Introduction
The available evidence indicates that a number of
interventions can produce a definite protection against myocardial
ischemia/reperfusion injury (IRI) (1). Notably, ischemic preconditioning
(IPC) can provide a powerful protection against myocardial IRI and
is commonly used as a gold standard for evaluating the
cardioprotective effect of interventions in experimental studies
(2). However, clinical application
of IPC is significantly hindered by ethical issues, including a
requirement of direct interventions on blood vessels of heart and
unpredictability of ischemic heart attack. Thus, it is generally
considered that postconditioning is the most valuable treatment of
myocardial IRI in clinical practice, especially pharmacological
postconditioning (3). In available
literatures, numerous drugs including α7 nicotinic acetylcholine
receptor (α7nAChR) agonists, anesthetics, opioid drugs,
rosuvastatin, atorvastatin, dexmedetomidine, endomorphin-1,
phosphodiesterase and caspase inhibitors have been used for
pharmacological postconditioning and have been demonstrated to
produce moderate protection against myocardial IRI in normal rats
(3,4).
It has been demonstrated that common comorbidities
of patients with ischemic heart diseases, such as
hypercholesterolemia, hypertension, myocardial hypertrophy,
diabetes, obesity and sensory neuropathy, can significantly affect
the cardioprotective effectiveness of various interventions
including IPC and ischemia postconditioning by different mechanisms
(5,6). Therefore, therapeutic interventions
cannot be used practically in a clinical setting until their
cardioprotection has been demonstrated in the presence of common
comorbidities of ischemic heart diseases (7). Because hypercholesterolemia is one of
most prevalent comorbidities of ischemic heart diseases, there have
been numerous experimental and clinical studies assessing its
effect on the cardioprotective potentials of drugs or ischemic
preconditioning and postconditioning (8,9).
Indeed, the majority of studies indicate that hypercholesterolemia
can abolish or attenuate the cardioprotection of IPC (10,11).
However, other studies instead indicate that IPC can still preserve
cardioprotective potential in hypercholesterolemic animals
(12,13). Notably, the detailed mechanisms of
how hypercholesterolemia affects cardioprotective effect of IPC
have yet to be fully revealed. Moreover, to the best of our
knowledge, there has been no study evaluating the effect of
hypercholesterolemia on the cardioprotection of postconditioning
with α7nAChR agonists. Thus, the present study was designed to
compare the cardioprotective effects, inflammatory responses and
changes of the PI3K/Akt/endothelial nitric oxide synthase (eNOS)
signaling pathway between normal and hypercholesterolemic rats
receiving the IPC and α7nAChR agonist postconditioning. The main
aims of the present study were to determine the effect of
hypercholesterolemia on the cardioprotective efficacy of IPC and
α7nAChR agonist postconditioning and to explore the potential
mechanisms through which hypercholesterolemia affected their
cardioprotection.
Materials and methods
Laboratory animals
The present experiment used 80 SPF-grade male
Sprague Dawley rats, aged ~1-month-old and weighing 130-150 g. All
animals were supplied by Beijing Vital River Laboratory Animal
Technology Co., Ltd. The rats were kept under controlled
environmental conditions at a temperature of 20±2˚C, a relative
humidity of 60±5% and a 12 h light dark cycle with free access to
water and food. After the protocol was approved by the Animal Care
and Use Committee of Plastic Surgery Hospital, Chinese Academy of
Medical Sciences [approval no. 2017(38); June 16, 2017; Beijing,
China], this experiment was conducted in accordance with our
institutional guidelines on the use of live animals for
research.
Establishment of hypercholesterolemic
rat model
As described in previous literature (14), the rats were fed with a
high-cholesterol diet containing 2% cholesterol and 0.5% bile salts
for 8 weeks. The control rats were fed with a normal diet for 8
weeks. Subsequently, serum total cholesterol (TC), triglyceride
(TG) and low-density lipoprotein (LDL) were measured using an AU480
Chemistry Analyzer (Beckman Coulter, Inc.).
Establishment of myocardial IRI rat
model
According to the method reported in our previous
work (4), a rat model of
myocardial IRI was performed. Briefly, the rat was anesthetized
with an intraperitoneal injection of 10% chloral hydrate 350 mg/kg,
and anesthetic was supplemented during the experiment if needed.
After the left thoracotomy and pericardiotomy, myocardial ischemia
was achieved by occlusion of the left anterior descending coronary
artery (LAD) with a 5-0 silk ligature. Successful occlusion of the
LAD was confirmed by the presence of ST segment elevation on
electrocardiogram (ECG) and a change in epicardial color from
fresh-red to dark-red or paleness of the myocardium. After the
ligature was released, adequate myocardial reperfusion of blood
flow was verified using epicardial hyperemia and reversion of ECG
changes, such as ST segment level in the reperfusion phase
descended >50% of ST segment in the ischemia period.
After the experiment was completed, the rats'
abdominal cavities were opened to determine whether intraperitoneal
injection of drug caused visceral injury or peritonitis. If so, the
animal was excluded from data analysis.
Experimental protocols
Using computer-generated random numbers 40
hypercholesterolemic rats and 40 normal rats were randomly divided
into four groups (n=10 per group) and received the following
different treatments and controls: i) Hypercholesterolemic control
(HC) and normal control (NC) groups; ii) hypercholesterolemic
ischemia/reperfusion (HI) and normal ischemia/reperfusion (NI)
groups; iii) hypercholesterolemic ischemic preconditioning (HIPC)
and normal ischemic preconditioning (NIPC) groups; and iv)
hypercholesterolemic PNU282987 postconditioning (HPNU) and normal
PNU282987 postconditioning (NPNU) groups.
In the HC and NC groups, animals were only subjected
to surgical manipulation without ischemia/reperfusion
interventions. In the other groups, animals received
ischemia/reperfusion interventions including a LAD occlusion for 30
min, followed by 120 min of reperfusion. In the HIPC and NIPC
groups, rats were first subjected to the classic IPC interventions
before ischemia/reperfusion interventions, namely 5 min of ischemia
followed by 5 min of reperfusion for three cycles. Apart from the
HPNU and NPNU groups, all animals were injected intravenously with
1 ml normal saline at the end of a 30-min ischemia. In the HPNU and
NPNU groups, a highly selective α7nAChR agonist, PNU282987 (cat.
no. 123464-89-1; Tocris Bioscience), was intravenously injected
immediately before a 120-min reperfusion. According to our previous
work (4), the dosage of PNU282987
used for pharmacological postconditioning was 2.0 mg/kg, and it was
diluted with 1 ml normal saline immediately before use.
At 120 min of reperfusion, sodium pentobarbital 25
mg/kg was intravenously administered to increase the depth of
anesthesia and then a 3-ml blood sample was collected in a tube
containing EDTA from the right carotid artery. After settling for
30 min, blood samples were centrifuged at 377.325 x g for 10 min at
4˚C. The supernatants were collected and stored at -80˚C until
future analysis. The serum concentrations of TC, TG, LDL, creatine
kinase isoenzyme MB (CK-MB) and cardiac troponin I (cTnI) were
assessed by using an AU480 Chemistry Analyzer (Beckman Coulter,
Inc.). The serum concentrations of tumor necrosis factor α (TNF-α)
and interleukin-6 (IL-6) concentrations were assessed using the
enzyme-linked immunosorbent assay (ELISA) kits (rat TNF-α and IL-6;
cat. nos. ab236712, and ab234570, respectively; both Abcam)
specific for rat factors, following the manufacturer's instructions
(MULTISKAN MK3; Thermo Fisher Scientific, Inc.).
