Introduction
In recent years, significant progress has been made
regarding systemic therapies for lung cancer. For patients with
advanced, epidermal growth factor receptor (EGFR) mutation-positive
non-small-cell lung cancer (NSCLC) in particular, EGFR tyrosine
kinase inhibitors are widely used as the first-line therapy and
provide significantly improved overall survival (OS) (1,2).
However, NSCLC frequently gains resistance to these drug therapies
during the course of treatment. In such cases, immune checkpoint
inhibitors (ICIs) with or without chemotherapy are alternatives for
treating NSCLC that is resistant to cytotoxic
chemotherapies/molecular targeted therapies or does not have any
EGFR mutations.
ICIs, including inhibitors of programmed cell death
protein 1 (PD-1), programmed death-ligand 1 (PD-L1) and cytotoxic
T-lymphocyte-associated protein 4 (CTLA-4), are widely used in
patients with advanced NSCLC. However, a variety of immune-related
adverse events (irAEs) after the administration of ICIs have been
reported. IrAEs occur in ~45% of patients with NSCLC. Endocrine,
gastrointestinal and dermatologic toxicities are common events
associated with irAE (3,4). The incidence of fatal irAEs is ~1%
(5).
In palliative radiotherapy (PRT), the delivered
doses are lower than the maximum tolerated doses of
gastrointestinal tissues [mean PRT equivalent dose in 2 Gy
fractions (EQD2), 36 Gy; maximum tolerance dose of small bowel, 50
Gy; maximum tolerance dose of large bowel, 55 Gy) (6). In PRT, the delivered doses (mean PRT
EQD2, 36 Gy) are lower than the maximum tolerated dose of
gastrointestinal tissue. However, there is a possibility that RT
toxicity in the bowel is enhanced when RT and ICIs are combined.
Regarding combination therapy of PRT and ICIs, certain studies
suggested that it is well-tolerated (7-9).
They reported that the incidence of colitis in patients treated
with PRT involving the bowel was 5% or less and gastrointestinal
toxicities did not increase. However, these studies were limited by
the heterogeneity of patients and treatments. Therefore, further
studies on the safety of PRT and ICI combination therapy are
required.
In a study of adjuvant ICIs with durvalumab after
definitive chemoradiotherapy for NSCLC (PACIFIC study), adjuvant
ICI therapy appeared to slightly increase the incidence of
pneumonitis (statistically not significant) (10). Patel et al (11) suggested that T- and natural killer
(NK) cell infiltration are enhanced in lesions treated with
low-dose radiotherapy. These results suggest that activated T- and
NK cells accumulate in normal tissue damaged by RT and these
accumulated T- and NK cells damage the tissue further. Based on
these studies, the administration of ICIs may have the potential to
enhance radiation toxicity. Despite the comparatively lower dose
and small irradiation field size, administration of ICIs may also
increase the toxicity of PRT. To the best of our knowledge, only a
small number of studies have investigated whether gastrointestinal
toxicities are associated with combination therapy of PRT and ICIs
(7). Therefore, the present
retrospective study aimed to investigate the occurrence of
radiation-induced enterocolitis (RIE) after the administration of a
combination therapy of PRT and ICIs in patients with metastatic
lung cancer.
Patients and methods
A total of 45 abdominal-pelvic metastatic lesions in
38 patients with lung cancer who were treated with PRT involving
the bowel and ICIs (a PD-1/L1 inhibitor and/or a CTLA-4 inhibitor)
between December 2015 and June 2021 were reviewed. Of these,
patients who did not undergo follow-up computed tomography (CT)
after treatment (n=12) and those in whom the interval between PRT
and closest administration of ICIs was more than one year (n=4)
were excluded from this study. Finally, the remaining 32 lesions in
28 patients were retrospectively evaluated. This retrospective
study was approved by the institutional review board (Shikoku
Cancer Center, Ehime, Japan). An opt-out form of consent was used
to obtain consent for this study.
PRT doses were determined at the discretion of each
physician and 30 Gy in 10 fractions was the most frequently used
regimen. To compare the different dose-fraction schedules, total
doses of PRT were calculated with EQD2 values using an α/β ratio of
3 for the bowel. PRT was performed using 6-10 MV linear
accelerators (Varian Medical Systems, Inc.) and the doses of the
target volumes were ≥90% of the PRT dose in principle. The
treatment of all lesions was planned using three-dimensional
conformal RT.
RIE after combination therapy of PRT and ICIs was
graded using the Common Terminology Criteria for Adverse Events
version 5.0(12). The definition
of RIE was ‘segmental and circumferential bowel wall thickening and
inflammatory stranding in the area of an irradiated field occurring
within 6 months after PRT on CT images’. The diagnosis of RIE was
based on the patient's symptoms, physical examination and CT
imaging and/or colonoscopy. The dose-volume parameters of the large
and small bowel were assessed using CT simulation images. The
dose-volume parameters of the large and small bowel were analyzed
to determine the absolute volume cubic centimeters (cc) receiving
doses from 10 to 20 Gy (V10 and V20), as well as the maximum dose
to 2 cc volume (D2cc) and D2cc per fraction (D2cc/fr).
Statistical analysis
Kaplan-Meier survival analysis was used to calculate
the OS rate and the duration of follow-up was calculated from the
initiation of PRT. The statistical significance of differences in
OS was evaluated using the generalized Wilcoxon test. The interval
between PRT and the closest administration of ICIs was calculated
from the date of initiation of PRT if ICIs were administered prior
to PRT and the date of completion of PRT if ICIs were administered
after PRT. Fisher's exact test was used to examine the relationship
between the incidence of RIE and the risk factors. P-values were
calculated by rounding to the nearest three decimal places and a
two-sided P≤0.05 was considered to indicate statistical
significance.
In addition, receiver operating characteristic (ROC)
analysis was performed to examine optimal cut-off values of the
interval between PRT and the closest administration of ICIs,
fraction dose evaluated at the isocenter, total EQD2, V10 Gy, V20
Gy, D2cc and D2cc/fr for the incidence of RIE. Statistical analyses
were performed using JMP software (version 14.3.0; SAS Institute,
Inc.).
