Introduction
Platelets are the smallest blood cells and function
as ‘bricks’ in the development of arterial thrombosis. When the
integrity of the vascular system is disrupted, circulating
platelets are activated by the exposure to subendothelial matrix
proteins [e.g., von Willebrand factor (vWF) and collagen)].
Glycoprotein Ib-IX-V complex on the platelet surface plays an
important role in slowing down and recruiting circulating platelets
to the injured sites by binding to vWF (1). ADP and thromboxane A2, released by
activated platelets, further amplify the recruitment process
(2). Thrombin is an important end
product of the coagulation cascade, and aside from directly
activating the platelets, it cleaves fibrinogen to promote platelet
aggregation (3). Beside the
critical role in physiological hemostasis, platelet hyperactivity
during arterial thrombosis caused by plaque rapture, immune
response infection or metastasis may cause life threatening
myocardial infarction and stroke (2). Antiplatelet agents, such as
clopidogrel, prasugrel, ticagrelor and acetylsalicylic acid, are
widely used in preventing cardiovascular events in clinical
practice (4,5). However, the application of
antithrombotic agents in patients with cardiovascular disease tends
to increase the bleeding risk. Thus, there is an increasing
interest in searching for natural food products and biologically
active ingredients for the prevention and treatment of thrombosis
(6).
Arctium lappa L. has been traditionally used
as a healthy and nutritive food (7). Extracts from Arctium lappa L.
root have been shown to reduce inflammation (8), fight against infection (9,10)
and prevent high glucose levels in diabetes (11,12).
Saccharides from Arctium lappa L. root (ALR-S) is a
high-purity fructosaccharide. The antioxidant effects of ALR-S have
been well documented and ALR-S has been shown to actively destroy
free radicals (13). Recently, it
was demonstrated that ALR-S reduced thrombosis in an ferric
chloride (FeCl3)-induced mouse arterial thrombosis model
by rebalancing the expression of thrombotic and antithrombotic
factors in endothelial cells (6).
However, the exact effects of ALR-S on platelet activation in
vitro and in vivo remain elusive.
Reactive oxygen species (ROS) that are produced
during vascular injury are key mediators of platelet function.
Although multiple ROS sources have been proposed following vascular
injury, including superoxide anion, hydroxyl radicals and hydrogen
peroxide (14,15), it has previously been identified
that NADPH oxidase (NOX)-dependent ROS generation is the critical
radical source regulating platelet function (16,17).
NOX-dependent ROS generation promotes platelet activation via p38
and ERK1/2 phosphorylation (15-17),
and specific deletion of p38α in platelets impairs thrombosis and
hemostasis by disturbing the p38α/MAPK-activated protein kinase
2/heat shock protein 27 (HSP27) signaling pathway (18).
The present study investigated the antiplatelet
effects of ALR-S using platelets isolated from healthy subjects and
a laser-induced mouse arterial thrombosis model. The results may
help to determine whether ALR-S could be used as a beneficial food
and beverage resource for reducing thrombotic risk.
Materials and methods
Materials
Collagen, thrombin, ADP and luciferin/luciferase
were provided by the Chrono-Log Corporation. FITC-phalloidin,
H2O2 and mepacrine were purchased from
Sigma-Aldrich; Merck KGaA. Antibodies against phospho-ERK1/2
(catalog no. 4370S), phospho-p38 (catalog no. 4511S), phospho-HSP27
(catalog no. 2401S), total-p38 (catalog no. 8690S), total-ERK
(catalog no. 4695S) and HSP27 (catalog no. 95357S) were purchased
from Cell Signaling Technology Inc. β-actin monoclonal antibody
(catalog no. 66009-1-Ig) was obtained from ProteinTech Group, Inc.
The antibody for integrin β3 (D-11) (catalog no. sc-365679) was
obtained from Santa Cruz Biotechnology Inc. PAC-1 antibodies
(catalog no. MA5-28564) were from Invitrogen; Thermo Fisher
Scientific, Inc., and CD62P antibodies (catalog no. 555524) were
from BD Biosciences.
Preparation of ALR-S
Crude extract of Arctium lappa L. root
(catalog no. wkq-08912) was obtained from Sichuan Weikeqi
Biological Technology Co., Ltd. The crude extract was refluxed with
anhydrous alcohol and subsequent acetone, and then it was
evaporated under reduced pressure. Briefly, Sephadex G-50 gel
(MilliporeSigma) in a chromatographic column (25x400 mm) was used
for gel filtration chromatography. The gel was washed with 20 ml
sterile distilled water, and then quantified with 10 mM Tris HCl
buffer (pH 7.4). Samples (5 g) were dissolved into distilled water
at 5% (w/v), and loaded into columns at a flow rate of 1 ml/min for
2 h at 40˚C. The saccharide fractions were collected and
concentrated using a freeze-dried evaporator (SCIENTZ-10N; SCIENTZ;
Ningbo Xinzhi Freeze Drying Equipment Co., Ltd.). ALR-S purity was
measured by a Shimadzu Prominence gel permeation chromatography
(GPC) system (Shim-pack GPC 803C column; Shimadzu UK Ltd.) at 40˚C
using chloroform as the eluent, and the purity was >95%.
Preparation of washed platelets
Washed platelets were obtained as previously
described (19). Briefly, blood
was acquired from healthy individuals (12 female and 9 male) who
had not taken any medication for at least 1 week. Platelet rich
plasma (PRP) was obtained by centrifugation of whole blood samples
at 150 x g for 20 min at room temperature. Platelets were
resuspended in modified Tyrode's buffer (138 mmol/l NaCl, 5 mmol/l
D-glucose, 5 mmol/l HEPES, 1 mmol/l MgCl2, 12 mmol/l
NaHCO3, 400 mmol/l Na2HPO4 and 2.7
mmol/l KCl) after centrifugation at 800 x g for 10 min at room
temperature. Platelet concentration was measured by a hemocytometer
(BC-2800Vet; Mindray Medical International Ltd.) and the washed
platelet concentration was adjusted to a final concentration of
3x108/ml, equivalent to the platelet concentration in a
healthy individual. Platelets were stimulated by the addition of
CaCl2 at a final concentration of 1 mmol/l. All
experiments were approved by the Ethics Committee of Zhengzhou
University for the Use of Human Subjects (approval no.
2020-KY-122).
Platelet aggregation and ATP release
assay
Platelet aggregation and ATP release were carried
out by optical aggregometry as described previously (19), using a lumi-aggregometer model 700
(Chrono-log Corporation), under continuous stirring at 1,200 rpm.
Washed platelets were treated with ALR-S (20, 60 and 200 µg/ml) or
a vehicle at 37˚C for 10 min. Luciferin/luciferase (10 µl) were
added, and then stimulation was performed with collagen (1 µg/ml),
thrombin (0.025 U/ml) or ADP (10 µM), respectively. Traces for
aggregation and ATP release were recorded for 10 min at 37˚C. For
H2O2 enhanced platelet aggregation, washed
platelets were treated with ALR-S or a vehicle at 37˚C for 10 min,
then the agonists were immediately joined into platelets after
H2O2 (20 µM) was added.
Flow cytometry
Activated platelet integrin αIIbβ3 and α-granule
secretion were measured by flow cytometric analysis as previously
described (19). Briefly, washed
platelets were adjusted to a concentration of 5x107
cells/ml, and 100 µl (5x106 cells) were stained with
PE-CD62P or FITC-PAC-1, respectively, for 30 min at room
temperature. Stained platelets were then incubated with ALR-S or
saline vehicle for 10 min at room temperature. Reactions were
started with collagen-related peptide (CRP, 2 µg/ml), thrombin
(0.025 U/ml) and ADP (10 µM) for 5 min at room temperature,
respectively. The reactions were stopped by addition of 500 µl PBS.