Evaluation of infarct size
After a reperfusion period of 120 min, five
anesthetized rats from each group were randomly selected. According
to the method reported in our previous work (4), the LAD was reoccluded and 1 ml of 2%
Evans blue dye was injected by the carotid artery. When the body
was stained blue, the rat was deeply anesthetized with intravenous
injection of sodium pentobarbital 100 mg/kg and then was euthanized
by intravenous injection of 10% potassium chloride 100 mg/kg.
Subsequently, the entire heart was excised, rinsed of excess blue
dye and the right ventricle and right and left atria trimmed off.
The remaining left ventricle was deep frozen at -20˚C.
Subsequently, the frozen left ventricle was cut into ~five slices
with 1 mm-thickness from apex to base and all tissue slices were
incubated in a 1% solution of 2,3,5-triphenyltetrazolium chloride
for 15 min at 37˚C. The infracted tissue stained a characteristic
white color, whereas the viable tissue stained red. After overnight
fixation at 4˚C in 10% formaldehyde, images of the slices were
digitally captured. The slices were analyzed using the Adobe
Photoshop CS6 (Adobe Systems, Inc.) by a blinded investigator who
assessed the area at risk (AAR) and the infarct size, which was
expressed as a percentage of the AAR.
Myocardial expressions of Akt,
phosphorylated (p)-Akt and eNOS by western blotting
After a reperfusion period of 120 min, the remaining
five rats in each group were deeply anesthetized with intravenous
injection of sodium pentobarbital 100 mg/kg and then were
euthanized with intravenous injection of 10% potassium chloride 100
mg/kg. The left ventricle was quickly removed and the myocardial
tissues from the ischemic area were cut into small pieces of the
same weight and stored at -80˚C. The proteins were extracted from
myocardial tissue by suspension in radioimmunoprecipitation assay
lysis buffer 9 (Beijing BLKW Biotechnology Co., Ltd.). Samples were
centrifuged at 28,341.3 x g at 4˚C for 20 min. The protein
concentration was measured using bicinchoninic acid assay. An equal
amount of protein (30 µg per well) in each group was
electrophoresed (SDS-PAGE, 10% of separation gel and 5% of
concentration gel) and transferred to a polyvinylidene fluoride
membrane. After blocking (5% skimmed milk at room temperature for 2
h) and eluting, the membranes were incubated overnight shaking at a
4˚C condition with monoclonal antibodies against AKT (1:1,000,
4685S; Cell Signaling Technology, Inc.), p-AKT (1:2,000, 4060S;
Cell Signaling Technology, Inc.), eNOS (1:1,000, 32027s; Cell
Signaling Technology, Inc.) and GAPDH (1:1,000; cat. no. 5174; Cell
Signaling Technology, Inc.), respectively. Then, the membranes were
washed with Tris-buffered saline with 0.1% Tween solution and
incubated with a horseradish peroxidase-conjugated second antibody
(1:10,000, goat anti-rabbit immunoglobulin G, 111-035-003, Jackson
ImmunoResearch Laboratories, Inc.) for 1 h at room temperature. The
antigen-antibody complexes in the membranes were visualized using
enhanced chemiluminescence and films were exposed in the darkroom.
The times of exposure, development and fixing were dependent on the
darkness of bands. The films were scanned and saved as TIF image
files. The band intensity was quantified using Gel Image system
version 4.00 Analysis software (Tanon Science and Technology Co.,
Ltd.). Finally, expression levels of proteins were acquired by
standardizing the grey levels of Akt, p-Akt and eNOS with
GAPDH.
Statistical analysis
The primary endpoint of this experiment was infarct
size. According to our previous study (15), infarct size was 71.6±8.7 and
36.0±12.5% in the normal rats receiving ischemia/reperfusion and
IPC, respectively. Sample size calculation indicated that a sample
size of at least 4 rats/group would be required, with a power of
80% and P-value of 0.05. More than 5 rats per group for each
observed variable were included in the experiment so as to ensure
enough data to fit the ANOVA models and to allow for comparisons
among other outcome variables of interest. Statistical analysis of
data was performed using SPSS (version 18.0; SPSS, Inc.). For
continuous variables, the normal distribution test and Levene test
were employed to test the normal distribution and the homogeneity
of variance. If the data were normally distributed and had
homogeneous variance, they were expressed as mean ± standard
deviation. The comparisons of serum TC, LDL and TG levels between
NC and HC groups were performed by the unpaired t-test. The
comparisons of serum myocardial injury biomarker and inflammatory
factor levels, infarct sizes and myocardial Akt and eNOS expression
levels among groups were performed using a two- or three-way
analysis of variance, as needed. Sidak's test was used for post-hoc
multiple comparisons. When data were not normally distributed or
had inhomogeneous variance, they were expressed as median
(interquartile range). The non-parametric test was employed for
statistical analysis of data. The Mann-Whitney U test was used for
comparison between groups, and the Kruskal-Wallis test was used for
comparisons among multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
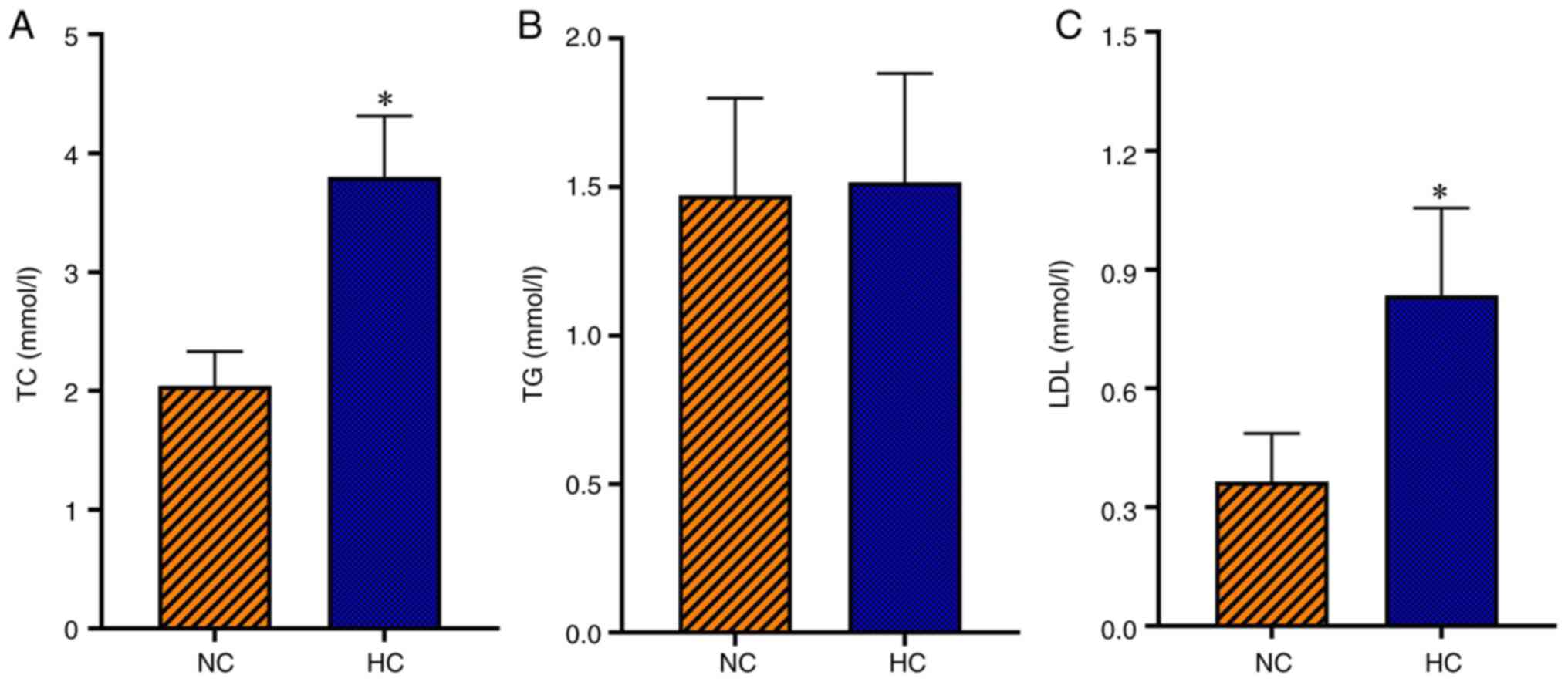
High cholesterol feed significantly
increases serum TC and LDL levels in rats
The serum TC and LDL levels were significantly
higher in the HC group compared with the NC group (P<0.05);
however, serum TG level was not significantly different between the
HC and NC groups (Fig. 1). This
suggested that a rat model of hypercholesterolemia was successfully
established.
