Introduction
The anatomic area represented by the nose, paranasal
sinuses and pharynx is frequently the site of appearance for tumors
(benign or malign) of various histopathologic origin. Some of these
tumors are, however, rare and unusual and require special attention
in diagnostic and therapeutic management. All cases presented are
of uncommon benign tumors of the nose, sinus or pharynx associated
with a high risk of changing from benign to malignant and of
unclear etiology. Environmental, and clinical risk factors cannot
always be ruled out and the clinician must always consider that
genetic predisposition is commonly augmented and complicated by
environmental factors (1).
Hemangiomas are benign tumors that originate from
vascular endothelial proliferation. They are relatively common in
the head and neck (>50%) but rare in the nasal cavity and
paranasal sinuses and can originate from vessels in numerous types
of tissue, such as the skin, mucosae, bone, muscle and glands
(2). The nasal cavity is
occasionally the site of appearance (2); hemangiomas represent ~20% of all
benign tumors of the nasal cavity and of these, 65% are located on
the septum, 18% on the lateral wall and 16% in the vestibule
(3). They typically arise from
soft tissue (skin, mucosa, vessels) and although they may cause
bony changes or destruction, rarely arise from bone (4). Although rare, this tumor must be
considered in the differential diagnosis of intra-nasal bleeding
mass (bleeding polyps of the septum, angio-fibromatous polyp)
(5).
Capillary hemangiomas are more frequently observed
than the cavernous type. The capillary type is more frequently
associated with the nasal septum site whereas the cavernous type
appears more frequently on the lateral wall of the nasal cavity
(5).
Pilomatrixoma, also termed calcifying epithelioma of
Malherbe or Epithelioma calcificans Malherbe, is a relatively
uncommon benign tumor of the skin derived from the hair matrix
cells. In 1880, when it was first described by Malherbe and
Chenantais (6) it was believed to
arise from sebaceous glands (7)
but in 1961, Forbis and Helwig (8)
discovered its origin in hair matrix cells and proposed the term
pilomatrixoma to avoid a connotation of malignancy. Similarly, to
the other two rare tumors in the present article, the tumor
commonly (but not exclusively) occurs in children as a hard
subcutaneous nodule or cyst with unremarkable overlying epidermis
0.5-3.0 cm in size, with the largest reported case at 24 cm
(9); it is typically located on
the scalp, face and upper extremities. Excluding lymph nodes, it is
the second most excised superficial mass in children after
epidermoid cysts (10). However,
pilomatrixomas can be easily misdiagnosed and/or missed in
differential diagnosis (6).
Clinical findings will aid in an accurate diagnosis. Surgical
removal is curative but incomplete excision can lead to recurrence,
although rare. Malignancy has been rarely reported (6). The tumor is also relatively frequent
in dogs; Kerry Blue and soft-coated Wheaton Terriers, standard
poodles and Old English sheepdogs exhibit increased susceptibility
(11).
Inverted Schneiderian papilloma has been considered
the best term to describe the tumor properties of inversion,
location, and distinctiveness of character (12). The first to describe it was Ward in
1854 but Billroth was credited with describing the first true
papilloma of the nasal cavity and called it villiform cancer
(13). Other names such as
fungiform papilloma, cylindrical or transitional papilloma have
also been used in literature. Papillomas are rare benign tumors
originating from the Schneiderian respiratory membrane and can be
classified into three distinctive types: Exophytic, oncocytic and
inverted papilloma (14-16).
The tumors are locally aggressive (bone destruction), have a high
recurrence rate if partially removed and exhibit a tendency for
malignant transformation into squamous cell carcinoma (likelihood,
≤20%) (17). The age of the
patients may vary from 10 to 87 but the majority of cases present
at 50-70 years with a male:female ratio of 3.3:1(18). The etiology remains controversial
but factors such as human papillomavirus infection, chronic
inflammation, allergy, occupational pollution (sulfur, tobacco) are
considered key (15,16,19).
The recommended treatment includes complete surgical resection and
life-long follow-up for potential recurrence (16,20-23).
The present study aimed to present the authors'
experience in treating this type of pathology as well as reporting
on curious turn of events that these tumors can take (e.g.,
auto-resection of hematoma) with the hope that it proves useful to
other ENT-HNS professionals.
Materials and methods
The present study was approved by Research Ethics
Committee of the Faculty of Medicine, Titu Maiorescu University
(approval no. 6/21.09.2021; Bucharest, Romania). All patients
provided written informed consent and approved the publication of
their data.
CT evaluation
All tumors were assessed by clinical, imagistic and
histopathological examination at the Ilfov County Clinical
University Hospital, Bucharest, Romania, between May 2015 and
August 2019. The patients underwent complete ear, nose and throat
(ENT) examination, complete with endoscopy (where possible). In two
of the presented cases, the hemangioma and inverted Schneiderian
papilloma underwent classic image evaluation via enhanced computed
tomography of the sinus and nasal cavity. The standard protocol was
applied: Patient in supine position, scout perpendicular to the
hard palate, tube voltage and tube current 125 kV and 80-160 mAs
with scan from the hard palate to above the end of the frontal
sinus. Scan direction was caudocranial (slice thickness,
0.625-1.000 mm) to obtain axial and coronal images (24).
Ultrasonography evaluation
The pilomatrixoma was assessed by an experienced ENT
specialist with ultrasonography competence using an Acuson 128XP
scanner (Siemens Medical Solutions) equipped with a 7- to 12-MHz
linear array transducer. The longitudinal and transverse scans of
the mass were obtained with gray scale and power Doppler
ultrasonography. Then two experienced radiologists evaluated the
printed images and gave the same description regarding tumor size,
shape, margin, echo texture, echogenicity, presence, shape, and
amount of calcification, presence of a hypoechoic rim and Doppler
flow pattern. Size was defined as the largest tumor diameter. Tumor
echo texture was described as homogeneous or heterogeneous, and
echogenicity was described as hyperechoic if tumor echogenicity was
higher than that of muscle.
Histopathological evaluation
All cases were histologically diagnosed by two
independent pathologists, under light microscope at 20, 100 and
200x magnifications, using hematoxylin-eosin staining. Fixation was
achieved with 10% formaldehyde buffered solution at room
temperature for 24 h. The slicing of wax-embedded material
(resected tumor) was performed by ultramicrotome at 3 µm. Staining
was performed according to the basic protocol: Dewaxing (xylene 3-5
min); Dehydration (ethanol at 100, 100 and 95% for 2 min each,
water wash for 2 min); hematoxylin (pre-prepared solution) staining
at room temperature (3-6 min depending on sample size followed by
water wash for 1 min); differentiation (mild acid for 1 min and
water wash for 1 min); bluing 1 min followed by water wash 1 min,
95% ethanol 1 min and water wash 1 min; eosin, 45 sec, dehydration
in ascending alcohol (95, 100 and 100% for 1 min each); clearing
(xylene 2-4 min) and cover-slipping (Canada Balsam). No
immunohistochemical studies were performed.
Literature review
Literature review was performed using search
engines, such as Web of Science, PubMed, NCBI, Wiley Online
Library, Sage Journals, Science Direct, Scopus and MEDLINE, using
the following key words: Cavernous nasal hemangioma, pilomatrixoma,
inverted Schneiderian papilloma, self-resection, trans-oral
approach, vascularization, differential diagnosis. Cases that
presented pediatric and congenital pathology as well as
localization other than the nasal cavity were not included.
Inclusion criteria were histological match, same localization, same
surgical technique and unusual tumor development.
