Introduction
Renal cell carcinoma (RCC) is the most fatal
genitourinary malignant tumor. Clear cell renal cell carcinoma
(ccRCC) is the most common type of RCC (1); however, the underlying mechanism
remains unclear. Although treatments for ccRCC have been explored
for years, there are no available approaches that provide
satisfactory results (2). The
5-year survival rate of patients with advanced tumors is only 23%
(3). Therefore, there is a need to
uncover the molecular mechanisms underlying the pathogenesis of
ccRCC and identify novel therapeutic targets.
Small proline-rich repeat protein 3 (SPRR3) is a
member of the small proline-rich protein family. SPRR3 has been
associated with the progression of multiple cancer types. SPRR3 was
reported as a tumor promoter in colorectal cancer, breast cancer,
glioblastoma multiforme and non-small-cell lung cancer (4-7),
while in esophageal cancer, it was revealed to be a tumor
suppressor (8). It also plays a
role in non-tumor cells. SPRR3 acts to promote cell survival in
vascular smooth muscle cells (9),
and proliferation and matrix synthesis of cardiac fibroblast
(10). However, the role of SPRR3
in ccRCC remains to be elucidated. In previous years, microRNA
(miRNA) dysfunction has been revealed to be involved in the
pathogenesis of various cancer types (11). MiRNAs can downregulate target gene
expression by specifically binding to the 3'untranslated region
(3'UTR). miR-338-3p inhibits the proliferation, migration and
invasion of ccRCC cells (12-14);
however, the underlying mechanism remains unclear, and to the best
of our knowledge no study has reported the association between
miR-338-3p and SPRR3. Activation of the PI3K/Akt signaling pathway
promotes ccRCC progression (15).
Furthermore, this pathway mediates the regulation of RCC cells by
miR-338-3p (16). However, it
remains unclear whether this pathway is regulated by SPRR3 in ccRCC
cells.
The present study aimed to evaluate the relationship
between SPRR3 expression and ccRCC prognosis and to detect its
expression in the normal human renal cell line HK-2 and ccRCC cell
lines. Furthermore, the current study aimed to evaluate the roles
of SPRR3 in the tumor phenotypes of 786-O cells, including their
proliferation, migration and invasion. Notably, this study aimed to
further investigate the upstream and downstream regulatory
mechanisms of SPRR3.
Materials and methods
Bioinformatics analysis
UALCAN (http://ualcan.path.uab.edu/) (17), a web portal for analyzing cancer
data (project ID: TCGA-KIRC) from The Cancer Genome Atlas TCGA
database (18), was used to assess
the relationship between SPRR3 expression and prognosis in patients
with ccRCC. The public prediction platform TargetScan (version 7.2;
http://www.targetscan.org/vert_72/)
was used to predict the potential miRNAs those target the 3'UTR of
SPRR3 mRNA (19).
Cell culture
HK-2 (cat. no. SCSP-511), 786-O (cat. no. TCHu186)
and CaKi-1 (cat. no. TCHu135) cell lines were purchased from the
National Collection of Authenticated Cell Cultures (Shanghai
China), whereas the UMRC-2 cell line (cat. no. HTX2941) was
purchased from Otwo Biotech Co., Ltd. The cells were cultured in
Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal
bovine serum (FBS) and 1% penicillin/streptomycin at 37˚C, under a
humidified atmosphere of 5% CO2. For each cell line,
three replicates were obtained using a parallel culture for
subsequent experiments (n=3). All cell culture reagents were
obtained from Gibco (Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from each sample (HK-2,
786-O, CaKi-1 and UMRC-2 cell lines) using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA for
SPRR3 and β-actin (ACTB) detection were synthesized using the
TransScript All-in-One First-Strand cDNA Synthesis SuperMix
(Beijing Transgen Biotech Co., Ltd.) following the manufacturer's
instructions, while qPCR was performed using TransStart®
Top Green qPCR SuperMix (Beijing TransGen Biotech Co., Ltd.). A
TaqMan miRNA assay kit (Ambion; Thermo Fisher Scientific, Inc.) was
used for the synthesis of cDNA for miR-338-3p and U6 detection and
RT-qPCR analysis. The reaction was carried out using the following
parameters: Initial denaturation at 94˚C for 10 min; 40 cycles of
denaturation at 94˚C for 5 sec; annealing at 60˚C for 15 sec;
extension at 72˚C for 10 sec; and dissociation. RT-qPCR was
performed using a LightCycler® 480 system (Roche
Diagnostics). SPRR3 and miR-338-3p levels were normalized to those
of ACTB and U6, respectively. The primers of miR-338-3p and U6 were
used following a previous study (20), and both reverse primers are
universal. The 2-∆∆Cq method was used to calculate the
relative mRNA expression levels of these genes (21). The primers used for qPCR are listed
in Table I. Each sample was run in
triplicates.
 | Table ISequences of primers used for reverse
transcription-quantitative PCR. |
Table I
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene | Forward (5'-3') | Reverse (5'-3') |
|---|
| SPRR3 |
CTTCTCTGCACAGCAGGTCC |
AGCAATTTAATGAGGGAAGAGC |
| ACTB |
CTCCATCCTGGCCTCGCTGT |
GCTGTCACCTTCACCGTTCC |
| miR-338-3p |
TGCGGTCCAGCATCAGTGAT |
CCAGTGCAGGGTCCGAGGT |
| U6 |
GCTCGCTTCGGCAGCACA |
GAGGTATTCGCACCAGAGGA |
Antibodies and drugs
The primary antibodies used for western blotting
were as follows: Anti-SPRR3 (1:1,000; cat. no. DF12751; Affinity
Biosciences, Ltd.), anti-β-actin (1:2,500; 60008-1-Ig; Proteintech
Group, Inc.), anti-phospho-pan-Akt (1:500; cat. no. AF0016;
Affinity Biosciences, Ltd.), and anti-pan-Akt (1:500; cat. no.
AF6261; Affinity Biosciences, Ltd.). The secondary HRP-conjugated
antibodies (1:5,000; goat anti-mouse, SA00001-1; goat anti-rabbit,
SA00001-2; Proteintech Group, Inc.) were used for western blotting.
Recombinant human insulin-like growth factor-1 (IGF-1) protein
(cat. no. 291-G1; R&D systems, Inc.), an agonist of the
PI3K/Akt pathway, was dissolved in DMEM and used at a concentration
of 100 ng/ml.
Western blotting
The cells were harvested and processed with RIPA
lysis buffer (CST Biological Reagents Co., Ltd.) supplemented with
phenylmethylsulphonyl fluoride (Thermo Fisher Scientific, Inc.),
protease inhibitor cocktail (TransGen Biotech Co., Ltd.) and
phosphatase inhibitor cocktail (TransGen Biotech Co., Ltd.).
Western blotting was performed as described below, and the protein
samples (20 µg) were separated on 10% gels using SDS-PAGE, and then
electro-transferred onto PVDF membranes (Immobilon-P;
MilliporeSigma). After blocking with 5% bovine serum albumin
(MilliporeSigma) in Tris-buffered saline containing 0.05% Tween 20
for 1 h at room temperature, the PVDF membranes were incubated
overnight with the primary antibodies at 4˚C. Following incubation
with the corresponding secondary antibodies for 1 h at room
temperature, protein chemiluminescence was detected using the
BeyoECL Plus kit (Beyotime Institute of Biotechnology) in a KETA GL
Imaging System (Wealtec Corp.), and the gray value of the band was
quantified using ImageJ (version 1.51; National Institutes of
Health). β-actin was used as the control.