Effects of IPC and PNU282987
postconditioning decreases elevation of myocardial injury
biomarkers, which are attenuated or eliminated by
hypercholesterolemia
In the normal rats, serum LDH, CK-MB and cTnI levels
were significantly elevated in the NI, NIPC and NPNU groups
compared with the NC group (P<0.05). Whereas serum LDH, CK-MB
and cTnI levels were significantly decreased in the NIPC and NPNU
groups compared with the NI group (P<0.05). Moreover, serum
CK-MB and cTnI levels were significantly elevated in the NPNU group
compared with the NIPC group (P<0.05) (Fig. 2).
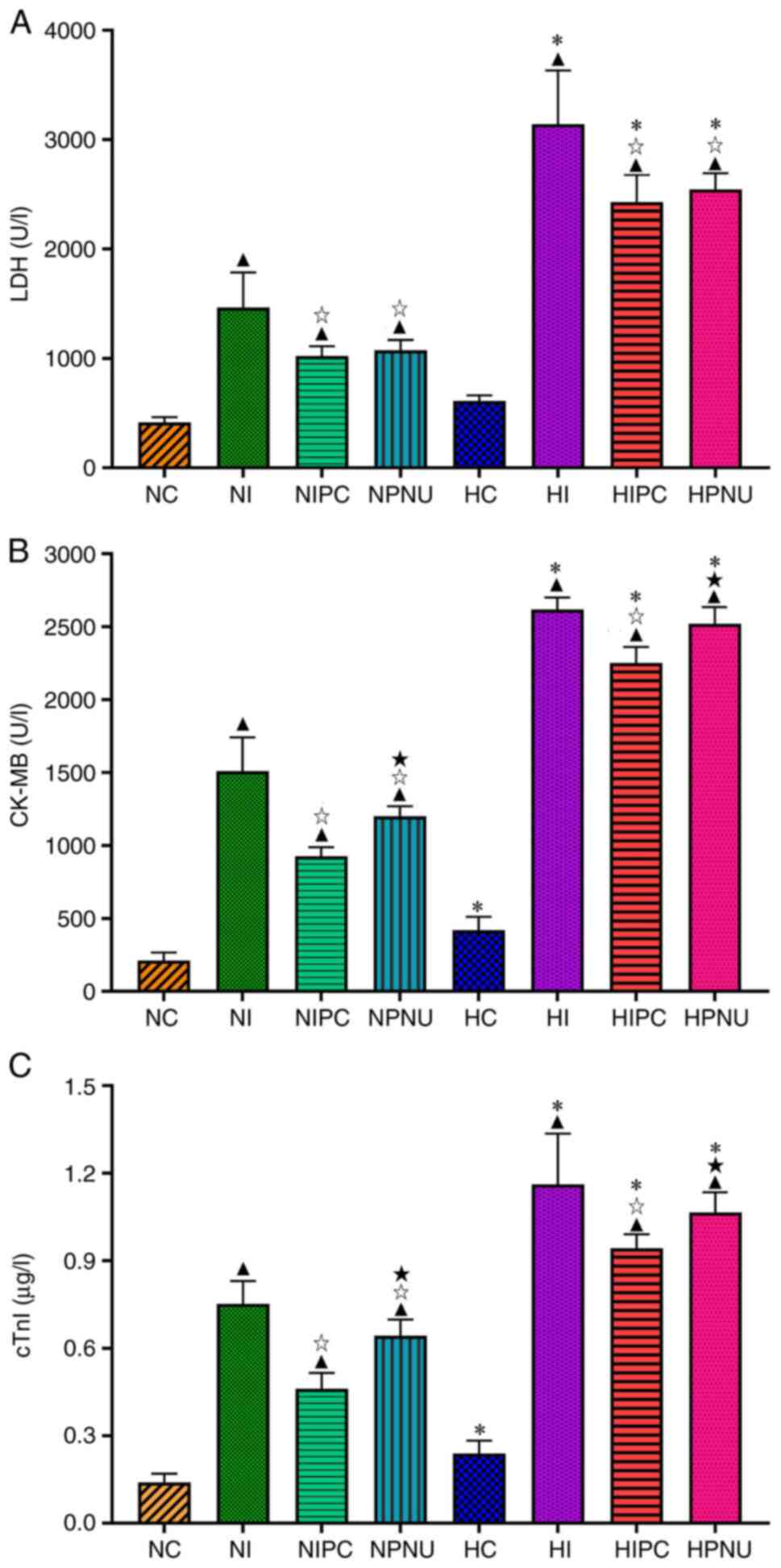 | Figure 2Comparisons of serum (A) LDH, (B)
CK-MB and (C) cTnI levels between normal and hypercholesterolemic
rats. Intra-group comparisons of normal and hypercholesterolemic
rats: ▲P<0.05 compared with the NC or HC group;
☆P<0.05 compared with the NI or HI group;
★P<0.05 compared with the NIPC or HIPC group.
Inter-group comparisons between corresponding treatment groups of
normal and hypercholesterolemic rats; namely,
*P<0.05, HC vs. NC, HI vs. NI, HIPC vs. NIPC and HPNU
vs. NPNU groups. LDH, lactate dehydrogenase; CK-MB, creatine kinase
isoenzyme MB; cTnI, cardiac troponin I; NC, normal control; NI,
normal ischemia/reperfusion; NIPC, normal ischemic preconditioning;
NPNU, normal PNU282987 postconditioning; HC, hypercholesterolemic
control; HI, hypercholesterolemic ischemia/reperfusion; HIPC,
hypercholesterolemic ischemic preconditioning; HPNU,
hypercholesterolemic PNU282987 postconditioning. |
In the hypercholesterolemic rats, serum LDH, CK-MB
and cTnI levels were significantly increased in the HI, HIPC and
HPNU groups compared with the HC group (P<0.05); serum LDH,
CK-MB and cTnI levels were significantly reduced in the HIPC and
HPNU groups compared with the HI group (P<0.05). In addition,
serum CK-MB and cTnI levels were significantly elevated in the HPNU
group compared with the HIPC group (P<0.05). Compared with the
normal rats, serum LDH, CK-MB and cTnI levels were significantly
elevated in the corresponding treatment groups of
hypercholesterolemic rats (HI vs. NC, HIPC vs. NIPC and HPNU vs.
NPNU groups; P<0.05) (Fig.
2).
