1. Introduction
Various nanomaterials have been linked to endocrine
disruptors or endocrine-disrupting chemicals (EDCs) which represent
a large, heterogeneous, yet incompletely understood group of
structures acting on normal and pathological body pathways such as
hormonal production, secretion, transport, activation/inactivation,
receptor binding and feedback regulation (1-3).
This is an ongoing topic of discussion due to a high variety of
nanotechnology-related molecules that mimic, block or antagonize
the organs or tissues of humans and/or animals and thus an increase
or a deficiency of physiological organism pathways of regulation is
identified (1-3).
EDCs are part of pollutants that dysregulate the functions of cells
representing an emerging public health issue (1-3).
However, a large amount of data remain a matter of debate or remain
incompletely known while a close collaboration of biotechnology
specialists with clinicians and researchers of different medical
fields is essential on this particular topic (1-4).
Other areas that actually have a lack of feasible information are
the methods and protocols existing thus far to routinely assess the
effect of EDCs on human and animal bodies (5). It only takes a small amount of a
certain EDC to alter an entire signal transduction network, and
methods used vary from traditional well-known instruments such as
enzyme-linked immunosorbent assay (ELISA) or high-performance
liquid chromatography (HPLC) to modern electrochemical detectors
based on biosensors using different nanomaterials (5).
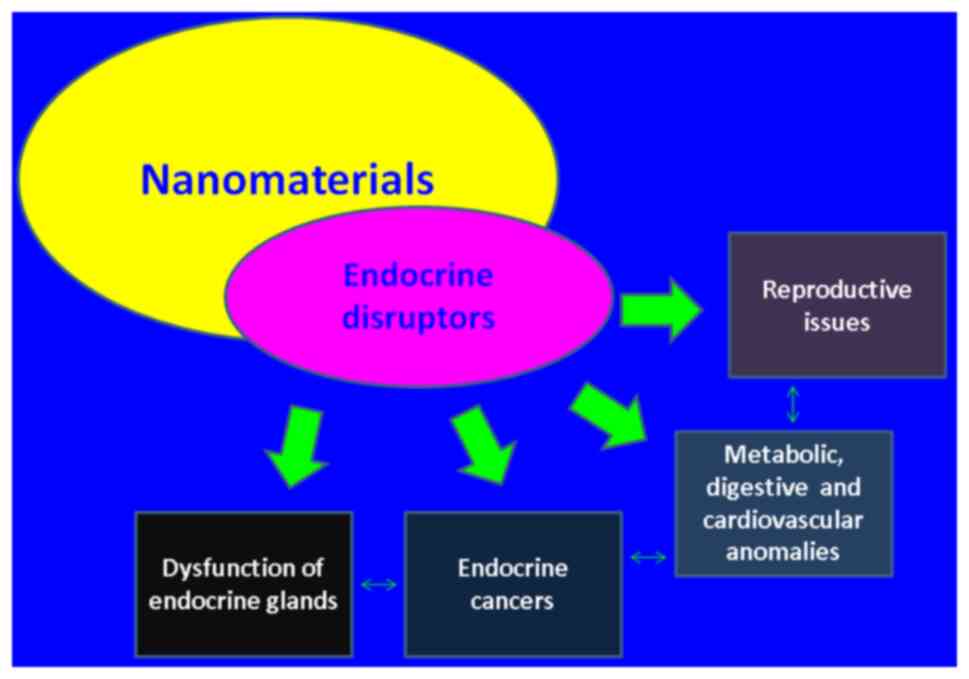
An extensive number of EDC-associated disorders vary
from digestive and endocrine cancers, inflammatory conditions to
autoimmune diseases, and DM but mostly the mechanisms coexist at
multiple levels. For instance, EDC-emerged inflammatory and
destructive antibodies in the pathogenesis of neoplasia,
infertility as well as DM are to mention but a few (1-3),
(Fig. 1). In fact, EDCs regulate
the microenvironment of both estrogenic and androgenic receptors
and the immune system activation through signal proteins such as
receptor for activated C kinase 1 (RACK1), a promoter of
carcinogenesis (1). Another largely
described family of EDCs acting as carcinogenic promoters is
represented by bisphenols which are identified in various products;
they impair multiple biological functions such as hormonal
secretion or cell growth, including steroid receptors (2). By contrast, enzymatic nanoreactors
such as virus-like particles (VLP) identify the EDC-induced damage
at the level of natural defense chains including activity of
cytochrome P450 enzymes involved in oxidative stress and glucose
metabolism (3). Exposure to
industry-derived palladium nanoparticles (Pd-NPs) reveals EDC
potential, particularly by impairing the gonadal axes (3). Wistar rats exposed to Pd-NPs for a
prolonged period of time in small amounts had higher levels of
follicle stimulating hormone (FSH), as negative feedback to ovarian
failure which decreases the reproductive potential (4). In general, a need for interventional
strategies of biophysics detection and medical risk evaluation of
nanomolecular-based EDCs is required knowing that actually most
advanced assessment methods are also based on nanotechnology
(1-5).
