Introduction
Hypoparathyroidism is an infrequent endocrine
deficiency disorder characterized by low levels of serum calcium,
increased levels of serum phosphorus and absent or inappropriately
low levels of serum parathyroid hormone (PTH) (1). Hypoparathyroidism usually occurs as a
result of post-surgical or autoimmune damage to the parathyroid
gland (2). The most common cause
is neck surgery, which accounts for 75% of all cases. The incidence
of hypoparathyroidism following neck surgery is ~8%; however, in
75% of these cases, the condition is temporary and is resolved
within 6 months. As a result, the incidence of persistent
hypoparathyroidism following neck surgery is ~2% (3).
The most common cause of non-surgical
hypoparathyroidism is autoimmune hypoparathyroidism. This disorder
can present on its own or as a part of autoimmune polyglandular
syndrome type I (4). The
destruction of the parathyroid glands can develop secondary to
infiltration in the context of granulomatous diseases, such as
sarcoidosis, amyloidosis and Riedel thyroiditis (5). Genetic causes account for <10% of
all cases of hypoparathyroidism, with DiGeorge syndrome the most
common genetic cause (6). The
destruction of the parathyroid glands leading to hypoparathyroidism
is rarely observed due to infiltrating metastatic cancer (7).
Hypoparathyroidism can also be caused by external or
internal radiation, although this is rarely mentioned due to the
fact that the parathyroid glands are extremely resistant to
radiation (8). Moreover,
hypoparathyroidism may develop due to the accumulation or
deposition of minerals, such as copper (Wilson's disease) and iron
(hemochromatosis) in the parathyroid gland (4). Furthermore, numerous mitochondrial
diseases have been found to be associated with hypoparathyroidism
(4). In addition,
hypoparathyoidism may develop with no obvious etiology. In such
cases, patients are most likely suffering from an autoimmune
disorder (8).
The novel coronavirus, severe acute respiratory
syndrome coronavirus 2 (SARS-CoV-2), which is responsible for
corona virus disease 2019 (COVID-19), has rapidly spread across the
globe, posing a severe threat to human health due to a pandemic
(9). The distribution of the
angiotensin converting enzyme 2 (ACE2) protein in human tissues,
identified by previous studies, has allowed researchers to
recognize probable infection pathways and speculate on the
pathogenetic consequences of SARS-CoV-2 infection. According to
these studies, ACE2 expression is highest in the lungs and small
intestine enterocytes, and lowest in the testes, thyroid, adipose
tissue, ovaries and endothelium. ACE2 has also been found in the
adrenals, prostate, pituitary and hypothalamus (10,11).
In addition, the increased expression of ACE2 receptors has been
found in acidophilic cells of the parathyroid glands (12).
Hypocalcemia has been identified as a prevalent
symptom of COVID-19, and it appears to be a biochemical trait that
distinguishes COVID-19 from other acute respiratory distress
syndromes (13). Furthermore, it
appears to be a predictor of the development of severe COVID-19
infection. These findings, however, appear to be predominantly
linked to vitamin D insufficiency (14).
Research into the effects of the novel coronavirus
on parathyroid glands is limited. Hypoparathyroidism due to
SARS-CoV-2 infection has been reported in a small number of cases
(15,16). The present study describes a rare
case of primary hypoparathyroidism induced by COVID-19.
Case report
A 53-year-old male patient, who was a non-smoker,
presented to the Emergency Department of Laiko General Hospital
(Athens, Greece) complaining of fever and cough over the past 10
days. He had a medical history of a surgically resected colon
cancer 10 years prior, with no relapse in follow-up. He was
receiving no medication.
A clinical examination revealed a febrile patient
with crackles on auscultation at the bases of both lungs. The
clinical evaluation of the other organs/systems did not reveal any
notable findings. Blood pressure was 130/80 mmHg, heart rate was 96
beats per minute, oxygen saturation was 92% in room air and body
temperature was 37.6˚C. An electrocardiography did not reveal any
abnormalities upon admission.