Cell transfection
Small interfering RNAs (siRNAs) for SPRR3 knockdown
and control-siRNAs (scrambled siRNAs) were purchased from Sangon
Biotech Co., Ltd. with the sequences listed in Table II. miR-338-3p-mimics (cat. no.
MC10716) for miR-338-3p overexpression and the corresponding
control-mimics (cat. no. 4464058) were purchased from Thermo Fisher
Scientific, Inc. The human SPRR3 gene (accession no. NM_005416.3)
was cloned into pcDNA 3.1/His B (cat. no. V385-20; Thermo Fisher
Scientific, Inc.) for protein overexpression (Fig. S1), and the empty vector was
transfected as a control. Lipofectamine 3000 transfection reagent
(L3000075; Thermo Fisher Scientific, Inc.) was used to transfect
these plasmids (1 µg/ml), siRNAs (50 nM) and miRNA-mimics (50 nM)
into 786-O cells following the manufacturer's instructions. After
transfection at 37˚C for 6 h, the culture medium was replaced with
fresh medium and cells were incubated at 37˚C for another 24 h.
 | Table IISequences of siRNAs. |
Table II
Sequences of siRNAs.
| siRNA | Forward
(5'-3') | Reverse
(5'-3') |
|---|
| Control-siRNA |
UUCUCCGAACGUGUCACGUTT |
ACGUGACACGUUCGGAGAATT |
| SPRR3-siRNA-1 |
CUGAAUUAAGCAGAAAGUCUUTT |
AAGACUUUCUGCUUAAUUCAGTT |
| SPRR3-siRNA-2 |
CCCAUCUGUUUCUGUGUCUUATT |
UAAGACACAGAAACAGAUGGGTT |
Cell Counting Kit-8 (CCK-8) assay
A CCK-8 assay was used to assess the proliferation
of 786-O cells. Viable cell counts were indirectly determined by
measuring optical density (OD) values. The cells were seeded at
3x103 cells per well in a 96-well culture plate,
excluding the use of external rows and columns to avoid the edge
effects. After complete cell attachment, the cells were processed
according to the experimental requirements. When needed, IGF-1 was
used at a concentration of 100 ng/ml. After treatment for 0, 24, 48
and 72 h, 10 µl of CCK-8 (APExBIO Technology LLC) solution was
added to each well. After incubation for 2 h at 37˚C, the
absorbance was measured at 450 nm using a microplate reader (Thermo
Fisher Scientific, Inc.). The CCK-8 assay results were expressed as
OD values.
Colony formation assay
A plate colony formation assay was used to assess
the proliferation of 786-O cells. The cells were plated in 12-well
plates at a density of 200 cells/well. When needed, IGF-1 was used
at a concentration of 100 ng/ml. After incubation for 14 days at
37˚C, the colonies were washed with PBS, fixed with methanol for 10
min at -20˚C and stained with 0.5% Crystal Violet Stain Solution
(Shanghai Yeasen Biotechnology Co., Ltd.) for 10 min at room
temperature, and the number of colonies (>50 cells/colony) was
counted using ImageJ software.
Wound healing assay
A wound healing assay was used to assess 786-O
migration. The cells were cultured in a 12-well culture plate in
DMEM containing 10% FBS until the confluence reached 100%. A 200 µl
pipette tip was used to create a scratch in the middle of each
well. The medium was replaced with serum-free DMEM. When needed,
IGF-1 was used at a concentration of 100 ng/ml. Images were
captured of three random fields of view using a light microscope at
0 and 24 h. Wound area was measured using ImageJ software. The
results were presented as migration rate (%)=(initial wound
area-wound area at 24 h)/initial wound area x100.
Transwell invasion assay
Matrigel (BD Biosciences) was applied to Transwell
plates (24-well, 8 µm; Millicell; MilliporeSigma) for 30 min at
37˚C for precoating. The cells (6x104) were seeded in
the upper chambers of the Transwell plates and incubated in
serum-free DMEM, while the lower chambers were supplied with DMEM
containing 10% FBS. When needed, IGF-1 was used at a concentration
of 100 ng/ml. After incubation for 24 h at 37˚C, non-invading cells
on the upper face of the membrane were removed with a cotton swab,
and the invaded cells were fixed with methanol for 10 min at -20˚C
and stained with Giemsa dye solution (Yuanye Biotech, Co., Ltd.)
for 20 min at room temperature. The images of each well were
captured at three random fields of view using a light microscope,
and the number of cells was counted using ImageJ software.
Luciferase reporter gene assay
The luciferase reporter plasmids of wild-type SPRR3
3'UTR (Luc-WT) or mutant SPRR3 3'UTR (Luc-Mut) were constructed
using pEZX-MT06 (Guangzhou iGene Biotechnology Co., Ltd.). Empty
vector, Luc-WT and Luc-Mut were co-transfected with control-mimics
or miR-338-3p-mimics into 786-O cells seeded in 96-well plates
using Lipofectamine® 3000 transfection reagent. After 48
h of transfection, the cells were lysed. The results were
determined using the Luc-Pair™ Duo-Luciferase HS Assay kit
(Guangzhou iGene Biotechnology Co., Ltd.). The data were normalized
by comparison with Renilla luciferase activity.
Statistical analysis
The quantified data were expressed as mean ±
standard deviation. Data were analyzed using one-way ANOVA followed
by post hoc Dunnet's or Sidak's test, and unpaired Student's t-test
with GraphPad Prism statistical package (version 7.00; GraphPad
Software Inc.). P<0.05 was considered to indicate a
statistically significant difference. All experiments were repeated
at least twice to verify the trends.
