Introduction
Inhibition and treatment of breast cancer remain a
major challenge in the scientific community and the primary goal of
breast cancer treatment is to induce cancer cell death and suppress
tumor proliferation (1,2). Recently, treatments for inhibiting
breast cancer are under several trials for drug resistance and
toxicity (3). New therapeutic
approaches with improved efficacy and fewer reversible effects are
needed to overcome this limitation.
Recently, a report was published on the possible
effects of nomifensine (NF) among anticancer substances targeting
estrogen signals as a result of large-scale screening in
silico (4). Previous research
has clearly shown that NF may be an effective new drug targeting
breast cancer (5). However, it is
necessary to improve its efficacy to lower the reversible effect.
NF is a norepinephrine-dopamine reuptake inhibitor that is marketed
as an antidepressant (6-8).
However, in 1986, the product license for NF (Merital®)
was voluntarily abandoned by the manufacturer for safety reasons
(9). In the 1970s, NF was shown to
be effective as an antidepressant in doses of 50-225 mg daily
(6). However, in the 1980s,
psychological dependence on drugs was reported when 400-600 mg was
administered daily in patients with a history of stimulant
addiction (10). Therefore, to
continue using this drug, there is a need for a strategy to reduce
the dosage and increase its efficacy.
Several pieces of research have been conducted for
increasing the efficacy of biomaterials using radiation
transformation technology (11,12).
In a previous study, the authors irradiated rotenone with gamma
rays and confirmed that its by-product, rotenoisin B, a radiolytic
derivative, was produced, which inhibited proliferation in
hepatocarcinoma (13). In
addition, it was confirmed that radiation-irradiated
Kenalog® also increased the apoptotic effect of melanoma
compared with Kanalog®, the original material (14). To date, there are no reports on the
effects of gamma irradiation on the amelioration of the
pharmacological effects of NF. The present study used radiation to
modify NF and develop a new candidate drug. It was hypothesized
that NF modified by ionizing radiation (IR)-NF might increase the
therapeutic efficacy in inhibiting breast cancer proliferation by
inducing cell death.
Materials and methods
Materials and reagents
All chemicals and reagents were used without further
purification. NF and Cell Counting Kit (CCK)-8 were procured from
MilliporeSigma. Anti-protein kinase B (AKT; cat. no. 4691),
anti-phospho (p)-AKT (cat. no. 4060), anti-glycogen synthase
kinase 3β (GSK3β; cat. no. 9315), anti-p-GSK3β (cat.
no. 9322), anti-β-catenin (cat. no. 8480), anti-BAD
(cat. no. 9292), anti-anti-apoptotic B cell lymphoma 2
(Bcl2; cat. no. 2870), anti-caspase 3 (cat. no.
9662), anti-cleaved caspase 3 (cat. no. 9664), anti-cyclin
D1 (cat. no. 2978), anti-extracellular signal-regulated
kinase (ERK; cat. no. 4695), anti-p-ERK (cat. no.
4377), anti-p38 (cat. no. 9212), anti-p-p38 (cat. no.
9215), anti-c-Jun N-terminal kinase (JNK; cat. no. 9252),
anti-p-JNK (cat. no. 9251), anti-α-tubulin (cat. no.
2144) and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH;
cat. no. 2118) were procured from Cell Signalling
Technology, Inc. Dulbecco's modified Eagle's medium (DMEM), RPMI
1640, penicillin/streptomycin and foetal bovine serum (FBS) were
procured from Lonza Group Ltd. Annexin V and cell cycle kits were
procured from MilliporeSigma.
Gamma irradiation and high-performance
liquid chromatography (HPLC)
A sample solution of NF (0.5 g) in methanol (200 ml)
in chapped vials was irradiated at 0-75 kGy (absorbed dose).
Irradiation was carried out at ambient temperature using a
cobalt-60 irradiator (point source AECL, IR-79, MDS Nordion
International Co. Ltd.) at the Advanced Radiation Technology
Institute (Jeongup, Korea). The source strength was ~320 kCi with a
dose rate of 10 kGy/h. Using gamma rays, NF in methanol solution
was directly irradiated and converted products were monitored using
an Agilent Technologies 1100 series HPLC system (Agilent
Technologies, Inc.). HPLC analysis was carried out on a YMC-Pack
ODS-A column (4.6x150 mm; YMC Korea Co., Ltd.) and was developed at
40˚C with 1% formic acid/methyl cyanide (1:1, flow rate: 1.0
ml/min, detection: 280 nm). The irradiated methanolic solution was
immediately evaporated to remove the solvent and lyophilized.
Cell culture
Human breast cancer MCF-7 cells, human lung cancer
A549 cells and human skin cancer SK-MEL-5 cells were procured from
American Type Culture Collection. The cells were cultured under
sterile conditions at 37˚C in a humid environment containing
CO2 (5%) and the culture medium comprised DMEM or RPMI
1640 medium supplemented with FBS (10%), glutamine (4 mM),
penicillin (100 U/ml) and streptomycin (100 µg/ml). The cultures
were regularly checked and trypsinised when the cell confluence
reached 85%.
Cell viability assay and crystal
violet assay
Cell viability was measured using CCK-8 Kit. Cells
were seeded in 96-well plates (1x104 cells/well) and
incubated for 24 h at 37˚C. The cells were treated with NF or IR-NF
(0, 62.5, 125 µg/ml), and incubated for 24 h at 37˚. A solution of
CCK-8 was added to each well and the plates were incubated for 1 h
at 37˚C to allow the reaction to take place before removal of the
culture medium. Cell viability was determined using a
spectrophotometer and the absorbance was measured at 450 nm (Tecan
Group, Ltd.). Cell viability for each group was expressed as a
percentage of that of the control group. To confirm that the growth
of MCF-7 cells was inhibited through induction by IR-NF, a crystal
violet assay was performed. Briefly, cells were seeded on sterile
coverslips in 12-well plates (1x104 cells/well) and
incubated for 48 h. The cells were incubated with NF or IR-NF for
24 h. Thereafter, the cells were stained with 0.1% crystal violet
solution for 20 min at 37˚C. After discarding the solution, the
coverslips were dried, and cells were observed using an inverted
phase-contrast microscope (Olympus IX71; Olympus Corporation). The
images were captured in 10 randomly selected fields (magnification
10X).