Results
Patients
Data from 32 lesions in 28 patients (one with SCLC
and 27 with NSCLC; male/female, 23/5; age 42-75 years, median age,
64 years) were included in the analysis dataset (Table I). Of these, two patients had
recurrent distant metastases that were not present at the initial
diagnosis, while the remaining 26 had distant metastases at the
initial diagnosis. The median follow-up time from the initiation of
PRT was nine months (range, 1-41 months).
 | Table ICharacteristics of the lesions. |
Table I
Characteristics of the lesions.
| Characteristic | Value (%) |
|---|
| Age, years
[range] | 64.0 [42-75] |
|
<65 | 17 (53.1) |
|
≥65 | 15 (46.9) |
| Sex | |
|
Male | 27 (84.4) |
|
Female | 5 (15.6) |
| PS (ECOG) | |
|
0 | 2 (6.3) |
|
1 | 18 (56.2) |
|
2 | 9 (28.1) |
|
3 | 1 (3.1) |
|
4 | 2 (6.3) |
| Primary cancer
histology | |
|
Non-small
cell lung cancer | 30 (93.8) |
|
Small cell
lung cancer | 2 (6.3) |
| PRT sites | |
|
Vertebral
bone | 13 (40.6) |
|
Pelvic
bone | 12 (37.5) |
|
Adrenal
gland | 3 (9.4) |
|
Lymph
node | 3 (9.4) |
|
Liver | 1 (3.1) |
| PRT dose, Gy (total
dose/number of fractions) | 30 [8-48] |
|
8.0/1 | 1 (3.1) |
|
20/5 | 4 (12.5) |
|
28.8/8 | 2 (6.3) |
|
30/10 | 20 (62.5) |
|
37.5/15 | 1 (3.1) |
|
40/16 | 1 (3.1) |
|
45/18 | 1 (3.1) |
|
45/15 | 1 (3.1) |
|
48/24 | 1 (3.1) |
| Chemotherapy | |
|
Yes | 30 (93.8) |
|
Administration
before PRT | 19 (59.4) |
|
Administration
after PRT | 28 (87.5) |
|
No | 2 (6.3) |
| Biotherapy | |
|
Yes | 9 (28.1) |
|
Administration
before PRT | 4 (12.5) |
|
Administration
after PRT | 6 (18.8) |
|
No | 23 (71.9) |
| ICIs therapy | |
|
Anti-PD-1
monotherapy | 22 (68.8) |
|
Anti-PD-L1
monotherapy | 5 (15.6) |
|
Anti-PD-1/PD-L1
+ anti-CTLA-4 | 5 (15.6) |
|
combination
therapy | |
| No. of ICI cycles
[range] | |
|
Anti-PD1/PD-L1
monotherapy | 4 [1-30] |
|
Anti-PD-1/PD-L1
+ anti-CTLA-4 | 4.0 [1.0-8] |
|
combination
therapy | |
| Interval between
PRT and the closest administration of ICIs, days | |
|
Administration
of ICIs before PRT | 11 (34.4) |
|
≤7 | 1 (3.1) |
|
8-14 | 1 (3.1) |
|
15-30 | 4 (12.5) |
|
31-90 | 4 (12.5) |
|
>90 | 1 (3.1) |
|
Administration
of ICIs after PRT | 17 (53.1) |
|
≤7 | 4 (12.5) |
|
8-14 | 2 (6.3) |
|
15-30 | 4 (12.5) |
|
31-90 | 4 (12.5) |
|
>90 | 3 (9.4) |
|
Administration
of ICIs before and after PRT | 4 (12.5) |
|
≤7 | 3 (9.4) |
|
8-14 | 1 (3.1) |
ROC analysis
The areas under the ROC curves for the incidence of
RIE were 0.56 (sensitivity, 100%; specificity, 47%) for the
interval between PRT and the closest administration of ICIs, 0.88
(sensitivity, 100%; specificity, 84%) for the fraction dose and
0.54 (sensitivity, 50%; specificity, 87%) for total EQD2. Regarding
the dose-volume parameters of the large bowel, the areas under the
ROC curve were 0.60 (sensitivity, 100%; specificity, 43%) for V10
Gy, 0.43 (sensitivity, 100%; specificity, 43%) for V20 Gy, 0.37
(sensitivity, 100%; specificity, 27%) for D2cc and 0.92
(sensitivity, 100%; specificity, 90%) for D2cc/fr. For the
dose-volume parameters of the small bowel, the areas under the ROC
curve were 0.65 (sensitivity, 100%; specificity, 53%) for V10 Gy,
0.58 (sensitivity, 100%; specificity, 50%) for V20 Gy, 0.63
(sensitivity, 100%; specificity, 60%) for D2cc and 0.63
(sensitivity, 50%; specificity, 43%) for D2cc/fr. For the incidence
of RIE, the interval between PRT and closest administration of ICIs
of 6-10 days, 3.6 Gy per fraction, total EQD2 of 28 Gy, V10 (large
bowel) of 67.2 cc, V20 (large bowel) of 49.6 cc, D2cc (large bowel)
of 21.2 Gy, D2cc/fr (large bowel) of 3.4 Gy, V10 (small bowel) of
43 cc, V20 (small bowel) of 7.8 cc, D2cc (small bowel) of 19.7 Gy
and D2cc/fr (small bowel) of 3.9 Gy corresponded to the maximum sum
of sensitivity and specificity.
Treatment
A total of 19 patients with 22 lesions received
anti-PD-1 (pembrolizumab or nivolumab) monotherapy, 5 patients with
5 lesions received anti-PD-L1 (durvalumab or atezolizumab)
monotherapy and 4 patients with 5 lesions received anti-PD-1/PD-L1
(nivolumab, pembrolizumab or durvalumab) and anti-CTLA-4
(ipilimumab) therapy. Furthermore, 19 lesions were treated with
ICIs prior to the initiation of PRT, 19 lesions were treated with
ICIs after the initiation of PRT and the remaining 4 lesions were
treated with ICIs both prior to and after PRT. The median interval
between PRT and the closest administration of ICIs was 20.5 days
(range, 1-212 days).
In addition, 17 patients with 19 lesions received
chemotherapy prior to PRT and 24 patients with 28 lesions received
chemotherapy after PRT. Furthermore, two patients with four lesions
(one, bevacizumab; one, erlotinib) and six patients with six
lesions (four, ramucirumab; two, bevacizumab) received biotherapy
prior to and after PRT, respectively.
The median PRT dose was 30 Gy (range, 8-48 Gy) and
the median total EQD2 was 36.0 Gy (range, 17.6-49.5 Gy). In
addition, the frequently used dose-fractionation schedules, in
sequential order, were as follows for the PRT dose (EQD2): 1x8 Gy
(17.6 Gy), 5x4 Gy (28.0 Gy), 8x3.6 Gy (38.0 Gy), 10x3 Gy (36.0 Gy),
15-18x2.5 Gy (41.3-49.5 Gy), 24x2 Gy (48.0 Gy) and 10x2 Gy + 5x3 Gy
(38.0 Gy). The irradiated sites were the vertebral bones (n=13),
pelvic bones (n=12), adrenal glands (n=3), lymph nodes (n=3) and
liver (n=1). The details of patients and lesions characteristics
are shown in Table I.
