Introduction
Mesenchymal stem cells (MSCs) are multipotent adult
stem cells that present self-renewal and differentiation potential
to bone cells, chondrocytes, and adipocytes (1-3).
MSCs can be isolated from various tissues or organs such as the
blood, bone marrow, adipose tissue, umbilical cord, placenta, lung,
liver, and skin (1,4). An abundant source of MSCs is adipose
tissue, from which MSCs can be easily isolated. Human
adipose-derived stem cells (hADSCs) are a widely used source in
tissue engineering repair and regeneration because of their
adipogenic, chondrogenic, and osteogenic potential (5-7).
Therefore, it is very important to clarify the molecular mechanism
of the osteogenic differentiation of hADSCs.
The regeneration of bones is essential for bone
development, continuous bone remodeling in adults, and bone damage
repair (8,9). Bone regeneration consists of a
cascade of precisely regulated biological processes involving
various cell types, and intracellular and extracellular signaling
pathways (9). The Wnt signaling
pathway is capable of mediating multiple biological activities
during bone damage repair and bone regeneration, including
osteoblast differentiation and bone formation (10,11).
It constitutes a typical β-catenin-dependent pathway and two
atypical β-catenin-independent pathways (the atypical Wnt/PCP
pathway and the Wnt/Ca2+ pathway) (12). The typical Wnt/β-catenin pathway
exerts a dominant role in regulating osteoblast differentiation
during bone fracture repair (11).
It inhibits the differentiation of multifunctional MSCs into
adipocytes and promotes their differentiation into osteoblasts
(13,14). Previous studies have shown that the
expression of Runx2, ALP, OCN and OPN changes significantly during
osteogenic differentiation, which could be the markers of
osteogenic differentiation (15,16).
MicroRNAs (MiRNAs) are small-chain, single-stranded
non-coding RNAs (approximately 22 nucleotides long). They
incompletely or completely bind to the 3' untranslated region
(3'-UTR) of target mRNAs, thus regulating cell signaling pathways
through negatively mediating post-transcriptional gene expression
or degrading mRNAs (17,18). Studies have shown that miR-216a-3p
promotes cancer (19-21),
and many reports showed that miR-216a-3p significantly influenced
the activity of Wnt/β-catenin signaling pathway. Song et al
(22). reported that knockdown of
miR-216a-3p induces differentiation of BMMSCs into ACE II cells
through the Wnt/β-catenin pathway, thereby alleviating NRDS. It was
also demonstrated that the BRD4/miR-216a-3p/Wnt/β-catenin pathway
regulates the stemness of gastric cancer cells (23). Its potential function in bone
formation, however, is largely unclear.
This article aimed to investigate the potential
function and mechanism of miR-216a-3p in the osteogenic
differentiation of hADSCs.
Materials and methods
Cell culture
hADSCs (Cat. No. 7510, hADSCs) were obtained from
ScienCell Company (Carlsbad, CA, USA), and the 293T cells were
purchased from Mingzhou Bio (Cat. No. MZ-0266, Ningbo, China).
Cells were cultured in Dulbecco's modified Eagle's medium (DMEM)
(Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal
bovine serum (FBS) and 100 µg/ml penicillin and streptomycin in a
humidified atmosphere of 5% CO2 at 37˚C.
In vitro osteogenic
differentiation
At 80-90% cell confluence, the medium was replaced
with osteogenesis induction medium (DMEM containing 10% FBS, 10 mM
β-glycerophosphate, 0.1 µM dexamethasone, and 0.2 mM ascorbic
acid). Cells were collected before osteogenic differentiation (day
0) and 3, 7 and 14 days after osteogenic differentiation.
RT-qPCR
Cells were digested in EDTA containing 0.25%
trypsin, washed in phosphate-buffered saline (PBS) 2-3 times, and
lysed in TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) to isolate cellular RNAs. After erasing gDNAs using the
PrimeScript™ RT reagent kit with gDNA Eraser (Takara Biotechnology
Co., Ltd.), RNAs were reversely transcribed to cDNAs at 37˚C for 15
min and 85˚C for 5 sec, and were maintained at 4˚C. Using the SYBR
Premix Ex Taq™ II Kit (Takara Bio, Inc.), cDNAs were subjected to
thermal cycles at 95˚C for 15 min, followed by 40 cycles at 95˚C
for 5 sec, 60˚C for 30 sec, 72˚C for 60 sec, and extension at 72˚C
for 10 min. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and U6
were used as internal references. Relative levels of Runx2, ALP,
OCN, OPN, COL1A1, Wnt3a, and miR-216a-3p were calculated by
2-ΔΔCq for three independent measurements (24). The sequences of the primers used in
RT-qPCR are listed in Table I.
 | Table ISequence of the forward and reverse
primers used for RT-qPCR. |
Table I
Sequence of the forward and reverse
primers used for RT-qPCR.