By contrast, various applications of nanoparticles
are currently under investigation, since the delivery of useful
drugs, particularly insulin for DM is essential for individuals
with insulin deficiency.
2. DM: Past and future
The present review aimed to introduce and assess
different plasticizers and nanomaterials with potential
applications in the everyday life of diabetic patients or
structures acting similarly to EDCs in association to human and
animal organisms, particularly at the level of glucose metabolism
impairment such as in DM. This a narrative review; 50 studies are
cited, published between 2014 and 2021. Full length English
articles were included. A comprehensive PubMed search was conducted
with the following search terms: ‘Endocrine disrupting chemicals’,
‘diabetes mellitus’, ‘nanomaterials’, and ‘plasticizers’ in
different combinations. The selection of cited studies was based on
the most relevant articles which highlight key transdisciplinary
information, combing through both clinical points and
chemical/biophysics features. Each key point is identified and
presented in the following subsections.
DM: A global concern
Glucose metabolism anomalies vary from impaired
glucose tolerance, impaired fasting glucose to frank DM of
different types such as type 1, type 2, and secondary type,
associated to endocrine conditions such as acromegaly or Cushing
syndrome (6). A significant number
of complications are associated with the disease including
cardiovascular risk, neurological conditions (such as stroke and
neuropathy), kidney failure, obesity, high blood pressure,
polycystic ovary syndrome, infertility, hypogonadism, sarcopenia,
higher risk of infections, certain malignancies, depression,
hypovitaminosis D, osteoporosis in addition to overall reduced
quality of life and increased mortality (6,7).
Diabetes is closely associated with obesity contributing to the
concept of ‘diabesity’ which, at least in pre-pandemic days, was
considered the true global pandemic of the world (8,9).
Diabetes is considered the true pandemic of the modern era, at
least before the Corona Virus Disease-19 (COVID-19) pandemic wave
that has affected the population worldwide (8,9).
The increasing incidence of DM over the last
decades, still highly underestimated in the general population, has
generated increased economic and social burden, thus rendering it
important to study the potential molecules involved in its
pathogeny and new efficient treatment options (8,9). A
total of 1/11 adults is diagnosed with DM (90% have type 2 DM)
(6,8,9).
Globally, in 2000 there were an estimated 151 million diabetic
individuals, while the prediction for 2030 was 324-366 million
individuals, depending on the source, but this was actually
demonstrated to be an underestimation since in 2015 there were
already 415 million individuals diagnosed with the condition
(6,8,9).
Multiple factors play a certain role in the
pathophysiological mechanisms of DM including lifestyle choices
(sedentary habits and junk food consumption), genetic
susceptibility (such as genes involved in glucose metabolism,
insulin control and secretion), chronic inflammation, oxidative
stress, growth hormone and cortisol axis anomalies which contribute
to insulin resistance and are a necessary step in type 2 DM
development (6,8,9). EDCs
have been demonstrated to be involved in the occurrence of DM based
on experimental and clinical studies (8-10).
For instance, bisphenol A (BPA) induces excessive insulin secretion
impairing the communication among fat-muscle-liver-pancreas
(10). Other molecules may display
a similar effect including pesticides, dioxins, and aromatic
polycyclic hydrocarbides (10).
Certain EDCs are nanomolecules with varying applications from
industry or medical fields (10).