Results
SPRR3 is a risk factor for the
survival of patients with ccRCC that is upregulated in ccRCC cell
lines
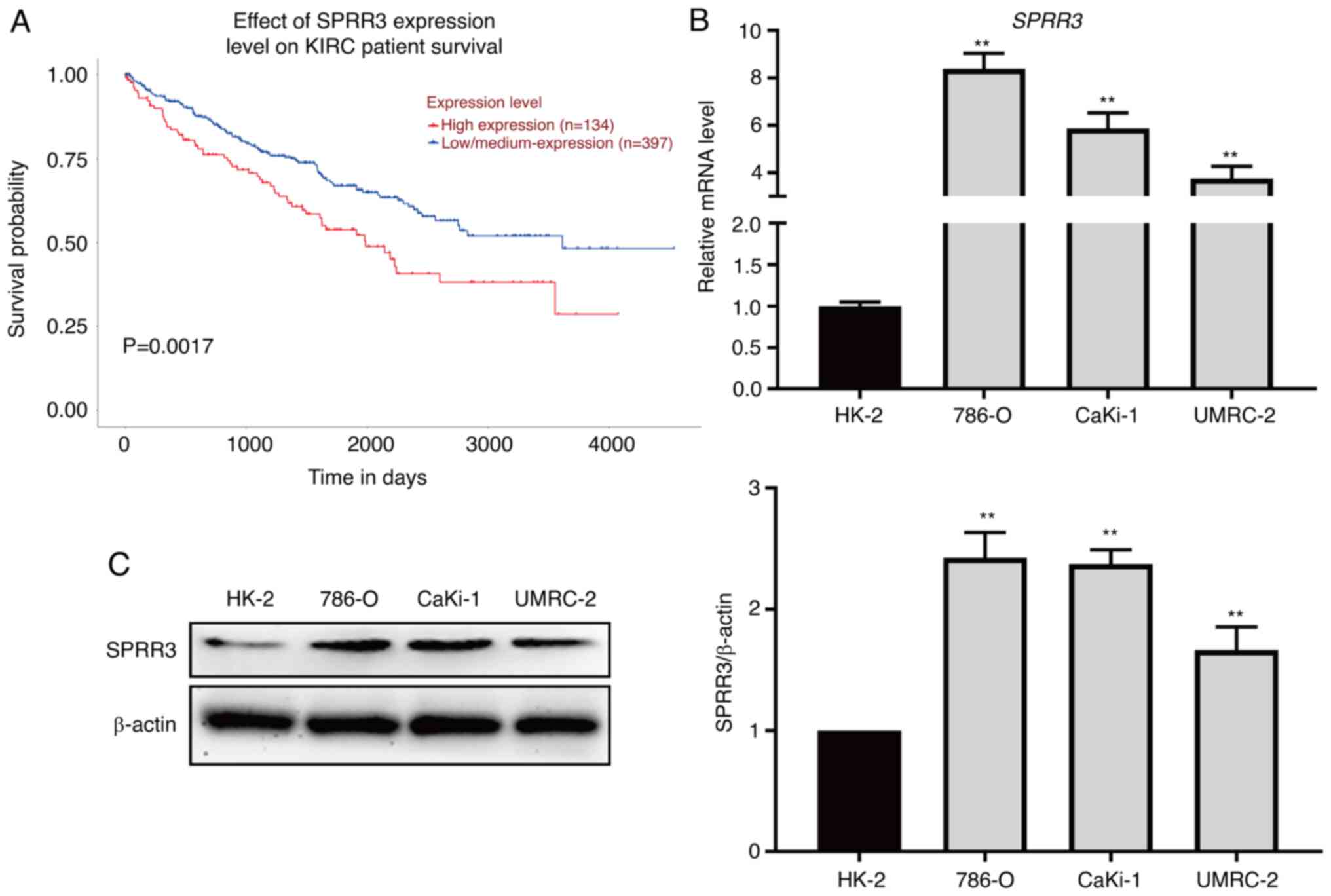
To evaluate the relationship between SPRR3
expression and the prognosis of ccRCC, a survival analysis was
performed using the UALCAN website based on the TCGA-KIRC database
(containing 531 samples). Patients with low or medium SPRR3
expression levels had improved prognosis compared with those with
high SPRR3 expression levels (P=0.0017; Fig. 1A). To further evaluate the SPRR3
expression levels in ccRCC, RT-qPCR and western blotting were
performed to detect the mRNA and protein levels of SPRR3,
respectively, in HK-2, 786-O, CaKi-1 and UMRC-2 cell lines. The
mRNA levels of SPRR3 were significantly higher in ccRCC cell lines
compared with that in the normal renal cell line HK-2 (Fig. 1B). The results of western blotting
were consistent with those of RT-qPCR (Fig. 1C). Overall, these data suggested
that SPRR3 was a risk factor for ccRCC and was upregulated in ccRCC
cell lines.
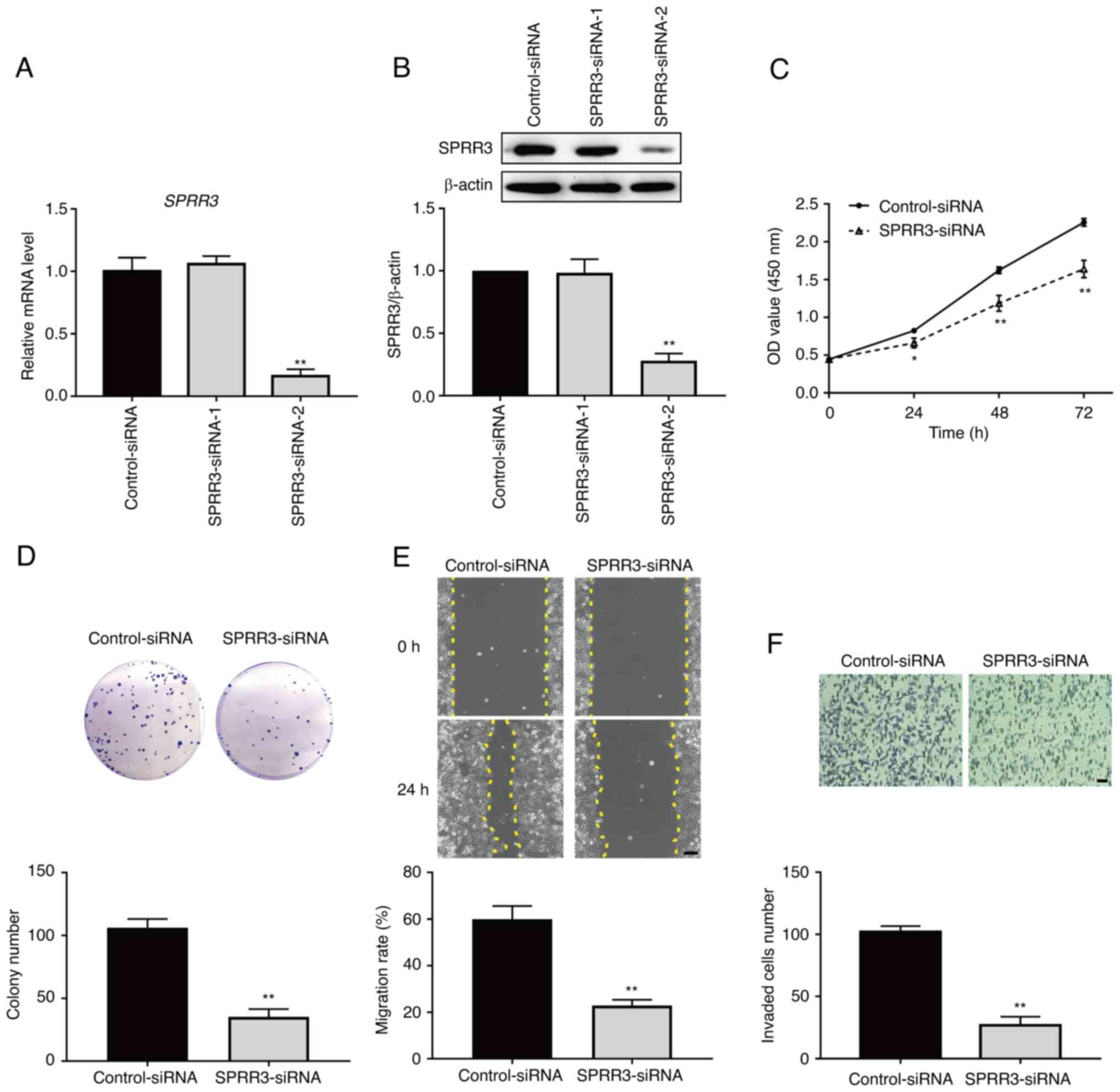
SPRR3 promotes the proliferation,
migration and invasion of ccRCC cells
To evaluate the role of SPRR3 in determining the
tumor phenotypes of ccRCC cells, the effects of SPRR3 knockdown on
the proliferation, migration and invasion of 786-O cells were
measured. The SPRR3-siRNA was initially transfected into 786-O
cells to knockdown SPRR3 expression. RT-qPCR and western blotting
were performed to screen for the effective siRNAs, and the results
showed that SPRR3-siRNA-2 significantly decreased the mRNA and
protein levels of SPRR3 (Fig. 2A
and B). Therefore, siRNA-2 was
selected to be used in all subsequent experiments. Next, the
results of the CCK-8 assay indicated that the viability of 786-O
cells was significantly decreased in the knockdown group compared
with the control group at each time point (Fig. 2C). The results of the colony
formation assay demonstrated that the colony number of 786-O cells
in the knockdown group was significantly decreased compared with
that in the control group (Fig.