| Gene | Forward (5' to
3') | Reverse (5' to
3') |
|---|
| miR-216a-3p |
TAATCTCAGCTGGCAACTGTGA |
TCACAGTTGCCAGCTGAGATTA |
| Runx2 |
CAAGGACAGAGTCAGATTAC |
GTGGTAGAGTGGATGGAC |
| ALP |
TAAGGACATCGCCTACCAGC |
TGGCTTTCTCGTCACTCTCA |
| OCN |
GGTGCAGCCTTTGTGTCCAAGC |
GTCAGCCAACTCGTCACAGTCC |
| OPN |
CTCCATTGACTCGAACGACTC |
CAGGTCTGCGAAACTTCTTAGAT |
| COL1A1 |
CAATGCTGCCCTTTCTGCTCCTTT |
ATTGCCTTTGATTGCTGGGCAGAC |
| Wnt3a |
CCATCCTCTGCCTCAAATTC |
TGGACAGTGGATATAGCAGCA |
| GAPDH |
GAAGGTGAAGGTCGGAGTC |
GAAGATGGTGATGGGATTTC |
| U6 |
GCTTCGGCAGCACATATACTAAAAT |
CGCTTCACGAATTTGCGTGTCAT |
Western blotting
Cells were washed in PBS and lysed in cell lysis
buffer (10 mM Tris-HCl, 1 mM MgCl2, 1% SDS, 1% NP-40, 1%
Triton X-100; pH 7.4) on ice. Protein concentrations were measured
using a bicinchoninic acid (BCA) Protein Assay Kit (Beyotime,
Jiangsu, China). The proteins were loaded at an amount of 10 µg,
and separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and electroblotted onto polyvinylidene
difluoride (PVDF) membranes (Millipore, USA). Following incubation
for 2 h at room temperature in Tris-buffered saline (TBS)
containing 0.1% Tween-20 (TBST) with 5% skim milk, immunoblotting
with primary antibodies (anti-Runx2, anti-ALP, anti-Wnt3a,
anti-OPN, anti-β-catenin, and anti-GAPDH; 1:1,000, ABclonal Biotech
Co., Ltd.) at 4˚C overnight and secondary antibodies (1:1,000,
ABclonal Biotech Co., Ltd.) for 1 h was performed. All of the
antibodies used in this manuscript were purchased from Abclonal,
Wuhan, China, and the catalog numbers were as listed: Runx2
(A11753), ALP (A0514), OPN (A19092), OCN (A6205), Wnt3a (A0642),
β-catenin (A19657), GAPDH (A19056), and the secondary antibody
(AS014). Band exposure was achieved by the enhanced
chemiluminescence (ECL) method, followed by measurement of grey
value analysis using the Quantity One® 1-D Analysis
Software (Bio-Rad, Hercules, CA, USA).
Lentivirus synthesis and
transfection
Lentivirus overexpression vectors GV287-miR-NC,
GV287-miR-216a-3p, GV287-anti-miR-NC, GV287-anti-miR-216a-3p,
GV287-Wnt3a and the control group GV287 were purchased from
GenePharma (Shanghai, China), and a 2nd generation system was used
to the package of lentivirus. The lentiviral plasmid, packaging
vector and envelope vector were mixed at a 4:3:2 ratio for a total
DNA mass of 20 µg. The mixture was firstly incubated with 1 ml
Lenti-Easy Packaging Mix (Shanghai GeneChem Co., Ltd.) for 15 min,
then incubated for another 20 min incubation with
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), and then added into 293T cell culture medium for
6 h at 37˚C. In brief, the 293T cells were seeded at a density of
2.5 * 105 cells/plate in a 10-cm plate and cultured to
80% confluence, then incubated in Opti-MEM for 4 h, and then
incubated with the transfection mixture as described. The
supernatant of the transfected 293T cells was collected after three
days by filtering through a 0.45-µm filter, and the viral particles
were concentrated by ultracentrifugation at 70,000 g for 2 h at
4˚C. hADSCs cells were infected with the lentivirus at a
multiplicity of infection of 5 and with polybrene (Sigma-Aldrich;
Merck KGaA) at a final concentration of 8 µg/ml at 37˚C with 5%
CO2 for 24 h. Fresh culture medium was then used to
replace the old medium. Fluorescence was measured 72 h
post-infection when the achieved infection efficiency was 80%.
Screening of stable cells using green fluorescent protein.
Dual-luciferase reporter assay
It was predicted using TargetScan 7.2 that Wnt3a is
the direct target of miR-216a-3p. Wnt3a 3' UTR or mutant sequences
were cloned into the pmirGLO vector (Promega, Beijing, China) for
the synthesis of pmirGLO-Wnt3a-WT and pmirGLO-Wnt3a-Mut. pRL-TK
vector (Takara, Dalian, Liaoning, China) was used as the negative
control. The cells seeded in a 6-well plate with higher than 80%
density were co-transfected with luciferase vector/pRL-TK vector
and miR-216a-5p mimic/negative control. After transfection for 48
h, relative firefly and Renilla luciferase activities were
measured using the Dual-Luciferase Reporter Gene Assay Kit
(Beyotime, Shanghai, China).
Statistical analysis
GraphPad PRISM 8.01 (GraphPad Software, Inc., La
Jolla, CA) was used for statistical analyses. The data were
obtained from three independent experiments and are expressed as
the mean ± standard deviation (SD). Differences between groups were
compared by the t-test, and those among groups were analyzed
by one-way analysis of variance (ANOVA), and followed by Tukey's or
Bonferroni's post hoc test. All experiments were performed in
triplicate and repeated three times. P<0.05 was considered as
statistically significant. All experiments were performed in
triplicate and repeated three times.
Results
Osteogenic differentiation of
hADSCs
The osteogenic differentiation potential of hADSCs
was determined by detecting relative levels of osteogenesis markers
at day 0, 3, 7 and 14, respectively. The mRNA levels of Runx2, ALP
and OPN were significantly upregulated at day 3, 7 and 14, and that
of OCN increased at day 7 and 14 (Fig.
1A-D). In addition, protein levels of Runx2, ALP, OCN and OPN
in hADSCs undergoing 0, 3, 7 and 14-day osteogenic differentiation
were detected by Western blot, and were significantly upregulated
(Fig. 1E-H).