The analysis of arterial blood gas revealed partial
pressure of oxygen (pO2) levels of 54 mmHg, pressure of
carbon dioxide (pCO2) levels of 41 mmHg, pH 7.47 and
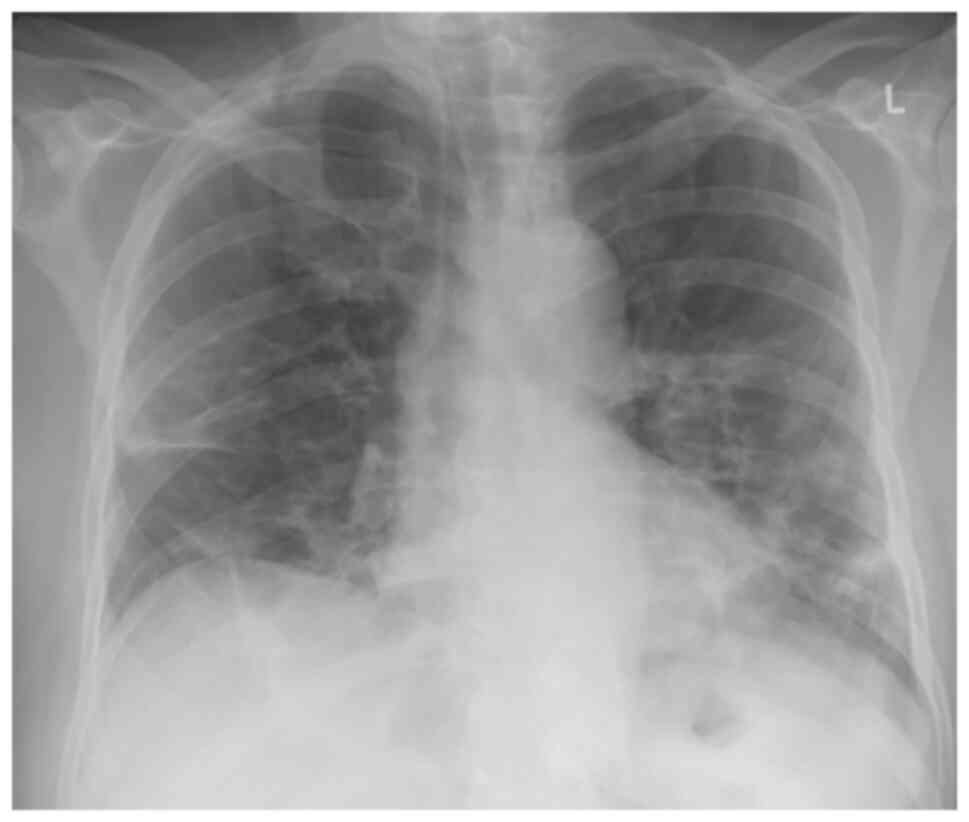
HCO3- at 29.8 mmol/l in room air. An X-ray of the chest revealed
patchy diffuse opacities in both lower lung lobes (Fig. 1).
Laboratory tests included a complete blood cell
count, biochemistry serum parameters and blood clotting testing.
The notable findings were a white blood cell count of
2.99x103/µl (normal count, 4-11x103/µl),
lymphocyte count of 0.58x103/µl (normal count,
1.2-3.4x103/µl), platelet count of 104x103/µl
(normal count, 140-440x103/µl), creatinine levels of
0.61 mg/dl (normal range, 0.6-1 mg/dl), serum calcium levels of 6.9
mg/dl (normal range, 8.6-10.2 mg/dl), serum phosphorus levels of
4.7 mg/dl (normal range, 2.5-4.5 mg/dl), serum albumin levels of
37.4 g/l (normal range, 35-50 g/l), C-reactive protein levels of
53.29 mg/l (normal levels, <6 mg/l) and ferritin levels of 759
ng/ml (normal range, 15-150 ng/ml).