Infarct size-limiting effect of IPC
are attenuated and effect of PNU282987 postconditioning is
eliminated by hypercholesterolemia
No myocardial infarction was observed in the HC and
NC groups. In the normal rats, infarct size was significantly
reduced in the NIPC and NPNU groups compared with the NI group
(P<0.05). In addition, infarct size was significantly increased
in the NPNU group compared with the NIPC group (P<0.05). In
hypercholesterolemic rats, infarct size was evidently reduced in
the HIPC group compared with the HI group (P<0.05), but was not
significantly changed in the HPNU group (P>0.05). Moreover,
infarct size was significantly increased in the HPNU group compared
with the HIPC group (P<0.05). Compared with normal rats, infarct
size was significantly increased in the corresponding treatment
groups of hypercholesterolemic rats (NI vs. HI, NIPC vs. HIPC and
NPNU vs. HPNU groups; P<0.05) (Fig.
3). These results indicated that both IPC and PNU282987
postconditioning provided a protection against myocardial IRI in
the normal rats, but this cardioprotective effect of IPC was
attenuated and that of PNU282987 postconditioning was eliminated in
the hypercholesterolemic rats.
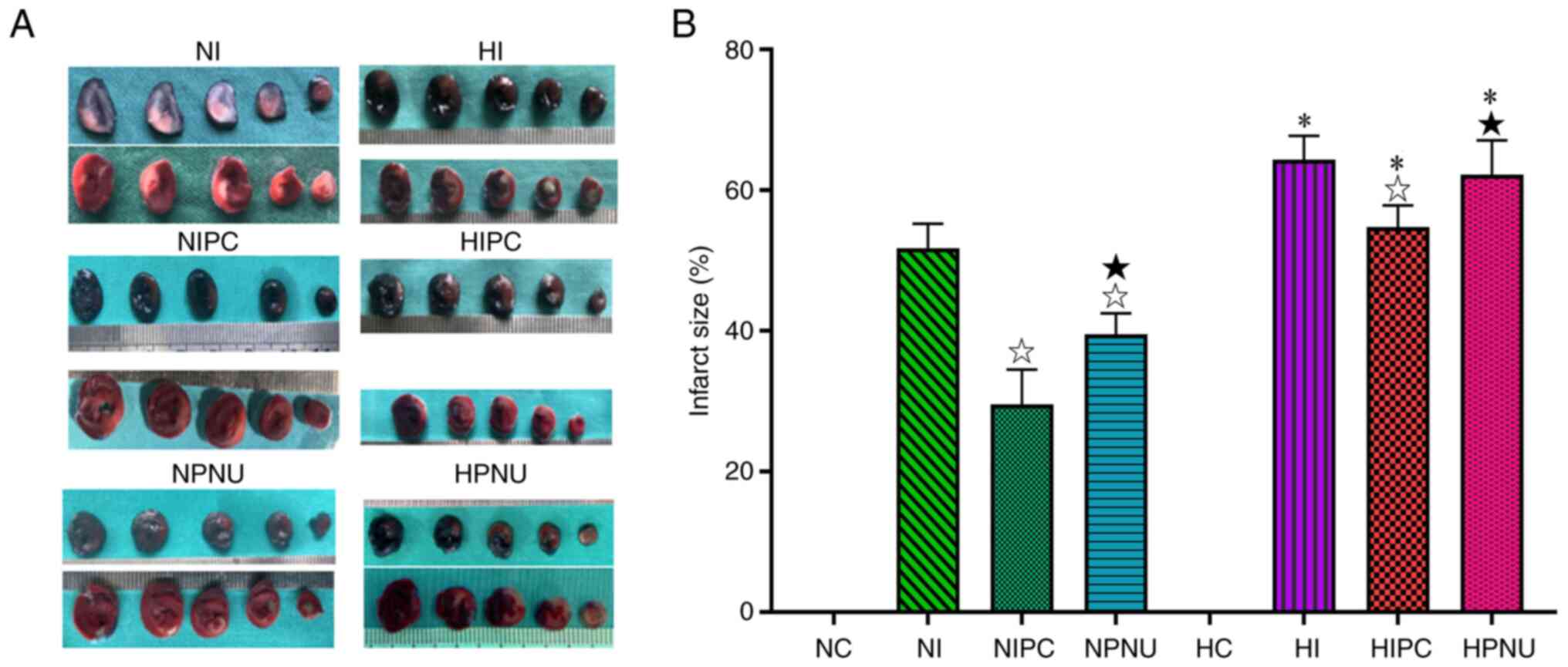 | Figure 3Representative images of (A) Evan's
blue staining and TTC staining of cardiac slices in normal and
hypercholesterolemic rats and (B) comparison of myocardial infarct
size (%). Intra-group comparisons of normal and
hypercholesterolemic rats: ☆P<0.05 compared with the
NI or HI group: ★P<0.05 compared with the NIPC or
HIPC group. Inter-group comparisons between corresponding treatment
groups of normal and hypercholesterolemic rats:
*P<0.05, HI vs. NI, HIPC vs. NIPC and HPNU vs. NPNU
groups. LDH, lactate dehydrogenase; CK-MB, creatine kinase
isoenzyme MB; cTnI, cardiac troponin I; NC, normal control; NI,
normal ischemia/reperfusion; NIPC, normal ischemic preconditioning;
NPNU, normal PNU282987 postconditioning; HC, hypercholesterolemic
control; HI, hypercholesterolemic ischemia/reperfusion; HIPC,
hypercholesterolemic ischemic preconditioning; HPNU,
hypercholesterolemic PNU282987 postconditioning. |
Inhibitive effects of IPC and
PNU282987 postconditioning on inflammation responses by myocardial
ischemia/reperfusion are attenuated or eliminated by
hypercholesterolemia
In the normal rats, serum TNF-α and IL-6 levels were
significantly increased in the NI, NIPC and NPNU groups compared
with the NC group (P<0.05); whereas these levels were
significantly reduced in the NIPC and NPNU groups compared with the
NI group (P<0.05). Serum TNF-α and IL-6 levels were
significantly increased in the NPNU group compared with the NIPC
group (P<0.05) (Fig. 4).
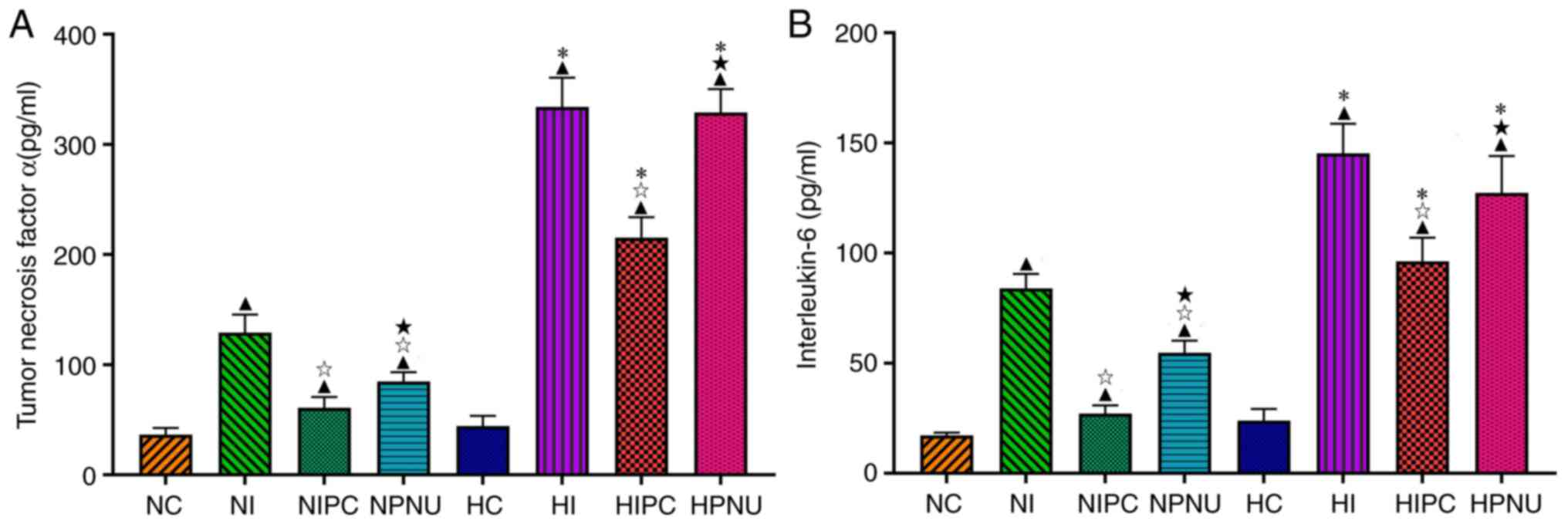 | Figure 4Comparisons of serum (A) IL-6 and (B)
TNF-α levels between normal and hypercholesterolemic rats.