Diabetes and nanotechnology
Nanotechnology has been linked to diabetes, either
as unwelcome side effects of nanomolecules that cause glucose
profile damage, or as applications, for instance, of insulin
delivery devices into the body or nanoparticles acting as hydrogels
that promote wound healing based on attached bioactive molecules
such as growth factors or proteins (11). Transdisciplinary nanotechnology
involving diabetes is expansively developing due to the major
epidemiological effect in the general population and it can be
applied through the use of traditional protocols of diagnosis and
therapy and also by using advanced computer-assisted drug design
(CADD) chemoinformatics instruments (12). However, numerous questions remain
unanswered on this topic, including the exact relationship between
endocrine disruptors and their effects on humans and animals, the
exposure time required in order to obtain a particular effect and
the interassociations between genetic background and environmental
exposure. In addition, the level of evidence is low in multiple
areas, particularly in type 2 DM at various ages, in relationship
to a certain anti-diabetic regime of medications, etc. Potential
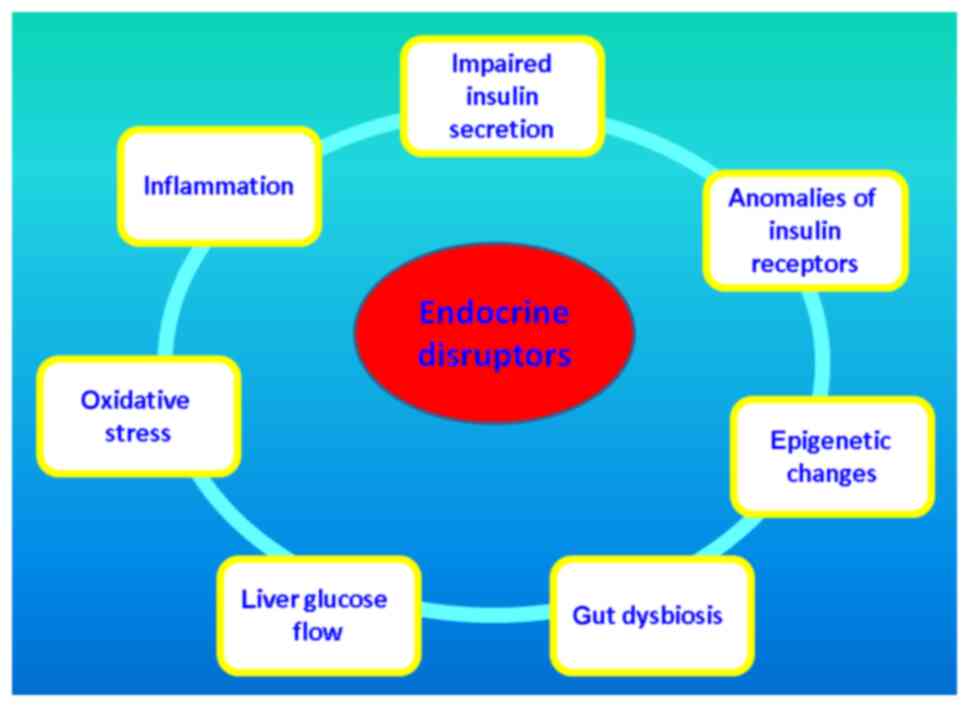
mechanisms of nanomaterial-based EDCs which damage glucose
metabolism are introduced in Fig.
2.
Selenium nanoparticles (SeNPs) and
insulin delivery
Among inorganic nanoparticles, selenium
incorporation represents an extension of their well-known standards
regarding reduced toxicity, increased bioactivity and improved
targeting (13). SeNPs may improve
the negative aspects of selenium delivery which include a limited
and narrowed therapeutic window and easily achievable toxicity
levels (13). However, this
essential trace element is very useful in the human organism since
it plays a major role in the active core of multiple enzymes called
selenoproteins which are mostly associated with oxido-reductase
functions (13). Thus, SeNPs are
currently being explored in applications for conditions related to
chronic exposure of oxidative stress as observed in chronic
inflammation such as cancer or diabetes (13). In addition, SeNPs may serve as
pharmacological carriers in various medical conditions even if the
exact kinetics of such mechanisms remain insufficiently known
(13). An example of this, is the
delivery of oral insulin in type 1 (insulin-dependent DM) or type 2
DM (13,14). Oral insulin has an extremely poor
bioavailability and insulin-loaded SeNPs may overcome the
absorption limits releasing a controllable amount of the hormone
(~9% bioavailability of subcutaneous insulin in murine experiments)
with high stability in the digestive environment (15).