Annexin V assay
The cells (1x106 cells/well) grown in a
65-mm culture dish were incubated with NF (125 µg/ml) or IR-NF
(31.2, 62.5 or 125.0 µg/ml) for 24 h at 37˚C. Thereafter, to
conduct the quantitative analysis of apoptotic and necrotic dead
cells, Muse Annexin V and Dead Cell Assay kits (MilliporeSigma)
were used. The cells were harvested and washed with Dulbecco's PBS
(DPBS). They were stained with Annexin V and the Dead Cell reagent
for 20 min at room temperature, and flow cytometric assessment was
performed using the Muse Cell Analyser (MilliporeSigma). The number
of apoptotic cells was expressed as the percentage of the live,
early/late apoptotic and dead cells, which was determined using the
Muse version 1.4 analysis software (MilliporeSigma).
Oxidative stress assay
MCF-7 cells (2x106 cells/well) grown in
12-well plates were incubated with 125 µg/ml of NF or IR-NF for 24
h at 37˚C. The oxidative stress assay was conducted by quantitative
measurement of cellular populations undergoing oxidative stress
using the Muse Cell Analyser and Muse Oxidative Stress kit
(MilliporeSigma). According to the manufacturer's protocol, the
cells were detached, resuspended to obtain 1x106
cells/ml and incubated at 37˚C for 30 min with the Muse Oxidative
Stress working solution. The number of oxidised cells were counted
using the Muse Cell Analyser based on the intensity of the red
fluorescence. The results were obtained from four independent
experiments.
Cell cycle assay
The cells (1x106 cells/well) grown in a
65-mm culture dish were incubated with 125 µg/ml of NF or IR-NF for
24 h at 37˚C prior to IR exposure. Thereafter, to conduct the
quantitative analysis of the cell cycle, Muse Cell Cycle Assay kits
(MilliporeSigma) were used. The cells were harvested, washed with
DPBS and fixed with ethanol (70% v/v). They were stained with
Annexin V and the Dead Cell reagent for 20 min and flow cytometric
assessment was performed using the Muse Cell Analyser. The number
of apoptotic cells was expressed as the percentage of the live,
early/late apoptotic and dead cells, as determined by the Muse
version 1.4 analysis software.
Confocal microscopic analysis
The cells (1x105 cells/well) were
prepared on sterilised glass coverslips (BD Biosciences) in
triplicate. Cells were incubated with 125 µg/ml of NF or IR-NF for
24 h at 37˚C prior to IR exposure. i) Immunofluorescence staining:
Following irradiation, the cells were fixed in 4% paraformaldehyde
in PBS for 10 min, permeabilized with 0.25% Triton X-100 in PBS for