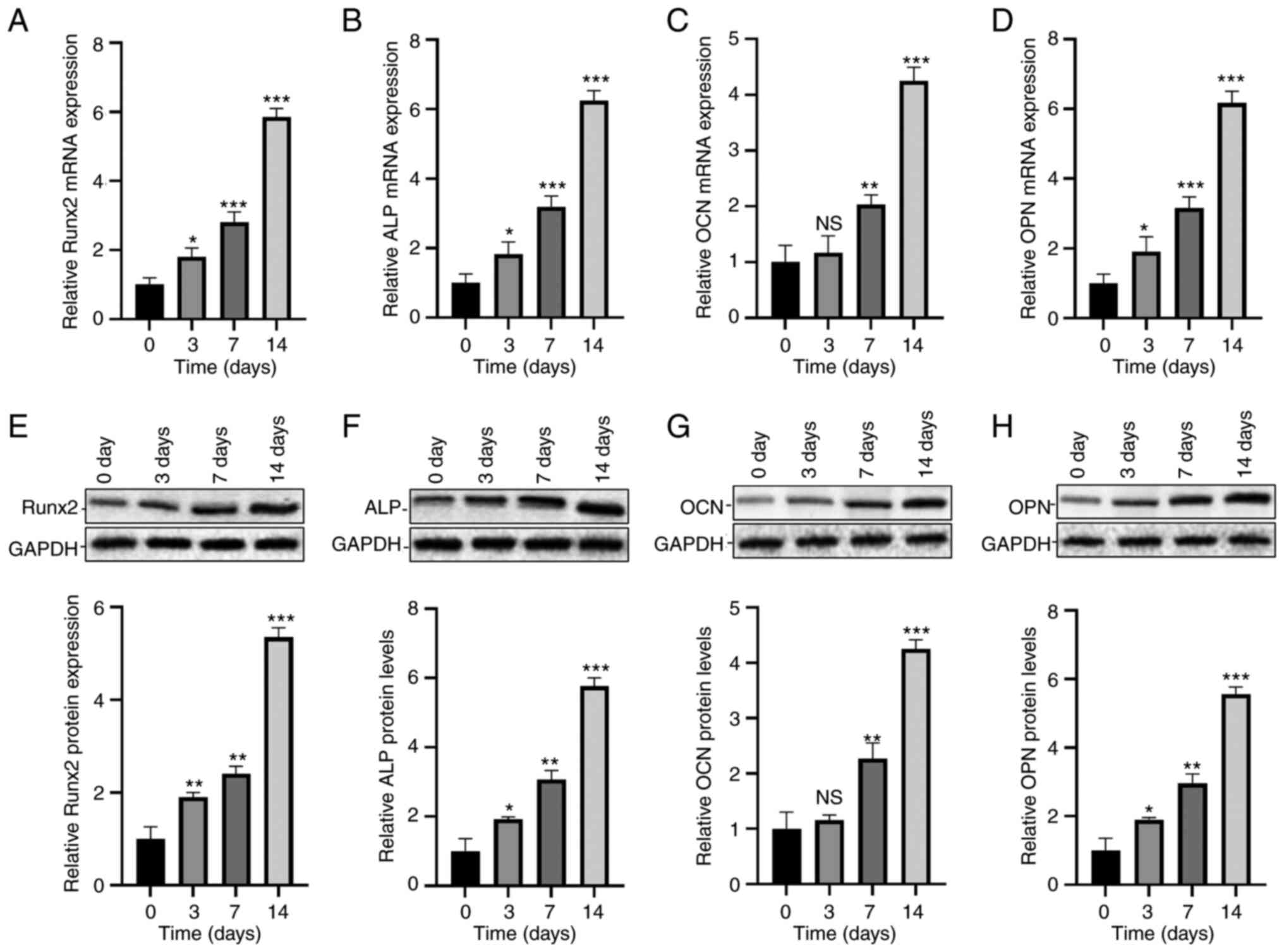 | Figure 1Osteogenic differentiation of hADSCs.
Relative mRNA expression levels of (A) Runx2, (B) ALP, (C) OCN and
(D) OPN in hADSCs on day 0, 3, 7, 14. Protein expression levels of
(E) Runx2, (F) ALP, (G) OCN and (H) OPN in hADSCs on day 0, 3, 7
and 14. *P<0.05, **P<0.01 and
***P<0.001 vs. day 0. ALP, alkaline phosphatase;
hADSCs, human adipose-derived stem cells; NS, no significance; OCN,
osteocalcin; OPN, osteopontin; Runx2, runt-related transcription
factor 2. |
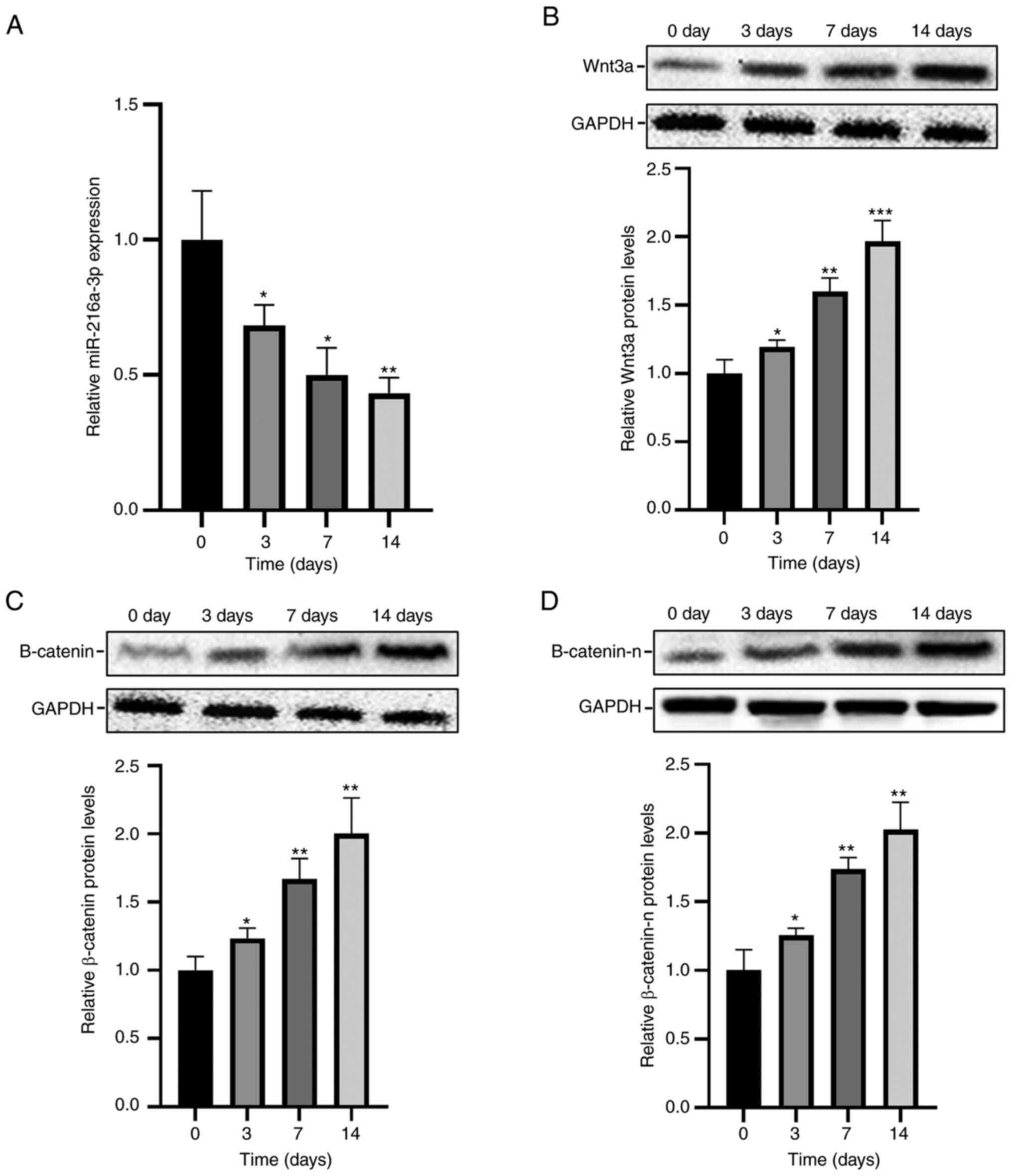
Dynamic expression changes of
miR-216a-3p and Wnt3a in osteogenic differentiation of hADSCs
Dynamic expression changes of miR-216a-3p during the
osteogenic differentiation of hADSCs were examined. Endogenous
miR-216a-3p was gradually downregulated, and showed a continuous
downward trend (Fig. 2A).
Conversely, the protein levels of Wnt3a, β-catenin, and β-catenin-n
(nuclear β-catenin) were gradually elevated, and the trend was
continuously upregulated (Fig.
2B-D).
Regulatory effect of miR-216a-3p on
osteogenic differentiation of hADSCs
The lentiviral vector carrying miR-216-3p affects
the miR-216-3p level, and the transfection efficacy was tested by
RT-qPCR (Fig. 3A and B). On the 3rd day of transfection, the
miR-216a-3p level in the miR-216a-3p overexpression group was 8-10