Case report
Case 1
A 72-year-old male patient presented in May 2015 in
the ENT-HNS Department of the Ilfov County Clinical University
Hospital, Bucharest, Romania, by referral from the Internal
Medicine Department, with a right nasal tumor. The patient
experienced intermittent right-sided minimal epistaxis for several
months in conjunction with progressive nasal obstruction. The
patient presented when the nasal obstruction became total (during
the last 2 months prior to admission) and when a tumor protruded
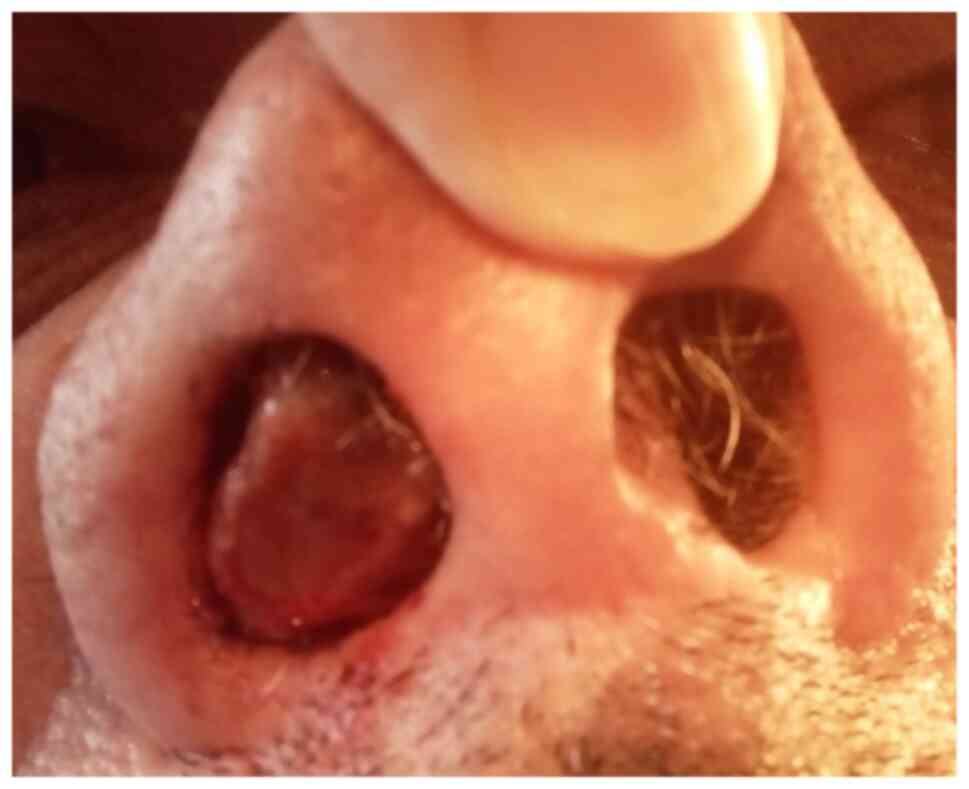
out of the right nostril (Fig. 1).
A nasal endoscopy examination was not possible since the tumor
obstructed the right narina and was clearly visible from the
outside. The mass was pinkish-red, necrotic, hard and bled easily
when handled. The site of origin in the nasal cavity was not clear.
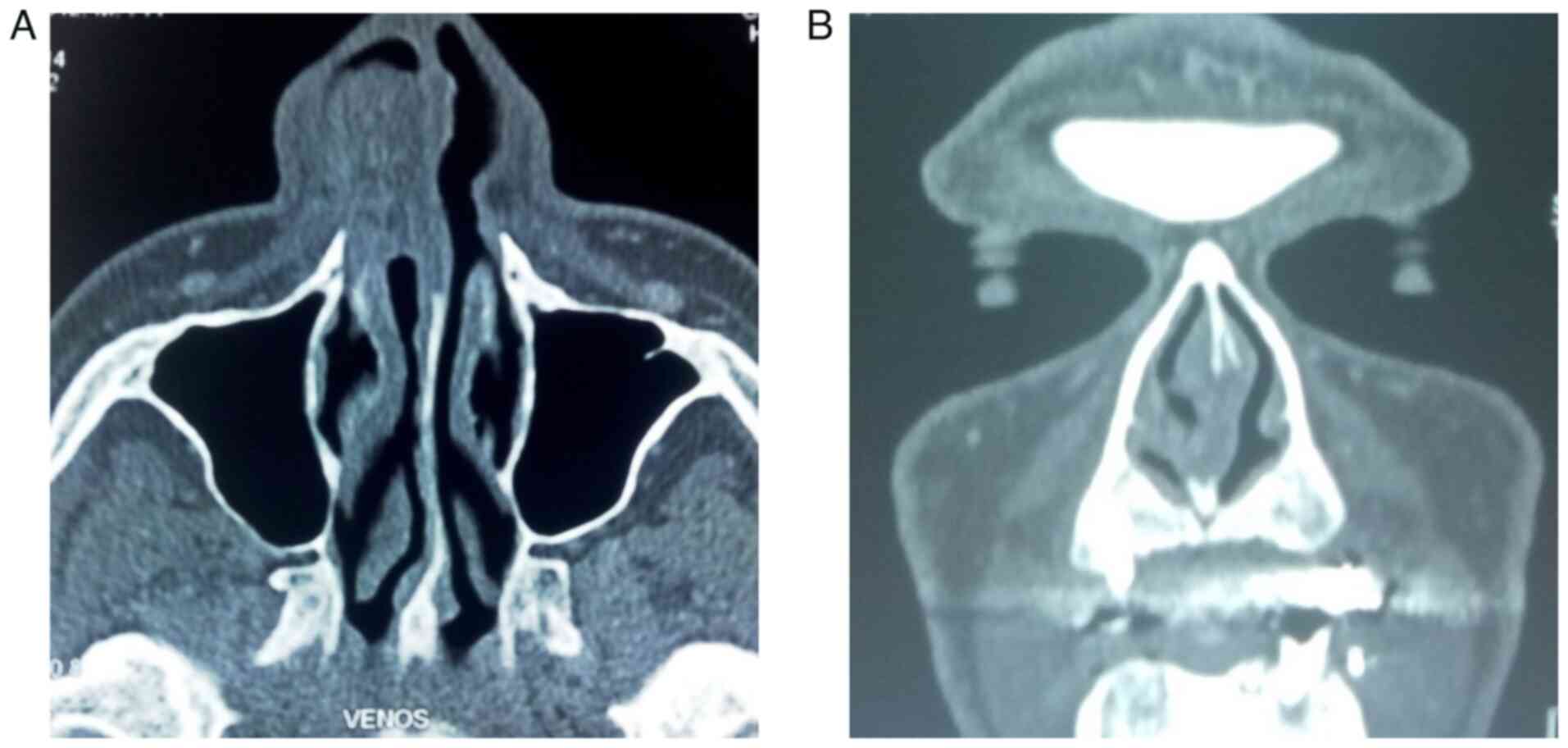
Enhanced axial computed tomography showed a homogeneous,
well-circumscribed enhancing mass ~32x17x28 mm in size that filled
the anterior part of the right nasal cavity, extending from the
lateral wall. The mass had a vascular pedicle on the lateral side
and contacted the anterior pole of the inferior turbinate without
modifying its osseous structure. The cartilaginous septum was
slightly deviated to the left but not perforated. The subcutaneous
tissue of the lateral nasal wall was also unaffected. The nasal
process of the maxillary bone was close to the mass but unaffected
(Fig. 2).
As endoscopic surgical approach was not possible,
lateral rhinotomy was planned but as the surgeon started handling
the tumor, it came out en bloc, much like a polyp, without
requiring force and without any bleeding. No incision or resection
from the surrounding tissue was necessary. The planned procedure
was therefore not performed as the tumor fell out (auto-resection).