10 min and incubated with the β-catenin primary antibody for 2 h at
room temperature. The cells were washed to remove the excess
primary antibody and incubated with the appropriate fluorescently
labelled secondary antibody for 1 h at room temperature. The nuclei
were stained with 4',6-diamidino-2-phenylindole (DAPI) and
incubated for 5 min. ii) Annexin V/propidium iodide (PI) staining:
Annexin V/PI (5 µl) were added to the culture dish and incubated at
room temperature in the dark for 10 min. iii) To measure
mitochondrial morphological changes, MitoTracker Red CMXRos
selective probe (Molecular Probes; Thermo Fisher Scientific, Inc.)
were used. The cells were incubated in a medium without FBS and
containing 100 nM MitoTracker for 30 min at 37˚C. The medium was
then replaced with a complete medium without MitoTracker, washed
twice and the nuclei were stained with DAPI. Fluorescence intensity
was analysed for each group. After mounting, fluorescence images
were captured using a confocal microscope (LSM 700; Zeiss AG). To
quantify the immune-probed cells, the fluorescence intensity was
measured in 10 randomly selected images (magnification, x40).
Statistical analysis
Each experiment was performed at least three times
and the results were expressed as the mean ± standard deviation.
For multiple comparisons, one-way analysis of variance (ANOVA) was
used followed by Tukey's multiple comparisons test. P<0.05 was
considered to indicate a statistically significant difference.
Results
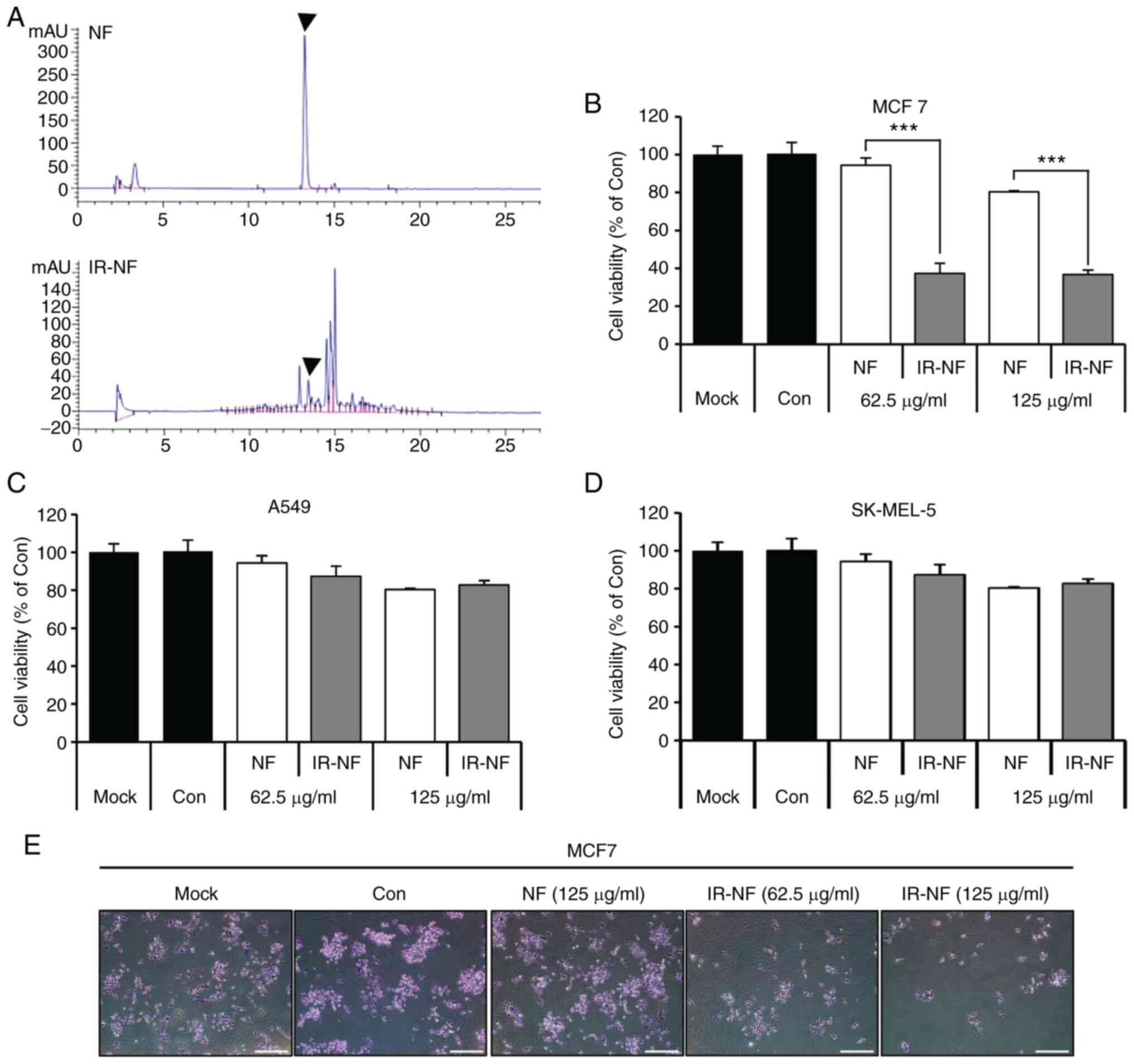
IR altered chemical properties of
NF
To detect the chemical structure of NF as changed by
IR, NF and IR-NF were dissolved in methanol and monitored by HPLC.
Fig. 1A showed that the original
peak of NF was lowered and new radiolytic peaks were generated from
12.6 to 15 min. The results indicated that NF transformed into a
completely different substance after irradiation. To investigate
the physiological activity of IR-NF, newly transformed by IR, three
tumor cells were treated with NF and IR-NF for 24 h, following
which cell viability was measured. Among the three cells treated
with IR-NF, in MCF-7 cells, the cell viability was significantly
decreased in the IR-NF-treated group compared with the group
treated with the same concentration of NF (Fig. 1B) The reduction reached 92.4±3.5%
in the 125 µg/ml NF-treated group, whereas 62.5 µg/ml IR-NF
treatment resulted in a reduction in the viability to 39.2±5.1% in
MCF-7 cells compared with the control. Crystal violet staining