A total number of 10,000 events per tube were collected in an
Accuri™ C6 flow cytometer (BD Biosciences) and analyzed by FlowJo
(version 10.0; Tree Star, Inc.).
Platelet spreading
Washed platelets were adjusted to 2x107
cells/ml, which is a proper concentration to avoid
platelet-platelet overlap on coverslip. Washed platelets were
preloaded with ALR-S (20, 60 and 200 µg/ml) or saline and then were
allowed to spread on fibrinogen-coated slides for 1 h at 37˚C.
Slides were washed with PBS 3 times for 10 sec, fixed with 1%
formaldehyde for 15 min, permeabilized with 0.1% Triton X-100 for
20 min and stained with FITC-phalloidin (catalog no. P5282;
MilliporeSigma) for 1 h at room temperature. Images of spreading
platelets were captured by a fluorescence microscope (BX53;
Olympus) with a charge-coupled device (CCD) camera (DP74; Olympus
Corporation). Platelet spreading area was evaluated using ImageJ
software (version 1.4; National Institutes of Health).
Clot retraction
PRP (3x108 cells/ml) was preincubated
with ALR-S (20, 60 and 200 µg/ml) or saline for 5 min. Addition of
thrombin (0.4 U/ml) at room temperature started clot retraction;
images were captured at 0, 20, 40 and 60 min, and evaluated by
ImageJ software (version 1.4).
Measurement of intracellular ROS
As previously reported (17), washed platelets (1x108
cells/ml) were incubated with fluorogenic probe
2',7'-dichlorodihydrofluorescein diacetate (H2DCFDA; 50
µmol/l) (catalog no. CA1410; Beijing Solarbio Science &
Technology Co., Ltd.) for 15 min at 37˚C in darkness, followed by
stimulation with CRP (2 µg/ml), thrombin (0.025 U/ml), or ADP (10
µM) for 5 min at 37˚C in darkness. Samples were then diluted with
10-fold HEPES/Tyrode's buffer containing 50 µmol/l
H2DCFDA and analyzed by flow cytometry immediately as
aforementioned.
Immunoblotting analysis
After aggregation of platelets in an aggregometer at
1,200 rpm, samples were lysed with 2X lysis buffer (50 mmol/l Tris
and 150 mmol/l NaCl; pH 7.4) containing 2X protease inhibitor
(catalog no. P1011; Beyotime Institute of Biotechnology) and 2X
phosphatase inhibitor (catalog no. P1081; Beyotime Institute of
Biotechnology). Proteins (20 µl) were separated by SDS-PAGE (10%
gels) and transferred onto PVDF membranes, and then blocked with 5%
BSA in Tris-buffered saline with Tween (TBST) (0.5%) for 1 h at
room temperature. The membranes were incubated with the indicated
antibodies at 4˚C overnight. Membranes were washed with TBST 3
times, then incubated with the corresponding anti-mouse (catalog
no. SA00001-1) or anti-rabbit (catalog no. SA00001-2) secondary
antibodies (ProteinTech Group, Inc.) (at a dilution of 0.5%) for 1
h at room temperature. After washing with TBST 3 times, proteins
were visualized by Tanon 4800 (Tanon Science and Technology Co.,
Ltd.) with ECL western blotting detection reagent
(MilliporeSigma).
Platelet adhesion to fibrillar
collagen under shear
Platelet adhesion was evaluated using a Bioflux-200
system (Fluxion Biosciences) as previously described (17). Briefly, bioflux plates were coated
with fibrillar collagen (50 µg/ml) overnight at 4˚C, and then
blocked with 0.5% BSA at room temperature for 30 min.
Heparin-treated (10 U/ml; catalog no. H3149; MilliporeSigma) human
whole blood was incubated with mepacrine (100 µM; catalog no.
Q3251; MilliporeSigma) for 30 min at 37˚C. Mepacrine-labeled blood
was allowed to adhere to a fibrillar collagen-coated plate under a
shear rate of 40 dynes/cm2 for 5 min using a Bioflux-200
system (Fluxion Biosciences). Images of adherent thrombi were
viewed and captured using an inverted fluorescence microscope
(IX73; Olympus) with a CCD camera (DP74; Olympus). Platelet-covered
area was calculated using Bioflux software (version 2.0.5.8;
Fluxion Biosciences).
Animal model of laser injury
thrombosis
C57BL/6 mice were provided by the Experimental
Animal Center of Zhengzhou University. The animal research protocol
was approved by the Ethics Committee of the First Affiliated
Hospital of Zhengzhou University (approval no. 2021-KY-256). A
total of 14 male mice (aged 8-12 weeks; weight, 20-25 g) were kept
at room temperature and atmosphere in a normal light/dark cycle
after acquisition. Groups of 4-5 mice were maintained in a single
cage and had free access to food and fresh water. Mice were
anesthetized with 40 mg/kg intraperitoneal sodium pentobarbital
(1%) and then 0.05 mg/kg intraperitoneal FITC-labeled antibody for
glycoprotein VI (GPVI; catalog no. M011-1; Emfret) was injected.
Mice were preloaded with different concentrations of ALR-S (0.2,
0.6 and 2 mg/kg) by intraperitoneal injection for 30 min. The mouse
cremaster muscle was exposed, and the connective tissue was cleaned
and maintained under a constant flow of saline at 37˚C. Arterioles
with diameter of 30-50 mm were visualized using a BX61WI microscope
(Olympus Corporation) with a 40X (0.9 NA) water-immersion objective
lens. When arterioles were injured with an SRS NL100 pulsed
nitrogen dye laser (440 nm), images were captured using a
Photometrics Cool Snap HQ CCD camera (Teledyne Photometrics). A
total of 4, 4, 3 and 3 mice were used for the vehicle group, and
the 0.2, 0.6 and 2 mg/kg groups, respectively. A total of six
arterioles for each group were captured, with a duration of 400 sec
at an interval of 8 sec. Mice were sacrificed by sodium
pentobarbital injection (90 mg/kg) followed by incubation in an
Isoflurane Vaporizer box (Matrx™ VIP 300) with 3% isoflurane after
the experiments.
Statistical analysis
All data were analyzed by GraphPad Prism (version
8.0.2; GraphPad Inc.) using one-way analysis of variance (ANOVA) to
compare normally distributed variables and Dunnett's post hoc test
for pairwise comparison between vehicle (saline) and ALR-S treated
groups. All data are expressed as the mean ± SEM. P<0.05 was
considered to indicate a statistically significant difference.