times higher than that of the control group, and it remained at a
high level until the 14th day (Fig.
3C). On the contrary, it was significantly downregulated in
cells transfected with anti-miR-216a-3p, at levels approximately
4.5 times lower than that of the controls, and it remained at a low
level on the 14th day (Fig. 3D).
Hence, the transfection efficacy of lentiviruses was verified.
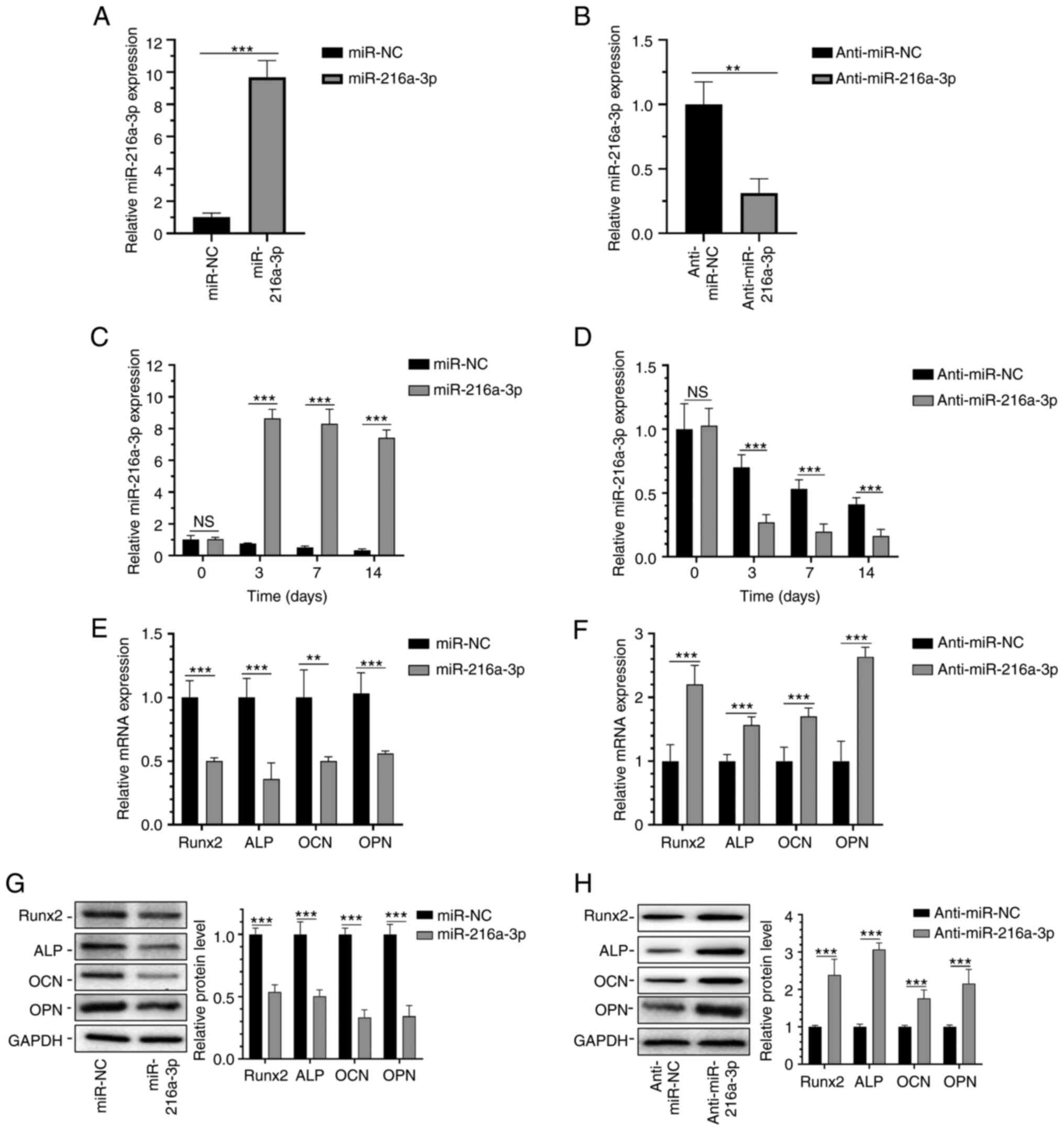 | Figure 3Regulatory effect of miR-216a-3p on
the osteogenic differentiation of hADSCs. Transfection efficiency
of (A) miR-216a-3p and (B) anti-miR-216a-3p plasmids. (C)
Expression level of miR-216a-3p in hADSCs transfected with (C)
miR-216a-3p and (D) anti-miR-216a-3p plasmids. mRNA expression of
Runx2, ALP, OCN and OPN in hADSCs on the 14th day after
transfection with (E) miR-216a-3p and (F) anti-miR-216a-3p
plasmids. Protein expression of Runx2, ALP, OCN and OPN in hADSCs
on the 14th day after transfection with (G) miR-216a-3p and (H)
anti-miR-216a-3p plasmids. **P<0.01 and
***P<0.001 as indicated. ALP, alkaline phosphatase;
hADSCs, human adipose-derived stem cells; miR, microRNA; NC,
negative control; NS, no significance; OCN, osteocalcin; OPN,
osteopontin; Runx2, runt-related transcription factor 2. |
Furthermore, the regulatory effects of miR-216a-3p