results showed that IR-NF inhibited the growth of MCF-7 cells in a
concentration-dependent manner. There were no significant
differences in A549 and SK-MEL cells (Fig. 1C and D). As shown in Fig. 1E, in the negative control group
(Mock) and the NF-treated group, mitosis was actively performed and
several colonies were formed. On the other hand, in the cells
treated with IR-NF, the relative growth percentage significantly
decreased as the treatment concentration increased.
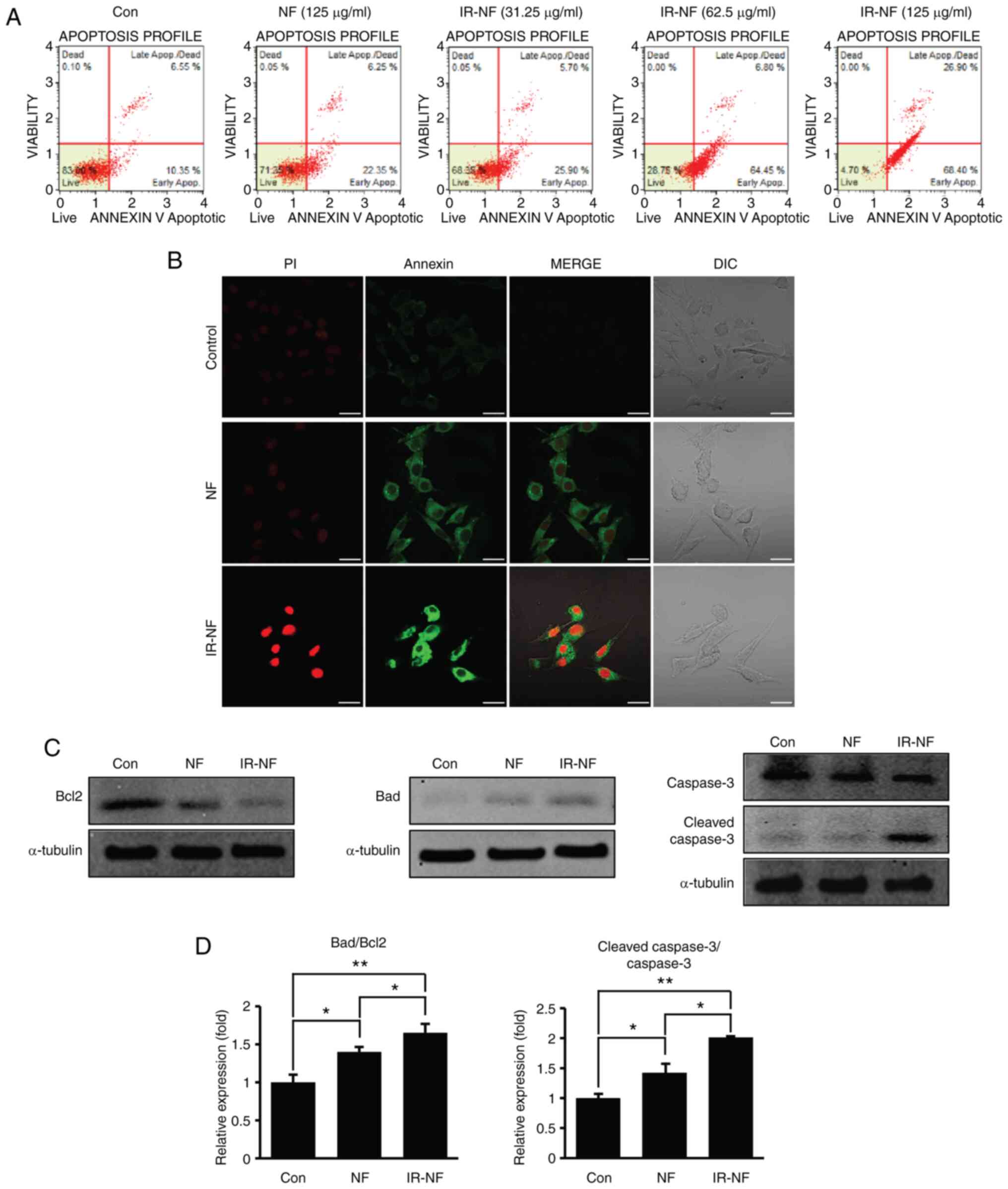
IR-NF induced apoptosis in the MCF-7
cell line
To verify whether the cell growth inhibition
affected by NF and IR-NF was induced through apoptosis, Annexin/PI
assay and the expression of apoptosis-related proteins were
observed. Similar tendencies were observed in the results of
Annexin/PI staining (Fig. 2A and
B). The total apoptosis rate of
the IR-NF-treated group significantly increased from 34.2 to 95% in
a concentration-dependent manner, whereas that of the NF-treated
group (125 µg/ml) increased slightly (Fig. 2A). In addition, Fig. 2B shows that Annexin V-positive
cells appeared prominently in the IR-NF-treated group. These
results suggested that IR-NF has a higher anti-proliferative effect
in MCF-7 cells than NF. The present study investigated changes in
the BAD/Bcl2 ratio following NF or IR-NF treatment. BAD is a
representative marker protein for pro-apoptotic proteins, whereas
Bcl2 is a marker protein for anti-apoptotic proteins. It was
observed that the ratio of BAD/Bcl2 significantly increased in the
IR-NF-treated group compared with the control and NF-treated groups
(Fig. 2C and D). In addition, as shown in Figs. 2C and D, the expression level of cleaved caspase
3 protein which is a marker protein of programmed cell death,
significantly increased compared with that in the NF-treated group.
Eventually, it was confirmed that the imbalance of mitochondrial
proteins induced by IR-NF treatment induces apoptosis.
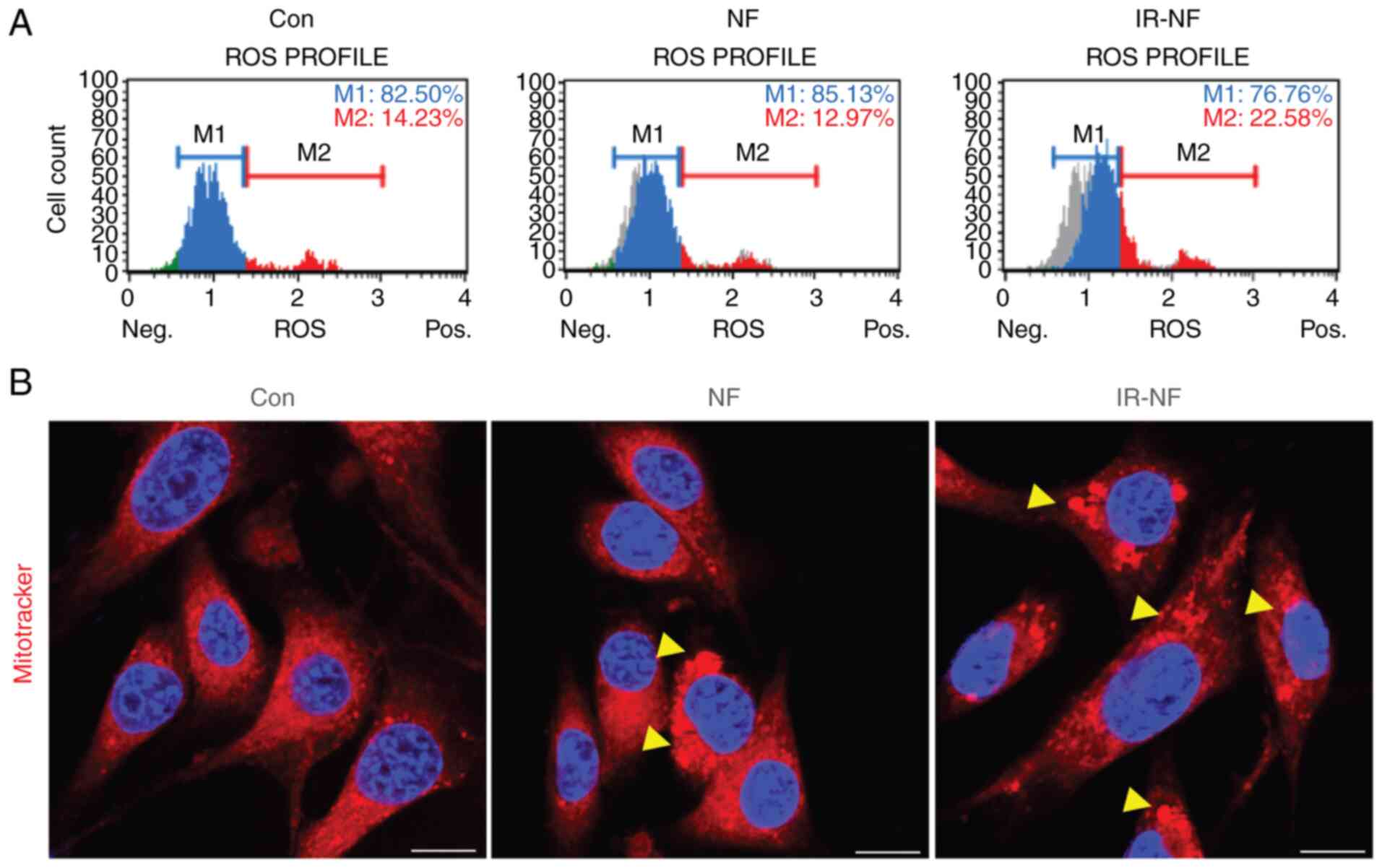
IR-NF induced reactive oxygen species
(ROS) accumulation and morphological changes of the
mitochondria
Further observations were made to confirm whether
the cytotoxicity induced by IR-NF was related to excessive ROS
production in the cytosol. Mitochondria-derived ROS can mediate
redox signalling or, in excess, cause cell injury and even cell
death (15). Notably, the number
of ROS-positive cells increased after 125 µg/ml of IR-NF treatment,
whereas there was no significant change after treatment with the
same concentration of NF (Fig.