2D). The results of the wound healing assay indicated that the
migration rate of 786-O cells in the knockdown group was
significantly decreased compared with that in the control group
(Fig. 2E). The Transwell invasion
assay indicated that the number of invaded 786-O cells per field in
the knockdown group was significantly decreased compared with that
in the control group (Fig. 2F).
These data demonstrated that SPRR3 promoted the proliferation,
migration and invasion of ccRCC cells.
SPRR3 regulates the tumor phenotypes
of ccRCC cells via the PI3K/Akt pathway
To further explore the downstream signaling pathway
responsible for theSPRR3-regulated phenotypes of ccRCC cells, the
present study investigated whether the PI3K/Akt pathway was
involved in this regulation. The level of phosphorylation of Akt,
measured based on the ratio of phosphorylated Akt (p-Akt)/total Akt
(t-Akt), was used as an indicator of the activation status of the
PI3K/Akt pathway. The effect of SPRR3 knockdown on the PI3K/Akt
pathway was initially examined. As presented in Fig. 3A, compared with the control group,
the level of p-Akt was significantly decreased, and no significant
change was observed in the level of t-Akt in the knockdown group.
The ratio of p-Akt/t-Akt was significantly decreased in the
knockdown group, suggesting that SPRR3 knockdown inhibited the
PI3K/Akt pathway in 786-O cells. Next, IGF-1, a known agonist of
the PI3K/Akt pathway, was used to verify whether this signaling
pathway was involved in SPRR3-regulated 786-O cell proliferation,
migration and invasion. As presented in Fig. 3B, IGF-1 significantly increased the
level of p-Akt/t-Akt compared with the control group, and
significantly reversed the decreased level of p-Akt/t-Akt led by
SPRR3 knockdown, thus confirming the validity of IGF-1. The results
of the CCK-8 assay demonstrated that activation of the PI3K/Akt
pathway significantly reversed the SPRR3 knockdown-induced
reduction in the viability of 786-O cells at each time point
(Fig. 3C). The results of the
colony formation assay indicated that activation of the PI3K/Akt
pathway significantly reversed the SPRR3 knockdown-induced decrease
in the colony number of 786-O cells (Fig. 3D). The results of the wound healing
assay showed that activation of the PI3K/Akt pathway significantly
reversed the SPRR3 knockdown-induced decrease in the migration rate
of 786-O cells (Fig. 3E). The
results of the Transwell invasion assay showed that activation of
the PI3K/Akt pathway significantly reversed the SPRR3
knockdown-induced decrease in invaded 786-O cells per field
(Fig. 3F). Overall, these data
demonstrated that SPRR3 regulated the tumor phenotypes of ccRCC
cells via the PI3K/Akt pathway.
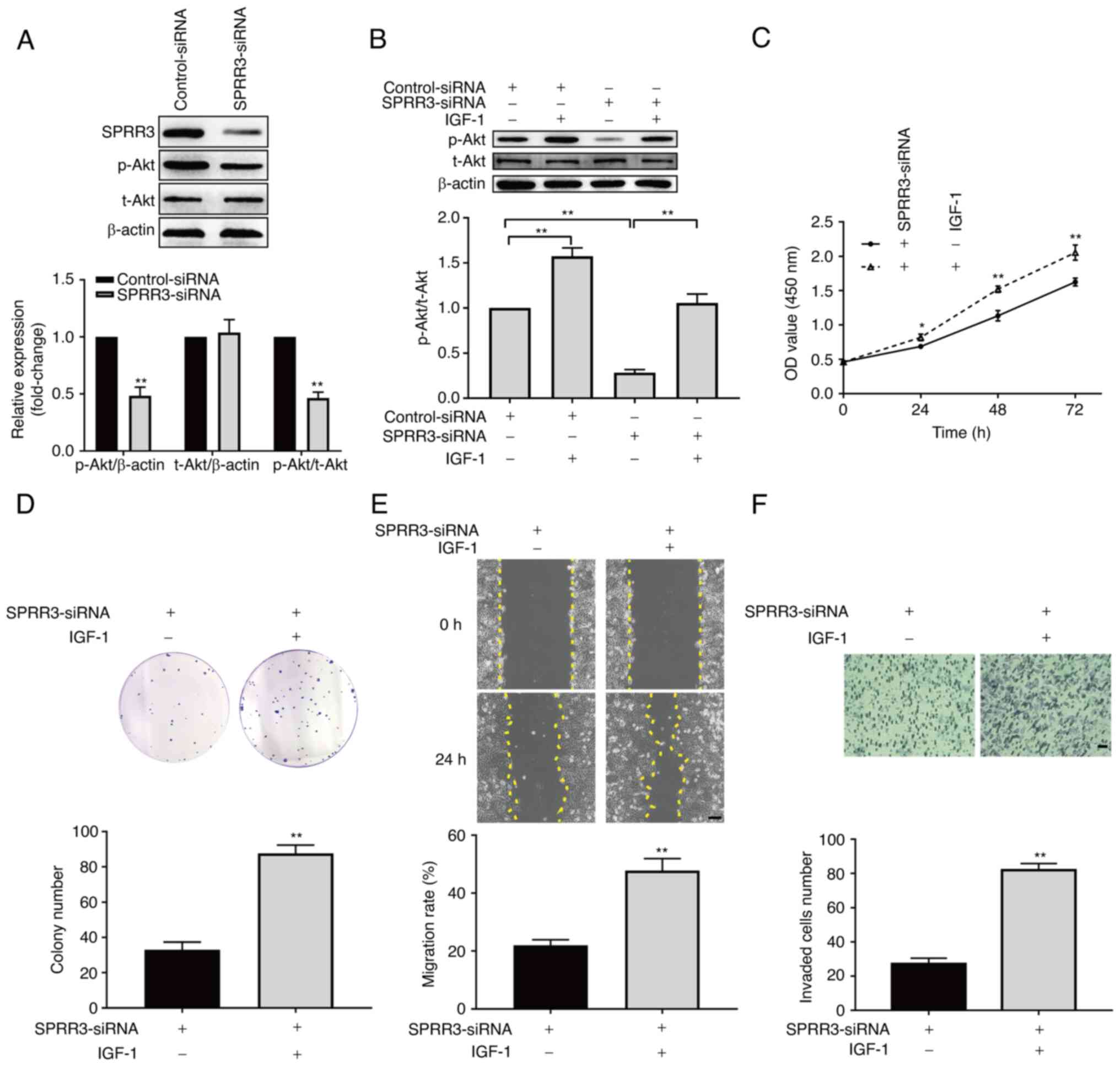 | Figure 3Involvement of the PI3K/Akt pathway in
SPRR3-mediated regulation of ccRCC cells. (A) Effects of SPRR3
knockdown on the PI3K/Akt pathway, as detected using western
blotting. (B) Confirming the validity of IGF-1 as an agonist of the
PI3K/Akt pathway, as detected using western blotting. Effects of
the PI3K/Akt pathway activation on SPRR3 knockdown-induced
inhibition of ccRCC cell proliferation, as detected using (C) Cell
Counting Kit-8 assay and (D) colony formation assay. (E) Effects of
the PI3K/Akt pathway activation on SPRR3 knockdown-induced
inhibition of ccRCC cell migration, as detected using wound healing
assay (yellow dashed lines denote the wound edge; scale bar, 100
µm). (F) Effects of the PI3K/Akt pathway activation on SPRR3
knockdown-induced inhibition of ccRCC cell invasion, as detected
using Transwell invasion assay (scale bar, 50 µm).