Since no bleeding was present, the site of implantation of the
pedicle was not readily apparent but was hypothesized to be the
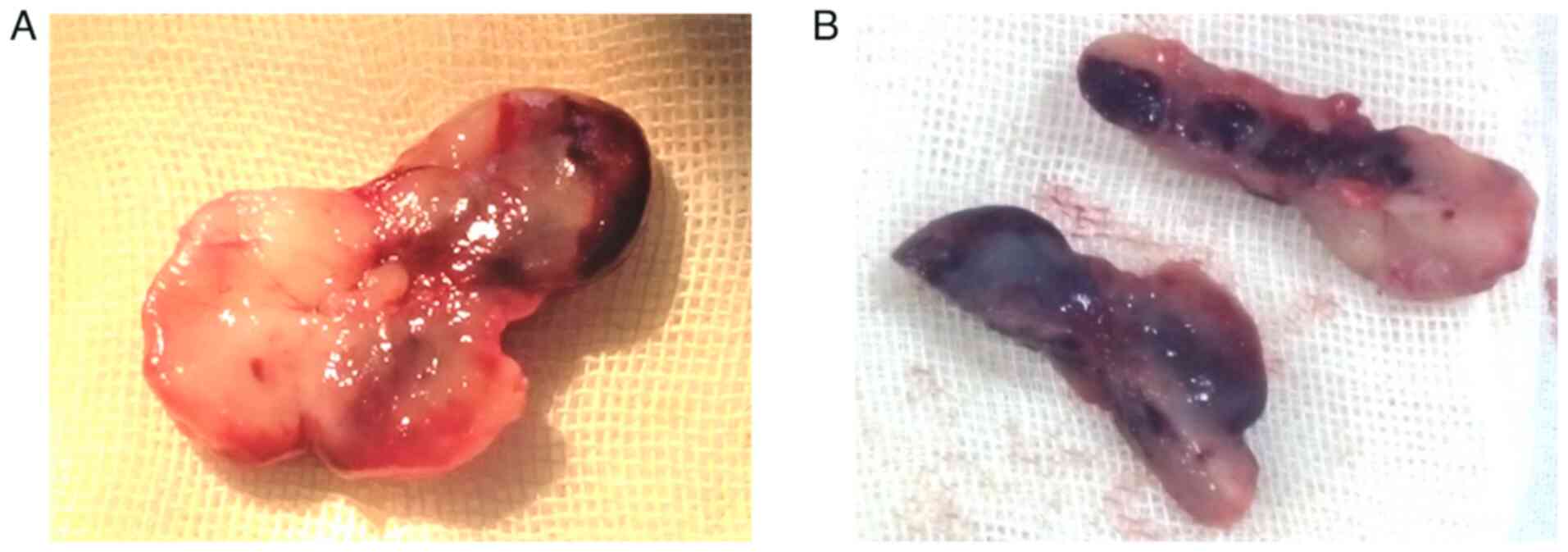
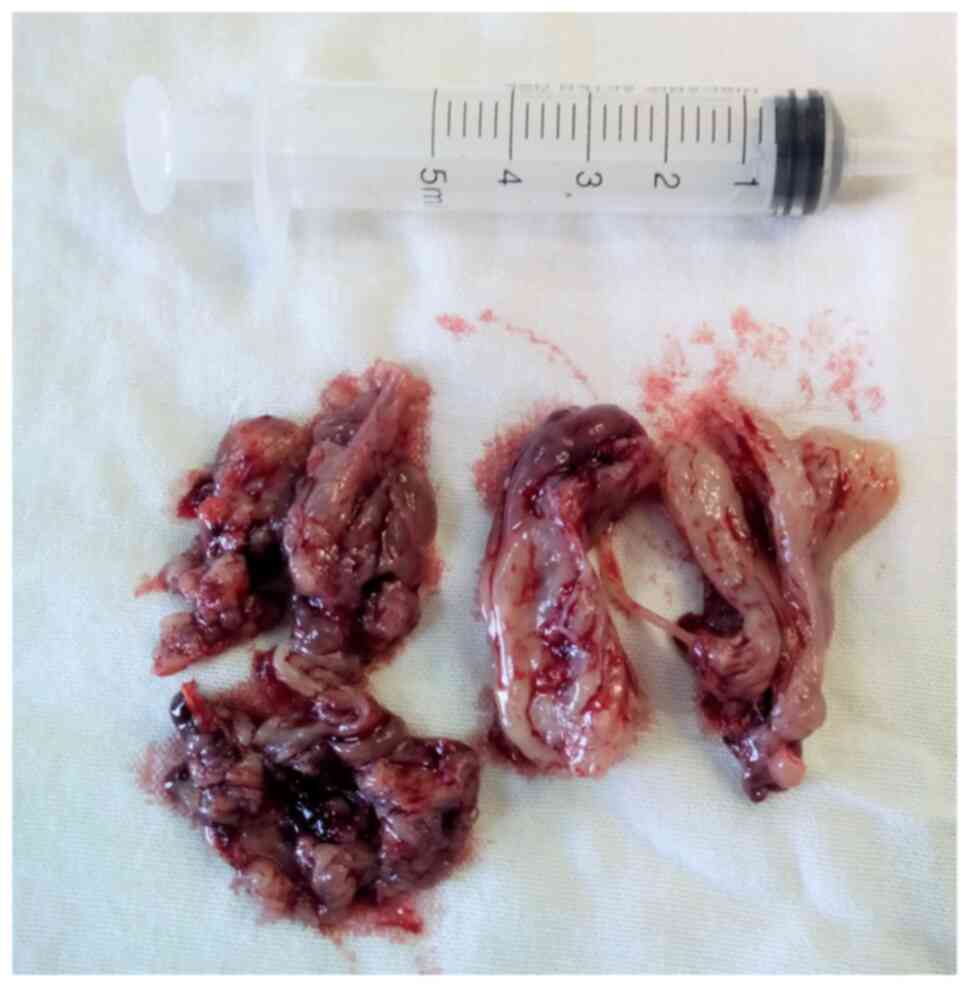
inferior turbinate. The surgical specimen consisted of a
yellow-pinkish, hard, cartilaginous-like lesion; when dissected, it
presented a purple-red, clot-like interior (Fig. 3).