on the expression levels of osteogenesis markers at day 7 of
osteogenic differentiation of hADSCs were determined. In hADSCs
overexpressing miR-216a-3p, mRNA levels of OCN, OPN, Runx2, and ALP
were significantly downregulated, and were upregulated in cells
with miR-216a-3p knockdown (Fig.
3E and G). As expected, the
protein levels of OCN, OPN, Runx2, and ALP were similarly regulated
by miR-216a-3p (Fig. 3G and
H).
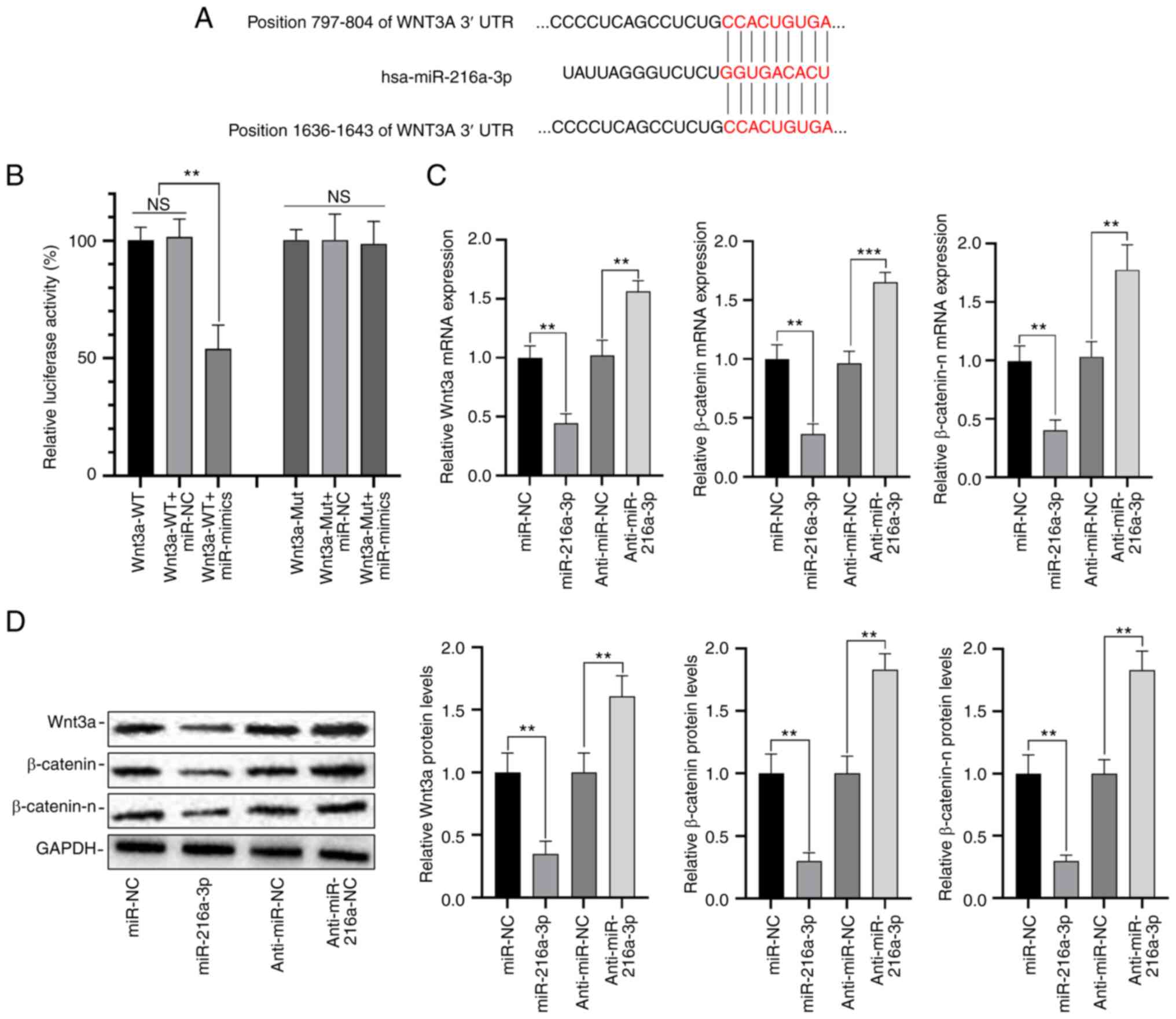
MiR-216a-3p directly targets
Wnt3a
Using TargetScan 7.1, it was predicted that
miR-216a-3p could target Wnt3a, and highest score (Fig. 4A). We thereafter performed
dual-luciferase reporter assays, and the data showed that compared
with the negative control, the luciferase activity in cells
co-transfected with pmirGLO-Wnt3a-WT and miR-216a-3p mimics was
reduced by 45%. However, no significant difference in luciferase
activity was detected between cells co-transfected with
pmirGLO-Wnt3a-Mut and miR-216a-3p mimic or Anti-miR-216a-3p, and
those of the controls (Fig. 4B);
overexpression of miR-216b-3p downregulated the level of Wnt3a,
β-catenin and β-catenin-n, while anti-miR-216b-3p upregulated the
level of Wnt3a, β-catenin and β-catenin-n (Fig. 4C and D). Therefore, it was proven that Wnt3a
was the target of miR-216a-3p.