*P<0.05 and **P<0.01 vs. corresponding
control. SPRR3, small proline-rich repeat protein 3; ccRCC, clear
cell renal cell carcinoma; siRNA, small interfering RNA; p-,
phosphorylated; t-, total; OD, optical density. |
miR-338-3p directly targets SPRR3 and
negatively regulates SPRR3 expression
To explore the upstream regulator of SPRR3,
TargetScan was used to predict the potential functional miRNAs. The
binding site between SPRR3 mRNA 3'UTR and miR-338-3p was predicted
using TargetScan (Fig. 4A). The
expression levels of miR-338-3p in HK-2, 786-O, CaKi-1 and UMRC-2
cell lines were determined using RT-qPCR, which revealed that the
levels of miR-338-3p were significantly lower in ccRCC cell lines
compared with HK-2 cells (Fig.
4B). Transfection of miRNA mimics for miR-338-3p overexpression
was used to verify its association with SPRR3 in 786-O cells. As
presented in Fig. 4C, the RT-qPCR
results showed that the level of miR-338-3p was significantly
increased in the mimics group compared with the control group,
which confirmed that the miR-338-3p-mimics were effective. The mRNA
and protein levels of SPRR3 were further investigated, which
revealed that the expression levels of SPRR3 mRNA and protein were
significantly decreased in the mimic groups compared with those in
the control group (Fig. 4D and
E), which indicated that
miR-338-3p inhibited SPRR3 expression in 786-O cells. WT and
mut-type SPRR3 3'UTR luciferase reporter vectors were used to
perform a dual-luciferase reporter assay to confirm the binding
relationship and position between SPRR3 mRNA and miR-338-3p
(Fig. 4A). As presented in
Fig. 4F, in the Luc-WT
transfection group, the relative luciferase activity of 786-O cells
transfected with miR-338-3p-mimics was significantly lower compared
with that of cells transfected with control-mimics; however, no
significant change was observed in the Luc-Mut transfection groups.
These data demonstrated that miR-338-3p directly targeted SPRR3 and
negatively regulated SPRR3 expression in ccRCC cells.
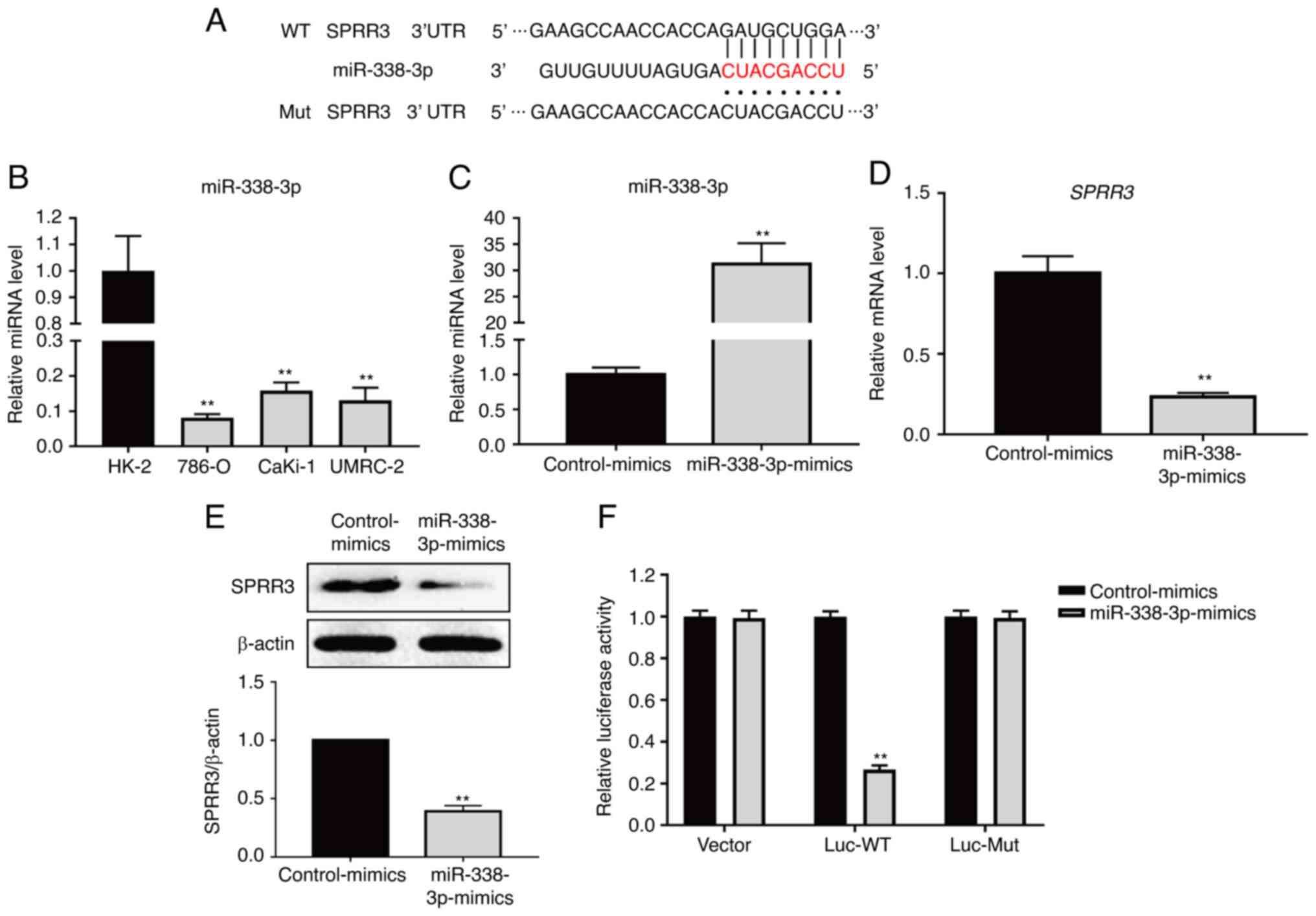 | Figure 4Relationship between miR-338-3p and
SPRR3 in ccRCC cells. (A) Sequence alignment of miR-338-3p with
SPRR3 3'UTR-WT and SPRR3 3'UTR-Mut. (B) miR-338-3p expression in
HK-2, 786-O, CaKi-1 and UMRC-2 cell lines, as detected using
RT-qPCR. (C) Confirming the validity of miR-338-3p mimics
transfection, as detected using RT-qPCR. Effects of miR-338-3p
overexpression on the SPRR3 expression in ccRCC cells, as detected
using (D) RT-qPCR and (E) western blotting. (F) Relative luciferase
activities were detected by the dual-luciferase reporter assay
system. **P<0.01 vs. corresponding control. RT-qPCR,
reverse transcription-quantitative PCR; SPRR3, small proline-rich
repeat protein 3; ccRCC, clear cell renal cell carcinoma; miR,
microRNA; UTR, untranslated region; WT, wild-type; Mut,
mutated. |
miR-338-3p inhibits the PI3K/Akt
pathway and tumor phenotypes of ccRCC cells by downregulating SPRR3
expression
Based on the aforementioned findings, the present
study further investigated whether miR-338-3p inhibited the
PI3K/Akt pathway and the tumor phenotypes of ccRCC cells by
downregulating SPRR3. The overexpression efficiency of SPRR3
plasmids was validated using RT-qPCR and western blotting, and the
results showed that the mRNA and protein levels of SPPR3 were
significantly increased in the overexpression group (Fig. 5A and B). The p-Akt/t-Akt ratio was
significantly decreased in the miR-338-3p-mimics group compared
with that in the control group, which confirmed that miR-338-3p
inhibited the PI3K/Akt pathway in 786-O cells (Fig. 5C). Rescue experiments were carried
out. SPRR3 overexpression significantly reversed the downregulation
of SPRR3 by the miR-338-3p-mimics, under which downstream pathway
and tumor phenotypes regulated by the miR-338-3p-mimics were
observed. As presented in Fig. 5C,
SPRR3 overexpression significantly reversed the reduction of
p-Akt/t-Akt by the miR-338-3p-mimics. Additionally, the results of
the CCK-8 assay showed that the overexpression of SPRR3
significantly reversed the miR-338-3p-mimics-induced inhibition of
786-O cell proliferation (Fig.