Results
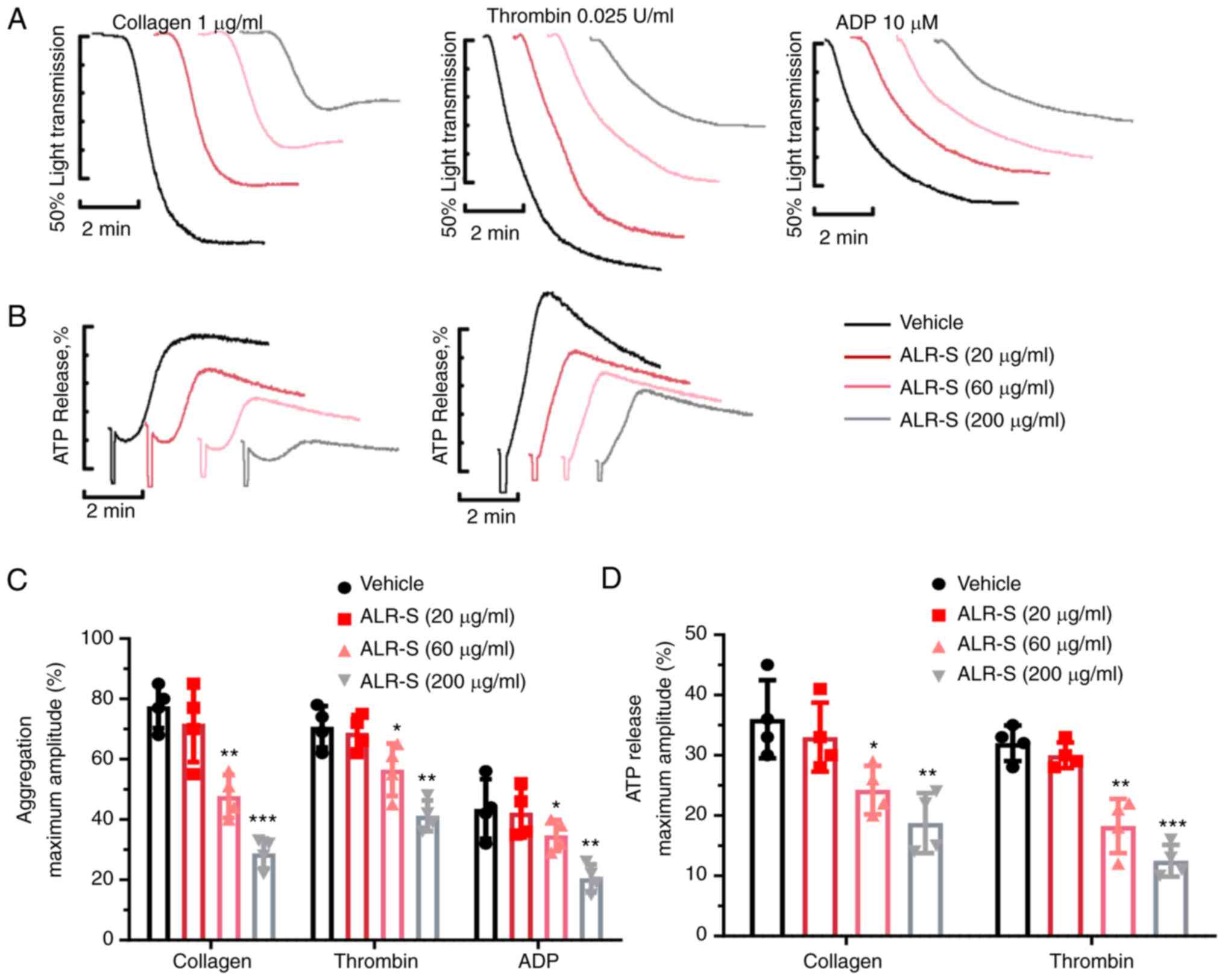
ALR-S decreases agonist-induced
platelet aggregation and ATP release
The effects of ALR-S on agonist-induced platelet
aggregation and ATP release were measured using a Chrono-log
aggregometer. It has been previously reported that ALR-S (200
µg/ml) could significantly reduce ROS generation in
FeCl3-treated aortic endothelial cells, and rebalance
thrombotic and antithrombotic factor expression and secretion in
endothelial cells (6). In the
present study, the experiments were performed using ALR-S at
concentrations of 20, 60 and 200 µg/ml (Fig. 1). The data revealed that ALR-S (60
and 200 µg/ml) inhibited platelet aggregation induced by collagen,
thrombin and ADP (Fig. 1A and
C). Meanwhile, ATP release induced
by thrombin and collagen and measured using a Luciferin/Luciferase
system showed that ALR-S (200 and 60 µg/ml) significantly
attenuated ATP release from dense granules of washed platelets
(Fig. 1B and D).
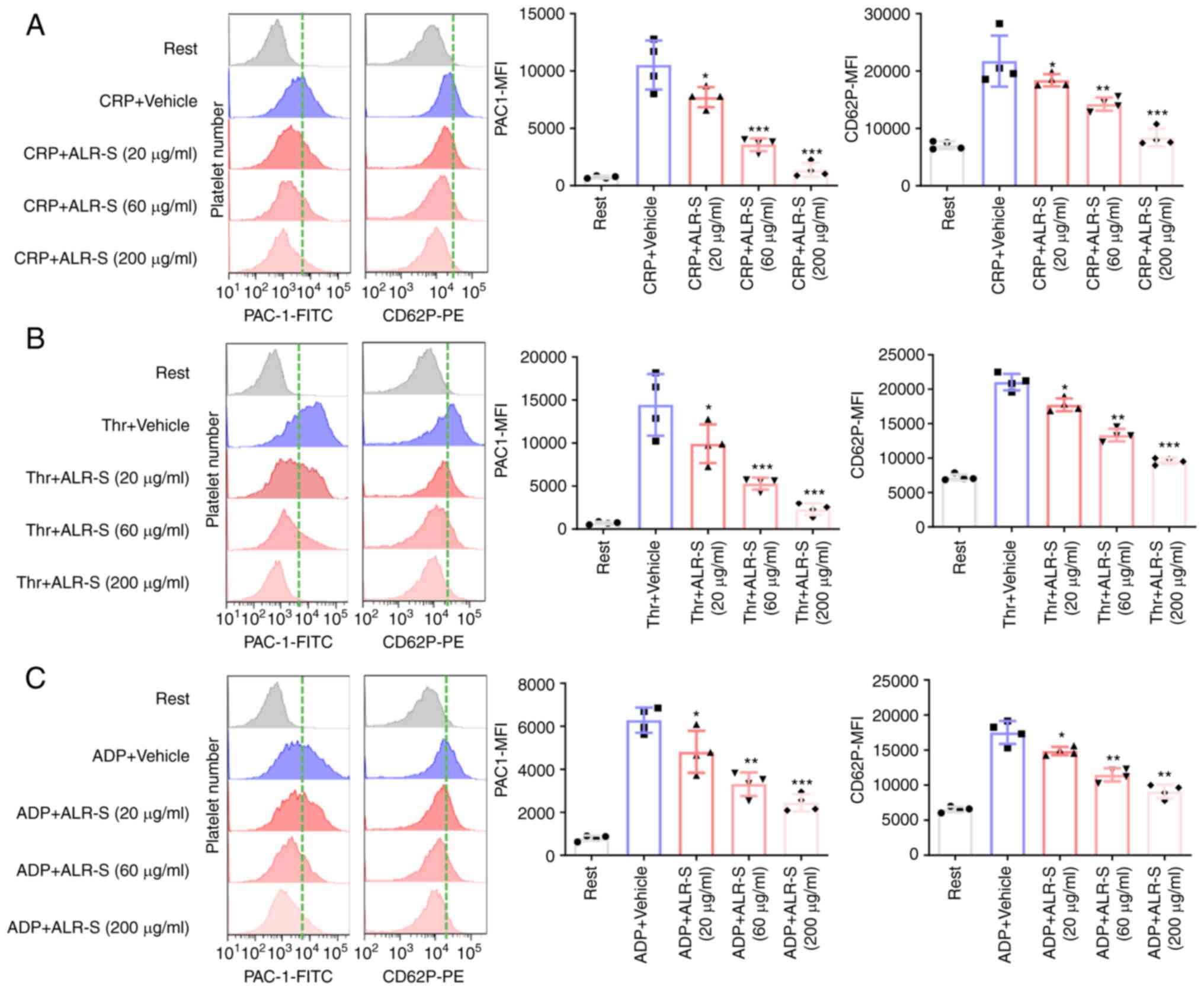
ALR-S attenuates agonist-induced
platelet αIIbβ3 activation and CD62P expression
It is notable that αIIbβ3 (also known as CD41/CD61)
is an important integrin on the platelet surface (20), while CD62P (also known as
P-selectin) is located in the inner surface of resting platelet
α-granules (21). Upon activation,
αIIbβ3 on the platelet surface is transformed into an active state
to bind fibrinogen, which subsequently mediates the ‘outside-in’
signaling and regulates platelet aggregation, while CD62P
translocates to the platelet surface. PE-conjugated CD62P antibody
(binding to released CD62P on the platelet surface) and
FITC-conjugated PAC-1 antibody (binding to an activation-induced
conformational epitope PAC-1 on αIIbβ3) were applied to investigate
the effects of ALR-S on single-platelet activation by flow
cytometry. CRP was used to substitute collagen in the flow
cytometry analysis. ALR-S (200 and 60 µg/ml) showed a potent
inhibitory effect on CRP, thrombin and ADP-induced αIIbβ3
activation and CD62P expression (Fig.