MiR-216a-3p mediated the osteogenic
differentiation of hADSCs through the Wnt3a/β-catenin signaling
pathway
Wnt3a was overexpressed by the transfection of
lentiviral vector, and the overexpression level was detected by
RT-qPCR (Fig. 5A); then the
miR-216a-3p overexpression plasmid was transfected and the
transfection level was tested (Fig.
5B); the results showed that overexpression of Wnt3a could
significantly reverse the effect of miR-216a-3p on regulation of
β-catenin and β-catenin-n (Fig. 5C
and D); moreover, miR-216a-3p
could reverse the effects of Runx2, ALP, OCN and OPN (Fig. 5E and F). These results indicate that Wnt3a is a
direct target of miR-216a-3p, and overexpression of Wnt3a can
effectively reverse the inhibition of miR-216a-3p on osteogenic
differentiation.
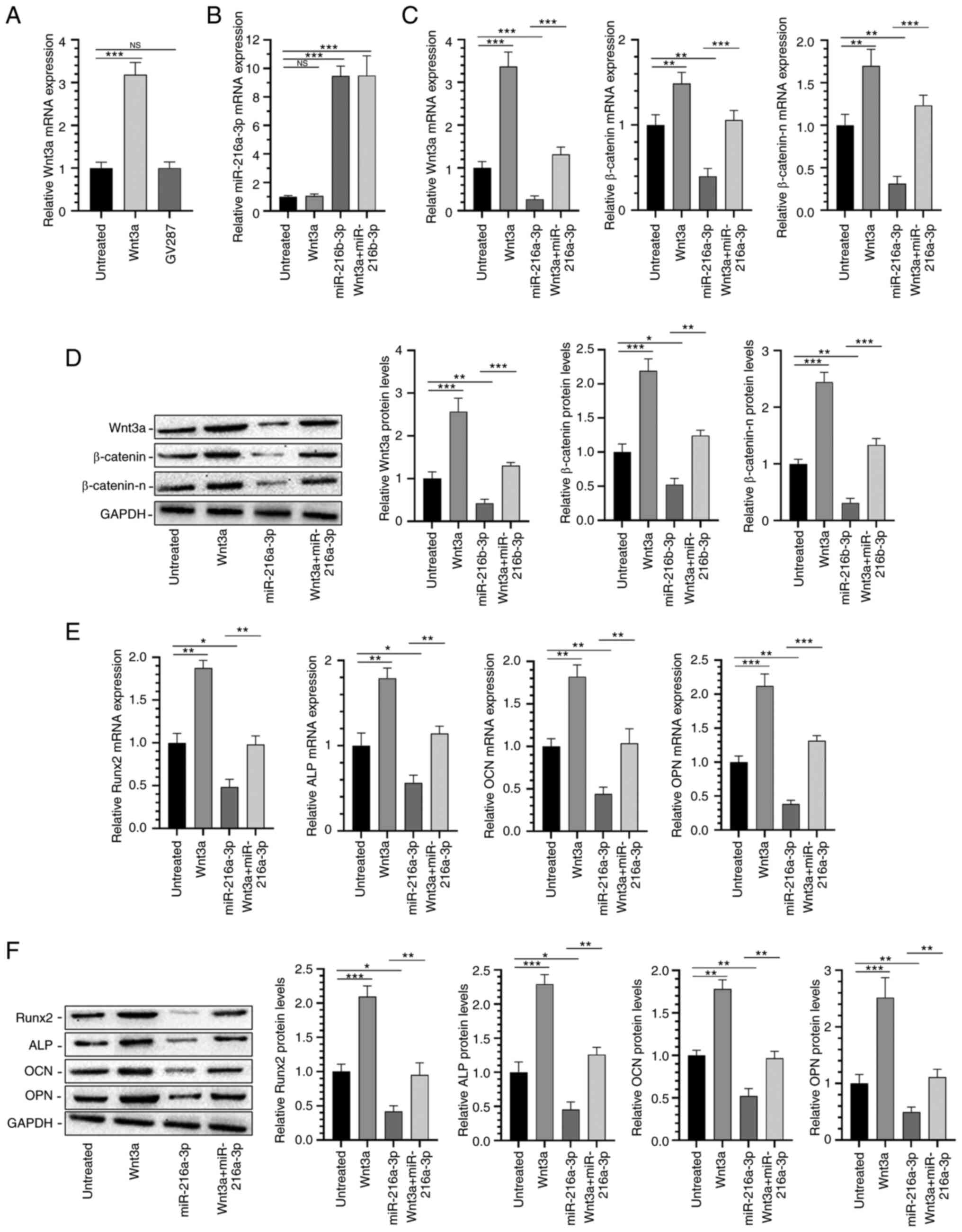 | Figure 5MiR-216a-3p mediates the osteogenic
differentiation of hADSCs through the Wnt3a/β-catenin signaling
pathway. Transfection efficiency of (A) Wnt3a and (B) miR-216a-3p
plasmids. Relative (C) mRNA and (D) protein expression levels of
Wnt3a, β-catenin and β-catenin-n in hADSCs. (E) Relative (E) mRNA
and (F) protein expression levels of Runx2, ALP, OCN and OPN in
hADSCs. *P<0.05, **P<0.01 and
***P<0.001 as indicated. NS, No significance. ALP,
alkaline phosphatase; hADSCs, human adipose-derived stem cells;
miR, microRNA; NS, no significance; OCN, osteocalcin; OPN,
osteopontin; Runx2, runt-related transcription factor 2;
β-catenin-n, β-catenin-nucleus. |
Discussion
As a type of MSC, characteristics of ADSCs are high
proliferation and strong lineage-specific differentiation (24). ADSCs are easily and abundantly
isolated with relatively low risk. Additionally, they are optimally
applied in bone regeneration and osteogenic tissue engineering
because they can be in vitro differentiated into lipocytes,
osteoblasts, chondrocytes, nerve cells, or myogenic cells under
certain conditions (24,25). In the present study, hADSCs were
cultivated in osteogenesis medium for in vitro osteogenic
differentiation. By examining the relative levels of osteogenesis
markers (Runx2, ALP, and OCN), we have proven that osteogenic
differentiation of hADSCs could be induced via inhibition of
miR-216a-3p.