A nasopharyngeal swab was obtained and the patient
tested positive for SARS-CoV-2 infection using reverse
transcription-polymerase chain reaction (RT-PCR). The patient had
not been vaccinated against SARS-CoV-2. He was transferred to the
COVID-19 unit and received oxygen therapy with a Venturi mask
delivering 50% oxygen. He also received intravenous dexamethasone,
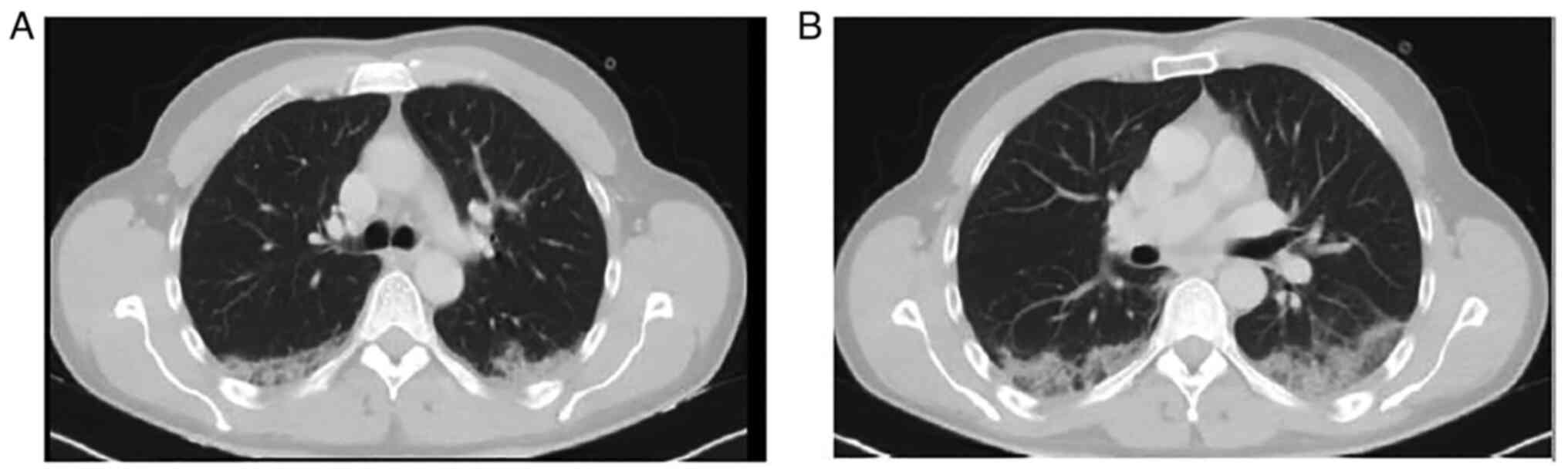
remdesivir and subcutaneous enoxaparin. A computed tomography (CT)
scan of the patient's chest revealed nodular ground glass opacities
in the posterior segments of the upper and lower lobes (Fig. 2).
Due to detected hypocalcemia and hyperphosphatemia,
a further laboratory investigation was performed, revealing PTH
levels of 11.7 pg/ml (normal range, 12-65 pg/ml) and 25
hydroxyvitamin D levels of 38.4 ng/ml (levels of sufficiency,
>30 ng/ml). The patient had no vitamin D deficiency and no
chronic renal insufficiency. Therefore, the presence of
hypocalcemia, hyperphosphatemia and inappropriately low serum
levels of PTH confirmed the diagnosis of primary
hypoparathyroidism.
The patient had no symptoms related to this
condition. In addition, he had a normal level of serum calcium at
8.9 mg/dl (normal range, 8.6-10.2 mg/dl) at ~6 months prior when
evaluated as a part of his general health checkup. This fact, along
with his age, ruled out a genetic cause of hypoparathyroidism.
Moreover, he had no history of surgery, trauma, infiltrative
disease or regional radiation. PTH-related peptide levels were
determined to be within the normal range, thus ruling out
paraneoplastic syndrome as a cause of low PTH levels. Autoantibody
screening for autoimmune diseases did not reveal any abnormal
findings.
The patient underwent a CT scan of the neck without
revealing any abnormal findings, which ruled out the infiltration
of the parathyroid glands by metastases. He also underwent an
abdominal CT scan with no abnormalities. Thus, primary
hypoparathyroidism was considered to have been caused by SARS-CoV-2
infection.
The patient received oral calcium supplementation.
After 5 days of hospitalization, his clinical condition and serum
calcium levels improved. At 1 month following discharge, he
presented with normal levels of serum calcium and serum
phosphorus.
Discussion
Researchers have reported a small number of cases of
primary hypoparathyroidism induced by SARS-CoV-2 infection and the
decompensation of old primary hypoparathyroidism during the course
of COVID-19 infection. In 2020, Elkattawy et al (15) reported the first case of primary
hypoparathyroidism induced by SARS-CoV2 infection in a 46-year-old
male patient with no marked past medical history, who was
hospitalized with hypoxic respiratory failure and had a prolonged
hospital stay. In that case, hyperphosphatemia and low PTH levels
were detected incidentally (15).