2A-C). Notably, ALR-S (20 µg/ml) was less effective in
inhibiting agonist-induced platelet activation compared with the
high concentrations.
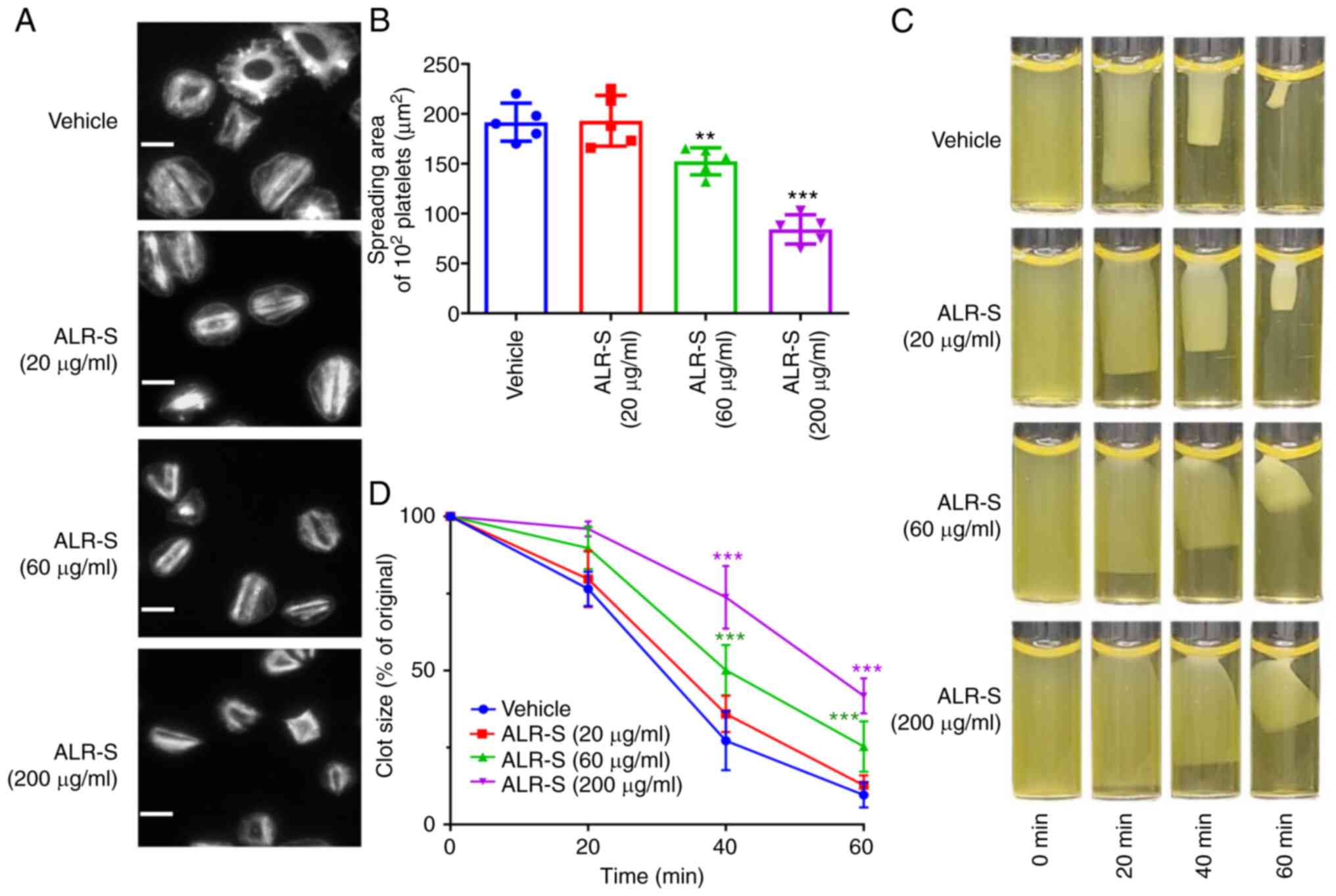
ALR-S inhibits platelet spreading on
immobilized fibrinogen and delays clot retraction
Integrin αIIbβ3-initiated ‘outside-in’ signaling
could promote platelet spreading on immobilized fibrinogen. ALR-S
was applied to determine whether it could affect ‘outside-in’
signaling. The average surface coverage for 100 spread platelets
was 192±19 µm2 in the absence of ALR-S. ALR-S at a
concentration of 60 µg/ml inhibited platelet spreading and the
average area of 100 spread platelets was reduced to 152±14
µm2 (Fig. 3A and
B). Consistently, clot retraction
in PRP requires platelet integrin αIIbβ3-mediated tight
interactions between the membrane and cytoskeleton. In
ALR-S-treated platelets, clot retraction was delayed. The maximum
clot retraction occurred at 60 min after stimulation with thrombin
(0.4 U/ml). By contrast, platelets treated with ALR-S (60 and 200
µg/ml) failed to form tight clots at 40 min and only partial clots
were observed at 60 min (Fig. 3C
and D).
ALR-S decreases ROS generation during
platelet activation
The antioxidant effects of ALR-S on platelets were
examined by flow cytometry. H2DCFDA-loaded platelets (50
µmol/l) were challenged with agonist in the absence or presence of
ALR-S. ALR-S repressed ROS generation during platelet activation in
a concentration-dependent manner (Figs. 4A and S1). Furthermore, ALR-S reduced ROS
induced intracellular signaling activation, and the phosphorylation
of p38 and ERK 1/2 was reduced by ALR-S (Fig. 4B). HSP27 could be activated by p38
phosphorylation (18). Consistent
with this previous report, HSP27 phosphorylation during platelet
activation was reduced in the presence of ALR-S in response to
various agonists in the present study (Fig. 4B). To further testify the
antioxidant effects of ALR-S on exogenous ROS,
H2O2 (20 µM) was added to platelets before
aggregation. Administration of exogenous H2O2
increased platelet aggregation in response to stimuli (Fig. 4C), while preincubation with ALR-S
showed decreased H2O2-mediated platelet
aggregation (Fig. 4C).