OS
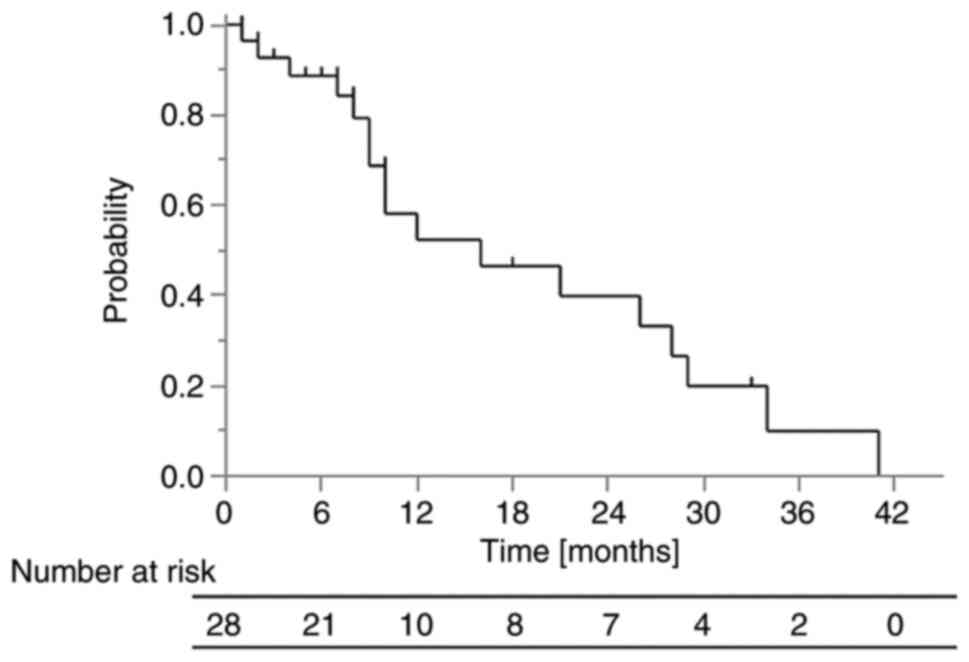
The 1-year OS rate was 53% (Fig. 1). The median survival time in all
patients was 10 months (range, 1-41 months) and the median
follow-up time in surviving patients was 7 months (range, 1-33
months). The 1-year OS rate in the group in which ICIs were
administered after PRT was 70%, while that in the group in which
ICIs were not administered after PRT was 38% (P=0.0091).
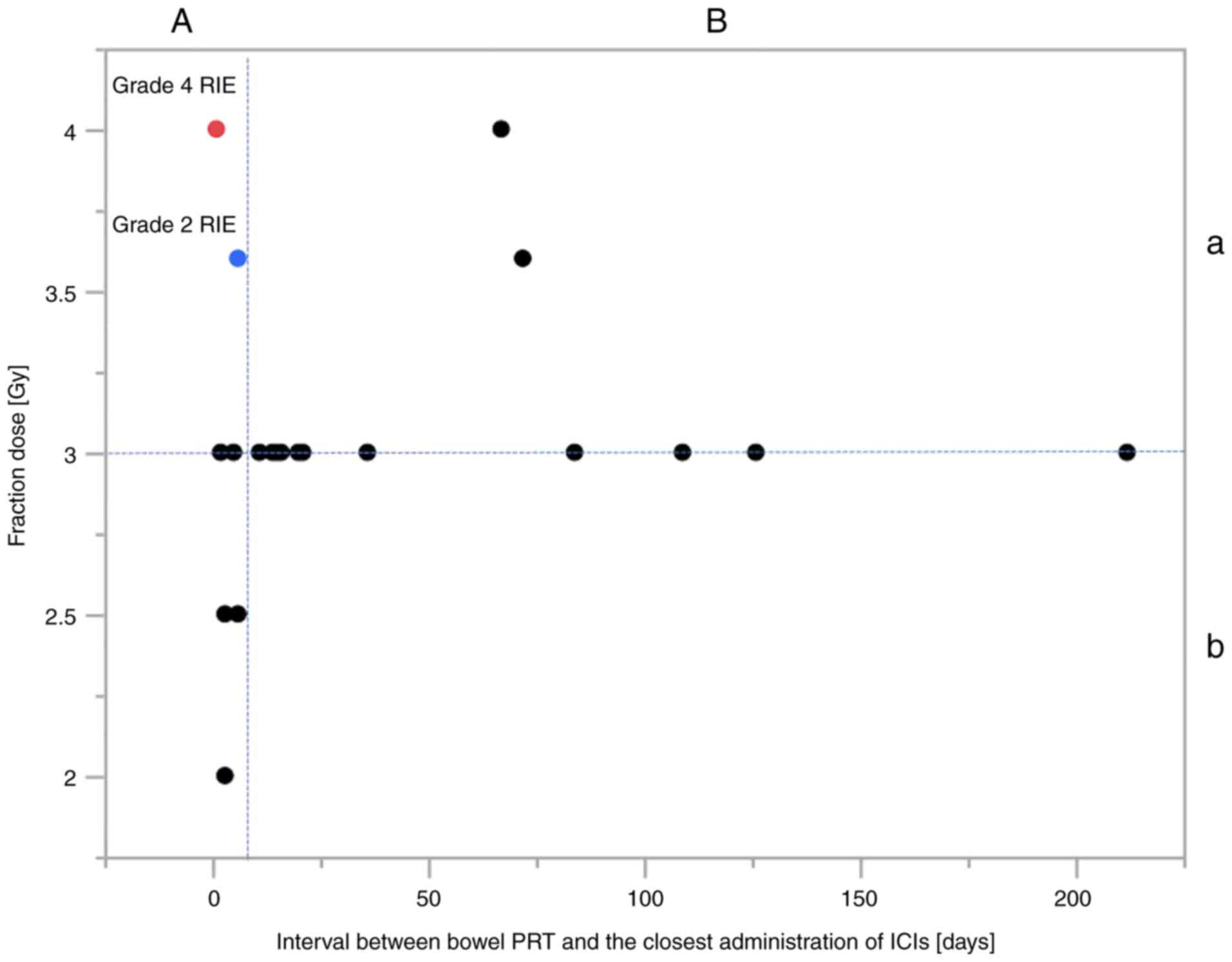
Factors affecting grade 2 or higher
RIE
Grade 2 or higher RIE was observed in 2 patients
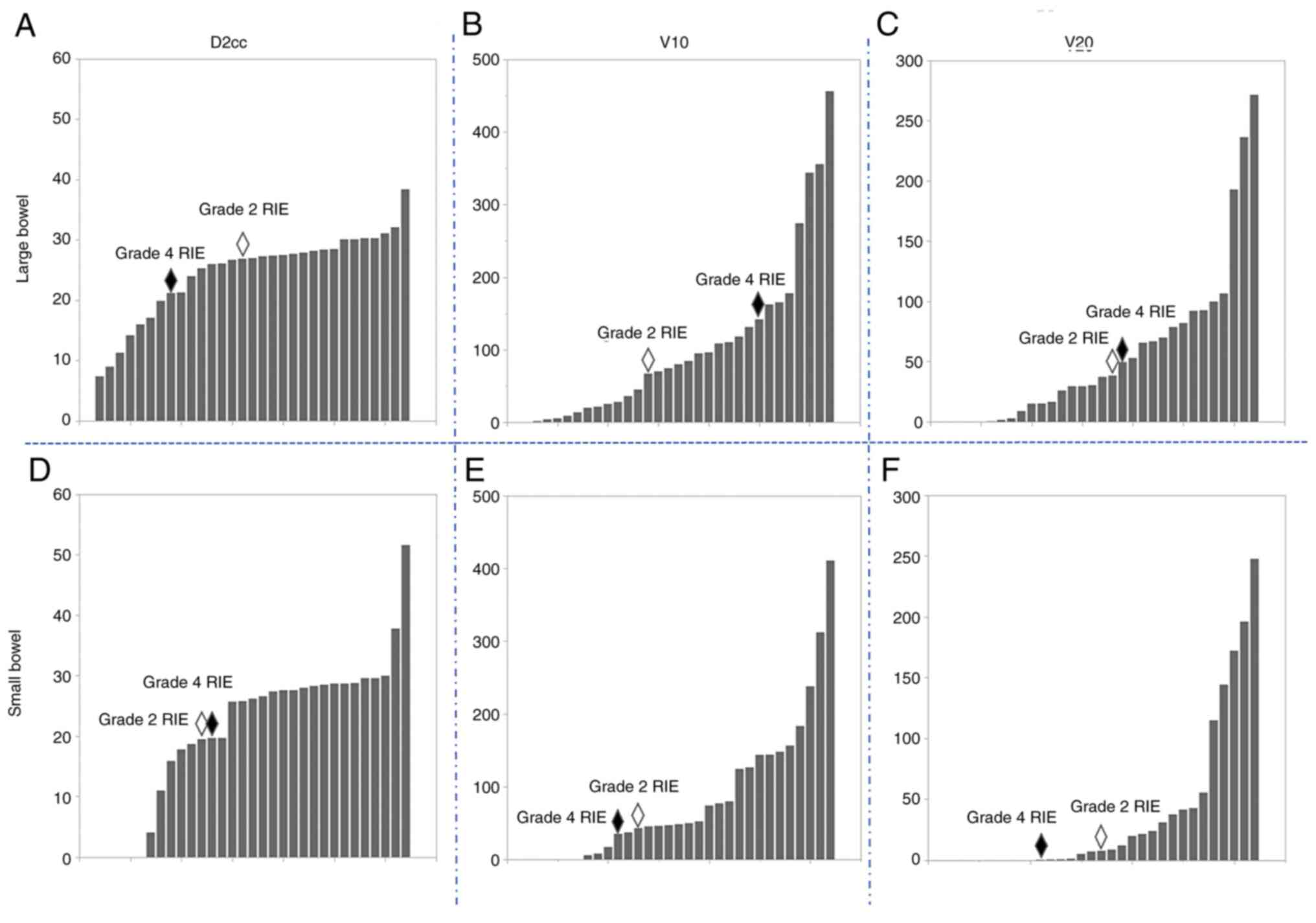
(2/28 patients, 7.1%; 2/32 lesions, 6.3%; Figs. 2 and 3). Regarding the fraction dose of PRT
evaluated at the isocenter, there was a significant difference in
the incidence of RIE between <3.6 and ≥3.6 Gy per fraction
(P=0.04, Table II). In addition,
there tended to be differences in the incidence of RIE between
administration of ICIs <7 and ≥7 days after PRT completion
(P=0.07). In 19 lesions that were treated with ICIs after PRT,
these two factors (<3.6 vs. ≥3.6 Gy per fraction and the
administration of ICIs <7 vs. ≥7 days after PRT completion) were
associated with significantly different incidences of RIE (P=0.04
and 0.05, respectively; Fig. 4,
Table II). In addition, D2cc/fr
of the large bowel (<3.4 vs. ≥3.4 Gy) had a significant
influence on the incidence of RIE (P=0.02, Table II). However, the other dose-volume
parameters of the bowel (V10, V20 and D2cc of small and large
bowel) were not associated with the incidence of RIE (Fig. 5, Tables II and III). In addition, age, sex, performance
status, PRT sites, total dose (EQD2), chemotherapy and biotherapy
were not associated with the incidence of RIE. The clinical and
treatment details of subgroups of patients that received >3 Gy
per fraction and/or were administered ICIs within seven days after
completing PRT are provided in Table
IV.
 | Table IIIncidence of grade 2 or higher
radiation-induced enterocolitis. |
Table II
Incidence of grade 2 or higher
radiation-induced enterocolitis.
| | Administration of
ICIs before and/or after PRT | Administration of
ICIs after PRT |
|---|
| Characteristic | No. of lesions | P-value | No. of lesions | P-value |
|---|