3A). In addition, aberrant mitochondrial morphology is tightly
coupled to excessive ROS accumulation, which may lead to functional
damage to the mitochondria. Therefore, mitochondria located around
the cell nucleus were also observed after NF or IR-NF treatment.
Following the addition of IR-NF, a dense precipitate was observed
in the mitochondria and the mitochondria were swollen. These
changes were more pronounced in the IR-NF group compared with the
NF group (Fig. 3B). These results
indicated that IR-NF induces structural and functional damage to
the mitochondria, resulting in apoptotic cell death.
The mitogen-activated protein kinase
(MAPK) (extracellular signal-regulated kinase 1/2, p38 and c-Jun NH
2-terminal kinase) pathway is involved in cell death-induced by
irradiated nomifensine
The stress-responsive MAPK serves an important role
in mammalian cells. In particular, MAPK is considered an important
prognostic factor for breast cancer (16). Thus, MAPK family proteins (ERK1/2,
p38 and JNK) were explored in IR-NF-treated MCF-7 cells using
western blotting analysis. As shown in Figs. 4A and B, after the addition of either substance,
phosphorylation of JNK and p38 was upregulated, whereas that of ERK
was downregulated. These phenomena were more noticeable in the
IR-NF-treated group than in the NF-treated group. These results
suggest that IR-NF influences cell death through the regulation of
the MAPK (ERK1/2, p38 and JNK) signalling pathways.
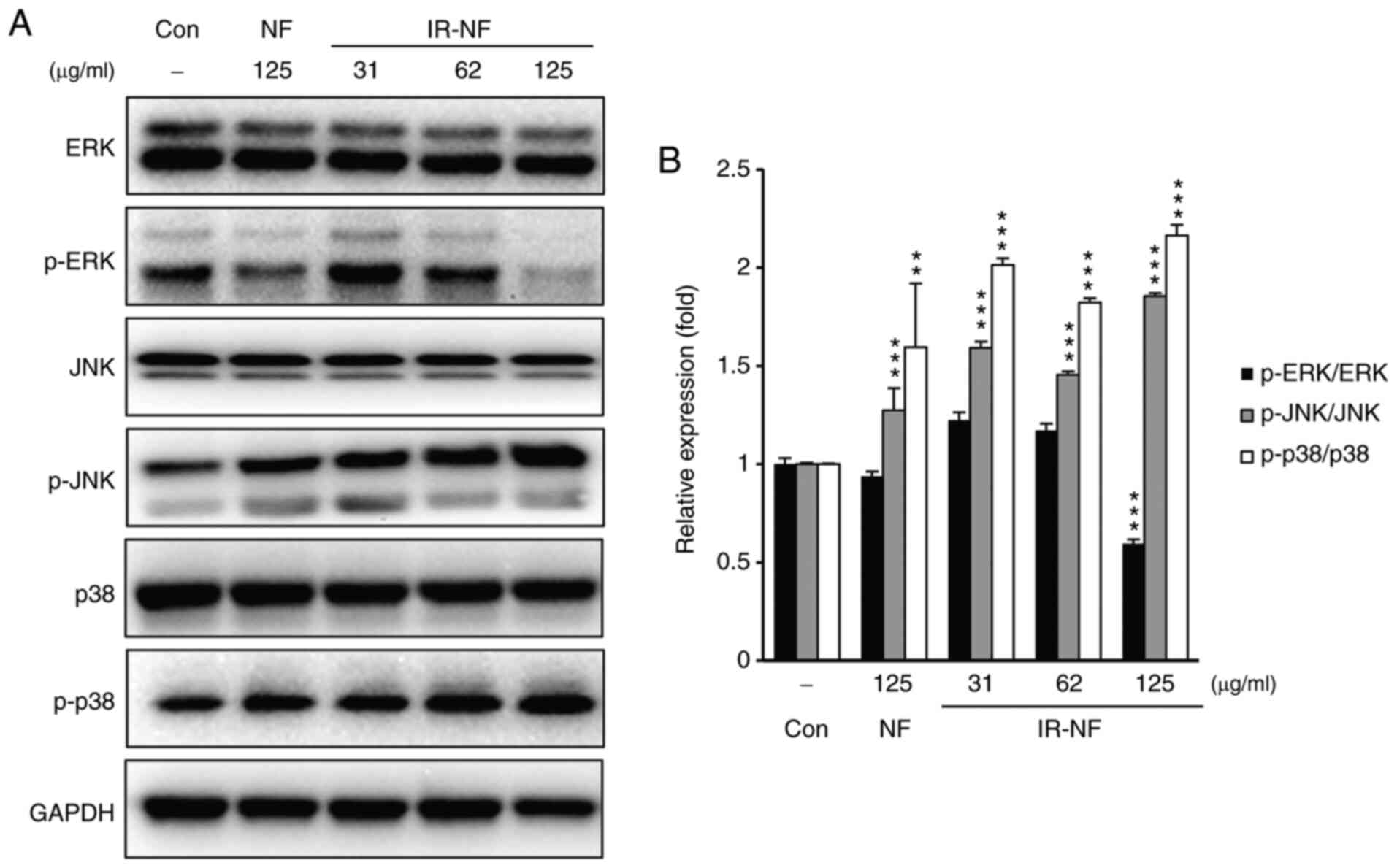 | Figure 4IR-NF stimulates the MAPK pathway in
MCF-7 cells. (A) Representative images of western blot analysis for
the protein expressions of JNK, p-JNK, p38, p-p38, ERK, p-ERK and
glyceraldehyde-3-phosphate dehydrogenase antibodies. (B)
Densitometry analysis. The data are presented as the mean ±
standard deviation. **P≤0.01 and ***P≤0.001
vs. control. IR-NF, irradiated nomifensine; MAPK, mitogen-activated
protein kinase; JNK, c-Jun NH 2-terminal kinase; p-,
phosphorylated; ERK, extracellular signal-regulated kinase; NF,
nomifensine; Con, control. |
IR-NF induced cell cycle arrest and
suppressed β-catenin translocation to the nucleus in MCF-7
Cells
The Wnt/β-catenin signalling pathway serves a key
role in diverse physiological processes, such as proliferation,
differentiation, migration, invasion, apoptosis and tissue
homeostasis (17). β-catenin, a
major transcription factor in the Wnt/β-catenin signalling pathway,
contributes to pivotal molecular mechanisms in cancer development
and progression (18,19). In the present study, MCF-7 cells
treated with IR-NF showed significant arrest at the
G0/G1 stage (Fig. 5A). Moreover, IR-NF not only
downregulated the expression of cyclin D1 (Fig. 5B and C) but also inhibited the nuclear
translocation of β-catenin (Fig.