Dianatfar et al (16)
described a 44-year old, previously healthy, female patient who was
hospitalized due to COVID-19, who then entered a depressive state,
and had one tonic-clonic seizure at ~1 week after being discharged
from the hospital. Laboratory tests revealed a low serum calcium
level and an increased serum phosphorus level. In addition, the
serum PTH level was significantly low, establishing the diagnosis
of primary hypoparathyroidism (16). The case described herein is the
third, to the best of our knowledge, to report primary
hypoparathyroidism induced by SARS-CoV-2 infection. The patient in
the present study, similar to the other cases (15,16)
developed hypocalcemia and hyperphosphatemia, with low levels of
PTH in the context of COVID-19 pneumonia. Malignancy and
paraneoplastic syndrome and autoimmune diseases were ruled out. The
patient had a positive response to therapy with oral calcium
supplementation. SARS-CoV-2 infection induced hypoparathyroidism,
which was not persistent.
COVID-19 disease has also been reported to induce
the decompensation of previously well-tolerated primary
hypoparathyroidism. Bossoni et al (17) reported a case of a 72-year-old
female patient with a past history of thyroidectomy who experienced
mild COVID-19 infection and presented with acute perioral
paresthesia and dysarthria. Laboratory investigations revealed a
low serum calcium level, an increased serum phosphorus level and a
low serum PTH level, suggesting that SARS-CoV-2 infection
precipitated severe hypocalcemia in the context of a subclinical
post-surgical hypoparathyroidism (17).
Pla et al (18) described a case of a 76-year-old
male patient with a known history of hypocalcemia who presented
with perioral paresthesia during COVID-19 infection, upper
extremity paresthesia, anorexia, and with hypocalcemia,
hyperphosphatemia and inappropriately normal levels of PTH,
establishing the decompensation of a well-tolerated primary
hypoparathyroidism due to SARS-CoV-2 infection. Moreover, Bonnet
et al (19) reported
decompensated primary hypoparathyroidism in an 82-year-old male
patient with COVID-19. The cases of primary hypoparathyroidism and
the decompensation of old primary hypoparathyroidism due to
SARS-CoV-2 infection in the literature are summarized in Table I.
 | Table ICases of primary hypoparathyroidism
and the decompensation of primary hypoparathyroidism due to
COVID-19 found in the literature. |
Table I
Cases of primary hypoparathyroidism
and the decompensation of primary hypoparathyroidism due to
COVID-19 found in the literature.
| Author/(Refs),
year | Age, years/sex | Symptoms/signs
related to hypoparathyroidism | Type of
dysfunction | Course of COVID-19
infection | Outcome |
|---|
| Elkattawy et
al (15), 2020 | 46/M | None, incidental
finding | Primary
hypoparathyroidism | Critical | Recovery |
| Dianatfar et
al (16), 2021 | 44/F | Tonic-clonic seizure;
depressed mood | Primary
hypoparathyroidism | Severe | Recovery |
| Bossoni et al
(17), 2020 | 72/F | Acute-onset
dysarthria; perioral paresthesia | Decompensation of
primary hypoparathroidism | Mild | Recovery |
| Pla et al
(18), 2021 | 76/M | Perioral paresthesia;
upper extremity paresthesia; anorexia; positive Trousseau sign | Decompensation of
primary hypoparathroidism | Severe | Recovery |
| Bonnet et al
(19), 2021 | 82/M | Prolongation of the
QTc interval at 470 msec on electrocardiography | Decompensation of
primary hypoparathroidism | Severe | Recovery |
| The present
study | 53/M | None, incidental
finding | Primary
hypoparathyroidism | Severe | Recovery |
The frequency of parathyroid dysfunction among
patients with COVID-19, as well as the exact underlying mechanisms,
duration and reversibility of this phenomenon, remain unclear. Due
to the paucity of data linking SARS-CoV-2 to parathyroid glands,
studies on the previous generation of coronavirus (SARS-Co-V),
which caused the SARS pandemic in 2003, may elucidate this topic.