| Age, years | | 1.00 | | 1.00 |
|
<65 | 1/17 | | 1/10 | |
|
≥65 | 1/15 | | 1/9 | |
| Sex | | 1.00 | | 1.00 |
|
Male | 2/27 | | 2/16 | |
|
Female | 0/5 | | 0/3 | |
| PS (ECOG) | | 0.13 | | 0.12 |
|
0-1 | 0/20 | | 0/12 | |
|
2-4 | 2/12 | | 2/7 | |
| PRT sites | | 0.40 | | 0.30 |
|
Bone | 1/25 | | 1/16 | |
|
Others | 1/6 | | 1/3 | |
| Total EQD2, Gy | | 1.00 | | - |
|
<28 | 0/1 | | 0/0 | |
|
≥28 | 2/31 | | 2/19 | |
| Fraction dose,
Gy | | 0.04 | | 0.04 |
|
<3.6 | 0/25 | | 0/15 | |
|
≥3.6 | 2/7 | | 2/4 | |
| Chemotherapy before
PRT | | 1.00 | | 1.00 |
|
Yes | 1/19 | | 1/9 | |
|
No | 1/13 | | 1/10 | |
| Chemotherapy after
PRT | | 1.00 | | - |
|
Yes | 2/28 | | 2/19 | |
|
No | 0/4 | | 0/0 | |
| Biotherapy before
PRT | | 1.00 | | 1.00 |
|
Yes | 0/4 | | 0/1 | |
|
No | 2/28 | | 2/18 | |
| Biotherapy after
PRT | | 1.00 | | 1.00 |
|
Yes | 0/6 | | 0/4 | |
|
No | 2/26 | | 2/15 | |
| Administration of
ICIs before PRT | | 0.49 | | 1.00 |
|
Yes | 0/15 | | 0/2 | |
|
No | 2/17 | | 2/17 | |
| Administration of
ICIs after PRT | | 0.53 | | 0.30 |
|
Yes | 2/21 | | 1/16 | |
|
No | 0/11 | | 1/3 | |
| ICIs
monotherapy | | 0.29 | | 0.30 |
|
Yes | 1/27 | | 1/16 | |
|
No | 1/5 | | 1/3 | |
| Interval between
the closest administration of ICIs and PRT, days | | 0.07 | | 0.05 |
|
<7 | 2/9 | | 2/5 | |
|
≥7 | 0/23 | | 0/14 | |
| V10 of the small
bowel | | 1.00 | | 1.00 |
|
<43 | 1/12 | | 1/6 | |
|
≥43 | 1/20 | | 1/13 | |
| V20 of the small
bowel | | 1.00 | | 1.00 |
|
<7.8 | 1/16 | | 0/4 | |
|
≥7.8 | 1/16 | | 2/15 | |
| D2cc of the small
bowel | | 1.00 | | 1.00 |
|
<19.7 | 1/12 | | 1/6 | |
|
≥19.7 | 1/20 | | 1/13 | |
| D2cc/fr of the
small bowel | | 0.12 | | 0.20 |
|
<3.9 | 1/30 | | 1/17 | |
|
≥3.9 | 1/2 | | 1/2 | |
| V10 of the large
bowel | | 0.50 | | 0.51 |
|
<67.2 | 0/13 | | 0/7 | |
|
≥67.2 | 2/19 | | 2/12 | |
| V20 of the large
bowel | | 1.00 | | 1.00 |
|
<49.6 | 1/18 | | 1/9 | |
|
≥49.6 | 1/14 | | 1/10 | |
| D2cc of the large
bowel | | 1.00 | | 1.00 |
|
<21.2 | 0/9 | | 1/11 | |
|
≥21.2 | 2/23 | | 1/8 | |
| D2cc/fr of the
large bowel | | 0.02 | | 0.02 |
|
<3.4 | 0/27 | | 0/16 | |
|
≥3.4 | 2/5 | | 2/3 | |
 | Table IIIDose-volume parameters for the
patients/lesions. |
Table III
Dose-volume parameters for the
patients/lesions.