5D). The results of the colony formation assay showed that the
overexpression of SPRR3 significantly reversed the
miR-338-3p-mimics-induced decrease in the colony number of 786-O
cells (Fig. 5E). The results of
the wound healing assay showed that the overexpression of SPRR3
significantly reversed the miR-338-3p-mimics-induced decrease in
the migration rate of 786-O cells (Fig. 5F). The results of the Transwell
invasion assay showed that SPRR3 overexpression significantly
reversed the miR-338-3p-mimics-induced decrease in the number of
invaded cells per field (Fig. 5G).
These data demonstrated that miR-338-3p inhibited the PI3K/Akt
pathway and the tumor phenotypes of ccRCC cells by downregulating
SPRR3.
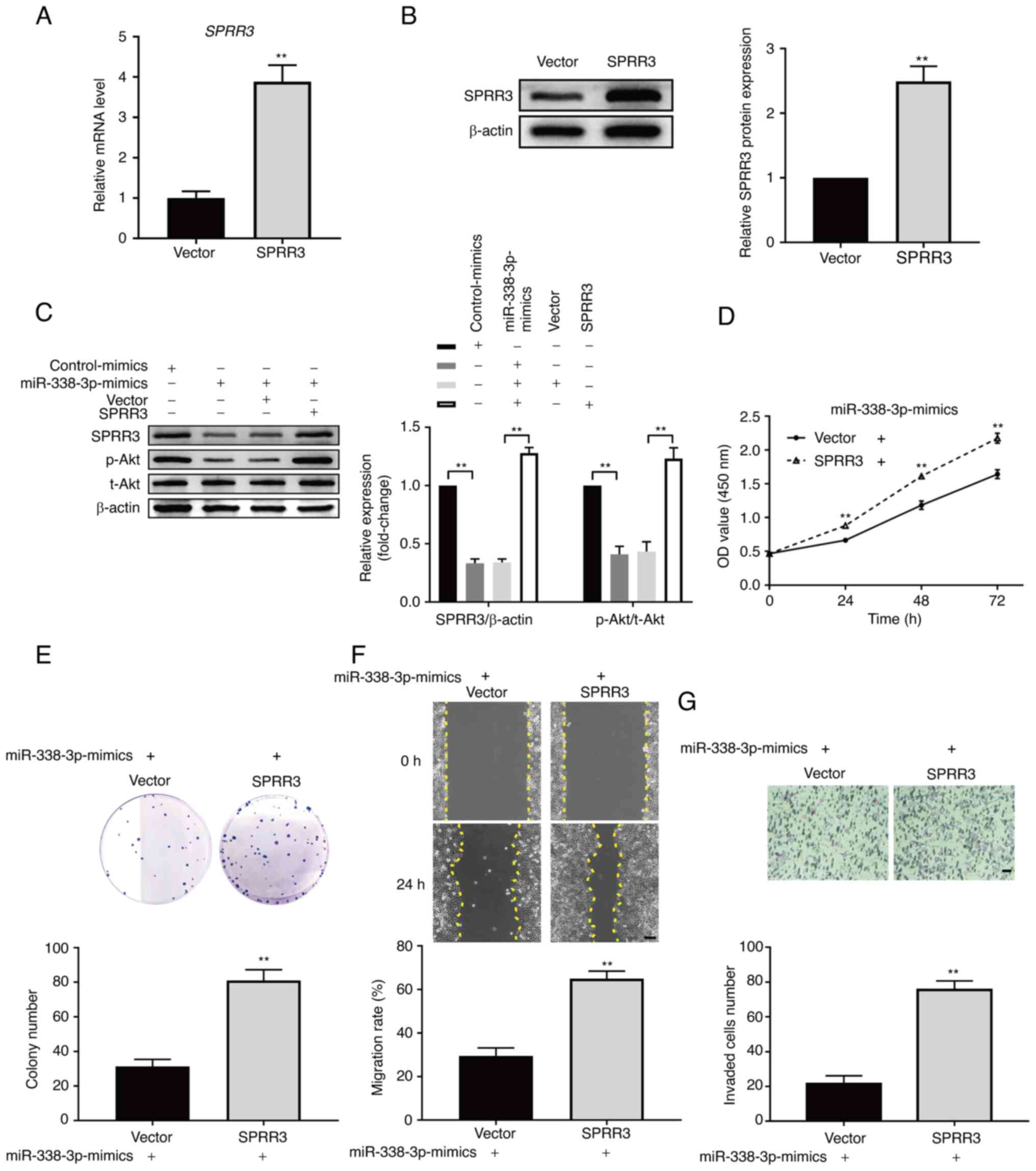 | Figure 5Role of miR-338-3p in SPRR3-mediated
regulation of ccRCC cells. Confirming the validity of SPRR3
overexpression plasmid, as detected using (A) reverse
transcription-quantitative PCR and (B) western blotting. (C)
Effects of upregulating miR-338-3p on the PI3K/Akt pathway, and
effects of SPRR3 overexpression on this regulation, as detected
using western blotting. Effects of SPRR3 overexpression on the
upregulation of miR-338-3p-induced inhibition of ccRCC cell
proliferation, as detected using (D) Cell Counting Kit-8 assay and
(E) colony formation assay. (F) Effects of SPRR3 overexpression on
upregulation of miR-338-3p-induced inhibition of ccRCC cell
migration, as detected using wound healing assay (yellow dashed
lines denote the wound edge; scale bar, 100 µm). (G) Effects of
SPRR3 overexpression on the upregulation of miR-338-3p-induced
inhibition of ccRCC cell invasion, as detected using Transwell
invasion assay (scale bar, 50 µm). **P<0.01 vs.
corresponding control. SPRR3, small proline-rich repeat protein 3;
ccRCC, clear cell renal cell carcinoma; miR, microRNA; p-,
phosphorylated; t-, total; OD, optical density. |
Discussion
To the best of our knowledge, this study
demonstrated the role of SPRR3 in determining the tumor phenotypes
of ccRCC cells for the first time, and further uncovered its
associated downstream and upstream mechanisms. SPRR3 as a tumor
promoter has been revealed to be upregulated in some types of
cancer. SPRR3 accelerates the proliferation and invasion of
colorectal cancer cells (4), the
proliferation of breast cancer cells (5), the proliferation and invasion of
glioblastoma multiforme cells (6)
and the proliferation and invasion of non-small-cell lung cancer
cells (7). These results are
similar to those reported in the present study, which suggested the
expression of SPRR3 as a poor prognostic factor and a potential
novel therapeutic target for ccRCC. By contrast, several studies on
esophageal cancer have revealed that low expression of SPRR3 is
associated with disease progression (8,22,23).