5D). In addition, IR-NF treatment suppressed its interaction
with the AKT/GSK3β cascade (Figs.
5D-F), followed by a decreased β-catenin expression. Therefore,
IR-NF was found to inhibit tumour development and progression by
blocking or interfering with the interaction between the
Wnt/β-catenin and phosphatidylinositol 3-kinase-AKT pathways.
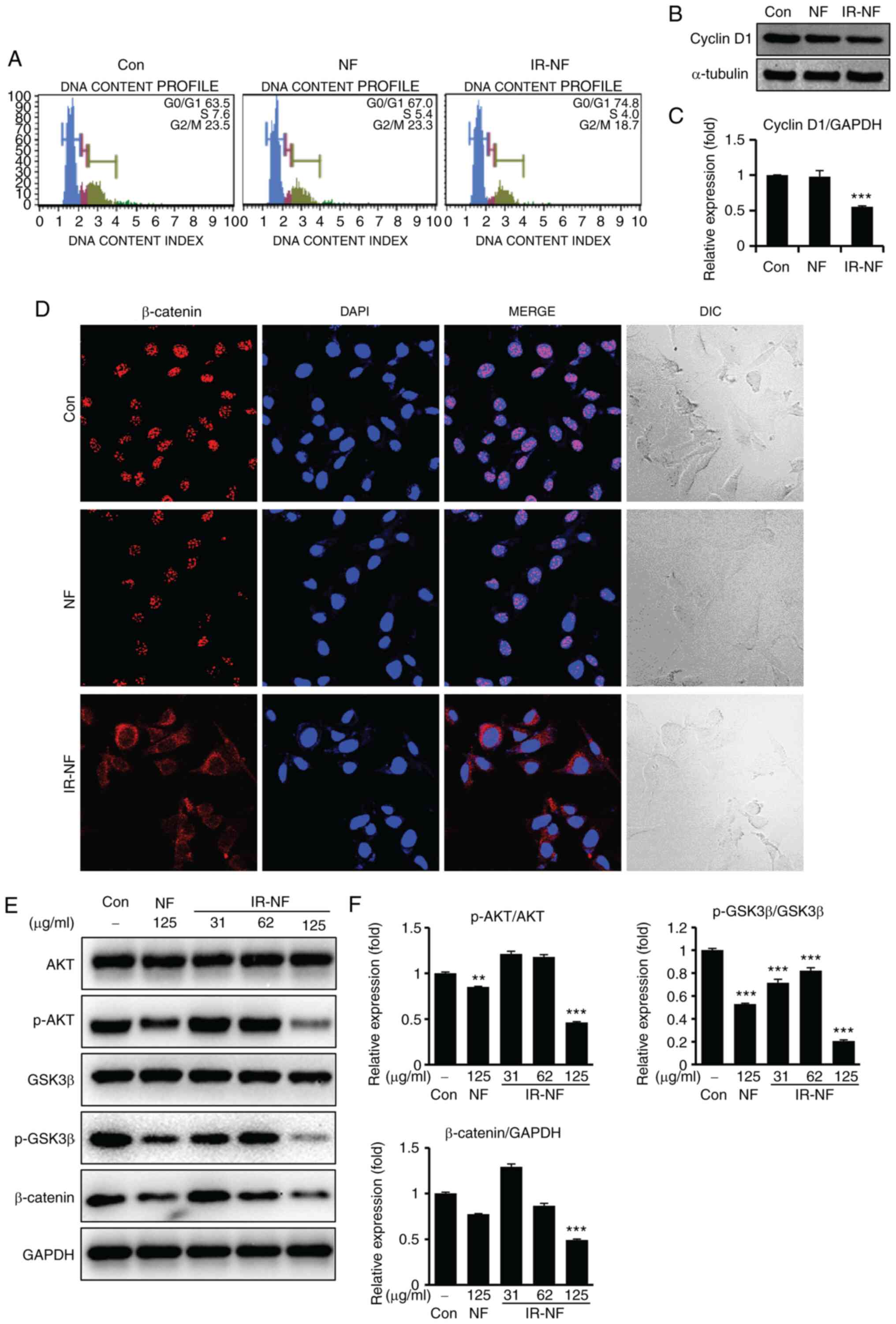 | Figure 5IR-NF induced cell cycle arrest and
suppressed β-catenin translocation to the nucleus in MCF-7 cells.
MCF-7 cells were treated with IR-NF (125 µg/ml) for 24 h. (A) Cells
were stained using the Cell Cycle Assay Kit and analyzed using the
Muse cell sorting system. (B) Whole cell lysates prepared and
immunoblotted with anti-cyclin B1. Anti-α-tubulin was used as
loading controls. (C) Densitometry analysis. Data are presented as
the mean ± standard deviation. ***P≤0.001 vs. control.
(D) Cells were stained with anti-β-catenin antibody and observed
using confocal microscopy. Scale bar=25 µm. (E) Whole cell lysates
were prepared and immunoblotted with anti-AKT, anti-p-AKT,
anti-GSK3β, anti-p-GSK3β, anti-β-catenin and anti-GAPDH were used
as loading controls. (F) The western blots were quantitatively
analyzed. Band intensities were normalized to those of the normal
form of each protein or GAPDH. Data are presented as the mean ±
standard deviation. **P≤0.01, ***P≤0.001 vs.