| | Large bowel | | Small bowel |
|---|
| Subgroup | EQD2 | Fraction doses | Grade of
enterocolitis | D2cc/fr | D2cc | V20 | V10 | D2cc/fr | D2cc | V20 | V10 |
|---|
| All | 36.0
(17.6-49.5) | 3.0 (2-8) | - | 2.8 (0-7.4) | 27.0 (0-38.4) | 33.9 (0-271.5) | 77.35
(0-456.2) | 2.8 (0-4.1) | 26.0 (0-51.6) | 7.6 (0-247.7) | 47.7 (0-410.9) |
| ICIs after PRT | 36.0
(28.0-49.5) | 3 (2-4) | - | 2.8 (1.6-4.2) | 26.9 (9-31.1) | 37.3 (0-236.4) | 74.6
(1.7-456.2) | 2.8 (0-3.9) | 25.7 (0-29.6) | 7.3 (0-247.7) | 48.4 (0-410.9) |
| No ICIs after
PRT | 36.0
(17.6-41.3) | 3 (2.5-8) | - | 2.8 (0-7.4) | 27.3 (0-38.4) | 29.5 (0-271.5) | 84.6 (0-355.7) | 2.9 (0-4.1) | 28.3 (0-51.6) | 19.9 (0-144.3) | 47.0 (0-183.3) |
| Patient 1 | 28.0 | 4 | 4 | 4.2 | 21.2 | 49.6 | 141.8 | 3.9 | 19.7 | 0.5 | 35.1 |
| Patient 2 | 38.0 | 3.6 | 2 | 3.4 | 26.9 | 38.4 | 67.2 | 2.4 | 19.5 | 7.8 | 43 |
 | Table IVCharacteristics of patients/lesions
given >3 Gy per fraction or ICIs within 7 days after PRT. |
Table IV
Characteristics of patients/lesions
given >3 Gy per fraction or ICIs within 7 days after PRT.
| A, Series with
grade 2 or higher RIE |
|---|
| Patient | Age | Sex | PS (ECOG) | Grade of
enterocolitis | PRT sites | Total dose in
Gy/fractions | ICIs therapy | Interval between
PRT and closest administration of ICIs | Timing of onset of
RIE symptoms | CT image | Max fraction dose
of descending colon, Gy (%) |
|---|
| 1 | 75 | Male | 2 | 4 | Adrenal gland | 20.0/5.0 | Anti-PD-L1
monotherapy | 1 day after
completion of PRT | 8 days after
completion of PRT | Descending
colitis | 4.28(107) |
| 2 | 58 | Male | 2 | 2 | Pelvic bone | 28.8/8.0 | Anti-PD-1 +
anti-CTLA-4 combination therapy | 6 days completion
after end of PRT | 7 days after
completion of PRT | Descending
colitis | 3.63(104) |
| B, series received
>3 Gy per fraction |
| Patient | Age | Sex | PS (ECOG) | Grade of
enterocolitis | PRT sites | Total dose in
Gy/fractions | ICIs therapy | Interval between
PRT and closest administration of ICIs | Timing of onset of
RIE symptoms | CT image | Max fraction dose
of descending colon, Gy (%) |
| 3 | 63 | Male | 2 | 0 | Pelvic bone | 20/5 | Anti-PD-1
monotherapy | 75 days before
initiation of PRT | - | No
enterocolitis | 4.12(103) |
| 5 | 65 | Male | 1 | 0 | Pelvic bone | 20/5 | Anti-PD-1
monotherapy | 67 days after
completion of PRT | - | No
enterocolitis | 4.24(106) |
| 4 | 63 | Male | 2 | 0 | Vertebral bone | 20/5 | Anti-PD-1
monotherapy | 82 days before
initiation of PRT | - | No
enterocolitis | 3.44(86) |
| 6 | 73 | Male | 1 | 0 | Vertebral bone | 28.8/8.0 | Anti-PD-1
monotherapy | 72 days after
completion of PRT | - | No
enterocolitis | 3.31(92) |
| 7 | 48 | Male | 1 | 0 | Vertebral bone | 8/1.0 | Anti-PD-L1
monotherapy | 183 days before
initiation of PRT | - | No
enterocolitis | 7.52(94) |
| C, Series received
the administration of ICIs within seven days after completion of
PRT |