Gold nanoparticles and insulin
delivery
Nanoparticles containing gold have the advantage of
reduced toxicity and increased surface area which render them
useful in drug delivery such as in insulin for glucose control or
growth factors for diabetic wound healing using various
nanotechnology-based devices (16).
This type of nanoparticle is listed, as well as SeNPs, among future
insulin nanocarriers, a large family that also includes liposomes,
dendrimers, niosomes, micelles, which are currently under
evaluation indicating a promising and remarkable option in the
management of DM (17). For
instance, a study from 2020 used gold nanoparticles based on acid
chloroauric reduction by modified apple polysaccharide (MAP) in
association with insulin, for oral delivery in rats with
streptozotocin-induced DM (18).
Nanotechnology-based insulin caused a 3.36-fold decrease in
glycemic levels within 240 min compared with oral non-conjugated
insulin, an effect that was consistent after 28 days in improvement
of other glucose-related parameters such as blood lipids and the
weight of rats (18).
Glucose-responsive insulin delivery
devices
Systems which are able to achieve a constant level
of glycemia by regulating insulin release appear to be more
promising options than those that exist to date, as far as DM
therapy is concerned (19). Such a
device is mesoporous silica nanoparticle (MSN)-based with
self-regulation (19). Insulin is
attached to the channels of MSNs via adsorption and it is released
depending on the needs of diabetic mice during a 12-h interval
(19). Integrating sensors of
real-time blood glucose (such as Alizarin complexone) to an
MSM-insulin system helps the adequate release of the hypoglycemic
hormone (20).
Glucagon-like peptide (GLP) analogues
and nanotechnology
GLP analogues (such as GLP-1) represent a new
generation of hypoglycemic agents with real benefits not only to
glucose control, but also to reduction of diabetes-associated
cardiovascular risk (21). This
class of drugs has a similar issue as human insulin, which is a low
bioavailability if the oral route is used, thus the need for new
means of delivery (21).
Nanotechnology has taken an important step in resolving this matter
by introducing carrier nanoparticles that increase bioavailability
of GLP-1 analogues (22). For
instance, cyclodextrin and liraglutide (a GLP-1 analogue)
containing nanocarriers exhibited a high stability in the
intestinal site offering a protection of GLP-1 analogue from enzyme
degradation up to 4 h in murine experiments (22).
Diabetes wound healing and
nanotechnology
DM is associated with a very high risk of wounds,
skin infections and foot ulcers, with poor healing due to a
multifactorial etiology (11).
Nanotechnology devices that deliver wound healing drugs are based
on nanoparticles/hydrogels in addition to bioactive pharmacological
products (11). For instance, a
recent in vivo study which incorporated silver ions to a
chitosan hydrogel to deliver epidermal growth factor with
antibacterial and cell growth effects in diabetic mice, translated
into encouraging clinical effects such as re-epithelization of the
area of the ulcer and increased collagen deposits in the same foot
region (23). An alternative for
chronic skin diabetic lesions is a system containing hyaluronic
acid in combination with oxidized hydroxymethyl propyl cellulose
and oridonin-loaded alginate microspheres, which promotes
fibroblastogenesis and angiogenesis and reduces inflammation by
inhibiting factors such as interleukin-6(24).
Silicon dioxide nanoparticles
(SiO2NPs) and insulin resistance
SiO2NPs, food industry-related EDCs, are studied in
murine experiments to induce insulin resistance (25). Oral intake of SiO2NPs (on doses
calculated based on the body weight of mice) induces hyperglycemia
after they are absorbed and act through glucose transporters and
insulin receptors at the level of organs involved in insulin
activity such as the liver, or insulin secretion such as the
pancreas (25). The underlying
mechanisms include upregulation of genes encoding reactive oxygen
species (ROS) production via increasing the level of stress at the
endoplasmic reticulum (ER) (25).
ROS activate the NF-kB pathway leading to increased cytokines that
promote inflammation further inducing insulin resistance due to
serine phosphorylation of insulin receptor substrate 1 (IRS1)
(25).