SARS-Co-V RNA and antigenic materials have been detected in
parathyroid gland acidophilic cells in tissue samples obtained from
patients with SARS who did not survive (20). In addition, the increased
expression of ACE2 receptors has been detected in acidophilic cells
of parathyroid glands (9).
Therefore, SARS-CoV-2 may potentially directly affect the
parathyroid glands by binding to the ACE2 receptors on acidophilic
cells (21).
Previous pathological research on SARS-CoV-1- or
SARS-CoV-2-infected patients has revealed a range of endocrine
tissue damage, including direct cell damage from viral entry and
replication, vasculitis, arterial and venous thrombosis, hypoxic
cell damage, immune response and the cytokine storm (22). Thrombosis is more common in
patients with COVID-19, particularly in small vessels and
extrapulmonary organs, than in patients with SARS (23). This SARS-CoV-2-specific
pathogenetic action may cause damage to highly vascularized organs,
such as the endocrine glands, and in particular those with a dense
vascular network (24).
Of note, a previous study demonstrated that chronic
respiratory alkalosis increased resistance of renal PTH receptors
to PTH, resulting in hypocalcemia and hyperphosphatemia with no
increase in PTH levels, indicating that chronic respiratory
alkalosis might lead to relative hypoparathyroidism (25). These findings suggest an additional
potential mechanism of indirect affection of parathyroid gland
function during COVID-19 disease, which is characterized by an
increased effort for respiration and carbon dioxide washout,
leading to respiratory alkalosis (25). Further studies are required to
investigate the causative association between SARS-CoV-2 infection
and the effects on parathyroid glands.
In conclusion, SARS-CoV-2 infection can lead to
multi-organ involvement, including the dysfunction of the endocrine
glands. The present study highlighted the effect of the novel
coronavirus on parathyroid glands. Clinicians should also consider
that although SARS-CoV-2 does not present a known tropism for the
parathyroid glands, it can cause the decompensation of old primary
hypoparathyroidism that was well-tolerated prior to infection.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PA, AB and PP conceptualized the study. VEG, EK and
CD advised on patient treatment, wrote and prepared the draft of
the manuscript and made a substantial contribution to data
analysis. AG and MSV analyzed patient data. NT and DAS analyzed
patient data and provided critical revisions. PS and DP obtained
the medical images and made substantial contributions to data
interpretation. VEG and AB confirm the authenticity of all the raw
data. All authors contributed to manuscript revision and have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and accompanying
images. A copy of the written consent is available for review by
the Editor-in-Chief of this journal on request.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have not competing
interests.
References
|
1
|
Bilezikian JP, Khan AA and Potts JT Jr:
Third International Workshop on the Management of Asymptomatic
Primary Hyperthyroidism. Guidelines for the management of
asymptomatic primary hyperparathyroidism: Summary statement from
the third international workshop. J Clin Endocrinol Metab.
94:335–339. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bilezikian JP, Khan A, Potts JT Jr, Brandi
ML, Clarke BL, Shoback D, Jüppner H, D'Amour P, Fox J, Rejnmark L,
et al: Hypoparathyroidism in the adult: Epidemiology, diagnosis,
pathophysiology, target-organ involvement, treatment, and
challenges for future research. J Bone Miner Res. 26:2317–2337.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Clarke BL, Brown EM, Collins MT, Jüppner
H, Lakatos P, Levine MA, Mannstadt MM, Bilezikian JP, Romanischen
AF and Thakker RV: Epidemiology and diagnosis of
hypoparathyroidism. J Clin Endocrinol Metab. 101:2284–2299.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Siraj N, Hakami Y and Khan A: Medical
hypoparathyroidism. Endocrinol Metab Clin North Am. 47:797–808.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Brinkane A, Peschard S, Leroy-Terquem E,
Bergheul S, Raheriarisoa H, Hubert N, Crickx L and Levy R:
Association rare d'une hypoparathyroïdie et d'une sarcoïdose
médiastino-pulmonaire: Rare association of hypoparathyroidism and
mediastinal-pulmonary sarcoidosis. Ann Med Interne (Paris).