Intra-group comparisons of normal and hypercholesterolemic rats:
▲P<0.05 compared with the NC or HC groups;
☆P<0.05 compared with the NI or HI groups;
★P<0.05 compared with the NIPC or HIPC groups.
Inter-group comparisons between corresponding treatment groups of
normal and hypercholesterolemic rats: *P<0.05, HC vs.
NC, HI vs. NI, HIPC vs. NIPC and HPNU vs. NPNU groups. LDH, lactate
dehydrogenase; CK-MB, creatine kinase isoenzyme MB; cTnI, cardiac
troponin I; NC, normal control; NI, normal ischemia/reperfusion;
NIPC, normal ischemic preconditioning; NPNU, normal PNU282987
postconditioning; HC, hypercholesterolemic control; HI,
hypercholesterolemic ischemia/reperfusion; HIPC,
hypercholesterolemic ischemic preconditioning; HPNU,
hypercholesterolemic PNU282987 postconditioning; IL, interleukin;
TNF-α, tumor necrosis factor α. |
In the hypercholesterolemic rats, serum TNF-α and
IL-6 levels were significantly increased in the HI, HIPC and HPNU
groups compared with the HC group (P<0.05); whereas serum TNF-α
and IL-6 levels were significantly reduced in the HIPC group
compared with the HI group (P<0.05), but did not significantly
change in the HPNU group (P>0.05). Moreover, compared with the
HIPC group, serum TNF-α and IL-6 levels were significantly
increased in the HPNU group (P<0.05) (Fig. 4).
There were no significant differences in serum TNF-α
and IL-6 levels between the NC and HC groups (P>0.05). However,
compared with the normal rats, serum TNF-α and IL-6 levels were
significantly increased in the corresponding treatment groups of
hypercholesterolemic rats (NI vs. HI, NIPC vs. HIPC and NPNU vs.
HPNU groups, P<0.05) (Fig. 4).
These results indicated that both IPC and PNU282987
postconditioning could inhibit inflammatory responses by myocardial
IRI in normal animals, but hypercholesterolemia significantly
attenuated anti-inflammatory effect of IPC and eliminated the
effect of PNU282987 postconditioning on inflammatory responses.
IPC and PNU282987 postconditioning
enhances myocardial Akt phosphorylation and eNOS expression, which
are attenuated or eliminated by hypercholesterolemia
In the normal rats, myocardial p-Akt/Akt and
eNOS/GAPDH ratios were significantly higher in the NI, NIPC and
NPNU groups compared with the NC group (P<0.05); these ratios
were also significantly higher in the NIPC and NPNU groups compared
with the NI group (P<0.05). Moreover, the myocardial p-Akt/Akt
ratio was significantly increased in the NPNU group compared with
the NIPC group (P<0.05) (Fig.
5).
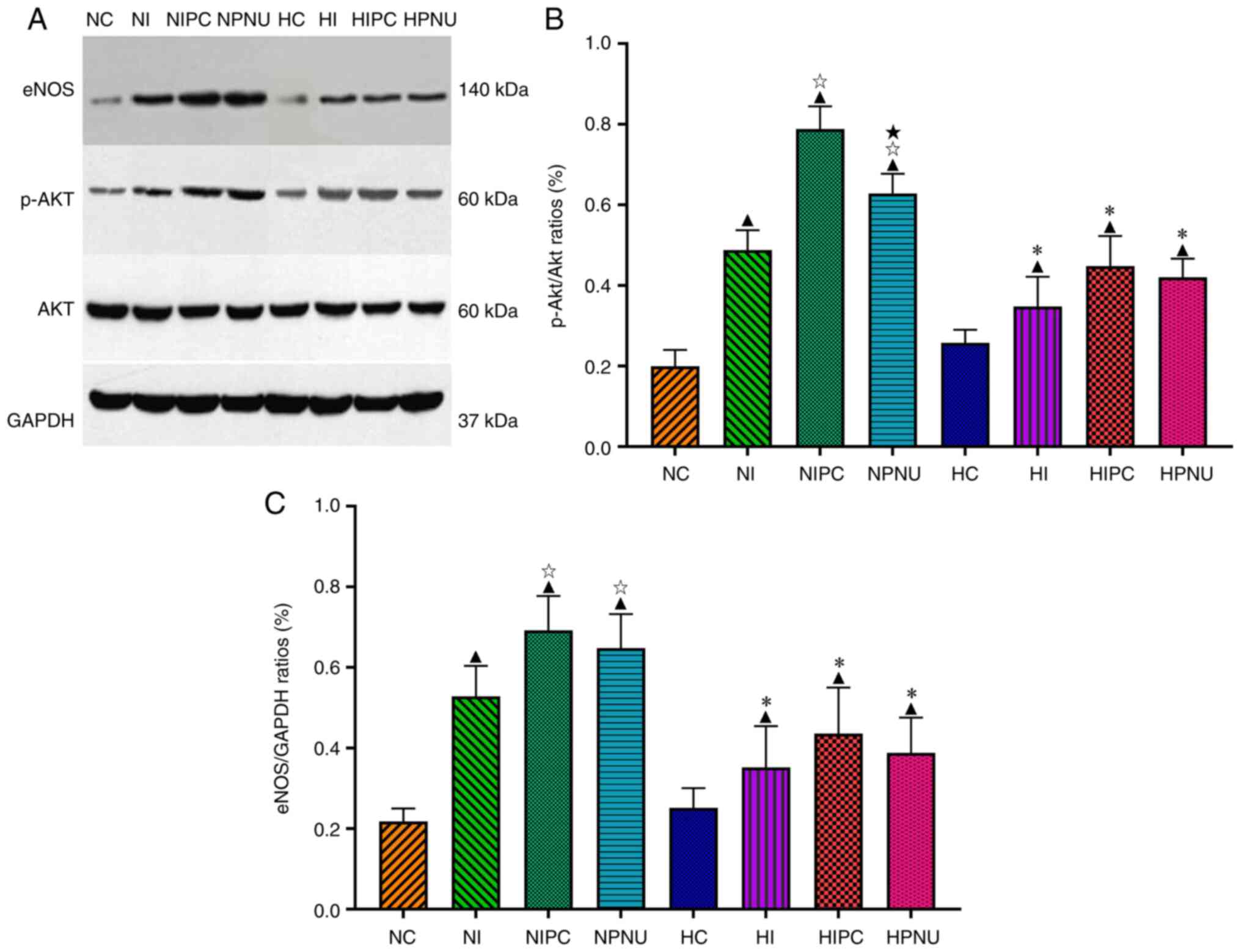 | Figure 5(A) Representative western blotting
images of myocardial p-Akt, Akt and eNOS expressions and
comparisons of (B) myocardial p-Akt/Akt and (C) eNOS/GAPDH ratios
between normal and hypercholesterolemic rats. Intra-group
comparisons of normal and hypercholesterolemic rats:
▲P<0.05 compared with the NC or HC group;
☆P<0.05 compared with the NI or HI group;
★P<0.05 compared with the NIPC or HIPC group.