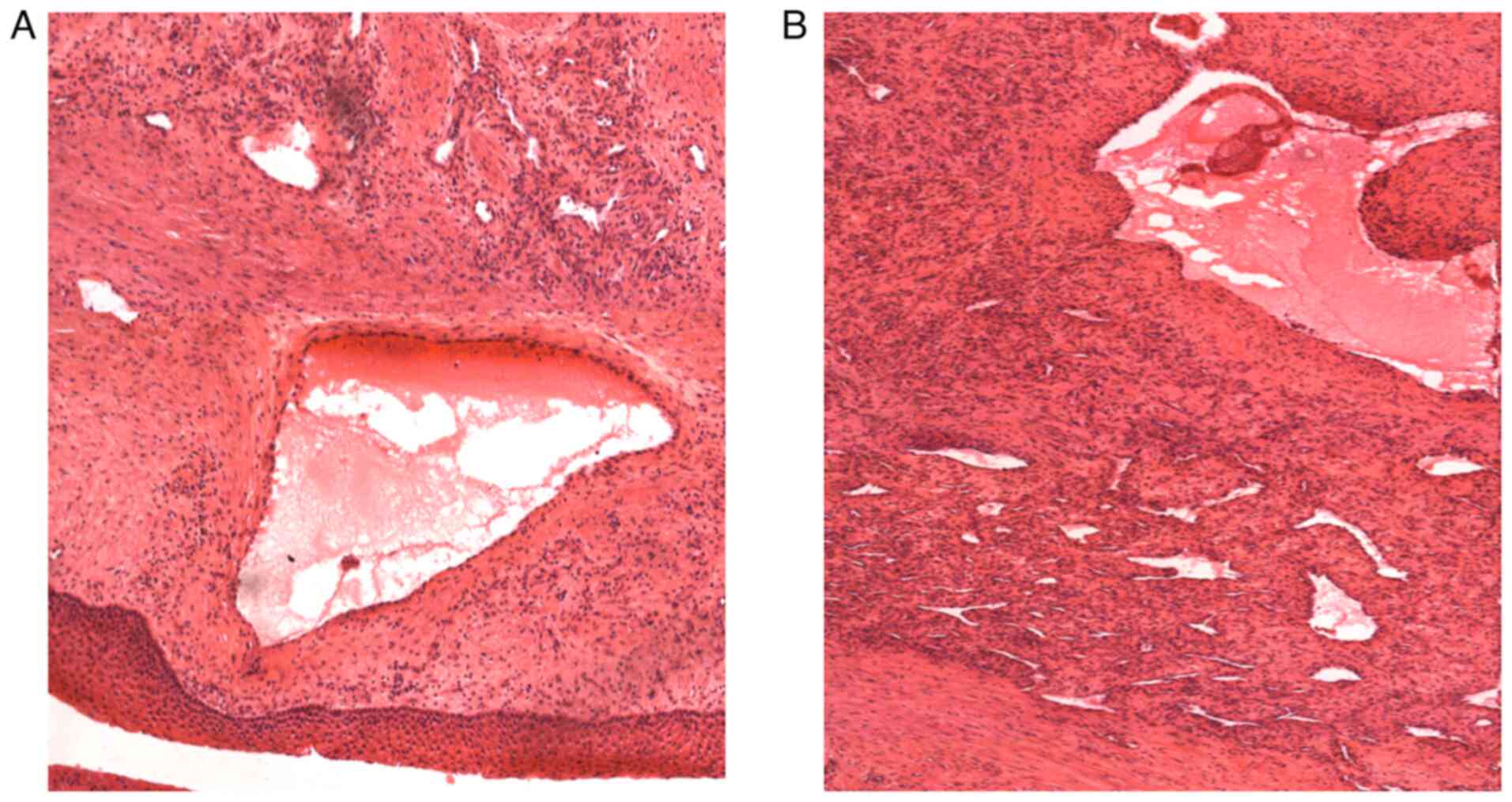
Histopathological examination showed large
blood-filled spaces lined with flattened endothelium and vessels of
different shapes and sizes with areas of oedema and hemosiderinic
pigment (Fig. 4). The tumor was a
cavernous hemangioma with no sign of malignancy. The patient
followed an uneventful post-operative course and was discharged
within 3 days. Follow-up at 30 days, 3 months and 1 year revealed
no sign of recurrence or residual disease.
Following discharge, the patient was referred to
Pulmonary Rehabilitation Clinic of the Iași Clinical Rehabilitation
Hospital, where he underwent pulmonary function tests for inclusion
in the respiratory recovery program. The patient performed the
respiratory rehabilitation program for 2 weeks in the hospital
under strict supervision of the rehabilitation team before
continuing permanently at home. During the program, the patient
performed breathing exercises, coughing, expectoration and
exercises to train the muscles of the chest, abdomen, neck, head,
limbs and respiratory system. The benefits of the respiratory
rehabilitation program were assessed at 3, 6 and 12 months and
consisted of increased exercise capacity evidenced by the 6-min
walk and oximetry test, decreased symptoms, including anxiety, with
rapid return to the social environment. The patient was advised to
continue the permanent respiratory rehabilitation program at home,
according to an established protocol, with periodic evaluation
every 3 months (25).
Case 2
A 26-year-old female patient presented in June 2017
in the ENT-HNS Department of the Ilfov County Clinical University
Hospital, Bucharest, Romania, with a hard subcutaneous nodule of
the left cheek. The tumor was superficial, mobile over the
underlying area, ~1 cm in diameter and exhibited no associated
tenderness. It had been present for several years with no
progression in size. The ultrasound examination described a
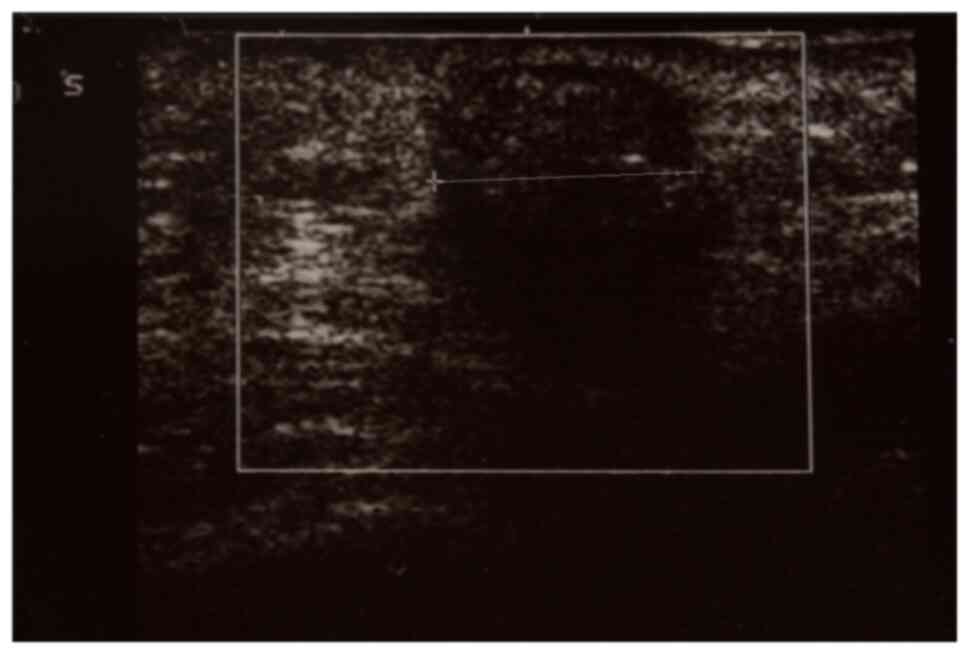
superficial, 10.7 mm hypoechoic tumor of the prezygomatic area. Its
margins were well defined and it presented no vascular signaling
upon Doppler examination (Fig. 5).
The patient refused classical external incision to avoid facial
scarring. Therefore, a trans-oral approach was performed, which
proved to be laborious since the tumor was superficially located.
Locating and resecting a mobile tumor trans-orally via mucosa
incision is more difficult than via tegument incision and the
potential for bleeding increases. Nevertheless, the resection was
successful and an oval shaped, hard tumor covered by a well-defined
connective tissue capsule and filled with reddish calcification
deposits was excised. The patient recovery was uneventful and there
were no signs of recurrence at 6 month and 1-year post-operative
follow-up. There was also no scarring of the cheek tegument.