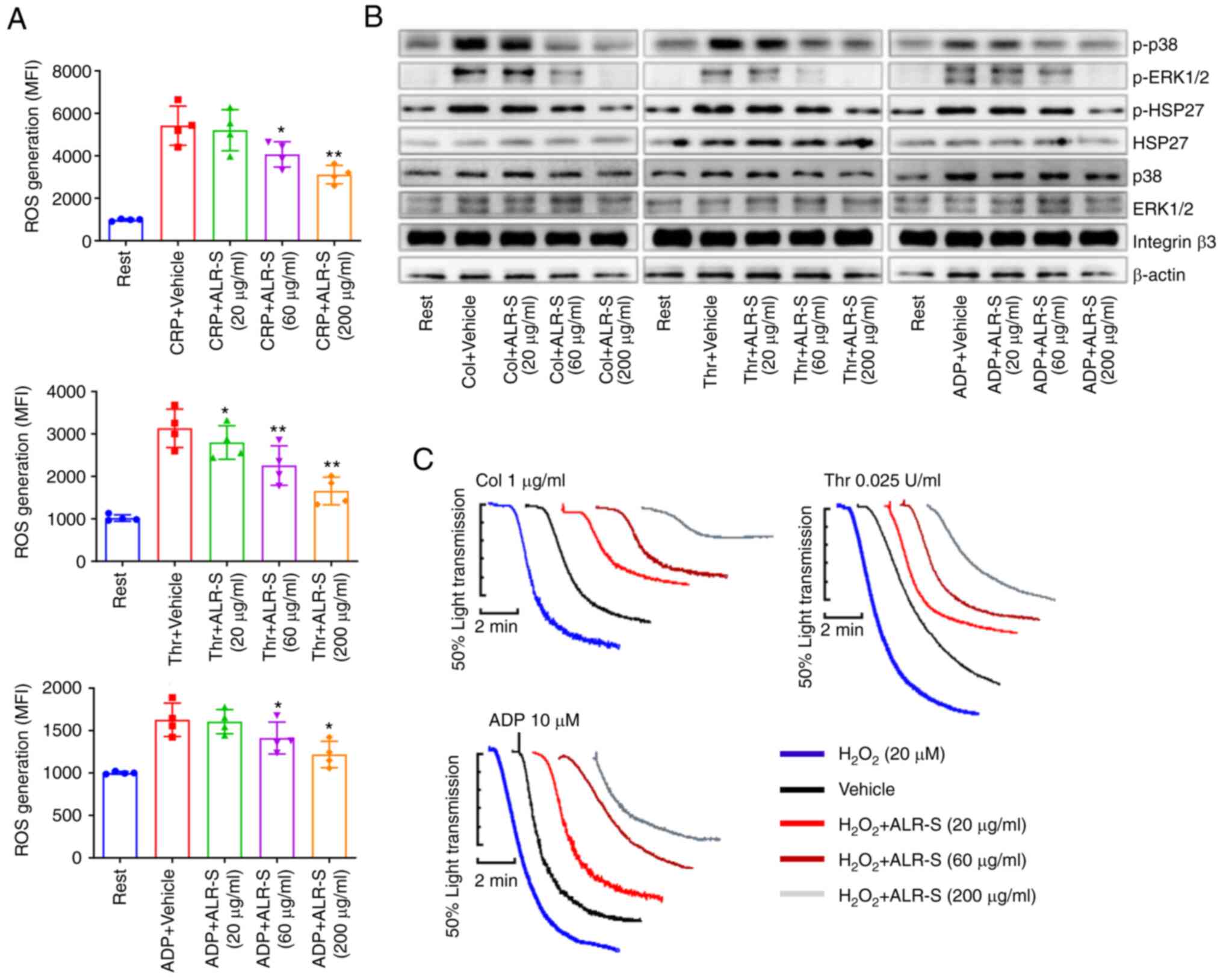 | Figure 4ALR-S decreases ROS generation during
platelet activation. (A) Platelet intracellular ROS level was
determined using flow cytometry. Platelets loaded with
H2DCFDA (50 µmol/l) were incubated with ALR-S or vehicle
for 10 min and then stimulated with CRP (2 µg/ml), thrombin (0.025
U/ml), or ADP (10 µM) for 5 min. *P<0.05 and
**P<0.01 vs. control. (B) Western blotting was
performed to analyze the effects of ALR-S on phosphorylated ERK1/2,
p38 and HSP27 during platelet activation in an aggregometer. (C)
Representative aggregation traces of washed platelets in response
to collagen, thrombin or ADP. Washed platelets were preincubated
with ALR-S (0, 20, 60 and 200 µg/ml), respectively.
H2O2 (20 µM) was added before aggregation was
started, and traces were recorded by a Chrono-log aggregometer
under stirring. ALR-S, saccharides from Arctium lappa L.
root; p38, p38 mitogen-activated protein kinase; HSP27, heat shock
protein 27; Col, collagen; Thr, thrombin; MFI, mean fluorescence
intensity; ROS, reactive oxygen species; CRP, collagen-related
peptide. |
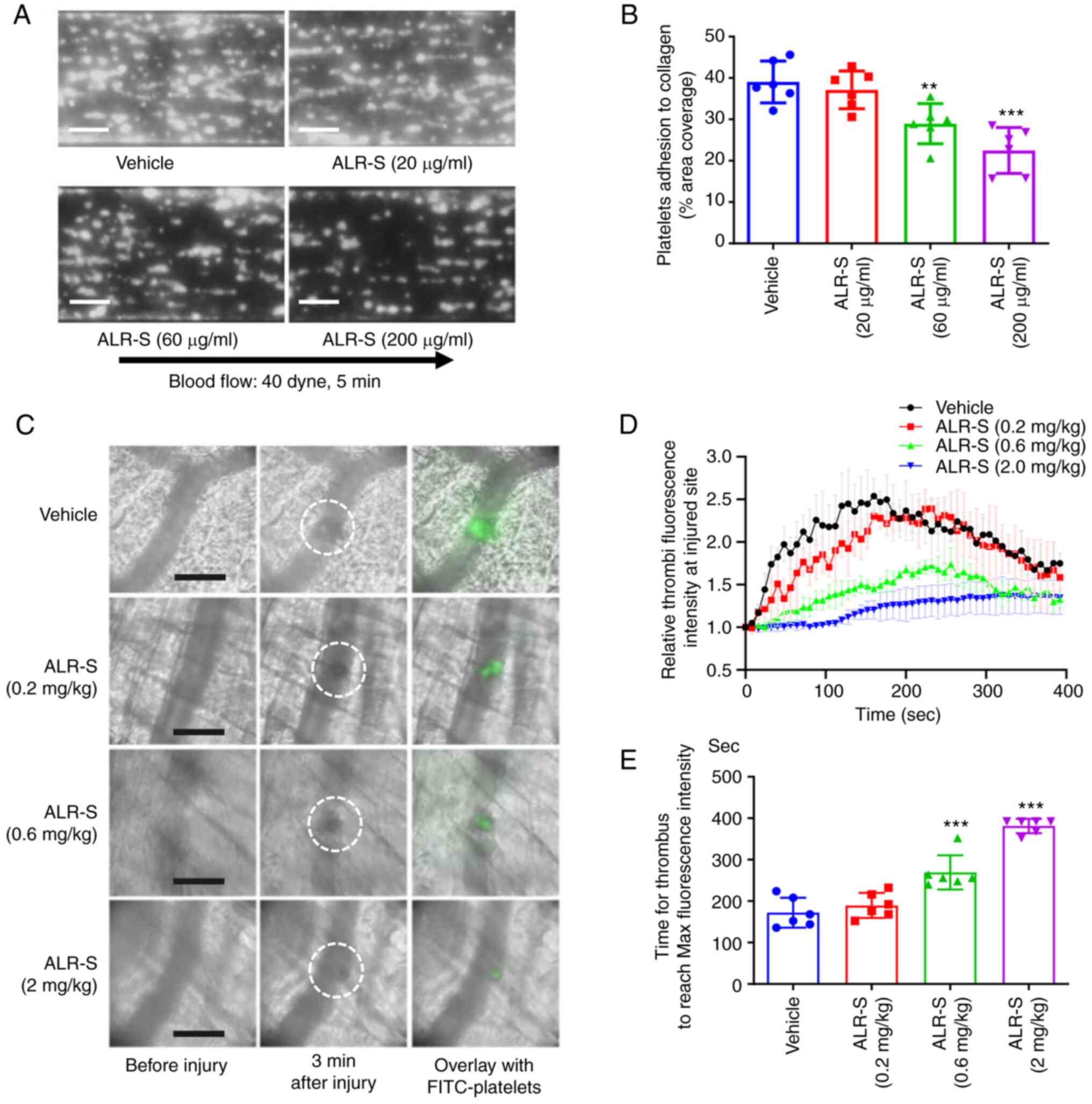
ALR-S inhibits thrombosis both in vivo
and ex vivo
Once endothelium cells are injured in vivo,
platelets are recruited to form stable adhesion on the initially
exposed collagen surface (22). To
clarify the role of ALR-S in platelet adhesion under shear, a
whole-blood microfluidic perfusion system was applied under an
arterial flow condition (40 dynes/cm2). ALR-S (60 and
200 µg/ml) significantly inhibited platelet adhesion over
collagen-coated surfaces as shown by the reduced area coverage of
adherent platelets (P=0.0063 and P<0.0001, respectively)
(Fig. 5A and B). These results confirmed that ALR-S
could reduce thrombosis by inhibiting platelet adhesion onto
exposed collagen. Using a laser injury thrombosis model, the
effects of ALR-S on thrombosis were examined and characterized by
real-time imaging. FITC-conjugated anti-GPVI antibody was
intravenously injected in the tail to label circulating platelets
in mice. ALR-S (0.6 and 2 mg/kg) pretreated mice showed delayed and
smaller thrombi (Fig. 5C and
D) as determined by fluorescence
intensity at the injured site. Additionally, the time for thrombi
to reach maximal fluorescence intensity was reduced by ALR-S
treatment of the same concentrations (0.6 and 2 mg/kg) (Fig. 5E).
Discussion
Medicinal plants are considered important sources of
functional foods to treat and prevent multiple diseases. Arctium
lappa L. is among the most popular plants in the traditional
Chinese pharmacopoeia. Extracts from Arctium lappa L.
exhibit a wide range of pharmacological effects on various
diseases, including hypertension, gout, arteriosclerosis and other
inflammatory disorders (7,23). These effects originate from the
biological activities of its components, such as caffeoylquinic
acid derivatives, lignans and various flavonoids (24). Water-soluble polysaccharide is an
important ingredient in the root extracts of Arctium lappa
L. Polysaccharide extracts from Arctium lappa L. root could
increase production of short chain fatty acids and improve the gut
microbiota environment in mice (25,26),
and administration of water-soluble saccharide could significantly
enhance activities of antioxidant enzyme (13). var. Herkules, a
low-molecular-weight fructofuranan from the roots of Arctium
lappa L. has exhibited significant bioactivity in treating
coughs (27). In terms of
hemostasis, Qiu et al (6)
showed that ALR-S could be protective against arterial thrombosis
risk in a mouse model of FeCl3-induced mesenteric
arterial injury by interfering with the endothelial
thrombotic/antithrombotic factor expression and secretion. In
addition to injured endothelial cells, multiple events
(e.g., platelet reactivity, inflammation, coagulation and
fibrinolysis) could participate in the complex hemostasis, in which
platelets play a vital role. The present study described the direct
effects of ALR-S on platelet activation. More specifically, ALR-S
directly inhibited platelet activation stimulated by exogenous
agonist, via aggregation, ATP secretion, CD62P expression and PAC-1
binding. Presence of ALR-S induced a decrease in platelet spreading