Inter-group comparisons between corresponding treatment groups of
normal and hypercholesterolemic rats: *P<0.05, HC vs.
NC, HI vs. NI, HIPC vs. NIPC and HPNU vs. NPNU. LDH, lactate
dehydrogenase; CK-MB, creatine kinase isoenzyme MB; cTnI, cardiac
troponin I; NC, normal control; NI, normal ischemia/reperfusion;
NIPC, normal ischemic preconditioning; NPNU, normal PNU282987
postconditioning; HC, hypercholesterolemic control; HI,
hypercholesterolemic ischemia/reperfusion; HIPC,
hypercholesterolemic ischemic preconditioning; HPNU,
hypercholesterolemic PNU282987 postconditioning; p-,
phosphorylated; eNOS, endothelial nitric oxide synthase. |
In the hypercholesterolemic rats, myocardial
p-Akt/Akt and eNOS/GAPDH ratios were significantly higher in the
HI, HIPC and HPNU groups compared with the HC group (P<0.05).
However, compared with the HI group, myocardial p-Akt/Akt and
eNOS/GAPDH ratios did not significantly change in the HIPC and HPNU
groups (P>0.05). Furthermore, there were no significant
differences in the myocardial p-Akt/Akt and eNOS/GAPDH ratios
between the HIPC and HPNU groups (Fig.
5).
The myocardial p-Akt/Akt and eNOS/GAPDH ratios were
not obviously different between the NC and HC groups (P>0.05).
However, myocardial p-Akt/Akt and eNOS/GAPDH ratios were
significantly reduced in corresponding treatment groups of
hypercholesterolemic rats compared with the normal rats (NI vs. HI,
NIPC vs. HIPC and NPNU vs. HPNU groups; P<0.05) (Fig. 5). These results indicated that both
Akt and eNOS were involved in the cardioprotective effects of IPC
and PNU282987 postconditioning against IRI in normal and
hypercholesterolemic rats.
Discussion
In the present study, after 1-month-old SD rats were
fed with a high-cholesterol diet for 8 weeks their serum TC and LDL
levels significantly increased, which indicated that a
hypercholesterolemic rat model had been successfully established
(16). Serum LDH, CK-MB and cTnI
levels were significantly increased in the normal and
hypercholesterolemic rats experiencing myocardial ischemia and
reperfusion. Furthermore, serum LDH, CK-MB and cTnI levels were
significantly higher in the hypercholesterolemic rats compared with
the normal rats (HI vs. NI groups), suggesting that
myocardial IRI was more serious in hypercholesterolemic rats. These
results were consistent with the findings of previous studies
(14,17), in which hypercholesterolemia can
aggravate myocardial IRI.
Furthermore, the present study demonstrated that in
the normal rats, both IPC and PNU282987 postconditioning
significantly decreased serum LDH, CK-MB and cTnI levels,
especially the IPC (NIPC and NPNU vs. NI groups, respectively).
These findings correspond with results of a previous study
(18). All of these findings in
the normal rats indicated that both IPC and α7nAChR agonist
postconditioning could provide significant protection against
myocardial IRI and the cardioprotection of IPC was stronger.
The main aim of the present study was to determine
effects of hypercholesterolemia on the cardioprotective efficacy of
IPC and α7nAChR agonist postconditioning. The results demonstrated
that the IPC significantly reduced serum LDH, CK-MB and cTnI levels
in the hypercholesterolemic rats, but the potency of IPC to
decrease these biomarkers was significantly weakened in the
hypercholesterolemic rats compared with the normal rats. These
results indicated that hypercholesterolemia attenuated the
protection of IPC against myocardial IRI. This is in accordance
with the findings of Ueda et al (10) where the cardioprotective effect of
IPC is decreased in hypercholesterolemic rabbit hearts subjected to
ischemia/reperfusion.
To the best of our knowledge, there has been no
study assessing the effect of hypercholesterolemia on
cardioprotection of α7nAChR agonist postconditioning. The present
experiment indicated that serum LDH, CK-MB and cTnI levels were not
significantly different between the HPNU and HI groups, indicating
that hypercholesterolemia eliminated the cardioprotection from
α7nAChR agonist postconditioning.
Infarct size is a gold standard parameter that
evaluates the severity of myocardial injury and cardioprotective
efficacy of interventions in the animal experiment (4). Consistent with the aforementioned
changes of myocardial injury biomarkers, the present study revealed
that in the normal rats subjected to myocardial IRI, both IPC and
PNU282987 postconditioning significantly reduced the infarct size
by 42.9 and 23.7% (NIPC and NPNU vs. NI groups), respectively.
These results agree with the findings of previous studies (4,18).
All of these support the aforementioned conclusions obtained by the
myocardial injury biomarkers that the two interventions can produce
a significant protection against myocardial IRI in the normal rats,
but the cardioprotective potency of IPC is stronger.
However, the infarct size was increased by 19.6% in
the hypercholesterolemic rats compared with the normal rats (HI vs.
NI groups). This further supports that hypercholesterolemic rats
are more vulnerable to myocardial IRI than normal rats. Similarly,
in hypercholesterolemic rats the IPC only reduced the infarct size
by 14.9% (HIPC vs. HI groups), which was significantly smaller
compared with that in the normal rats (NIPC vs. NI groups; 42.9%).
This indicated that hypercholesterolemia significantly decreased
the infract size-limiting effect of IPC. The present result that
hypercholesterolemia attenuated cardioprotection of IPC was in line
with the results of Ueda et al (10) in the hypercholesterolemic rabbit
heart subjected to ischemia/reperfusion and with the results of
Ungi et al (19) in
patients undergoing coronary angioplasty. Moreover, Kocić et
al (20) demonstrated that
hypercholesterolemia completely abolishes the cardioprotective
effect of IPC in isolated stunned papillary rat muscle.
However, in the available literature on the effect
of hypercholesterolemia on cardioprotection of IPC other studies
report different findings. Iliodromitis et al (12) demonstrated that IPC preserves its
cardioprotection in the myocardial IRI model of
hypercholesterolemic rabbits (infract size, 55.2±5.9 and 17.9±4.2%
in control and IPC groups, respectively). Furthermore, Jung et
al (13) confirmed that
experimental hypercholesterolemia does not affect the infarct size
sparing of IPC in the rabbit heart subjected to
ischemia/reperfusion (63±3 and 21±3% in control and IPC groups,
respectively). These inconsistent results may be mainly
attributable to the differences among various studies in the
experimental designs, timings of IPC implementation, durations and
cycle numbers of IPC, study objects and methods of making a
hypercholesteremic model. For example, in studies by Iliodromitis
et al (12) and Jung et
al (13), IPC intervention
includes two cycles of 5 min ischemia separated by 10 min
reperfusion before the index ischemia. In the present experiment,
IPC intervention was performed using the classic scheme, including
three cycles of 5 min ischemia followed by 5 min reperfusion before
the index ischemia. Notably, hypercholesterolemic rabbit and rat
IRI models are applied in both the previous works and this present
study, though different animals share various anatomical and
physiological characteristics of hearts.
Interestingly, the present results indicated that
PNU282987 postconditioning did not significantly reduce the infarct
size in the hypercholesterolemic rats (HPNU vs. HI groups),
suggesting that the infarct size-limiting effect of PNU282987
postconditioning is completely abolished by hypercholesterolemia.