| Patient | Age | Sex | PS (ECOG) | Grade of
enterocolitis | PRT sites | Total dose in
Gy/fractions | ICIs therapy | Interval between
PRT and closest administration of ICIs | Timing of onset of
RIE symptoms | CT image | Max fraction dose
of descending colon, Gy (%) |
| 8 | 73 | Male | 3 | 0 | Pelvic bone | 30/10 | Anti-PD-1
monotherapy | 2 days after
completion of PRT | - | No
enterocolitis | 2.58(86) |
| 9 | 67 | Male | 0 | 0 | Lymph node | 48/24 | Anti-PD-L1
monotherapy | 3 days after
completion of PRT and 26 days before initiation of PRT | - | No
enterocolitis | 1.21(60) |
| 10 | 64 | Female | 1 | 0 | Pelvic bone | 40/16 | Anti-PD-1
monotherapy | 3 days after
completion of PRT and 20 days before initiation of PRT | - | No
enterocolitis | 2.29(92) |
| 11 | 70 | Male | 1 | 0 | Pelvic bone | 30/10 | Anti-PD-1
monotherapy | 5 days after
completion of PRT | - | No
enterocolitis | 3.08(103) |
| 12 | 57 | Male | 0 | 0 | Adrenal gland | 45/18 | Anti-PD-1
monotherapy | 6 days after
completion of PRT and 5 days before initiation of PRT | - | No
enterocolitis | 1.76(70) |
Cases with grade 2 or higher RIE
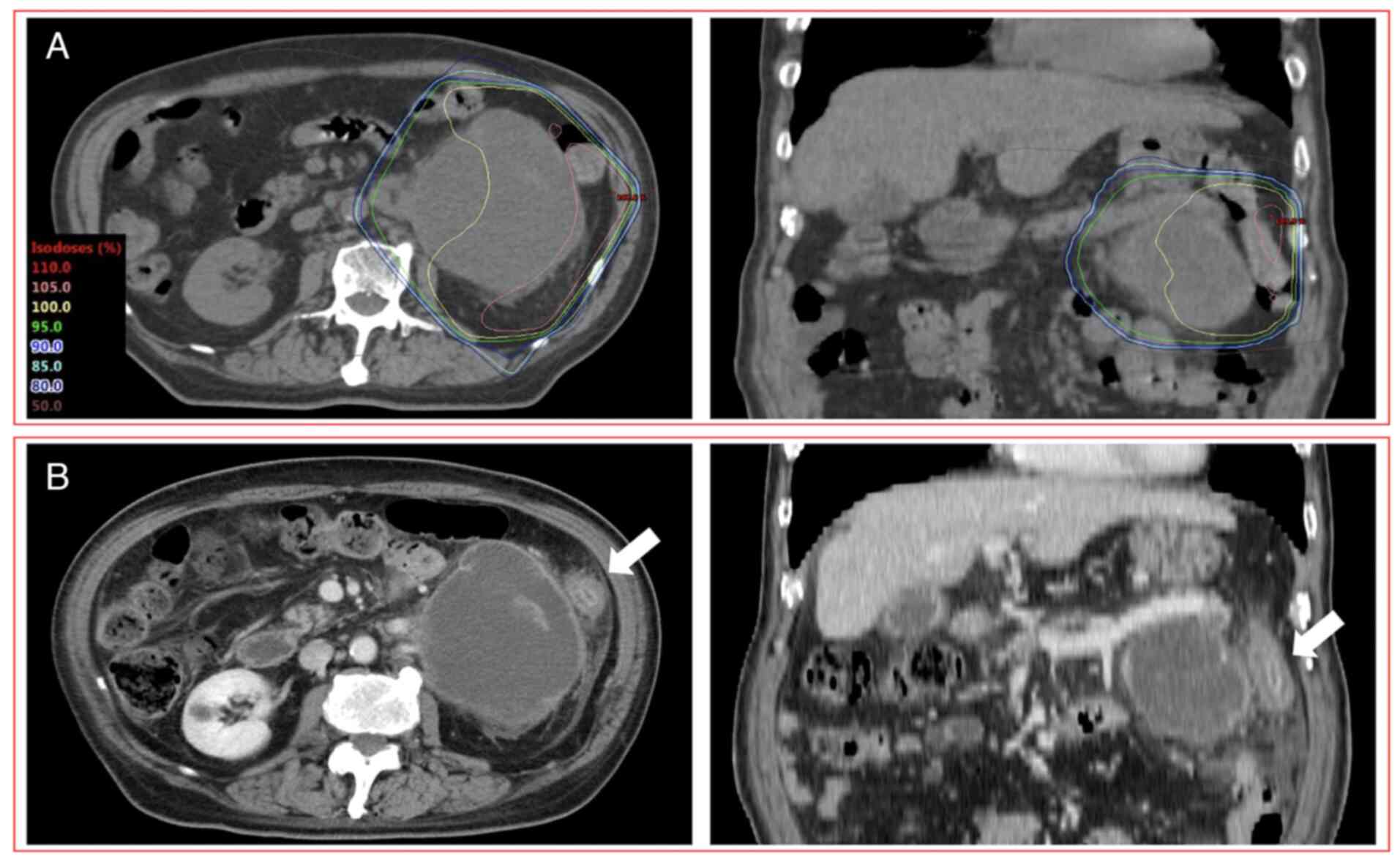
Grade 4 RIE was reported in one patient (75 years,
male) who received anti-PD-L1 (atezolizumab) monotherapy with
chemotherapy (carboplatin and paclitaxel) one day after completing
PRT (5x4 Gy) (Fig. 2). After
completing PRT, this patient had diarrhea and abdominal pain after
8 days and hematochezia after 18 days. CT images acquired 28 days
after the completion of PRT indicated enterocolitis limited to the
irradiated field. These symptoms were improved 49 days after the
completion of PRT. However, after the third administration of
anti-PD-L1 (atezolizumab) monotherapy, enterocolitis deteriorated
98 days after the completion of PRT (11 days after the third ICI
administration). Eventually, as colonoscopy performed 128 days
after the completion of PRT revealed erosion and angiectasis of the
descending colon limited to the irradiated field without
neutrophilic infiltration of the intra-epithelial compartment or
formation of neutrophilic crypt abscess, this patient was diagnosed
with RIE and colostomy was performed. The dose-volume parameters of
D2cc, V10 and V20 of the large bowel were 21.2 Gy, 141.8 cc and
49.6 cc, respectively.
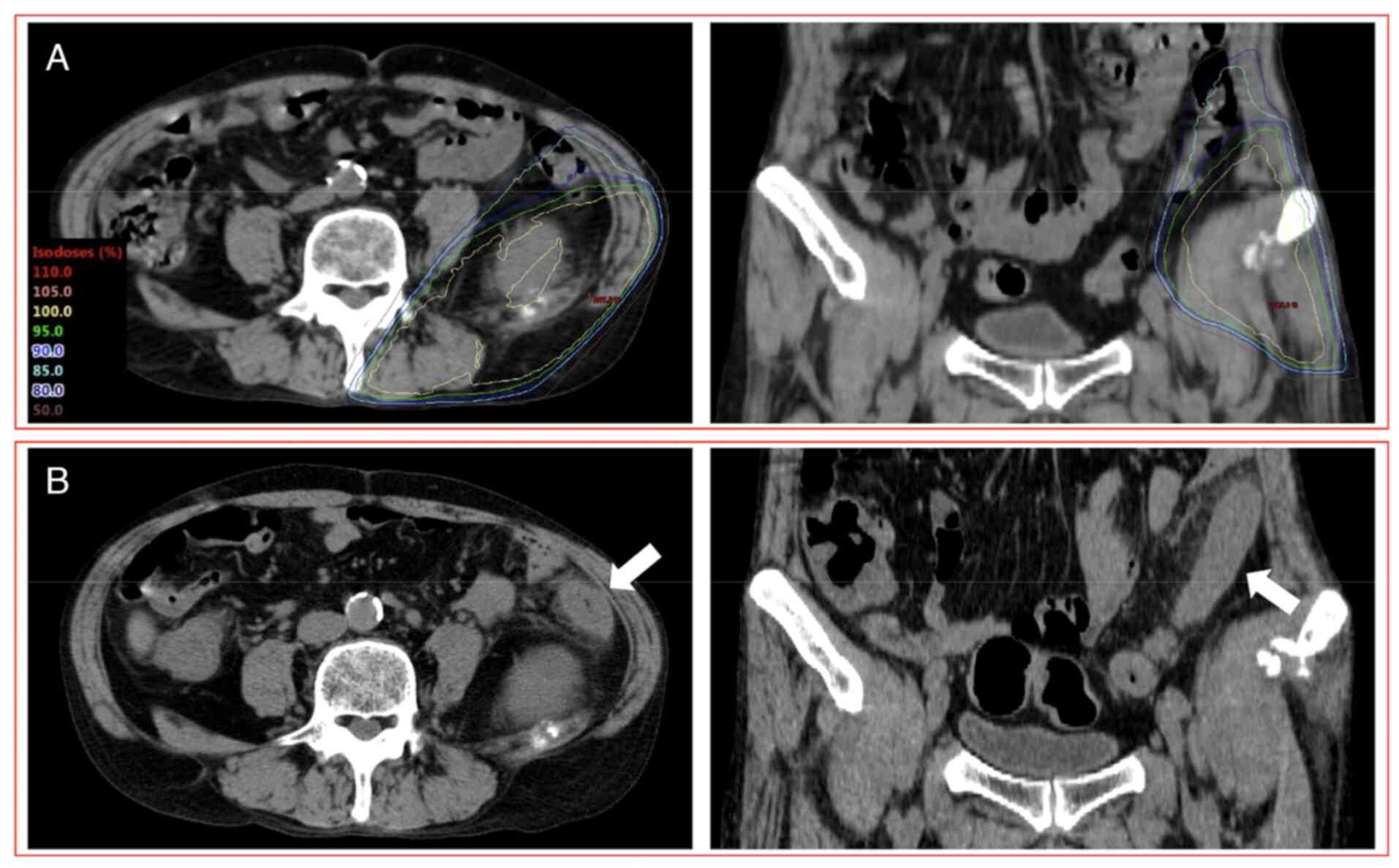
Another patient (58 years, male) who had grade 2 RIE
was administered anti-PD-1 (nivolumab) plus anti-CTLA-4
(ipilimumab) combination therapy with chemotherapy (carboplatin and
pemetrexed) 6 days after the completion of PRT (8x3.6 Gy) (Fig. 3). This patient had diarrhea and
abdominal pain 7 days after the completion of PRT. CT images
acquired 17 days after the completion of PRT revealed findings of
enterocolitis limited to the irradiated field. Biopsy was not
performed. The dose-volume parameters of D2cc, V10 and V20 of the
large bowel were 26.9 Gy, 67.2 cc and 38.4 cc, respectively.