Gut microbiome as a target of EDCs and
promoter of metabolic anomalies
Previous studies have indicated that gut dysbiosis
is an important source of metabolic anomalies including DM,
obesity, immune and autoimmune conditions as well as cancer
(25,26). Due to the multitude of
microorganisms that inhabit the gut microbiome, this environment is
extremely dynamic and heterogeneous and it is targeted by molecules
originating from outside of the body, including EDCs, which
stimulate the negative effects associated to microorganism
proliferation and virulence, including the production of
lipopolysaccharides or the induction of epigenetic changes in host
human organisms (26).
BPA
BPA is a well-known EDC and certain studies have
revealed that even at nanomolecular doses it induces negative
effects on human and animal organisms (27,28).
BPA is a chemical used worldwide; however, the exposure varies with
regard to the source, environment, time of exposure and age of the
organism (27,28). Daily exposure is mostly associated
to food and drink containers sold on the market, as well as
absorption through the skin or through the maternal placenta or
breast milk which are risk factors for the fetus and for the
newborn baby (27,29). It exerts an estrogen-like profile
but it also induces insulin resistance by impairing peripheral
insulin receptors or it decreases insulin secretion by acting on
insulin-secreting pancreatic β-cells (27,30).
Insulin resistance as well as impaired pancreatic insulin secretion
are key pathogenic elements in type 2 DM (27,31).
BPA may also be a missing element in the complex pathogenesis of
obesity, also with a major epidemiological impact in modern
society, which currently has yet to be completely elucidated
(27,32).
The chemical xenoestrogen, a major component of
multiple plastic products of various domestic and industrial
sources, aggravates glucose control in addition to fatty liver
disease and impairment of hypothalamic pathways involved in the
regulation of energy balance through food intake (33,34).
Other EDCs with central effects are phthalates, biphenyls and
tributyltin (35,36). The effect of epoxy resins and
polycarbonate plasticizers containing BPA may be quantified by
assessment of free BPA levels in urine samples based on different
methods such as solid-phase extraction (37,38).
Higher levels in diabetic patients are an indicator of their role
in the pathogeny of diabetes (39,40).
However, according to current knowledge, DM, as well as obesity are
regarded as pluri-factorial and systemic conditions, and the role
of BPA needs to be integrated in a more complex frame of genetic,
epigenetic and environmental elements (41,42).
Prenatal exposure to phthalates
Phthalates, compounds of flexible plastics, act as
EDCs via their human metabolites such as diethyl phthalate and
derivative monoethyl phthalate (43,44).
It has been indicated that they participate in inducing type 2 DM
as well as gestational DM and weight gain/obesity during pregnancy
(45,46). However, the exact pathogenic
mechanisms remain a matter of debate; for instance, the Center for
the Health Assessment of Mothers and Children of Salinas (CHAMACOS)
cohort study published in 2021 on 415 pregnant women, based on the
assessment of 11 urinary metabolites (using mass spectrometry),
revealed that the direct association with the glucose profile was
not statistically significant, however it was with weight gain
which is an indirect contributor to insulin resistance as a
potential contributor to DM (47).
Murine experiments revealed another mechanism involving
insulin-secreting pancreatic β-cells: Di-n-butyl phthalate
upregulated the expression of phosphorylated signal transducer and
activator of transcription 1 (pSTAT1) which inhibited Forkhead box
protein M1 (FoxM1), as a toxicity mediator of β-cell dysfunction
(48). Additional effects of
plastic bottles and plastic containers of foods and drinks are
transferred to the fetus producing disturbances in embryonic
development and growth restriction, due to the epigenetic role of
phthalates ingested by pregnant women (49). In addition, the phthalate-related
risk is identified later in life; for example, pubertal mice with
type 2 DM displayed neurotoxicity when exposed to oral ingestion of
di-2-ethylhexyl phthalate for three weeks; the presence of glucose
anomalies being prone to further neuronal damage (50).
3. Conclusions
The vast field of plasticizers and nanomolecules
acting as endocrine disruptors is widely linked to the clinical
aspects of DM, a serious condition with a major impact worldwide.