and coverage areas on immobilized fibrinogen, and adhesion on
collagen under shear, respectively. In a laser injury thrombosis
model, exogenous uptake of ALR-S significantly inhibited
thrombosis. Reduced ROS generation and subsequent MAPK
phosphorylation during platelet activation were responsible for
hampered thrombus formation.
Platelet surface receptors are glycosylated
(28), and both N- and
O-glycosylation of platelets play critical roles in the hemostatic
system, including receptor expression, platelet clearance and
signal transduction (29).
Defective platelet surface glycosylation has been reported to be
significantly associated with coronary heart disease and type 2
diabetes mellitus (30,31). Notably, there are several
lectin-like receptors linking platelet activation with sulfated
polysaccharides belonging to the dextran and fucoidan families,
such as C-type lectin-like type II (32), platelet endothelial aggregation
receptor-1 (PEAR1) (33), galectin
1(34), and galectin 8(35). Heterogeneous fucose-containing
sulfated polysaccharides show complex and controversial effects on
hemostasis. Fucosylated glycosaminoglycan, saccharides that were
initially found in the body wall of echinoderms, may lead to
platelet aggregation, possibly depending on the structural
interaction with platelet αIIbβ3(36). Synthetic glycopolymers and natural
fucoidans promote platelet aggregation via PEAR1 and glycoprotein
Ibα (33). In line with the
previous report from Qiu et al (6), the present study showed that
water-soluble saccharides from Arctium lappa L. root
significantly decreased platelet aggregation in response to various
stimuli accompanied by reduced ROS generation. The effects of
saccharides on platelet activation vary due to chain length,
branching, and degree of sulfation (33,37,38).
The radical-scavenging activity of polysaccharides
may depend on the saccharide spectrum and molecular weight
(39). It has been reported that
mannose and glucose, rather than galactose content are highly
associated with the antioxidant activity of polysaccharide from
Parthenocissus tricuspidata (40). Considering the results of previous
reports (39,41), the antioxidant activity of ALR-S
could be attributed to its high mannose content or suitable ratios
of different monosaccharides.
Elderly individuals are vulnerable to undesired
thrombosis, including myocardial infarction, cerebral ischemia and
venous thrombosis, representing the common causes of morbidity and
mortality for that age group (42). There is increasing evidence
suggesting that the aging-related prothrombotic state is derived
from increased oxidative stress (43). Oxidative stress triggers platelet
hyperreactivity and thrombotic susceptibility by decreasing nitric
oxide bioavailability (42). A
gain in platelet function during aging increases the thrombotic
risk, and modulating oxidative stress in platelets is likely a
beneficial approach to counterbalance the hypercoagulation state in
the elderly. In the present study, it was shown that the exogenous
uptake of ALR-S significantly inhibited platelet activation, with
decreased ROS generation, indicating that applying ALR-S as a
beverage in daily life may improve vascular behavior and reduce
thrombotic risk.
Although the antioxidant activity of ALR-S has been
indicated in previous studies (8,39,44),
another molecular basis may underlie the antithrombotic ability of
ALR-S. For instance, glycosaminoglycan has been indicated to
non-selectively inhibit the coagulation cascade and platelet
activation (45,46). Heparin, a negatively charged
sulfated glycosaminoglycan, is the most widely used anticoagulant
saccharide in clinical practice (47). The anticoagulant ability of heparin
derives from its affinity to several serine proteases of the
coagulation cascade in plasma, especially thrombin, factor Xa and
factor IXa. Notably, in addition to the classic anticoagulant
properties, heparin also modulates vascular cell behavior by
binding to angiogenic growth factors, including VEGF and basic
fibroblast growth factor, through their heparin binding sites
(48). Thus, the structural basis
for antithrombotic activity and other beneficial effects of ALR-S
for cardiovascular disease should be further investigated.