MiRNAs are widely involved in the osteogenic
differentiation of ADSCs. Zhang et al (26) showed that through the k-Ras/MEK/ERK
signaling pathway, knockdown of miR-143 triggers osteogenic
differentiation of hADSCs. In Fan's study (27), miR-450b is critically important for
accelerating in vitro osteogenic differentiation of hADSCs
and in vivo bone formation by targeting BMP3. Ai et
al (28) found that the
upregulation of microRNA-196a promoted the osteogenic
differentiation of ACS, but the downregulation of microRNA-196a
induced adipogenic differentiation, promoted the osteogenic
differentiation of adipose stem cells, and inhibited adipogenic
differentiation by regulating the β-catenin pathway. The results of
Yang et al (29) showed
that MiR-100-5p is upregulated in NONFH exosomes, and by targeting
BMPR2 and inhibiting the BMPR2/SMAD1/5/9 signaling pathway, it
leads to NONFH-like damage, thereby inhibiting the osteogenesis of
hBMSCs.
Our results clarified that there is significant
downregulation of miR-216a-3p during osteogenic differentiation of
hADSCs. Overexpression of miR-216a-3p markedly downregulated
osteogenesis markers, including OCN, OPN, COL1A1, Runx2, and ALP,
which were upregulated following miR-216a-3p knockdown. We
concluded that miR-216a-3p plays an important role in the
osteogenic differentiation of hADSCs.
There is a clear correlation between the
Wnt/β-catenin signaling pathway, bone development, and osteogenic
differentiation of MSCs (8,11).
The study by Fan et al (27) also supported the fact that the
Wnt/β-catenin signaling pathway is responsible for senescence and
osteogenesis of hADSCs mediated by miR-1292. In the present
research, Wnt3a was upregulated during the process of osteogenic
differentiation of hADSCs. Wnt3a is a classic ligand and stimulator
of the Wnt/β-catenin signaling pathway (22,30).
Consistent with previously reported findings, miR-216a-3p possessed
the ability to mediate the Wnt/β-catenin signaling pathway by
targeting Wnt3a (22,23). However, since the current research
does not include any experiments or data of cellular
immunohistochemistry, more in-depth research remains to be carried
out, which is a major limitation of this study.
MiR-216a-3p plays an important role in the
osteogenic differentiation of hADSCs and is downregulated during
the osteogenesis process. By targeting Wnt3a, it was determined
that miR-216a-3p exerts an active role in the osteogenic
differentiation of hADSCs through mediating the Wnt/β-catenin
signaling pathway. Collectively, miR-216a-3p mediates the
osteogenic differentiation of hADSCs by targeting Wnt3a, thus
negatively regulating the Wnt/β-catenin signaling pathway.
Acknowledgements
Not applicable.
Funding
Funding: This project was funded by Guangdong medical science
and Technology Research Fund (grant no. A2019289).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DL, GS and ZZ conceived and designed the study, and
acquired, analyzed and interpreted the data. All of the authors
agreed to be accountable for all aspects of the work in ensuring
that questions related to the accuracy and integrity of any part of
the work are appropriately investigated and resolved. All authors
confirm the authenticity of all the raw data, and read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen Q, Shou P, Zheng C, Jiang M, Cao G,
Yang Q, Cao J, Xie N, Velletri T, Zhang X, et al: Fate decision of
mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death
Differ. 23:1128–1139. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Atashi F, Modarressi A and Pepper MS: The
role of reactive oxygen species in mesenchymal stem cell adipogenic
and osteogenic differentiation: A review. Stem Cells Dev.
24:1150–1163. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Si YL, Zhao YL, Hao HJ, Fu XB and Han WD:
MSCs: Biological characteristics, clinical applications and their
outstanding concerns. Ageing Res Rev. 10:93–103. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
da Silva Meirelles L, Chagastelles PC and
Nardi NB: Mesenchymal stem cells reside in virtually all post-natal
organs and tissues. J Cell Sci. 119 (Pt 11):2204–2213.
2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gastaldi G, Asti A, Scaffino MF, Visai L,
Saino E, Cometa AM and Benazzo F: Human adipose-derived stem cells
(hASCs) proliferate and differentiate in osteoblast-like cells on
trabecular titanium scaffolds. J Biomed Mater Res A. 94:790–799.