The importance of understanding and using these agents and
applications is reflected in saving numerous human lives.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
PP and ITD revised the manuscript and are the
corresponding authors. MC summarized the literature findings and
wrote the manuscript. MJT, MG, CNC, AT, AGN, LMG and OAD revised
the literature data. MG, CNC, AT, AGN, LMG, OAD, AV and AG
researched the articles that were included as references. MJT, PP,
ITD, MG, CNC, AT, AGN, LMG and OAD reviewed the literature
findings, critically revised the manuscript and approved the
current form of the review. All authors read and approved the
published version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Buoso E, Masi M, Racchi M and Corsini E:
Endocrine-Disrupting Chemicals' (EDCs) effects on tumour
microenvironment and cancer progression: Emerging contribution of
RACK1. Int J Mol Sci. 21(9229)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
González-Davis O, Chauhan K, Zapian-Merino
SJ and Vazquez-Duhalt R: Bi-enzymatic virus-like bionanoreactors
for the transformation of endocrine disruptor compounds. Int J Biol
Macromol. 146:415–421. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pellerin E, Caneparo C, Chabaud S, Bolduc
S and Pelletier M: Endocrine-disrupting effects of bisphenols on
urological cancers. Environ Res. 195(110485)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Leso V, Fontana L, Marinaccio A, Leopold
K, Fanali C, Lucchetti D, Sgambato A and Iavicoli I: Sub-chronic
palladium nanoparticle effects on the endocrine reproductive system
of female Wistar rats: Preliminary data. Toxicol Ind Health.
35:403–409. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jaffrezic-Renault N, Kou J, Tan D and Guo
Z: New trends in the electrochemical detection of endocrine
disruptors in complex media. Anal Bioanal Chem. 412:5913–5923.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
American Diabetes Association.
Classification and Diagnosis of Diabetes: Standards of medical care
in diabetes-2020. Diabetes Care. 43 (Suppl 1):S14–S31.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Radu L, Carsote M, Gheorghisan-Galateanu
AA, Preda SA, Calborean V, Stanescu R, Gheorman V and Albulescu DM:
Blood parathyrin and mineral metabolism dinamics: A clinical
analyze. Rev Chim. 69:2754–2758. 2018.
|
|
8
|
Zimmet PZ: Diabetes and its drivers: The
largest epidemic in human history? Clin Diabetes Endocrinol.
3(1)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zheng Y, Ley SH and Hu FB: Global
aetiology and epidemiology of type 2 diabetes mellitus and its
complications. Nat Rev Endocrinol. 14:88–98. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vann R, Bussuan RM, Rombaldi RL and Arbex
AK: Endocrine disruptors and the induction of insulin resistance.
Curr Diabetes Rev. 17(e102220187107)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bai Q, Han K, Dong K, Zheng C, Zhang Y,
Long Q and Lu T: Potential applications of nanomaterials and
technology for diabetic wound healing. Int J Nanomedicine.
15:9717–9743. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kaur P and Khatik G: An overview of
computer-aided drug design tools and recent applications in
designing of antidiabetic agents. Curr Drug Targets. 22:1158–1182.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Al-Quraishy S, Dkhil MA and Abdel Moneim
AE: Anti-hyperglycemic activity of selenium nanoparticles in
streptozotocin-induced diabetic rats. Int J Nanomedicine.
10:6741–6756. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Khurana A, Tekula S, Saifi MA, Venkatesh P
and Godugu C: Therapeutic applications of selenium nanoparticles.
Biomed Pharmacother. 111:802–812. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Deng W, Xie Q, Wang H, Ma Z, Wu B and
Zhang X: Selenium nanoparticles as versatile carriers for oral
delivery of insulin: Insight into synergic antidiabetic effect and
mechanism. Nanomedicine. 13:1965–1974. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kumari S, Kamboj VK, Rajpoot D, Teotia AK,
Verma PK and Singh GN: The Unprecedented role of gold nanomaterial
in diabetes management. Recent Pat Drug Deliv Formul. 13:219–227.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mohsen AM: Nanothecnology advanced
strategies for the management of diabetes mellitus. Curr Drug
Targets. 20:995–1007. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kumari Y, Singh SK, Kumar R, Kumar B, Kaur
G, Gulati M, Tewari D, Gowthamarajan K, Karri VVSNR, Ayinkamiye C,
et al: Modified apple polysaccharide capped gold nanoparticles for
oral delivery of insulin. Int J Biol Macromol. 149:976–988.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hou L, Zheng Y, Wang Y, Hu Y, Shi J, Liu
Q, Zhang H and Zhang Z: Self-Regulated carboxyphenylboronic
acid-modified mesoporous silica nanoparticles with ‘touch switch’
releasing property for insulin delivery. ACS Appl Mater Interfaces.