These findings indicate that the role of SPRR3 may vary in
different cancer types. Additionally, both loricrin and SPRR3 are
cornified cell envelope precursor proteins, and their genes locates
in the same cluster on chromosome 1q21(24). Loricrin is reported to be
associated with tumor metastatic spread. This may indicate that
loricrin is involved in SPRR3-mediated regulation in ccRCC
(25). However, the role of SPRR3
in other diseases still needs to be explored further.
miR-338-3p is downregulated in RCC (26), which was also confirmed by the
ccRCC cell lines in the present study. Furthermore, previous
studies have uncovered several downstream molecules involved in
miR-338-3p-mediated inhibition of tumor phenotypes in ccRCC cells.
Tong et al (12) revealed
that miR-338-3p inhibits the proliferation and invasion of 786-O
and Caki-1 cells by targeting SOX-4. Yang et al (13) revealed that miR-338-3p inhibits the
proliferation, migration and invasion of ccRCC cells by
downregulating the expression of ETS1. Zhu et al (14) revealed that CAV-1 is also a
downstream target of miR-338-3p in ccRCC cells. The present study
demonstrated that SPRR3 was a novel direct target of miR-338-3p,
and that miR-338-3p inhibited the proliferation, migration and
invasion of ccRCC cells by downregulating SPRR3. In addition to
these findings, more research is needed to investigate the how the
downstream mechanism of miR-338-3p involved in the development of
ccRCC. The association between miR-338-3p and survival of patients
with ccRCC also warrants further investigation.
The aberrant activation of the PI3K/Akt signaling
pathway promotes the proliferation, migration and invasion of ccRCC
cells (15). SPRR3 activates the
PI3K/Akt pathway in colorectal and breast cancer cells (4,5).
Additionally, SPRR3 activates this pathway in vascular smooth
muscle cells and cardiac fibroblasts (9,10).
miR-338-3p inhibits the PI3K/Akt pathway in RCC (16), which is consistent with the results
of the present study showing miR-338-3p-mediated inhibition of the
PI3K/Akt pathway in 786-O cells. Moreover, miR-338-3p exerts its
anti-tumor effects by inhibiting the PI3K/Akt pathway in breast
cancer and neuroblastoma cells (27,28).
The present study demonstrated that the PI3K/Akt pathway was
involved in the regulation of ccRCC cell tumor phenotypes via the
miR-338-3p/SPRR3 axis, which presented a new model of the mechanism
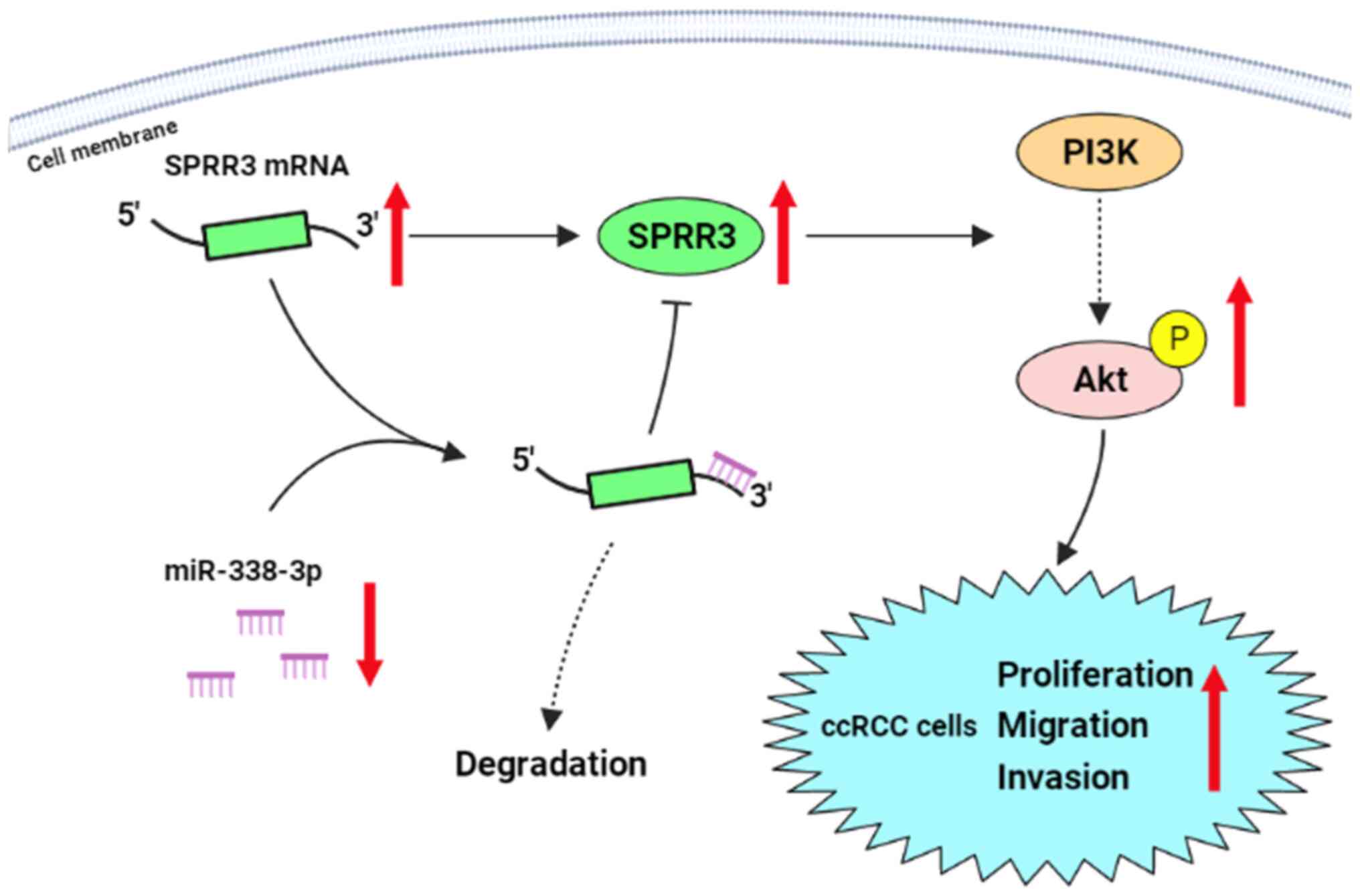
driving ccRCC cell development (Fig.