Con. IR-NF, irradiated nomifensine; IR-NF, irradiated nomifensine;
AKT, protein kinase B; p-, phosphorylated; anti-protein kinase B;
GSK3β, glycogen synthase kinase 3β; Con: control; DIC, differential
interference contrast. |
Discussion
NF was used as an antidepressant; however, its use
was discontinued due to a history of drug dependence when the drug
was used in very high doses. However, a recent large-scale
screening in silico has suggested its pharmacological
potential as an anticancer agent (4). A translational approach to medical
drug candidates with pharmacological effects is as valuable as
discovering new drug candidates. However, even if the drug is used
for other purposes, the concerns of previously reported reversible
effects cannot be ignored. As there is controversy over the
application of NF in humans, there might be an alternative strategy
that reduces the dosage in humans by increasing the efficacy of the
pharmacological effect of NF. The present study explored the
anticancer mechanism of NF and found that IR increased its
efficacy.
IR was used to change the chemical properties of NF.
The main peak of NF decreased following IR, which occurred
simultaneously with the detection of novel peaks. IR produced
marked modifications in the chemical properties of NF. In addition,
as a result of conducting a toxicity evaluation on several cancer
cell lines (A549, SK-MEL-5, MCF-7) using NF, it was confirmed that
the toxicity was observed only in the MCF-7 cell line. In addition
to a previous report which stated that NF can inhibit cell growth
in breast cancer cells, it was confirmed that the change in the
characteristics of NF induced by irradiation enhanced its
anti-proliferative efficacy (5).
The present study demonstrated that IR-NF
effectively inhibited cell proliferation at a lower concentration
compared with NF. IR-NF dysregulated mitochondrial formation,
increased ROS accumulation, induced caspase 3 dependent apoptosis,
stimulated MAPK (ERK, JNK, p38) pathways and suppressed the cell
cycle.
Mitochondria serve an important role in triggering
and regulating apoptosis (20). In
particular, regulation of the cellular balance between pro- and
anti-apoptotic mitochondrial proteins is pivotal for the
determination of cell fate by either promoting survival or
mitochondrial-mediated apoptosis (21). Under stressful conditions, such as
intracellular ROS, physical/chemical stimuli, mitochondrial
deoxyribonucleic acid damage and hypoxia, mitochondrial membrane
permeability and release of pro-apoptotic proteins from the
intermembrane space of the mitochondria into the cytosol increases
(22,23). In addition, stresses due to these
various internal/external stimuli increase the cellular expression
of pro-apoptotic proteins relative to anti-apoptotic proteins,
inducing apoptosis (21). From
this point of view, the data suggested that IR-NF treatment in
MCF-7 cells induced apoptosis by inducing functional damage to the
mitochondria.
On the other hand, the present study demonstrated
that IF-NF upregulated the phosphorylation of JNK and p38 proteins
and downregulated the phosphorylation of the ERK protein in MCF-7
cells. The MAPK pathway serves a crucial role in most cellular
functions and can mediate different anti-proliferative events, such
as apoptosis, autophagy and senescence, depending on the cell type
and stimulus (24-27).
In a number of previous studies, it has been suggested that MAPKs
(ERK1/2, p38 and JNK) are involved in inhibiting the onset of
breast tumors, inhibiting tumor metastasis and being an implicit
parameter for sensitization to tumor suppression and apoptosis
(28-30).
Additionally, previous studies reported that activation of Wnt
signaling increases the transcription of a number of genes,
including cyclin D1, in breast cancer (31,32).
Overexpression of cyclin D1 occurs in >50% of breast cancers
(33). Taken together, the results
showed trends similar to those of previous studies, suggesting that
MAPK pathways and β-catenin signaling are involved in the apoptosis
of MCF-7 cells caused by IR-NF treatment.
In the present study, IR was used because of its
potential to introduce energy into a material to create new
polarity or characteristics. In addition, materials irradiated with
sufficiently high energy can decompose, creating highly reactive
intermediate molecules or forming new molecules. However, the
varying possibilities of material variations induced by IR can
raise concerns about reproducibility. For irradiation technology to
be used as a new platform for drug development, repetitive
reproducibility and yield will be an important issue. In a
pre-experiment of the present study, it was found that the
reproducibility of radiolytic NF and its derivatives were
represented when NF solutions with different concentrations and
volumes were irradiated by IR at a total dose of 75 kGy. This
indicated that gamma irradiation is an effective way to produce NF
derivatives with improved efficacy and has provided new insights
into the development of effective new drugs. The present study
investigated the anticancer effects of NF derivatives on breast
cancer cells and demonstrated that the potential effects of these
derivatives serve an important role in the inhibition of MCF-7
proliferation. Although not all derivatives were isolated, it would
be a valuable discovery if some derivatives showed improved
cytotoxicity in one compound compared with the original
material.
In conclusion, the present study provided the first
evidence that IR-induced modification significantly contributed to
improving the pharmacological properties and anticancer efficacy of
NFs for the treatment of breast cancer. The results will provide an
opportunity for re-evaluation of a number of drugs whose use has
been restricted due to serious adverse effects or drug resistance.
Although the present study reached meaningful conclusions from
cellular-level studies, the diversity of environments in humans and
animals is insufficient to make a strong claim that IR-NF is a
valuable cancer-associated marker. The physiological effects of
IR-NF on the human will be meaningful through animal and clinical
studies and will be clarified through ongoing research. In
addition, separating individual fractions to analyze key single
compounds and confirm their effectiveness will have to be carried
out in further studies.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Convergence Research
Group project (grant no. CRC21021-300) of the National Research
Council of Science and Technology, Republic of Korea.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SHK conceived the present study and wrote the
original draft. DHB performed formal analysis and investigation.
HWB and BYC performed the statistical analysis and revised the
manuscript critically for important intellectual contents. SHK and
DHB confirmed the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sledge GW, Mamounas EP, Hortobagyi GN,
Burstein HJ, Goodwin PJ and Wolff AC: Past, present and future
challenges in breast cancer treatment. J Clin Oncol. 32:1979–1986.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Razak NA, Abu N, Ho WY, Zamberi NR, Tan
SW, Alitheen NB, Long K and Yeap SK: Cytotoxicity of eupatorin in
MCF-7 and MDA-MB-231 human breast cancer cells via cell cycle
arrest, anti-angiogenesis and induction of apoptosis. Sci Rep.