Taken together, the results of the present study
described the antithrombotic effects of ALR-S in vitro and
in vivo. These findings indicate that water-soluble ALR-S
may serve as a useful antithrombotic agent via its antioxidant
activity. With possible use as an accessible beverage in daily
life, further follow-up studies may be required to dissect the
exact effects of ALR-S on cardiovascular health for elderly
individuals.
Supplementary Material
Representative histograms of platelet
ROS generation induced by CRP (2 μg/ml), thrombin (0.025
U/ml) and ADP (10 μM). H2DCFDA-loaded (50 μmol/l)
platelets were incubated with or without ALR-S (20, 60 and 200
μg/ml) and stimulated with CRP, thrombin or ADP for 5 min at
37˚C in darkness. Samples were then diluted with 10-fold
HEPES-Tyrode's buffer containing 50 μmol/l H2DCFDA and
analyzed by flow cytometry immediately. ALR-S, saccharides from
Arctium lappa L. root; H2DCFDA, 2',7'-dichlorodihydrofluorescein
diacetate; CRP, collagen-related peptide.
Acknowledgements
The authors would like to thank Dr Jianlin Qiao from
Xuzhou Medical University (Xuzhou, China) for providing CRP.
Funding
Funding: This study was financially supported by the National
Natural Science Foundation of China (grant no. 81903603), the China
Postdoctoral Science Foundation (grant no. 2020M6722920, the Henan
Province Medical Science and Technology Key Project (Union
construction) (grant no. 2018020067), the Natural Science
Foundation of Henan Province (Youth Project) (grant no.
202300410396) and the Young Talent Promotion Project from Henan
Province (grant no. 2021HYTP043).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YR designed and performed experiments, and analyzed
data. YD conducted flow cytometry. MW and ML helped to maintain the
C57BL mice and performed animal experiments. LW contributed to the
western blotting. XL, CZ and JX helped with the chromatographic gel
filtration of ALR-S. YL and JD designed the research and wrote the
manuscript. LH, XZ and ZD helped to design the animal experiments,
and provided critical advice to optimize the experiment protocol in
the laser induced thrombosis injury. All the authors read and
approved the final manuscript. YR and YL confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
Written informed consent was provided before blood
sampling from human volunteers who claimed no underlying disease.
The blood collection procedure was approved by the Ethics Committee
of the First Affiliated Hospital of Zhengzhou University for Use of
Human Subjects (approval no. 2020-KY-122). The animal research
protocol was carried out in compliance with the guidelines of the
International Society on Thrombosis and Hemostasis and was approved
by the Ethics Committee of the First Affiliated Hospital of
Zhengzhou University (approval no. 2021-KY-256).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li Z, Delaney MK, O'Brien KA and Du X:
Signaling during platelet adhesion and activation. Arterioscler
Thromb Vasc Biol. 30:2341–2349. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Stevens H and McFadyen JD: Platelets as
central actors in thrombosis-reprising an old role and defining a
new character. Semin Thromb Hemost. 45:802–809. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mosesson MW: Fibrinogen and fibrin
structure and functions. J Thromb Haemost. 3:1894–1904.
2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Petzold T, Thienel M, Dannenberg L,
Mourikis P, Helten C, Ayhan A, M'Pembele R, Achilles A, Trojovky K,
Konsek D, et al: Rivaroxaban reduces arterial thrombosis by
inhibition of FXa-driven platelet activation via protease activated
receptor-1. Circ Res. 126:486–500. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mackman N, Spronk HMH, Stouffer GA and Ten
Cate H: Dual anticoagulant and antiplatelet therapy for coronary
artery disease and peripheral artery disease patients. Arterioscler
Thromb Vasc Biol. 38:726–732. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Qiu T, Zhou H, Li S, Tian N, Li Z, Wang R,
Sun P, Peng J, Du J, Ma X, et al: Effects of saccharides from
Arctium lappa L. Root on FeCl3-induced arterial
thrombosis via the ERK/NF-κB signaling pathway. Oxid Med Cell
Longev. 2020(7691352)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chan YS, Cheng LN, Wu JH, Chan E, Kwan YW,
Lee SM, Leung GP, Yu PH and Chan SW: A review of the
pharmacological effects of Arctium lappa (burdock).
Inflammopharmacology. 19:245–254. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Maghsoumi-Norouzabad L, Alipoor B, Abed R,
Eftekhar Sadat B, Mesgari-Abbasi M and Asghari Jafarabadi M:
Effects of Arctium lappa L. (Burdock) root tea on
inflammatory status and oxidative stress in patients with knee
osteoarthritis. Int J Rheum Dis. 19:255–261. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rajasekharan SK, Ramesh S, Satish AS and
Lee J: Antibiofilm and Anti-β-lactamase activities of burdock root
extract and chlorogenic acid against klebsiella pneumoniae. J
Microbiol Biotechnol. 27:542–551. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rajasekharan SK, Ramesh S, Bakkiyaraj D,
Elangomathavan R and Kamalanathan C: Burdock root extracts limit
quorum-sensing-controlled phenotypes and biofilm architecture in
major urinary tract pathogens. Urolithiasis. 43:29–40.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li X, Zhao Z, Kuang P, Shi X, Wang Z and
Guo L: Regulation of lipid metabolism in diabetic rats by
Arctium lappa L. polysaccharide through the PKC/NF-κB
pathway. Int J Biol Macromol. 136:115–122. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tousch D, Bidel LP, Cazals G, Ferrare K,
Leroy J, Faucanié M, Chevassus H, Tournier M, Lajoix AD and
Azay-Milhau J: Chemical analysis and antihyperglycemic activity of
an original extract from burdock root (Arctium lappa). J
Agric Food Chem. 62:7738–7745. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu W, Wang J, Zhang Z, Xu J, Xie Z,
Slavin M and Gao X: In vitro and in vivo antioxidant activity of a
fructan from the roots of Arctium lappa L. Int J Biol
Macromol. 65:446–453. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pratico D, Iuliano L, Ghiselli A,
Alessandri C and Violi F: Hydrogen peroxide as trigger of platelet
aggregation. Haemostasis. 21:169–174. 1991.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xu Z, Liang Y, Delaney MK, Zhang Y, Kim K,
Li J, Bai Y, Cho J, Ushio-Fukai M, Cheng N and Du X: Shear and
integrin outside-in signaling activate NADPH-oxidase 2 to promote
platelet activation. Arterioscler Thromb Vasc Biol. 41:1638–1653.
2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Delaney MK, Kim K, Estevez B, Xu Z,
Stojanovic-Terpo A, Shen B, Ushio-Fukai M, Cho J and Du X:
Differential roles of the NADPH-Oxidase 1 and 2 in platelet
activation and thrombosis. Arterioscler Thromb Vasc Biol.
36:846–854. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu Y, Hu M, Luo D, Yue M, Wang S, Chen X,
Zhou Y, Wang Y, Cai Y, Hu X, et al: Class III PI3K positively
regulates platelet activation and thrombosis via PI(3)P-directed
function of NADPH oxidase. Arterioscler Thromb Vasc Biol.