Discussion
The present study indicated that the combination of
PRT involving the bowel and ICIs were well tolerated by a majority
of patients. However, RIE of grade 2 or higher was observed in 6.3%
(2/32) of patients. In all of these cases, the interval between the
administration of ICIs and the completion of PRT was within 7 days
and fraction doses were >3.6 Gy (evaluated at the isocenter),
and D2cc/fr ≥3.4 Gy. A clear relationship between grade 2 or higher
RIE and other dose-volume parameters of the bowel was not observed
in patients who received PRT in combination with ICIs. However, it
was indicated that a larger fraction dose of PRT and a shorter
interval between the administration of ICIs and PRT may affect the
incidence of grade 2 or higher RIE.
RIE is typically associated with progressive
occlusive vasculitis. Although the role of ICIs in RIE remains
elusive, PRT alone, as it involves a comparatively low dose, is
unlikely to cause severe RIE (6,13).
Bang et al (7) reported
that mild colitis was observed in 4% of the patients who received
ICIs and PRT to the bowel. The present results also suggested that
the incidence of enterocolitis was not high after combination
therapy with PRT and ICIs. By contrast, Bang et al (7) indicated that irAEs occurred more
frequently when ICIs were administered within 14 days prior to and
after PRT compared to when ICIs were administered 14 days or more
after PRT (statistically not significant). In the present study,
patients who experienced grade 2 or higher RIE received ICIs within
7 days after the completion of PRT. Although the optimal intervals
between RT and ICIs to achieve a systemic effect of RT and ICIs
remained to be determined (14),
the administration of ICIs immediately after PRT may also be a
potential risk factor for severe RIE.
In addition, several studies suggested that moderate
hypofractionated regimens (6-8 Gy per fraction) may increase the
synergistic effect of ICIs (15,16).
In the present study, the fraction doses (3.6 and 4 Gy per
fraction) in the two patients with RIE were lower than this
fraction dose. A fraction dose of >3 Gy (D2cc/fr of large bowel
≥3.4 Gy) may be associated with the risk of severe RIE with PRT and
ICI combination therapy. Thus, for combination therapy with PRT and
ICIs, two factors, namely the fraction dose and interval between
PRT and ICIs, may be important. Furthermore, the interaction
between a higher fraction dose of PRT and the interval between PRT
and administration of ICIs may be significant in the development of
grade 2 or higher RIE.
In addition, elevated levels and imbalance of
several cytokines generally result in various symptoms in advanced
cancers (17). The RT-induced
inflammatory response in the bowel involves the recruitment of
activated inflammatory cells (18). These immune cells synthesize and
release several different cytokines, inflammatory mediators and
reactive oxygen metabolites (19).
In addition to the RT-induced inflammatory response, ICIs also
promote the activity of immune cells and facilitate autoimmune
responses against any organ (20).
The combination of these two factors may lead to RIE even when PRT
is administered.
In the present study, one patient (3.1%) experienced
grade 4 RIE after combination therapy with PRT and ICIs. In this
patient, the interaction between PRT and ICIs may have induced
severe RIE. Although RIE was initially alleviated in this patient,
it worsened again and grade 4 RIE was developed after the
subsequent administration of ICIs. Radiation recall phenomenon is
an inflammatory reaction that manifests within a previously
irradiated field after the administration of a variety of
pharmacological agents (21). This
grade 4 RIE may have been caused by a radiation recall phenomenon
associated with the subsequent administration of ICIs.
There were certain limitations to the present study
owing to its retrospective nature and small sample size. Selection
bias and confounding factors must also be considered. In addition,
based on symptoms alone, accurate differentiation between RIE and
irAE is difficult in numerous cases, as RIE and irAE enterocolitis
exhibit similar symptoms. Therefore, the present study focused on
the importance of CT images in addition to the symptoms of
enterocolitis. Although the incidence of mild irAEs in the bowel,
such as diarrhea, abdominal pain and nausea, is 12.1-13.7% for
anti-PD-1 and 30.2-35.4% for anti-CTLA-4, the incidence of severe
irAEs of the bowel (enterocolitis) is 0.7-1.6% for anti-PD-1,
5.7-9.1% for anti-CTLA-4 and 13.6% for the combination of both
therapies (22,23). In the present study, the incidence
of RIE was similar to the incidence of irAEs of the bowel. However,
as the CT images of RIE (grade 2 and 4) were consistent with the
irradiated fields and the histology of grade 4 RIE was not typical
for an irAE of the bowel, these two cases were diagnosed as RIE.
Although irAE enterocolitis and RIE may not be completely
separated, the results of the present study suggested that RIE may
appear even after administering PRT in combination with ICIs.
Severe RIE may at times be induced by PRT involving
the bowel and ICI administration. Although further studies are
required, administration of ICIs immediately after PRT with a
higher fraction dose (at the isocenter) was indicated to be a risk
factor for severe RIE. However, a relationship between dose-volume
parameters other than D2cc/fr and RIE was not observed in the
present study.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KM, YH, HK, and KN were involved in the conception
and design of the study. KM, YH, HK and KN collected patient data
and drafted the manuscript. KM, YH, HK, KN, YS, TN, DH and TK
interpreted the data. KM and YH prepared the manuscript and HK, KN,
YS and TK edited the manuscript. All authors confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were conducted in accordance with the ethical
standards of the institutional research committee and with the 1964
Declaration of Helsinki and its later amendments or comparable
ethical standards. This retrospective study was approved by the
institutional review board (Shikoku Cancer Center, Ehime,
Japan).