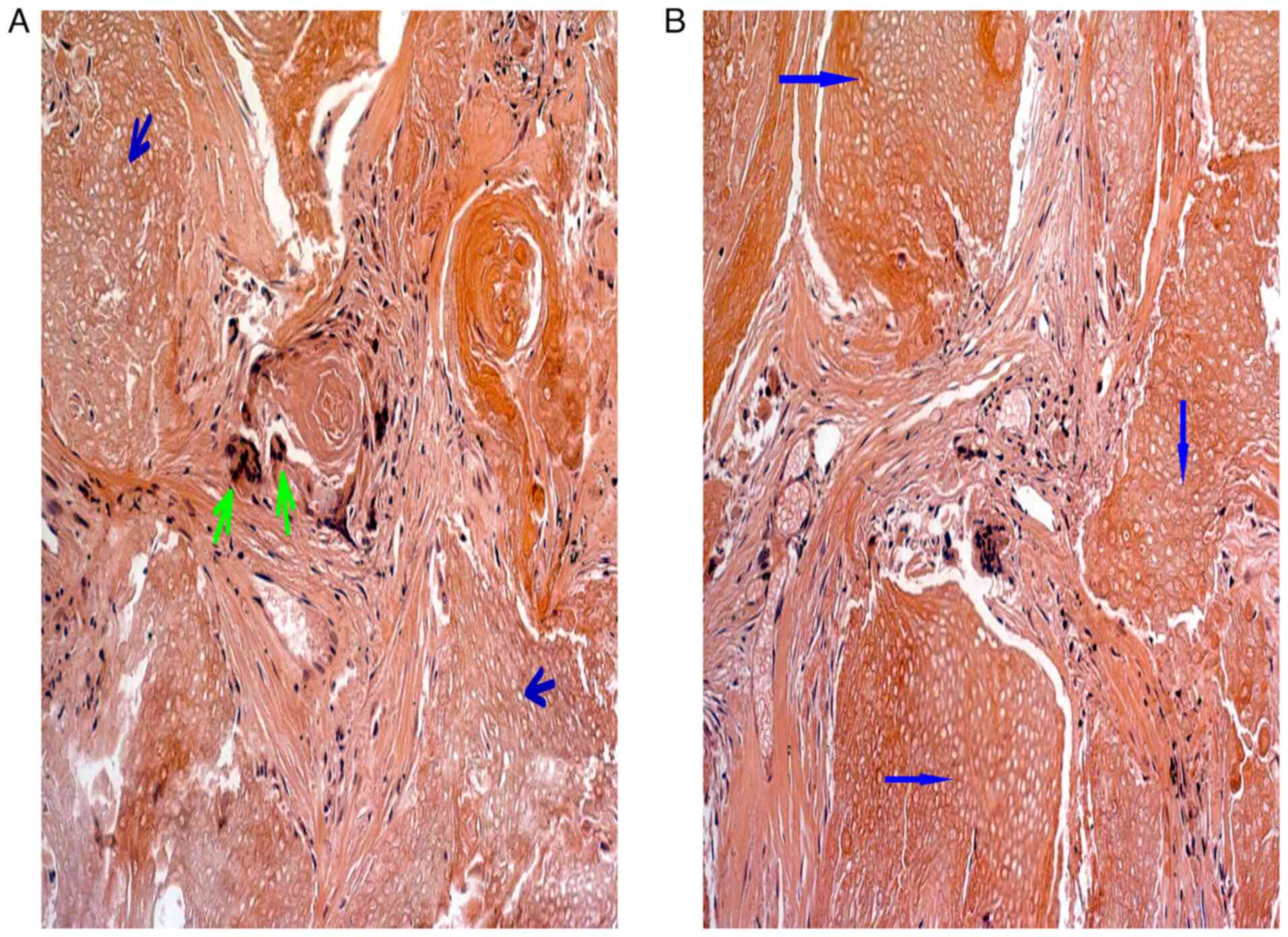
The histological examination with hematoxylin-eosin
staining showed the typical pilomatrixoma characteristics of shadow
or ghost cells with a central unstained area representing the
shadow of a lost nucleus. Basaloid cells with an elongated
basophilic nucleus and scant cytoplasm at the periphery of
epithelial islands were also present, along with calcium deposits
and foreign body reaction (giant cells; Fig. 6).
Case 3
A 68-year-old male patient presented in August 2018
in the ENT-HNS Department of the Ilfov County Clinical University
Hospital, Bucharest, Romania, with bilateral evolving nasal
obstruction, bilateral purulent blood-tinged rhinorrhea, hyposmia
and fluctuating headaches and facial pressure. Numerous types of
medication, such as antibiotics, anti-inflammatory agents and nasal
decongestant, achieved no improvement. The patient was evaluated
first at Internal Medicine Clinic of the Ilfov County Clinical
University Hospital, Bucharest, Romania, for headaches. The
differential diagnosis included arterial hypertension and sleep
apnea syndrome. The evaluation protocol included complete blood
work, cranio-facial CT and complete cardiological, neurological and
ENT examination with endoscopic examination of the nasal fossa. The
endoscopic examination revealed large pink-yellowish masses that
almost totally obstructed the nasal airways bilaterally. This was
confirmed by imaging. The masses were excised under endoscopic
control with excision into healthy tissue. Post-operatively, nasal
breathing was possible almost immediately after removing the nasal
packing, which was kept in place for 48 h, and recovery was
uneventful. The patient was advised to attend ENT examinations
every month for the 2 years and has been symptom-free for the past
5 years. The macroscopic aspect of the polypoid mass was reddish
yellow with firm, cartilage-like consistency and irregular surface.
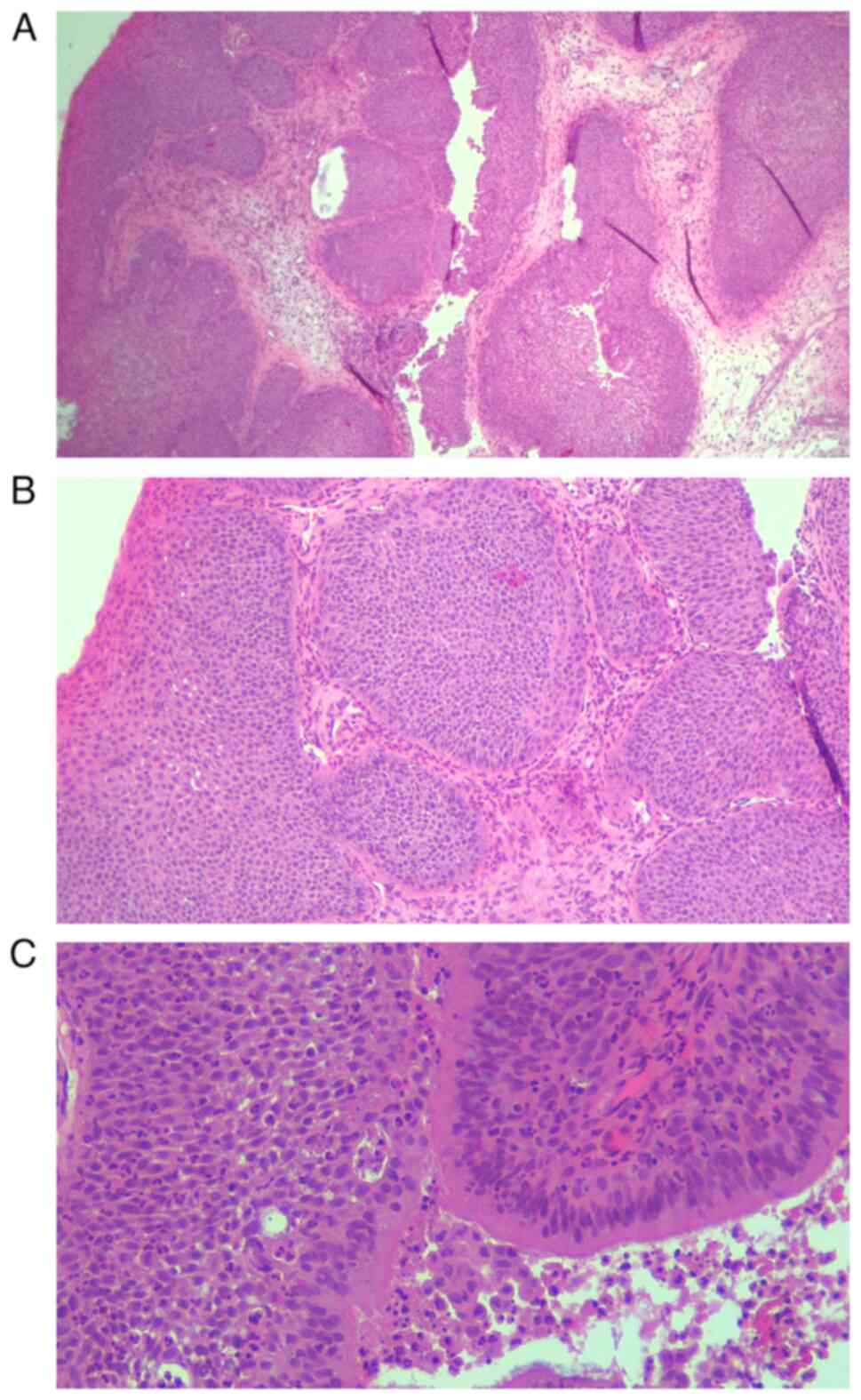
The masses were large (≤5 cm in length; Fig. 7). The histopathological examination
revealed papillary proliferation within the mucosa with connective
vascular axis covered by squamous epithelium. These findings
suggested inverted Schneiderian papilloma (Fig. 8).