This supports the above findings from myocardial injury
biomarkers.
The available evidence indicates that IRI is a
result of complex interactions of multiple pathogenic factors
(3). Of them, inflammation is an
important pathogenic factor, involving numerous cytokines, adhesion
molecules, activation of complement cascade system and toll-like
receptors (21). As IL-6 and TNF-α
are important cytokines that can accurately reflect the development
and severity of inflammatory responses, they are commonly used as
the indicators that assess the characteristics of inflammatory
responses during myocardial IRI process (22). The present study demonstrated that
in the normal rats, both IPC and PNU282987 postconditioning
significantly reduced serum IL-6 and TNF-α levels, but the ability
of IPC to decrease serum levels of two cytokines was significantly
stronger compared with that of PNU282987 postconditioning. In
available literature, inhibition of two interventions on
inflammatory responses induced by myocardial IRI has been
considered as a notable mechanisms for their cardioprotection
(4,15). However, the present experiment
demonstrated that serum IL-6 and TNF-α levels in the HIPC group
were significantly lower compared with those in the HI group, but
were higher compared with those in the NIPC group. These results
suggested that the inhibitory effect of IPC on the inflammatory
responses induced by myocardial IRI were significantly weakened in
the presence of hypercholesterolemia. In addition, serum IL-6 and
TNF-α levels were not significantly different between the HI and
HPNU groups, indicating that hypercholesterolemia completely
eliminates the inhibitory effect of PNU282987 postconditioning on
inflammatory responses induced by myocardial ischemia/reperfusion.
Therefore, it is concluded that hypercholesterolemia can
significantly attenuate inhibitive effect of cardioprotective
interventions on the inflammatory responses induced by myocardial
ischemia/reperfusion. This may be one of the reasons why
cardioprotection of the interventions including IPC is decreased in
the presence of hypercholesterolemia.
PI3K/Akt is a signaling pathway widely present in
cells and is involved in inflammation and cell activation, survival
and apoptosis (23). It is
generally considered that activation of the PI3K/Akt signaling
pathway can protect the myocardium from lethal IRI (24). eNOS, which is continuously
expressed in mammalian cardiomyocytes, is a downstream effector of
Akt and is regulated by the PI3K/Akt signaling pathway. The
available evidence indicates that the PI3K/Akt/eNOS signaling
pathway plays an important role in the mechanisms of
cardioprotection by various interventions such as delayed
preconditioning, dexmedetomidine and baicalin (25-27).
It is reported that a specific knock-out of eNOS gene can
significantly increase the sensitivity of myocardium to IRI and
eliminate the protection of IPC against myocardial IRI (28). Furthermore, hypercholesterolemia
can downregulate the expression of eNOS and thus decrease the
generation of nitric oxide to induce vascular endothelial
dysfunction (29), which may
affect the function of coronary artery in the myocardium.
The present study demonstrated that after
ischemia/reperfusion, myocardial p-Akt/Akt and eNOS/GAPDH ratios
were significantly decreased in the hypercholesterolemic rats
compared with the normal rats, suggesting that myocardial Akt
phosphorylation and eNOS expression are significantly inhibited in
the presence of hypercholesterolemia. Specifically, the present
experiment demonstrated that PNU282987 postconditioning enhanced
myocardial Akt phosphorylation and eNOS expression, and reduced
serum myocardial injury biomarker levels and infarct size in the
normal rats, but it did not lead to significant changes in the
infarct size, Akt phosphorylation and eNOS expression in the
hypercholesterolemic rats. Based on these findings, it is
hypothesized that hypercholesterolemia abolishes the
cardioprotection of α7nAChR agonist postconditioning by eliminating
inflammatory inhibition and inhibiting activation of PI3K/Akt/eNOS
signaling pathway. In the hypercholesterolemic rabbit heart
subjected to ischemia/reperfusion, Ueda et al (10) demonstrated that pravastatin can
restore the cardioprotective effect of IPC by activating
ecto-5'-nucleotidase. Thus, the present study considered that both
restoring regulation of the cholinergic anti-inflammatory pathway
on inflammatory responses and provoking activation of myocardial
PI3K/Akt/eNOS signaling pathway may be feasible strategies to
improve the cardioprotection of α7nAChR agonist postconditioning in
the presence of hypercholesterolemia (7). However, these results deserve further
studies.
The present study indicated that in
hypercholesterolemic rats, IPC did not significantly enhance
activation of the PI3K/Akt/eNOS signaling pathway in the ischemic
myocardium, and inhibition of IPC on the inflammatory responses
induced by myocardial ischemia/reperfusion was significantly
weakened. However, notably, differing from the result that
hypercholesterolemia completely eliminated cardioprotection from
PNU282987 postconditioning, the IPC still exerted a certain level
of protection against myocardial IRI in the hypercholesterolemic
rats, though cardioprotective potency of IPC was significantly
weakened. This may be because that beside inhibition of
inflammatory responses and activation of the PI3K/Akt/eNOS
signaling pathway, cardioprotection of IPC is also attributable to
other mechanisms. The available evidence indicates that activation
of reperfusion injury salvage kinase pathway, survival activating
factor enhancement pathway, Janus activated kinase signal
transducer and activator of transcription pathway, 70 ribosomal
protein S6 kinase and glycogen synthase kinase 3β, opening of
mitochondrial permeability transition pore and ATP-sensitive
K+ channels and inhibition of apoptosis all are involved
in the protection of IPC against myocardial IRI (2,7,23,24).
Furthermore, it has been indicated that hypercholesterolemia can
inhibit the opening of mitochondrial ATP-sensitive K+
channels in the rabbit heart subjected to ischemia/reperfusion
(30). In fact, opening of
mitochondrial ATP-sensitive K+ channels is considered as
a major component involved in the cardioprotection of IPC (23). Thus, the detailed roles of these
factors in the mechanisms that hypercholesterolemia affects the
cardioprotective effectiveness of IPC also deserves further
studies.
In summary, the present study demonstrated that
hypercholesterolemia could significantly aggravate myocardial IRI,
weaken cardioprotection of IPC and eliminate cardioprotection of
α7nAChR agonist postconditioning by enhancing inflammatory
responses and inhibiting activation of PI3K/Akt/eNOS signaling
pathway. Thus, both enhancing inhibition of inflammatory responses
and facilitating activation of the PI3K/Akt/eNOS signaling pathway
may be the useful measures to improve cardioprotective efficacy of
two interventions in the presence of hypercholesterolemia.
Acknowledgements
Not applicable.
Funding
Funding: This work was performed with the support of Youth
Innovation Project fund of Plastic Surgery Hospital, Chinese
Academy of Medical Sciences (grant no. Q2017002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW and FSX conceived and designed the experiments.
CW, YHW, XL and JHJ performed the experiments. CW, XL and FSX
analyzed and interpreted the results of the experiments. CW wrote
the manuscript. WC and FSX confirm the authenticity of all the raw
data. FSX revised manuscript. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study protocol was approved by the
Animal Care and Use Committee of Plastic Surgery Hospital, Chinese
Academy of Medical Sciences [approval no. 2017(38); June 16, 2017;
Beijing, China].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rossello X, Lobo-Gonzalez M and Ibanez B:
Editor's choice-pathophysiology and therapy of myocardial
ischaemia/reperfusion syndrome. Eur Heart J Acute Cardiovasc Care.