152:63–64. 2001.PubMed/NCBI(In French).
|
|
6
|
Goddard CJ, Mbewu A and Evanson JM:
Symptomatic hypocalcaemia associated with metastatic invasion of
the parathyroid glands. Br J Hosp Med. 43(72)1990.PubMed/NCBI
|
|
7
|
Bilezikian JP: Hypoparathyroidism. J Clin
Endocrinol Metab. 105:1722–1736. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shoback D: Clinical practice.
Hypoparathyroidism. N Engl J Med. 359:391–403. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Georgakopoulou VE, Avramopoulos P,
Papalexis P, Bitsani A, Damaskos C, Garmpi A, Gkoufa A, Garmpis N,
Mantzouranis K, Chlapoutakis S, et al: Exacerbation of
bronchiectasis by Pseudomonas putida complicating COVID-19
disease: A case report. Exp Ther Med. 22(1452)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen Y, Guo Y, Pan Y and Zhao ZJ:
Structure analysis of the receptor binding of 2019-nCoV. Biochem
Biophys Res Commun. 525:135–140. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lazartigues E, Qadir MMF and
Mauvais-Jarvis F: Endocrine significance of SARS-CoV-2's reliance
on ACE2. Endocrinology. 161(bqaa108)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
He L, Ding Y, Zhang Q, Che X, He Y, Shen
H, Wang H, Li Z, Zhao L, Geng J, et al: Expression of elevated
levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+
cells in SARS patients: relation to the acute lung injury and
pathogenesis of SARS. J Pathol. 210:288–297. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Di Filippo L, Formenti AM, Rovere-Querini
P, Carlucci M, Conte C, Ciceri F, Zangrillo A and Giustina A:
Hypocalcemia is highly prevalent and predicts hospitalization in
patients with COVID-19. Endocrine. 68:475–478. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu J, Han P, Wu J, Gong J and Tian D:
Prevalence and predictive value of hypocalcemia in severe COVID-19
patients. J Infect Public Health. 13:1224–1228. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Elkattawy S, Alyacoub R, Ayad S, Pandya M
and Eckman A: A novel case of hypoparathyroidism secondary to
SARS-CoV-2 infection. Cureus. 12(e10097)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dianatfar M, Sanjari M and Dalfardi B:
Hypoparathyroidism after COVID-19 Pneumonia. Shiraz E-Med J.
22(e115832)2021.
|
|
17
|
Bossoni S, Chiesa L and Giustina A: Severe
hypocalcemia in a thyroidectomized woman with Covid-19 infection.
Endocrine. 68:253–254. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pla B, Silva M, Arranz A and Marazuela M:
Hipocalcemia severa y resistente al tratamiento en paciente con
neumonía bilateral COVID-19. Severe and treatment-resistant
hypocalcemia in patient with bilateral COVID-19 pneumonia.
Endocrinol Diabetes Nutr. 68:518–519. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bonnet JB, Berchoux E and Sultan A:
Decompensated primary hypoparathyroidism in a patient with
COVID-19. Ann Endocrinol (Paris). 82:123–124. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ding Y, He L, Zhang Q, Huang Z, Che X, Hou
J, Wang H, Shen H, Qiu L, Li Z, et al: Organ distribution of severe
acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV)
in SARS patients: Implications for pathogenesis and virus
transmission pathways. J Pathol. 203:622–630. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Abobaker A and Alzwi A: The effect of
COVID-19 on parathyroid glands. J Infect Public Health. 14:724–725.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Guo Y, Korteweg C, McNutt MA and Gu J:
Pathogenetic mechanisms of severe acute respiratory syndrome. Virus
Res. 133:4–12. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu
SC, Mou HM, Wang LH, Zhang HR, Fu WJ, et al: A pathological report
of three COVID-19 cases by minimal invasive autopsies. Zhonghua
Bing Li Xue Za Zhi. 49:411–417. 2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
24
|
Piticchio T, Le Moli R, Tumino D and
Frasca F: Relationship between betacoronaviruses and the endocrine
system: A new key to understand the COVID-19 pandemic-A
comprehensive review. J Endocrinol Invest. 44:1553–1570.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Krapf R, Jaeger P and Hulter HN: Chronic
respiratory alkalosis induces renal PTH-resistance,
hyperphosphatemia and hypocalcemia in humans. Kidney Int.
42:727–734. 1992.PubMed/NCBI View Article : Google Scholar
|