9(1514)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang X, Zhang H and Chen X: Drug
resistance and combating drug resistance in cancer. Cancer Drug
Resist. 2:141–160. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tong XY, Quan Y and Zhang HY: NUDT5 as a
novel drug target and prognostic biomarker for ER-positive breast
cancer. Drug Discov Today. 26:620–625. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tong XY, Liao X, Gao M, Lv BM, Chen XH,
Chu XY, Zhang QY and Zhang HY: Identification of NUDT5 inhibitors
from approved drugs. Front Mol Biosci. 7(44)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kinney JL: Nomifensine maleate: A new
second-generation antidepressant. Clin Pharm. 4:625–636.
1985.PubMed/NCBI
|
|
7
|
Robinson S, Cheney D and Costa EJN-Ssaop:
Effect of nomifensine and other antidepressant drugs on
acetylcholine turnover in various regions of rat brain. Naunyn
Schmiedebergs Arch Pharmacol. 304:263–269. 1978.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Katz NS, Guiard BP, El Mansari M and Blier
P: Effects of acute and sustained administration of the
catecholamine reuptake inhibitor nomifensine on the firing activity
of monoaminergic neurons. J Psychopharmacol. 24:1223–1235.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
CSM Update: Withdrawal of nomifensine. Br
Med J (Clin Res Ed). 293(41)1986.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Böning J and Fuchs G: Nomifensine and
psychological dependence-a case report. Pharmacopsychiatry.
19:386–388. 1986.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Badaboina S, Bai HW, Na YH, Park CH, Kim
TH, Lee TH and Chung BY: Novel radiolytic rotenone derivative,
rotenoisin B with potent anti-carcinogenic activity in hepatic
cancer cells. Int J Mol Sci. 16:16806–16815. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee EH, Park CH, Choi HJ, Kawala RA, Bai
HW and Chung BY: Dexamethasone modified by gamma-irradiation as a
novel anticancer drug in human non-small cell lung cancer. PLoS
One. 13(e0194341)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bak DH, Kang SH, Park CH, Chung BY and Bai
HW: A novel radiolytic rotenone derivative, rotenoisin A, displays
potent anticarcinogenic activity in breast cancer cells. J
Radiation Res. 62:249–258. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kawala RA, Ramadhani FJ, Choi HJ, Lee EH,
Park CH, Chung BY and Bai HW: Kenalog modified by ionizing
radiation induces intrinsic apoptosis mediated by elevated levels
of reactive oxygen species in melanoma cancer. Oncol Rep.
41:1837–1850. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ježek J, Cooper KF and Strich R: Reactive
oxygen species and mitochondrial dynamics: The Yin and Yang of
mitochondrial dysfunction and cancer progression. Antioxidants
(Basel). 7(13)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Derin D, Eralp Y, Ozluk Y, Yavuz E, Guney
N, Saip P, Igci A, Ozmen V, Kücücük S, Aslay I, et al: Lower level
of MAPK expression is associated with anthracycline resistance and
decreased survival in patients with hormone receptor negative
breast cancer. Cancer Invest. 26:671–679. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang Y and Wang X: Targeting the
Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol.
13(165)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yu WK, Xu ZY, Yuan L, Mo S, Xu B, Cheng XD
and Qin JJ: Targeting β-catenin signaling by natural products for
cancer prevention and therapy. Front Pharmacol.
11(984)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bugter JM, Fenderico N and Maurice MM:
Mutations and mechanisms of WNT pathway tumour suppressors in
cancer. Nat Rev Cancer. 21:5–21. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tait SWG and Green DR: Mitochondrial
regulation of cell death. Cold Spring Harb Perspect Biol.
5(a008706)2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Redza-Dutordoir M and Averill-Bates DA:
Activation of apoptosis signalling pathways by reactive oxygen
species. Biochim Biophys Acta. 1863:2977–2992. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zorov DB, Juhaszova M and Sollott SJ:
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS
release. Physiol Rev. 94:909–950. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Webster KA: Mitochondrial membrane
permeabilization and cell death during myocardial infarction: Roles
of calcium and reactive oxygen species. Future Cardiol. 8:863–884.
2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yue J and López JM: Understanding MAPK
signaling pathways in apoptosis. Int J Mol Sci.
21(2346)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chang H and Zou Z: Targeting autophagy to
overcome drug resistance: Further developments. J Hematol Oncol.
13(159)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Munshi A and Ramesh R: Mitogen-activated
protein kinases and their role in radiation response. Genes Cancer.
4:401–408. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Davidson B, Konstantinovsky S, Kleinberg
L, Nguyen MT, Bassarova A, Kvalheim G, Nesland JM and Reich R: The
mitogen-activated protein kinases (MAPK) p38 and JNK are markers of
tumor progression in breast carcinoma. Gynecol Oncol. 102:453–461.
2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Whyte J, Bergin O, Bianchi A, McNally S
and Martin F: Key signalling nodes in mammary gland development and
cancer. Mitogen-activated protein kinase signalling in experimental
models of breast cancer progression and in mammary gland
development. Breast Cancer Res. 11(209)2009.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Kawiak A, Domachowska A, Krolicka A,
Smolarska M and Lojkowska E: 3-chloroplumbagin induces cell death
in breast cancer cells through MAPK-mediated Mcl-1 inhibition.
Front Pharmacol. 10(784)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
He Y, Liu Z, Qiao C, Xu M, Yu J and Li G:
Expression and significance of Wnt signaling components and their
target genes in breast carcinoma. Mol Med Rep. 9:137–143.
2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Guo L, Yilamu D, Sun L, Liu S and Ma F:
Association among the expression of β-catenin, cyclin D1 and
estrogen receptor-β in human breast cancer. Exp Ther Med.
10:1423–1428. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Velasco-Velázquez MA, Li Z, Casimiro M,
Loro E, Homsi N and Pestell RG: Examining the role of cyclin D1 in
breast cancer. Future Oncol. 7:753–765. 2011.PubMed/NCBI View Article : Google Scholar
|