8:443–456. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hausenloy DJ and Yellon DM: Ischaemic
conditioning and reperfusion injury. Nat Rev Cardiol. 13:193–209.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wu Y, Liu H and Wang X: Cardioprotection
of pharmacological postconditioning on myocardial
ischemia/reperfusion injury. Life Sci. 264(118628)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Xiong J, Yuan YJ, Xue FS, Wang Q, Cheng Y,
Li RP, Liao X and Liu JH: Postconditioning with α7nAChR agonist
attenuates systemic inflammatory response to myocardial
ischemia-reperfusion injury in rats. Inflammation. 35:1357–1364.
2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sack MN and Murphy E: The role of
comorbidities in cardioprotection. J Cardiovasc Pharmacol Ther.
16:267–272. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Andreadou I, Schulz R, Badimon L, Adameová
A, Kleinbongard P, Lecour S, Nikolaou PE, Falcão-Pires I, Vilahur
G, Woudberg N, et al: Hyperlipidaemia and cardioprotection: Animal
models for translational studies. Br J Pharmacol. 177:5287–5311.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bøtker HE: The future of
cardioprotection-pointing toward patients at elevated risk as the
target populations. J Cardiovasc Pharmacol Ther. 25:487–493.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Andreadou I, Iliodromitis EK, Lazou A,
Görbe A, Giricz Z, Schulz R and Ferdinandy P: Effect of
hypercholesterolaemia on myocardial function, ischaemia-reperfusion
injury and cardioprotection by preconditioning, postconditioning
and remote conditioning. Br J Pharmacol. 174:1555–1569.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
D'Annunzio V, Donato M, Buchholz B, Pérez
V, Miksztowicz V, Berg G and Gelpi RJ: High cholesterol diet
effects on ischemia-reperfusion injury of the heart. Can J Physiol
Pharmacol. 90:1185–1196. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ueda Y, Kitakaze M, Komamura K, Minamino
T, Asanuma H, Sato H, Kuzuya T, Takeda H and Hori M: Pravastatin
restored the infarct size-limiting effect of ischemic
preconditioning blunted by hypercholesterolemia in the rabbit model
of myocardial infarction. J Am Coll Cardiol. 34:2120–2125.
1999.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tang XL, Takano H, Xuan YT, Sato H, Kodani
E, Dawn B, Zhu Y, Shirk G, Wu WJ and Bolli R: Hypercholesterolemia
abrogates late preconditioning via a tetrahydrobiopterin-dependent
mechanism in conscious rabbits. Circulation. 112:2149–2156.
2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Iliodromitis EK, Zoga A, Vrettou A,
Andreadou I, Paraskevaidis IA, Kaklamanis L and Kremastinos DT: The
effectiveness of postconditioning and preconditioning on infarct
size in hypercholesterolemic and normal anesthetized rabbits.
Atherosclerosis. 188:356–362. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jung O, Jung W, Malinski T, Wiemer G,
Schoelkens BA and Linz W: Ischemic preconditioning and infarct
mass: The effect of hypercholesterolemia and endothelial
dysfunction. Clin Exp Hypertens. 22:165–179. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang JT, Wang J, Zhou XR, Xiao C, Lou YY,
Tang LH, Zhang FJ and Qian LB: Luteolin alleviates cardiac
ischemia/reperfusion injury in the hypercholesterolemic rat via
activating Akt/Nrf2 signaling. Naunyn Schmiedebergs Arch Pharmacol.
391:719–728. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang JQ, Wang Q, Xue FS, Li RP, Cheng Y,
Cui XL, Liao X and Meng FM: Ischemic preconditioning produces more
powerful anti-inflammatory and cardioprotective effects than limb
remote ischemic postconditioning in rats with myocardial
ischemia-reperfusion injury. Chin Med J (Engl). 126:3949–3955.
2013.PubMed/NCBI
|
|
16
|
Low LD, Lu L, Chan CY, Chen J, Yang HH, Yu
H, Lee CGL, Ng KH and Yap HK: IL-13-driven alterations in hepatic
cholesterol handling contributes to hypercholesterolemia in a rat
model of minimal change disease. Clin Sci (Lond). 134:225–237.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
He JY, Fan SX, Ma YL, Cao XF, Tian T and
Liu Y: Hypercholesterolemia abolishes the protective effect of
ischemic preconditioning on myocardial ischemia-reperfusion injury
in rats via PI3K/Akt pathway. Chin Rem Clin. 19:4024–4027.
2019.
|
|
18
|
Cui X, Wang S, Xue F, Yang G, Li H, Liu Y
and Liao X: Mechanism underlying inhibition of inflammatory
responses induced by α7nAChR agonist postconditioning alone or in
combination with remote limb ischemic postconditioning during
myocardial I/R in rats: The relationship with GSK-3β (Chinese).
Chin J Anesthesiol. 38:78–82. 2018.
|
|
19
|
Ungi I, Ungi T, Ruzsa Z, Nagy E,
Zimmermann Z, Csont T and Ferdinandy P: Hypercholesterolemia
attenuates the anti-ischemic effect of preconditioning during
coronary angioplasty. Chest. 128:1623–1628. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kocić I, Konstański Z, Kaminski M,
Dworakowska D and Dworakowski R: Experimental hyperlipidemia
prevents the protective effect of ischemic preconditioning on the
contractility and responsiveness to phenylephrine of rat-isolated
stunned papillary muscle. Gen Pharmacol. 33:213–219.
1999.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Vincent A, Lattuca B, Merlet N,
Sportouch-Dukhan C and Barrère-Lemaire S: New insights in research
about acute ischemic myocardial injury and inflammation.
Antiinflamm Antiallergy Agents Med Chem. 12:47–54. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Steffens S, Montecucco F and Mach F: The
inflammatory response as a target to reduce myocardial ischaemia
and reperfusion injury. Thromb Haemost. 102:240–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rosenberg JH, Werner JH, Moulton MJ and
Agrawal DK: Current modalities and mechanisms underlying
cardioprotection by ischemic conditioning. J Cardiovasc Transl Res.
11:292–307. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hausenloy DJ and Yellon DM: Reperfusion
injury salvage kinase signalling: Taking a RISK for
cardioprotection. Heart Fail Rev. 12:217–234. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
He X, Zhao M, Bi XY, Yu XJ and Zang WJ:
Delayed preconditioning prevents ischemia/reperfusion-induced
endothelial injury in rats: Role of ROS and eNOS. Lab Invest.
93:168–180. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sun Y, Jiang C, Jiang J and Qiu L:
Dexmedetomidine protects mice against myocardium
ischaemic/reperfusion injury by activating an AMPK/PI3K/Akt/eNOS
pathway. Clin Exp Pharmacol Physiol. 44:946–953. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bai J, Wang Q, Qi J, Yu H, Wang C, Wang X,
Ren Y and Yang F: Promoting effect of baicalin on nitric oxide
production in CMECs via activating the PI3K-AKT-eNOS pathway
attenuates myocardial ischemia-reperfusion injury. Phytomedicine.
63(153035)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jones SP, Girod WG, Palazzo AJ, Granger
DN, Grisham MB, Jourd'Heuil D, Huang PL and Lefer DJ: Myocardial
ischemia-reperfusion injury is exacerbated in absence of
endothelial cell nitric oxide synthase. Am J Physiol.
276:H1567–H1573. 1999.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shah DI and Singh M: Possible role of Akt
to improve vascular endothelial dysfunction in diabetic and
hyperhomocysteinemic rats. Mol Cell Biochem. 295:65–74.
2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Genda S, Miura T, Miki T, Ichikawa Y and
Shimamoto K: K(ATP) channel opening is an endogenous mechanism of
protection against the no-reflow phenomenon but its function is
compromised by hypercholesterolemia. J Am Coll Cardiol.
40:1339–1346. 2002.PubMed/NCBI View Article : Google Scholar
|