The patient followed the respiratory rehabilitation
program in the same rehabilitation clinic as Case 1, for 2 weeks in
the hospital and then permanently at home with evaluation every 3
months and exhibited improvements in quality of life, exercise
capacity, decreased of symptoms and fast social reintegration
(25,26).
Discussion
Case 1
Hemangiomas of the nasal cavity are rare, benign
vascular tumors. The exact etiology is unclear but it has been
hypothesized that hemangiomas are a type of tumor since they are
destructive and have blood vessels that exhibit tumor-like aspects
(27). Alternatively, hemangioma
has been described not as a tumor but as a hamartoma or congenital
anomaly that may be caused by opening of blood vessels previously
closed at birth (28). The
proliferation of local blood vessels and increased regional
hydrostatic pressure caused by repeated local stimulation affect
the occurrence of hemangioma (2).
The histological sub-typing of hemangioma classifies
them according to histological appearance as capillary, cavernous,
mixed or hypertrophic (5).
Capillary hemangioma, also known as lobular
capillary hemangioma or pyogenic granuloma, is the most common type
of hemangioma and typically arises from the anterior cartilaginous
nasal septum (5). Capillary
hemangioma is composed of capillary sized vessels lined with
flattened epithelium separated by collagen stroma (2).
Cavernous hemangioma is rare; it occurs on the
osseous septum or lateral nasal wall and is composed of large
endothelium-lined vascular spaces (2). Cavernous hemangioma of the nose and
paranasal sinus is uncommon; most cases arise, as in the present
case, from the inferior turbinate. Other structures that support
this type of growth are vomer, lamina perpendicularis ossi
ethmoidalis and maxillary sinus (5). The incidence of cavernous hemangioma
is the same for men and women and the mean age of presentation is
40 years (29). The mixed type of
hemangioma exhibits proliferation of endothelium-lined, thin-walled
blood vessels of different sizes (2).
The present case included all the aforementioned
characteristics of this hemangioma: Unilateral, red or purple, not
painful, slowly growing hemorrhagic mass. It also produced
progressive nasal obstruction and epistaxis. The tumor was
necrotic, which eventually led to auto-resection due to decreased
blood supply to the pedicle. To the best of our knowledge,
auto-resecting tumor, nasal hemangioma or otherwise, has not been
previously reported. The term auto-resection was used to explain an
uncommon pathogenic mechanism, which would profit from further
study regarding tumoral blood supply. Since no surgical action was
taken to resect the hemangioma, the term auto-resection was
considered to best describe the unexpected outcome, which was of
novel clinical significance and it represent a better solution for
an otherwise clear surgical indication. The problem resides in the
rarity and unpredictability of such a development. There were no
clear signs that the tumor would auto-resect or that
vascularization of the area was afflicted in any way. The
advantages of auto-resection are that it spares the patient a
physically and psychologically traumatic experience, especially in
the case of an open approach (lateral rhinotomy or midfacial
degloving).
Differential diagnoses of hemangioma include benign
(angiofibroma, venous hemangioma, hemangioendothelioma, angiomatous
glomus tumor, lymphangioma) and malignant tumors
(hemangiopericytoma, hemangiosarcoma, squamous cell carcinoma,
adenocarcinoma, metastatic malignancy) of the nasal cavity. The
definitive diagnosis is given by histological confirmation.
The tumor typically obstructs the nasal cavity,
which makes endoscopic examination impossible, as in the present
case. Therefore, CT scan is key in planning the course of
treatment. CT scan typically reveals anatomical location and extent
of the tumor. The underlying bone is usually normal but may be
deformed by adjacent long-term pressure from the expanding mass
(30,31). The present case, like most reports
of nasal hemangioma, involved a mass that bled easily when touched.
Thus, although biopsy provides key information, the risk of
bleeding is high and must be considered. Since the case presented
an unusual solution (auto-resection) that involved circulation to
the area, angiography and/or magnetic resonance angiography are
recommended to diagnose potential vascularization problems and
predict auto-resection. Features such as poor vascularization of
the mucosa or turbinate area or vascular malformations of the
sino-nasal region may suggest eventual auto-resection. However,
this hypothesis requires further study.
The treatment of choice for nasal hemagioma is
surgical excision. In extensive tumors, the treatment of choice is
complete excision with preoperative embolization (32). Other effective methods for
treatment of hemangioma include sclerotherapy, cryotherapy,
corticosteroid treatment and resection by YAG-laser (33). The surgical approach (midfacial
degloving, lateral rhinotomy, trans-palatal and trans-antral
approach and LeFort I osteotomy) depends on location and extent of
the tumor. The minimal invasive, trans-nasal endoscopic approach
has also been suggested by other studies (29,32)
but is not always available due to economic reasons, especially in
developing countries. The planned surgical option for the present
patient was lateral rhinotomy but this was not required.
Case 2
Pilomatrixomas are of ectodermal origin that arise
in the lower dermis from the outer root sheath cell of the hair
follicle (6,33) and form a connective tissue capsule.
A characteristic diagnostic sign is that the tumor slides freely
over the underlying area; this has been described as the ‘tent
sign’ as the irregular surface of the mass can be felt by
stretching the skin over the tumor (34). There is no associated
lymphadenopathy. The skin of the cheek and periorbital area are the
most common locations. A blue discoloration of the overlying
tegument has sometimes been reported (6). There may also be a history of
regional trauma (≤9%) prior to developing the tumor (4,6). The
significance of this is yet unknown.
Histologically, pilomatrixomas consist of anucleate
squamous (called ghost or shadow cells) with a central unstained
area representing a shadow of a lost nucleus, benign viable
squamous and foreign body giant cells. These neoplasms exhibit
characteristic transition of cells (6). The lining of the cyst consists of
basaloid cells with a round or elongated basophilic nucleus and
scant cytoplasm at the periphery of epithelial islands (35) that mature into eosinophilic
anucleated squamous cells. Calcium deposits and foreign body
reaction commonly occur (36) and
ossification has been reported (37). Multiple pilomatrixomas have been
associated with numerous genetic and non-genetic disorders such as
Gardner, Turner and Rubinstein-Taybi syndrome, trisomy 9, Steinert
disease, myotonic dystrophy and sarcoidosis (6,37-39).
Pilomatrixoma, which presents as an irregular nodule
on the skin, is differentiated from epidermal cysts, which are
firm, round and mobile and occur primarily in adolescents and
adults, and dermoid cysts, which are firmly attached to underlying
tissue. A differential diagnosis should be made with
pilomatrix-carcinoma, a rare malign tumor of hair matrix cells and
that arises from pilomatrixoma (6). Black et al (37) reported that clinical behavior of
pilomatrix carcinoma in adults resembles that of basal cell
carcinoma in its potential to metastasize. Treatment for the malign
tumor is wide local excision (37).
Diagnostic tests and imaging studies are often
unnecessary in workup of a superficial, benign skin lesion such as
pilomatrixoma (6). However, tests
are sometimes performed to exclude diagnosis of malignancy or to
determine the depth of a lesion (4). Pilomatrixoma in the parotid or
preauricular region may require further imagistic examination and
dissection from the parotid gland. Fine-needle aspiration may
reveal the presence of ghost and basaloid cells and calcium
deposition in the mass, which are diagnostic of pilomatrixoma
(40). However, without the
presence of ghost cells in the aspirate, the diagnosis may be
misleading (40). Ultrasound
diagnosis is helpful for diagnosis and easy to perform. Other
imagistic methods, such as CT and magnetic resonance imaging,
provide detail of the surrounding structure and depth of the lesion
but are too expensive to use in an otherwise simple diagnosis
(6).
