. 2019.PubMed/NCBI View Article : Google Scholar
|