2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dicker A, Le Blanc K, Astrom G, van
Harmelen V, Götherström C, Blomqvist L, Arner P and Rydén M:
Functional studies of mesenchymal stem cells derived from adult
human adipose tissue. Exp Cell Res. 308:283–290. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mitchell R, Mellows B, Sheard J, Antonioli
M, Kretz O, Chambers D, Zeuner MT, Tomkins JE, Denecke B, Musante
L, et al: Secretome of adipose-derived mesenchymal stem cells
promotes skeletal muscle regeneration through synergistic action of
extracellular vesicle cargo and soluble proteins. Stem Cell Res
Ther. 10(116)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Regard JB, Zhong Z, Williams BO and Yang
Y: Wnt signaling in bone development and disease: Making stronger
bone with Wnts. Cold Spring Harb Perspect Biol.
4(a007997)2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dimitriou R, Jones E, McGonagle D and
Giannoudis PV: Bone regeneration: Current concepts and future
directions. BMC Med. 9(66)2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jin H, Wang B, Li J, Xie W, Mao Q, Li S,
Dong F, Sun Y, Ke HZ, Babij P, et al: Anti-DKK1 antibody promotes
bone fracture healing through activation of β-catenin signaling.
Bone. 71:63–75. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang T, Zhang X and Bikle DD: Osteogenic
differentiation of periosteal cells during fracture healing. J Cell
Physiol. 232:913–921. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kim JH, Liu X, Wang J, Chen X, Zhang H,
Kim SH, Cui J, Li R, Zhang W, Kong Y, et al: Wnt signaling in bone
formation and its therapeutic potential for bone diseases. Ther Adv
Musculoskelet Dis. 5:13–31. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ross SE, Hemati N, Longo KA, Bennett CN,
Lucas PC, Erickson RL and MacDougald OA: Inhibition of adipogenesis
by Wnt signaling. Science. 289:950–953. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang Y, Zhang X, Shao J, Liu H, Liu X and
Luo E: Adiponectin regulates BMSC osteogenic differentiation and
osteogenesis through the Wnt/β-catenin pathway. Sci Rep.
7(3652)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wei Y, Ma H, Zhou H, Yin H, Yang J, Song Y
and Yang B: MiR-424-5p shuttled by bone marrow stem cells-derived
exosomes attenuates osteogenesis via regulating WIF1-mediated
Wnt/β-catenin axis. Aging (Albany NY). 13:17190–17201.
2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sun X, Cao J, Han J, Jia B, Wang J, Lian
J, Gao J, Liu S and Xiao H: Experimental Study of lncRNA
RP11-815M8.1 Promoting osteogenic differentiation of human bone
marrow mesenchymal stem cells. Biomed Res Int.
2021(5512370)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297.
2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Martin EC, Qureshi AT, Dasa V, Freitas MA,
Gimble JM and Davis TA: MicroRNA regulation of stem cell
differentiation and diseases of the bone and adipose tissue:
Perspectives on miRNA biogenesis and cellular transcriptome.
Biochimie. 124:98–111. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhao J, Li L and Yang T: MiR-216a-3p
suppresses the proliferation and invasion of cervical cancer
through downregulation of ACTL6A-mediated YAP signaling. J Cell
Physiol. 235:9718–9728. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wan Z, Liu T, Wang L, Wang R and Zhang H:
MicroRNA-216a-3p promotes sorafenib sensitivity in hepatocellular
carcinoma by downregulating MAPK14 expression. Aging (Albany NY).
12:18192–18208. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang D, Li Y, Zhang C, Li X and Yu J:
MiR-216a-3p inhibits colorectal cancer cell proliferation through
direct targeting COX-2 and ALOX5. J Cell Biochem. 119:1755–1766.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Song H, Lu HN, Chen X, Jiang XF, Yang Y
and Feng J: MiR-216a-3p promotes differentiation of BMMSCs into ACE
II cells via Wnt/β-catenin pathway. Eur Rev Med Pharmacol Sci.
22:7849–7857. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Song H, Shi L, Xu Y, Xu T, Fan R, Cao M,
Xu W and Song J: BRD4 promotes the stemness of gastric cancer cells
via attenuating miR-216a-3p-mediated inhibition of Wnt/β-catenin
signaling. Eur J Pharmacol. 852:189–197. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mohamed-Ahmed S, Fristad I, Lie SA,
Suliman S, Mustafa K, Vindenes H and Idris SB: Adipose-derived and
bone marrow mesenchymal stem cells: A donor-matched comparison.
Stem Cell Res Ther. 9(168)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Guasti L, New SE, Hadjidemetriou I,
Palmiero M and Ferretti P: Plasticity of human adipose-derived stem
cells-relevance to tissue repair. Int J Dev Biol. 62
(6-7-8):431–439. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang Y, Zhou K, Wu L, Gu H, Huang Z and
Xu J: Downregulation of microRNA143 promotes osteogenic
differentiation of human adipose-derived mesenchymal stem cells
through the k-Ras/MEK/ERK signaling pathway. Int J Mol Med.
46:965–976. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fan L, Fan J, Liu Y, Li T, Xu H, Yang Y,
Deng L, Li H and Zhao RC: MiR-450b Promotes osteogenic
differentiation in vitro and enhances bone formation in vivo by
targeting BMP3. Stem Cells Dev. 27:600–611. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ai G, Meng M, Wang L, Shao X, Li Y, Cheng
J, Tong X and Cheng Z: MicroRNA-196a promotes osteogenic
differentiation and inhibit adipogenic differentiation of adipose
stem cells via regulating β-catenin pathway. Am J Transl Res.
11:3081–3091. 2019.PubMed/NCBI
|
|
29
|
Yang W, Zhu W, Yang Y, Guo M, Qian H,
Jiang W, Chen Y, Lian C, Xu Z, Bai H, et al: Exosomal miR-100-5p
inhibits osteogenesis of hBMSCs and angiogenesis of HUVECs by
suppressing the BMPR2/Smad1/5/9 signalling pathway. Stem Cell Res
Ther. 12(390)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Park HW, Kim YC, Yu B, Moroishi T, Mo JS,
Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, et al: Alternative
Wnt Signaling Activates YAP/TAZ. Cell. 162:780–794. 2015.PubMed/NCBI View Article : Google Scholar
|