The treatment of choice and standard therapy for
benign pilomatrixoma is complete surgical excision. If the
overlying tegument is adherent to the tumor, it may also require
excision (6). Morales and McGoey
(41) advocated incision and
curettement for cosmetic preservation in large tumors or for those
in exposed areas and found no recurrence. Danielson-Cohen (6) noted 4% recurrence following complete
surgical excision. However, in certain cases, such as the one
presented, classical external incision of the skin, especially in
the cheek, is not an option; the trans-oral approach is feasible
but more laborious and prone to recurrence. This risk arises from
poorer exposure of the incision, increased mobility and bleeding
and therefore higher likelihood of remnant tissue. No recurrence
was present in this case at 6 months and 1 year postoperatively
which brings into consideration the assumption that both treatment
and technique coordinate the doctor to a successful performance and
that, occasionally, medical research can require a high degree of
theorizing, abstraction and innovation (42).
Case 3
Inverted Schneiderian papillomas are benign tumors
of the nasal and sinus area most commonly approached endoscopically
(43,44) and have been discussed in literature
for over a century (18). Due to
their rarity and confusing nomenclature, they remain a topic of
controversy (45). The reported
incidence of papilloma is 1.7-7.0% (46). They are typically located
unilaterally, whereas the present case involved bilateral
localization. A similar bilateral case was reported by Neagos et
al (47) in 2014. The
recurrence rate is 0-27% and the endoscopic approach is recommended
in cases with limited invasion of the nasal fossa and ethmoid cells
(43,44). The decision to resort to open
surgery depends on the size, localization and histopathological
tumor type (48,49). Cortisone and antibiotic treatment
are also paramount for postoperative care (48,49).
The inverted Schneiderian papilloma has a peak
incident in individuals aged 50-69 years, as proven by numerous
studies (2,12,15,18,47).
The retrospective study by Bielamowicz et al (50) on 61 cases reported a mean age of 63
years and a male:female ratio of 2:1. There are, however, reports
on papilloma in patients aged 5-25 years (51-54).
Malignant transformation in recurrent cases, as well as coexisting
inverted papilloma and squamous cell carcinoma, have been
documented (55,56).
The present hemangioma case involved a rare benign
tumor which appeared to auto-resect due to loss of blood supply. It
was hypothesized that the pedicle necrotized over time and cut off
blood supply to the mass. This allowed the surgeon to remove it
with ease and left no bleeding, but it is possible that the mass
would have fallen out.
Pilomatrixoma, also termed calcifying epithelioma of
Malherbe, is a relatively uncommon benign tumor of ectodermal
origin derived from the hair matrix cells. The most common
misdiagnosis is a dermoid cyst. Typical diagnostic methods include
clinical examination, ultrasound and histopathology. Surgical
removal is curative. The recurrence frequency reported is ~5%. The
present patient refused an external approach due to esthetic
reasons. Therefore, trans-oral resection was performed. This
approach, although possible, is counterintuitive, more laborious
and prone to recurrence due to incomplete resection of the
pilomatrixoma. There was no recurrence in the present case at 6
months and 1 year postoperatively. Nevertheless, the classical
external approach is recommended when possible.
Inverted papillomas have a high recurrence rate and
propensity for malignant change. The present case was distinctive
due to its rare bilateral nature. The endoscopic approach is the
most successful in terms of functional, aesthetic results, short
hospitalization period and improving quality of life. For large,
invading tumors, open surgery is recommended to minimize recurrence
and malignant development risk.
In conclusion, ENT tumors benefit from
multidisciplinary approach to diagnosis and treatment and, in
addition to ENT specialists, often require attention from general
and vascular surgeons, pneumologists and rehabilitation specialists
to provide a high quality of life following removal and complete
healing.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HM, PAP and AN conceived the study, performed
patient selection, care and operations, collected data and edited
the manuscript. PAP was responsible for designing, performing and
supervising the pulmonary rehabilitation evaluation program. AIM,
CM and IS made substantial contributions to acquisition, analysis
and interpretation of data. AIM, CM and AN performed data analysis
and prepared figures. HM and PAP confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by Research Ethics
Committee of the Faculty of Medicine, Titu Maiorescu University
(approval no. 6/21.09.2021; Bucharest, Romania). All patients
provided informed written consent.
Patient consent for publication
All patients provided informed consent and approved
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
HM, ORCID no. 0000-0002-9708-8285; AIM, ORCID no.
0000-0003-0725-2131; CM, ORCID no. 0000-0003-1362-6427; IS, ORCID
no. 0000-0003-3697-5611; PAP, ORCID no. 0000-0003-1789-4277; AN,
ORCID no. 0000-0002-1481-2822.
References
|
1
|
Neagu A, Mocanu AI, Bonciu A, Coadă G and
Mocanu H: Prevalence of GJB2 gene mutations correlated to presence
of clinical and environmental risk factors in the etiology of
congenital sensorineural hearing loss of the Romanian population.
Exp Ther Med. 21(612)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Batsakis JG and Rice DH: The pathology of
head and neck tumors: Vasoformative tumors, part 9A. Head Neck
Surg. 3:231–239. 1981.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hoffmann DF and Israel J: Intraosseous
frontal hemangioma. Head and Neck. 12:160–163. 1990.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Takeda K, Takeda Y and Hashimoto M:
Intraosseous hemangioma of the inferior turbinate. Case Rep Med.
2010(409429)2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Arhontaki M, Stamou AK, Hajioannou JK,
Kalomenoupoulou M, Korkolis DP and Kyrmizakis DE: Cavernous
hemangioma of the left nasal cavity. Acta Otolaryngol Ital.
28:309–311. 2008.PubMed/NCBI
|
|
6
|
Danielson-Cohen A, Lin SJ, Hughes CA, An
YH and Maddalozzo J: Head and neck pilomatrixoma in children. Arch
Otolaryngol Head Neck Surg. 127:1481–1483. 2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Julian CG and Bowers PW: A clinical review
of 209 pilomatricomas. J Am Acad Dermatol. 39 (2 Pt 1):191–195.
1998.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Forbis R Jr and Helwig EB: Pilomatrixoma
(calcifying epithelioma). Arch Dermatol. 83:606–617.
1961.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gongidi P, Meshekow J, Holdbrook T and
Germaine P: Giant pilomatrixoma presenting in the posterior thorax,
a rare location and the largest described. Case Rep Radiol.
2015(590742)2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Knight PJ and Reinerm CB: Superficial
lumps in children: What, when and why? Pediatrics. 72:147–153.
1983.PubMed/NCBI
|
|
11
|
Lee EJ, Kim AY, Lee EM, Park JK and Jeong
KS: Malignant pilomatricoma in a young dog. Acta Vet. 66:556–561.
2016.
|
|
12
|
Vrabec PD: The inverted schneiderian
papilloma: A 25-year study. Laryngoscope. 104 (5 Pt 1):582–604.
1994.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Brown B: The papillomatous tumours of the
nose. J Laryngol Otol. 78:889–905. 1964.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cheung FM, Lau TW, Cheung LK, Li AS, Chow
SK and Lo AW: Schneiderian papillomas and carcinomas: A
retrospective study with special reference to p53 and p16 tumour
suppressor gene expression and association with HPV. Ear Nose
Throat J. 89:E5–E12. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
von Buchwald C and Bradley PJ: Risks of
malignancy in inverted papilloma of the nose and paranasal sinuses.
Curr Opin Otolaryng Head Neck Surg. 15:95–98. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Eggers G, Mühling J and Hassfeld S:
Inverted papilloma of paranasal sinuses. J Craniomaxillofac Surg.