Patient consent for publication
Patients consented in writing to the possibility of
the use of their anonymous data for research at the time of tissue
collection. In addition, the Opt-out method was used to obtain
consent for this study.
Competing interests
DH received honoraria from MSD, Ono, Kyowa Hakko
Kirin, AstraZeneca, Boehringer Ingelheim, TOWA, Chugai, TAIHO, and
Eli Lilly, and received research funding MSD, Chugai, AstraZeneca,
Eli Lilly, Pfizer, BMS, Novartis, Kissei and Takeda. TK received
honoraria from MSD, Ono, Kyowa Hakko Kirin, AstraZeneca, Boehringer
Ingelheim, Chugai, TAIHO, Eli Lilly, Bristol Myers Squibb, Pfizer,
Merck Biopharma, Nippon Kayaku, Novartis, Daiichi-Sankyo, AbbVie
and Bayer, and received research funding MSD, Kyowa Hakko Kirin,
AstraZeneca, Eli Lilly, Pfizer, BMS, Novartis, Kissei, Takeda,
Chugai, TAIHO, Bristol-Myers, Merck Biopharma, Daiichi-Sankyo,
AbbVie and AMGEN. All other authors declare that they have no
competing interests.
References
|
1
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ramalingam SS, Vansteenkiste J, Planchard
D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y,
Chewaskulyong B, et al: Overall survival with Osimertinib in
untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 382:41–50.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Grangeon M, Tomasini P, Chaleat S, Jeanson
A, Souquet-Bressand M, Khobta N, Bermudez J, Trigui Y, Greillier L,
Blanchon M, et al: Association between immune-related adverse
events and efficacy of immune checkpoint inhibitors in
non-small-cell lung cancer. Clin Lung Cancer. 20:201–207.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ricciuti B, Genova C, De Giglio A,
Bassanelli M, Dal Bello MG, Metro G, Brambilla M, Baglivo S, Grossi
F and Chiari R: Impact of immune-related adverse events on survival
in patients with advanced non-small cell lung cancer treated with
nivolumab: Long-term outcomes from a multi-institutional analysis.
J Cancer Res Clin Oncol. 145:479–485. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Emami B, Lyman J, Brown A, Coia L, Goitein
M, Munzenrider JE, Shank B, Solin LJ and Wesson M: Tolerance of
normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol
Phys. 21:109–122. 1991.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bang A, Wilhite TJ, Pike LRG, Cagney DN,
Aizer AA, Taylor A, Spektor A, Krishnan M, Ott PA, Balboni TA, et
al: Multicenter evaluation of the tolerability of combined
treatment with PD-1 and CTLA-4 immune checkpoint inhibitors and
palliative radiation therapy. Int J Radiat Oncol Biol Phys.
98:344–351. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Qin R, Olson A, Singh B, Thomas S, Wolf S,
Bhavsar NA, Hanks BA, Salama JK and Salama AK: Safety and efficacy
of radiation therapy in advanced melanoma patients treated with
ipilimumab. Int J Radiat Oncol Biol Phys. 96:72–77. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Barker CA, Postow MA, Khan SA, Beal K,
Parhar PK, Yamada Y, Lee NY and Wolchok JD: Concurrent radiotherapy
and ipilimumab immunotherapy for patients with melanoma. Cancer
Immunol Res. 1:92–98. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, et
al: Overall survival with durvalumab after chemoradiotherapy in
stage III NSCLC. N Engl J Med. 379:2342–2350. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Patel RR, He K, Barsoumian HB, Chang JY,
Tang C, Verma V, Comeaux N, Chun SG, Gandhi S, Truong MT, et al:
High-dose irradiation in combination with non-ablative low-dose
radiation to treat metastatic disease after progression on
immunotherapy: Results of a phase II trial. Radiother Oncol.
162:60–67. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
National Cancer Institute: Common
terminology criteria for adverse events (CTCAE) v.5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
Accessed July 22, 2021.
|
|
13
|
Sprave T, Verma V, Förster R, Schlampp I,
Bruckner T, Bostel T, Welte SE, Tonndorf-Martini E, El Shafie R,
Nicolay NH, et al: Radiation-induced acute toxicities after
image-guided intensity-modulated radiotherapy versus
three-dimensional conformal radiotherapy for patients with spinal
metastases (IRON-1 trial): First results of a randomized controlled
trial. Strahlenther Onkol. 194:911–920. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bassanelli M, Ricciuti B, Giannarelli D,
Cecere FL, Roberto M, Giacinti S, Barucca V, Santarelli M, Ruggeri
EM, Marchetti P, et al: Systemic effect of radiotherapy before or
after nivolumab in lung cancer: An observational, retrospective,
multicenter study. Tumors 3008916211004733, 2021 (Epub ahead of
print).
|
|
15
|
Schaue D, Ratikan JA, Iwamoto KS and
McBride WH: Maximizing tumor immunity with fractionated radiation.
Int J Radiat Oncol Biol Phys. 83:1306–1310. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dewan MZ, Galloway AE, Kawashima N,
Dewyngaert JK, Babb JS, Formenti SC and Demaria S: Fractionated but
not single-dose radiotherapy induces an immune-mediated abscopal
effect when combined with anti-CTLA-4 antibody. Clin Cancer Res.
15:5379–5388. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dunlop RJ and Campbell CW: Cytokines and
advanced cancer. J Pain Symptom Manag. 20:214–232. 2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Uchida A, Mizutani Y, Nagamuta M and
Ikenaga M: Effects of X-ray irradiation on natural killer (NK) cell
system. II. Increased sensitivity to natural killer cytotoxic
factor (NKCF). Immunopharmacol Immunotoxicol. 11:521–534.
1989.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Agrawal A, Chandra D and Kale RK:
Radiation induced oxidative stress: II studies in liver as a
distant organ of tumor bearing mice. Mol Cell Biochem. 224:9–17.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Azria D, Magné N, Zouhair A, Castadot P,
Culine S, Ychou M, Stupp R, Van Houtte P, Dubois JB and Ozsahin M:
Radiation recall: A well recognized but neglected phenomenon.
Cancer Treat Rev. 31:555–570. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Marthey L, Mateus C, Mussini C, Nachury M,
Nancey S, Grange F, Zallot C, Peyrin-Biroulet L, Rahier JF,
Bourdier de Beauregard M, et al: Cancer immunotherapy with
anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel
disease. J Crohns Colitis. 10:395–401. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Soularue E, Lepage P, Colombel JF, Coutzac
C, Faleck D, Marthey L, Collins M, Chaput N, Robert C and Carbonnel
F: Enterocolitis due to immune checkpoint inhibitors: A systematic
review. Gut. 67:2056–2067. 2018.PubMed/NCBI View Article : Google Scholar
|