37:2075–2086. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shi P, Zhang L, Zhang M, Yang W, Wang K,
Zhang J, Otsu K, Huang G, Fan X and Liu J: Platelet-Specific p38α
deficiency improved cardiac function after myocardial infarction in
mice. Arterioscler Thromb Vasc Biol. 37:e185–e196. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang S, Liu Y, Wang X, Yang L, Li H, Wang
Y, Liu M, Zhao X, Xie Y, Yang Y, et al: SARS-CoV-2 binds platelet
ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol.
13(120)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ma YQ, Qin J and Plow EF: Platelet
integrin alpha(IIb)beta(3): Activation mechanisms. J Thromb
Haemost. 5:1345–1352. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Merten M and Thiagarajan P: P-selectin
expression on platelets determines size and stability of platelet
aggregates. Circulation. 102:1931–1936. 2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Varga-Szabo D, Pleines I and Nieswandt B:
Cell adhesion mechanisms in platelets. Arterioscler Thromb Vasc
Biol. 28:403–412. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lin SC, Lin CH, Lin CC, Lin YH, Chen CF,
Chen IC and Wang LY: Hepatoprotective effects of Arctium
lappa Linne on liver injuries induced by chronic ethanol
consumption and potentiated by carbon tetrachloride. J Biomed Sci.
9:401–409. 2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ferracane R, Graziani G, Gallo M, Fogliano
V and Ritieni A: Metabolic profile of the bioactive compounds of
burdock (Arctium lappa) seeds, roots and leaves. J Pharm
Biomed Anal. 51:399–404. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang N, Wang Y, Kan J, Wu X, Zhang X,
Tang S, Sun R, Liu J, Qian C and Jin C: In vivo and in vitro
anti-inflammatory effects of water-soluble polysaccharide from
Arctium lappa. Int J Biol Macromol. 135:717–724.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang X, Zhang N, Kan J, Sun R, Tang S,
Wang Z, Chen M, Liu J and Jin C: Anti-inflammatory activity of
alkali-soluble polysaccharides from Arctium lappa L. and its
effect on gut microbiota of mice with inflammation. Int J Biol
Macromol. 154:773–787. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kardosova A, Ebringerova A, Alfoldi J,
Nosal'ova G, Franova S and Hribalova V: A biologically active
fructan from the roots of Arctium lappa L., var. Herkules.
Int J Biol Macromol. 33:135–140. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
King SL, Joshi HJ, Schjoldager KT, Halim
A, Madsen TD, Dziegiel MH, Woetmann A, Vakhrushev SY and Wandall
HH: Characterizing the O-glycosylation landscape of human plasma,
platelets, and endothelial cells. Blood Adv. 1:429–442.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Toonstra C, Hu Y and Zhang H: Deciphering
the roles of N-glycans on collagen-platelet interactions. J
Proteome Res. 18:2467–2477. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li L, Qu C, Lu Y, Gong Y, You R, Miao L
and Guo S: The platelet surface glycosylation caused by glycosidase
has a strong impact on platelet function. Blood Coagul
Fibrinolysis. 30:217–223. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li L, Qu C, Wu X, Dai J, Lu Y, Gong Y, You
R and Liu Y: Patterns and levels of platelet glycosylation in
patients with coronary heart disease and type 2 diabetes mellitus.
J Thromb Thrombolysis. 45:56–65. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Suzuki-Inoue K, Fuller GL, Garcia A, Eble
JA, Pöhlmann S, Inoue O, Gartner TK, Hughan SC, Pearce AC, Laing
GD, et al: A novel Syk-dependent mechanism of platelet activation
by the C-type lectin receptor CLEC-2. Blood. 107:542–549.
2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kardeby C, Falker K, Haining EJ, Criel M,
Lindkvist M, Barroso R, Påhlsson P, Ljungberg LU, Tengdelius M,
Rainger GE, et al: Synthetic glycopolymers and natural fucoidans
cause human platelet aggregation via PEAR1 and GPIbα. Blood Adv.
3:275–287. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pacienza N, Pozner RG, Bianco GA, D'Atri
LP, Croci DO, Negrotto S, Malaver E, Gómez RM, Rabinovich GA and
Schattner M: The immunoregulatory glycan-binding protein galectin-1
triggers human platelet activation. FASEB J. 22:1113–1123.
2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Romaniuk MA, Tribulatti MV, Cattaneo V,
Lapponi MJ, Molinas FC, Campetella O and Schattner M: Human
platelets express and are activated by galectin-8. Biochem J.
432:535–547. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lin L, Yang L, Chen J, Zhou L, Li S, Gao N
and Zhao J: High-molecular-weight fucosylated glycosaminoglycan
induces human platelet aggregation depending on
alphaIIbβ3 and platelet secretion. Platelets.
32:975–983. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tengdelius M, Kardeby C, Falker K,
Griffith M, Påhlsson P, Konradsson P and Grenegård M:
Fucoidan-mimetic glycopolymers as tools for studying molecular and
cellular responses in human blood platelets. Macromol Biosci.
17:2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang Z, Till S, Jiang C, Knappe S,
Reutterer S, Scheiflinger F, Szabo CM and Dockal M:
Structure-activity relationship of the pro- and anticoagulant
effects of Fucus vesiculosus fucoidan. Thromb Haemost. 111:429–437.
2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jiang YY, Yu J, Li YB, Wang L, Hu L, Zhang
L and Zhou YH: Extraction and antioxidant activities of
polysaccharides from roots of Arctium lappa L. Int J Biol
Macromol. 123:531–538. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liang X, Gao Y, Fei W, Zou Y, He M, Yin L,
Yuan Z, Yin Z and Zhang W: Chemical characterization and
antioxidant activities of polysaccharides isolated from the stems
of Parthenocissus tricuspidata. Int J Biol Macromol. 119:70–78.
2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Meng L, Sun S, Li R, Shen Z, Wang P and
Jiang X: Antioxidant activity of polysaccharides produced by
Hirsutella sp. and relation with their chemical characteristics.
Carbohydr Polym. 117:452–457. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fuentes E and Palomo I: Role of oxidative
stress on platelet hyperreactivity during aging. Life Sci.
148:17–23. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Dayal S, Wilson KM, Motto DG, Miller FJ
Jr, Chauhan AK and Lentz SR: Hydrogen peroxide promotes
aging-related platelet hyperactivation and thrombosis. Circulation.
127:1308–1316. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Tian X, Sui S, Huang J, Bai JP, Ren TS and
Zhao QC: Neuroprotective effects of Arctium lappa L. roots
against glutamate-induced oxidative stress by inhibiting
phosphorylation of p38, JNK and ERK 1/2 MAPKs in PC12 cells.
Environ Toxicol Pharmacol. 38:189–198. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sheehan JP and Walke EN: Depolymerized
holothurian glycosaminoglycan and heparin inhibit the intrinsic
tenase complex by a common antithrombin-independent mechanism.
Blood. 107:3876–3882. 2006.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Xiao C, Zhao L, Gao N, Wu M and Zhao J:
Nonasaccharide inhibits intrinsic factor Xase complex by binding to
factor IXa and disrupting factor IXa-factor VIIIa interactions.
Thromb Haemost. 119:705–715. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Torri G and Naggi A: Heparin centenary-an
ever-young life-saving drug. Int J Cardiol. 212 (Suppl 1):S1–S4.
2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zieris A, Prokoph S, Levental KR, Welzel
PB, Grimmer M, Freudenberg U and Werner C: FGF-2 and VEGF
functionalization of starPEG-heparin hydrogels to modulate
biomolecular and physical cues of angiogenesis. Biomaterials.
31:7985–7994. 2010.PubMed/NCBI View Article : Google Scholar
|