35:21–29. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Suarez PA, Adler-Storthz K, Luna MA,
El-Naggar AK, Abdul-Karim FW and Batsakis JG: Papillary squamous
cell carcinomas of the upper aerodigestive tract: A
clinicopathologic and molecular study. Head Neck. 22:360–368.
2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jagtap SV, Nikumbh DB, Chavan SH, Jain G
and Havale AD: Inverted sinonasal schneiderian papilloma with
malignant transformation. J Clin Diagn Res. 5:1275–1277. 2011.
|
|
19
|
Batsakis JG and Suarez P: Schneiderian
papillomas and carcinomas: A review. Adv Anat Pathol. 8:53–64.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Perez-Ordonez B: Hamartomas, papillomas
and adenocarcinomas of the sinonasal tract and nasopharynx. J Clin
Pathol. 62:1085–1095. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lee TJ, Huang CC, Chen YW, Chang KP, Fu CH
and Chang PH: Medially originated inverted papilloma. Otolaryngol
Head Neck Surg. 140:324–329. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lawson W, Kaufman MR and Biller HF:
Treatment outcomes in the management of inverted papilloma: An
analysis of 160 cases. Laryngoscope. 113:1548–1556. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mirza S, Bradley PJ, Acharya A, Stacey M
and Jones NS: Sinonasal inverted papillomas: Recurrence, and
synchronous and metachronous malignancy. J Laryngol Otol.
121:857–864. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Som MP and Curtin HD: Head and Neck
Imaging. Vol 2. 5th edition. Mosby, St. Louis, MO, 2021.
|
|
25
|
Postolache P and Marciniuk D: Handbook of
Pulmonary Rehabilitation. Nova Science Publishers, New York, NY,
pp11-13, 2021.
|
|
26
|
Soare I: Insurance Medicine. Etna
Publishing House, Bucharest, pp43-61, 2017.
|
|
27
|
Ash JE and Old JW: Hemangiomas of the
nasal septum. Trans Am Acad Opthalmol Otolaryngol. 54:350–356.
1950.PubMed/NCBI
|
|
28
|
Willis RA and Collins WH: Pathology of
tumors. Br J Surg. 35(446)1948.
|
|
29
|
Iwata N, Hattori K, Nakagawa T and
Tsujimura T: Hemangioma of the nasal cavity: A clinicopathologic
study. Auris Nasus Larynx. 29:335–339. 2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dillon WP, Som PM and Rosenau W:
Hemangioma of the nasal vault: MR and CT features. Radiology.
180:761–765. 1991.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Itoh K, Nishimura K, Togashi K, Fujisawa
I, Nakano Y, Itoh H and Torizuka K: MR imaging of cavernous
hemangioma of face and neck. J Comput Assist Tomogr. 10:831–835.
1986.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Azzolini A, Bertani A and Riberti C:
Superselective embolization and immediate surgical treatment: our
present approach to treatment of large vascular hemangioma of the
face. Ann Plastic Surg. 9:42–60. 1982.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Howerd LL: Laser in endonasal surgery.
Otolaryngol Clin North Am. 30:451–455. 1997.PubMed/NCBI
|
|
34
|
Jungheim M and Chilla R: The monthly
interesting case-case no. 64. cavernous hemangioma.
Laryngorhinootologie. 83:665–668. 2004.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
35
|
Fink AM and Berkowitz RG: Sonography in
preauricular pilomatrixoma of childhood. Ann Otol Rhinol Laryngol.
106:167–169. 1997.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Graham JL and Merwin CF: The tent sign of
pilomatricoma. Cutis. 22:577–580. 1978.PubMed/NCBI
|
|
37
|
Black SJ, Marble BF and Vuitch F: Multiple
giant pilomatrix carcinomas of the head and neck. Otolaryngol Head
Neck Surg. 109:543–547. 1993.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Orlando RG, Rogers GL and Bremer DL:
Pilomatricoma in a pediatric hospital. Arch Ophthalmol.
101:1209–1210. 1983.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Urvoy M, Legall F, Toulemont PJ and
Chevrant-Breton J: Multiple pilomatricoma. Apropos of a case. J Fr
Ophthalmol. 19:464–466. 1996.PubMed/NCBI(In French).
|
|
40
|
Domanski HA and Domanski AM: Cytology of
pilomatrixoma (calcifying epithelioma of Malherbe) in fine needle
aspirates. Acta Cytol. 41:771–777. 1997.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Morales A and McGoey J: Pilomatricoma:
Treatment by incision and curettement. J Am Acad Dermatol. 2:44–46.
1980.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Alecu I, Mocanu H and Călin IE:
Intellectual mobility in higher education system. Rom J Mil Med
CXX. 2:16–21. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kim WS, Hyun DW, Kim CH and Yoon JH:
Treatment outcomes of sinonasal inverted papillomas according to
surgical approaches. Acta Otolaryngol. 130:493–497. 2010.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Osuch-Wójcikiewicz E, Wojas O, Nyckowska
J, Checiński P, Sielska-Badurek E, Bruzgielewicz A, Szwedowicz P
and Niemczyk K: Management of recurrent sinonasal inverted
papilloma in the experience of ENT Department Medical University of
Warsaw. Otolaryngol Pol. 64:73–76. 2010.PubMed/NCBI View Article : Google Scholar : (In Polish).
|
|
45
|
Lyngdoh NC, Ibohal TH and Marak IC: A
study on the clinical profile and the management of inverted
papilloma. Indian J Otolaryngol Head Neck Surg. 58:41–45.
2006.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Benninger MS, Robert JK, Sibek BA, Levine
HL, Tucker HM and Lavertu P: Inverted papilloma and associated
squamous cell carcinoma. Otolaryngol Head Neck Surg. 103:457–461.
1990.
|
|
47
|
Neagos A, Cirticioiu A, Duca D and Csiszer
I: Inverted papilloma of the nasal cavity-case report. Rom J
Rhinol. 4:55–58. 2014.
|
|
48
|
Llorente JL, Deleyiannis F, Rodrigo JP,
Nuñez F, Ablanedo P, Melón S and Suárez C: Minimally invasive
treatment of the nasal inverted papilloma. Am J Rhinol. 17:335–341.
2003.PubMed/NCBI
|
|
49
|
Schlosser RJ, Mason JC and Gross CW:
Aggressive endoscopic resection of inverted papilloma: An update.
Otolaryngol Head Neck Surg. 125:49–53. 2001.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Bielamowicz S, Calcaterra TC and Watson D:
Inverting papilloma of the head and neck: The UCLA update.
Otolaryngol Head Neck Surg. 109:71–76. 1993.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lund VJ: Optimum management of inverted
papilloma. J Laryngol Otol. 114:194–197. 2000.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Mohanty R, Dubey KP, Das SK and Chawla SC:
Sinonasal inverted Schneiderian papilloma. Indian J Otolaryngol
Head Neck Surg. 56:161–163. 2004.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Eavey RD: Inverted papilloma of the nose
and paranasal sinuses in childhood and adolescence. Laryngoscope.
95:17–23. 1985.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Mitskavich MT, Carrau RL, Snyderman CH,
Weissman JL and Fagan JJ: Intranasal endoscopic excision of a
juvenile angiofibroma. Auris Nasus Larynx. 25:39–44.
1998.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Kashima HK, Kessis T, Hruban RH, Wu TC,
Zinreich SJ and Shah KV: Human papillomavirus in sinonasal
papillomas and squamous cell carcinoma. Laryngoscope. 102:973–976.
1992.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Furuta Y, Shinohara T, Sano K, Nagashima
K, Inoue K, Tanaka K and Inuyama Y: Molecular pathologic study of
the human papillomavirus infection in inverted papilloma and
squamous cell carcinoma of the nasal cavities and paranasal
sinuses. Laryngoscope. 101 (1 Pt 1):79–85. 1991.PubMed/NCBI View Article : Google Scholar
|