10:21927–21938. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zou Z, He D, Cai L, He X, Wang K, Yang X,
Li L, Li S and Su X: Alizarin complexone functionalized mesoporous
silica nanoparticles: A smart system integrating glucose-responsive
double-drugs release and real-time monitoring capabilities. ACS
Appl Mater Interfaces. 8:8358–8366. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Iorga RA, Bacalbasa N, Carsote M, Bratu
OG, Stanescu AMA, Bungau S, Pantis C and Diaconu CC: Metabolic and
cardiovascular benefits of GLP-1 agonists, besides the hypoglycemic
effect (Review). Exp Ther Med. 20:2396–2400. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Presas E, Tovar S, Cuñarro J, O'shea JP
and O'driscoll CM: Pre-Clinical evaluation of a modified
cyclodextrin-based nanoparticle for intestinal delivery of
liraglutide. J Pharm Sci. 110:292–300. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee YH, Hong YL and Wu TL: Novel silver
and nanoparticle-encapsulated growth factor co-loaded chitosan
composite hydrogel with sustained antimicrobility and promoted
biological properties for diabetic wound healing. Mater Sci Eng C
Mater Biol Appl. 118(111385)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang L, Zhang L, Hu J, Wang W and Liu X:
Promote anti-inflammatory and angiogenesis using a hyaluronic
acid-based hydrogel with miRNA-laden nanoparticles for chronic
diabetic wound treatment. Int J Biol Macromol. 166:166–178.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hu H, Fan X, Guo Q, Wei X, Yang D, Zhang
B, Liu J, Wu Q, Oh Y, Feng Y, et al: Silicon dioxide nanoparticles
induce insulin resistance through endoplasmic reticulum stress and
generation of reactive oxygen species. Part Fibre Toxicol.
16(41)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rosenfeld CS: Gut dysbiosis in animals due
to environmental chemical exposures. Front Cell Infect Microbiol.
7(396)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Farrugia F, Aquilina A, Vassallo J and
Pace NP: Bisphenol a and type 2 diabetes mellitus: A review of
epidemiologic, functional, and early life factors. Int J Environ
Res Public Health. 18(716)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bakoyiannis I, Kitraki E and Stamatakis A:
Endocrine-disrupting chemicals and behaviour: A high risk to take?
Best Pract Res Clin Endocrinol Metab. 35(101517)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Khan NG, Correia J, Adiga D, Rai PS,
Dsouza HS, Chakrabarty S and Kabekkodu SP: A comprehensive review
on the carcinogenic potential of bisphenol A: Clues and evidence.
Environ Sci Pollut Res Int. 28:19643–19663. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Vom Saal FS and Vandenberg LN: Update on
the health effects of bisphenol A: Overwhelming evidence of harm.
Endocrinology. 162(bqaa171)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Campbell JE and Newgard CB: Mechanisms
controlling pancreatic islet cell function in insulin secretion.
Nat Rev Mol Cell Biol. 22:142–158. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wei Q, Qi L, Lin H, Liu D, Zhu X, Dai Y,
Waldron RT, Lugea A, Goodarzi MO, Pandol SJ and Li L: Pathological
mechanisms in diabetes of the exocrine pancreas: What's known and
what's to know. Front Physiol. 11(570276)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Marraudino M, Bonaldo B, Farinetti A,
Panzica G, Ponti G and Gotti S: Metabolism disrupting chemicals and
alteration of neuroendocrine circuits controlling food intake and
energy metabolism. Front Endocrinol (Lausanne).
9(766)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Nadal A, Quesada I, Tudurí E, Nogueiras R
and Alonso-Magdalena P: Endocrine-disrupting chemicals and the
regulation of energy balance. Nat Rev Endocrinol. 13:536–546.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Graceli JB, Dettogni RS, Merlo E, Niño O,
Da Costa CS, Zanol JF, Ríos Morris EA, Miranda-Alves L and Denicol
AC: The impact of endocrine-disrupting chemical exposure in the
mammalian hypothalamic-pituitary axis. Mol Cell Endocrinol.