6). Based on these findings, it was hypothesized that this
regulation may occur in other tumors, although this needs to be
verified. Additionally, further in vivo animal experiments
are needed to verify the present findings.
In conclusion, the current study demonstrated that
the upregulation of SPRR3 played a promotive role in the
proliferation, migration and invasion of ccRCC cells in
vitro, which was directly regulated by the downregulation of
miR-338-3p; moreover, the activation of the PI3K/Akt pathway was
involved in this regulation. This finding not only enriches the
understanding of the mechanism understanding ccRCC development, but
also presents a potential novel therapeutic target for this
disease.
Supplementary Material
Construction of the SPRR3 expression
plasmid. SPRR3, small proline.rich repeat protein 3.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the PhD Research Project of
Jilin Engineering Normal University (grant no. BSKJ201923).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW and XL conceived and designed the study. MW and
QG performed the experiments, which were guided and supervised by
LW and XL. MW and XL performed the data analysis. MW completed the
first draft of the manuscript. LW and XL revised the final version
of the manuscript. MW, XL and LW confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
R, Torre L and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Powles T, Albiges L, Staehler M, Bensalah
K, Dabestani S, Giles RH, Hofmann F, Hora M, Kuczyk MA, Lam TB, et
al: Updated European association of urology guidelines:
Recommendations for the treatment of first-line metastatic clear
cell renal cancer. Eur Urol. 73:311–315. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ljungberg B, Bensalah K, Canfield S,
Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L,
Merseburger AS, et al: EAU guidelines on renal cell carcinoma: 2014
update. Eur Urol. 67:913–924. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cho DH, Jo YK, Roh SA, Na YS, Kim TW, Jang
SJ, Kim YS and Kim JC: Upregulation of SPRR3 promotes colorectal
tumorigenesis. Mol Med. 16:271–277. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kim JC, Yu JH, Cho YK, Jung CS, Ahn SH,
Gong G, Kim YS and Cho DH: Expression of SPRR3 is associated with
tumor cell proliferation in less advanced stages of breast cancer.
Breast Cancer Res Treat. 133:909–916. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu Q, Zhang C, Ma G and Zhang Q:
Expression of SPRR3 is associated with tumor cell proliferation and
invasion in glioblastoma multiforme. Oncol Lett. 7:427–432.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li Q, Wang Y, Hu R and Yang G:
Dysregulation of SPRR3/miR-876-3p axis contributes to tumorigenesis
in non-small-cell lung cancer. Onco Targets Ther. 13:2411–2419.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang Y, Feng YB, Shen XM, Chen BS, Du XL,
Luo ML, Cai Y, Han YL, Xu X, Zhan QM, et al: Exogenous expression
of Esophagin/SPRR3 attenuates the tumorigenicity of esophageal
squamous cell carcinoma cells via promoting apoptosis. Int J
Cancer. 122:260–266. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Segedy AK, Pyle AL, Li B, Zhang Y, Babaev
VR, Jat P, Fazio S, Atkinson JB, Linton MF and Young PP: .
Identification of small proline-rich repeat protein 3 as a novel
atheroprotective factor that promotes adaptive Akt signaling in
vascular smooth muscle cells. Arterioscler Thromb Vasc Biol.
34:2527–2536. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Saraswati S, Lietman C, Li B, Mathew S,
Zent R and Young P: Small proline-rich repeat 3 is a novel
coordinator of PDGFRβ and integrin β1 crosstalk to augment
proliferation and matrix synthesis by cardiac fibroblasts. FASEB J.
34:7885–7904. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Harrandah A, Mora R and Chan E: Emerging
microRNAs in cancer diagnosis, progression, and immune
surveillance. Cancer Lett. 438:126–132. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tong Z, Meng X, Wang J and Wang L:
MicroRNA-338-3p targets SOX4 and inhibits cell proliferation and
invasion of renal cell carcinoma. Exp Ther Med. 14:5200–5206.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yang X, Zhang Y and Fan H: Downregulation
of SBF2-AS1 functions as a tumor suppressor in clear cell renal
cell carcinoma by inhibiting miR-338-3p-targeted ETS1. Cancer Gene
Ther. 28:813–827. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhu Q, Zhan D, Zhu P, Chong Y and Yang Y:
CircAKT1 acts as a sponge of miR-338-3p to facilitate clear cell
renal cell carcinoma progression by up-regulating CAV1. Biochem
Biophys Res Commun. 532:584–590. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Peng X, Yang J, Qiang Y, Sun R, Cao Y,
Zheng LS, Peng LX, Lang YH, Mei Y, Li CZ, et al: PTPN3 inhibits the
growth and metastasis of clear cell renal cell carcinoma via
inhibition of PI3K/AKT signaling. Mol Cancer Res. 18:903–912.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li G, Chong T, Yang J, Li H and Chen H:
Kinesin motor protein KIFC1 is a target protein of miR-338-3p and
is associated with poor prognosis and progression of renal cell
carcinoma. Oncol Res. 27:125–137. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cancer Genome Atlas Research Network.
Comprehensive molecular characterization of clear cell renal cell
carcinoma. Nature. 499:43–49. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Agarwal V, Bell G, Nam J and Bartel D:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4(e05005)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang L, Sui M and Wang X: miR-338-3p
suppresses the malignancy of T-cell lymphoblastic lymphoma by
downregulating HOXA3. Mol Med Rep. 20:2127–2134. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen BS, Wang MR, Cai Y, Xu X, Xu ZX, Han
YL and Wu M: Decreased expression of SPRR3 in Chinese human
oesophageal cancer. Carcinogenesis. 21:2147–2150. 2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
de A Simão T, Souza-Santos PT, de Oliveira
DS, Bernardo V, Lima SC, Rapozo DC, Kruel CD, Faria PA, Ribeiro
Pinto LF and Albano RM: Quantitative evaluation of SPRR3 expression
in esophageal squamous cell carcinoma by qPCR and its potential use
as a biomarker. Exp Mol Pathol. 91:584–589. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hohl D, de Viragh P, Amiguet-Barras F,
Gibbs S, Backendorf C and Huber M: The small proline-rich proteins
constitute a multigene family of differentially regulated cornified
cell envelope precursor proteins. J Invest Dermatol. 104:902–909.
1995.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ellis R, Tang D, Nasr B, Greenwood A,
McConnell A, Anagnostou ME, Elias M, Verykiou S, Bajwa D, Ewen T,
et al: Epidermal autophagy and beclin 1 regulator 1 and loricrin: A
paradigm shift in the prognostication and stratification of the
American joint committee on cancer stage I melanomas. Br J
Dermatol. 182:156–165. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Huang Y, Wu Y, Zeng L, Shan W and Huang L:
The tumor suppressor role of microRNA-338-3p in renal cell
carcinoma. Oncol Lett. 16:2195–2200. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
He J, Wang J, Li S, Li T, Chen K and Zhang
S: Hypoxia-inhibited miR-338-3p suppresses breast cancer
progression by directly targeting ZEB2. Cancer Sci. 111:3550–3563.
2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Xu Z, Sun Y, Wang D, Sun H and Liu X:
SNHG16 promotes tumorigenesis and cisplatin resistance by
regulating miR-338-3p/PLK4 pathway in neuroblastoma cells. Cancer
Cell Int. 20(236)2020.PubMed/NCBI View Article : Google Scholar
|