518(110997)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cocolos AM, Dumitru N, Petrova EN, Cocolos
I, Tiglis M, Dragomirescu RFI, Olaru M, Dumitru A and Ghemigian AM:
Endocrine disrupting chemicals-the X factor in different
pathologies. Rev Chim. 69:136–139. 2018.
|
|
37
|
Murphy L, Mérida-Ortega Á, Cebrián ME,
Hernández-Garciadiego L, Gómez-Ruiz H, Gamboa-Loira B and
López-Carrillo L: Exposure to bisphenol A and diabetes risk in
Mexican women. Environ Sci Pollut Res Int. 26:26332–26338.
2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Battal D, Cok I, Unlusayin I, Aktas A and
Tunctan B: Determination of urinary levels of Bisphenol A in a
Turkish population. Environ Monit Assess. 186:8443–8452.
2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Caban M and Stepnowski P: The
quantification of bisphenols and their analogues in wastewaters and
surface water by an improved solid-phase extraction gas
chromatography/mass spectrometry method. Environ Sci Pollut Res
Int. 27:28829–28839. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kahn LG, Philippat C, Nakayama SF, Slama R
and Trasande L: Endocrine-disrupting chemicals: Implications for
human health. Lancet Diabetes Endocrinol. 8:703–718.
2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lee MR, Kim JH, Choi YH, Bae S, Park C and
Hong YC: Association of bisphenol A exposure with overweight in the
elderly: A panel study. Environ Sci Pollut Res Int. 22:9370–9377.
2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Mileva G, Baker SL, Konkle AT and Bielajew
C: Bisphenol-A: Epigenetic reprogramming and effects on
reproduction and behavior. Int J Environ Res Public Health.
11:7537–7561. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Velmurugan G, Ramprasath T, Gilles M,
Swaminathan K and Ramasamy S: gut microbiota, endocrine-disrupting
chemicals, and the diabetes epidemic. Trends Endocrinol Metab.
28:612–625. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
James-Todd TM, Meeker JD, Huang T, Hauser
R, Ferguson KK, Rich-Edwards JW, Mcelrath TF and Seely EW:
Pregnancy urinary phthalate metabolite concentrations and
gestational diabetes risk factors. Environ Int. 96:118–126.
2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chevalier N and Fénichel P: Endocrine
disruptors: New players in the pathophysiology of type 2 diabetes?
Diabetes Metab. 41:107–115. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Shaffer RM, Ferguson KK, Sheppard L,
James-Todd T, Butts S, Chandrasekaran S, Swan SH, Barrett ES,
Nguyen R, Bush N, et al: Study team. Maternal urinary phthalate
metabolites in relation to gestational diabetes and glucose
intolerance during pregnancy. Environ Int. 123:588–596.
2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zukin H, Eskenazi B, Holland N and Harley
KG: Prenatal exposure to phthalates and maternal metabolic outcomes
in a high-risk pregnant Latina population. Environ Res.
194(110712)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chen M, Zhao S, Guo WH, Zhu YP, Pan L, Xie
ZW, Sun WI and Jiang JT: Maternal exposure to Di-n-butyl phthalate
(DBP) aggravate gestational diabetes mellitus via FoxM1 suppression
by pSTAT1 signalling. Ecotoxicol Environ Saf.
205(111154)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Rolfo A, Nuzzo AM, De Amicis R, Moretti L,
Bertoli S and Leone A: Fetal-maternal exposure to endocrine
disruptors: Correlation with diet intake and pregnancy outcomes.
Nutrients. 12(1744)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Feng W, Liu Y, Ding Y, Mao G, Zhao T, Chen
K, Qiu X, Xu T, Zhao X, Wu X and Yang L: Typical neurobehavioral
methods and transcriptome analysis reveal the neurotoxicity and
mechanisms of di(2-ethylhexyl) phthalate on pubertal male ICR mice
with type 2 diabetes mellitus. Arch Toxicol. 94:1279–1302.
2020.PubMed/NCBI View Article : Google Scholar
|