Introduction
Dilated cardiomyopathy (DCM) is a type of heart
disease characterized by ventricular dilation along with impaired
contractility, chronic inflammation and subsequent heart failure
(1-3).
There are no effective therapeutics for the disease thus far.
Long-term use of anthracycline antibiotics and cytotoxic
(antineoplastic) antitumor agents, such as doxorubicin (Dox), are
major causes of the development of DCM, even 10-20 years after Dox
chemotherapy has ceased (4).
Gradual loss of cardiomyocytes over time and compensatory
hypertrophic remodeling may contribute to the delayed onset of DCM.
It has been documented that Dox enhances oxidation and DNA damage,
and increases the accumulation of cytochrome c, myocardial
apoptosis and pyroptosis. The expression of NAD(P)H oxidase,
hydrogen peroxide, pro-apoptotic caspase-9, caspase-3, caspase-1,
high-mobility group box 1 (HMGB1) and NLRP3 are increased in
Dox-treated cardiomyocytes (5-8).
However, the expression of anti-apoptotic Bcl-xL is reduced in
Dox-treated cardiomyocytes (9-11).
Cluster of differentiation 47 (CD47) is a
transmembrane glycoprotein that plays a complex role in the
modulation of stem cells (12) and
endothelial cell renewal (13). In
addition, CD47 is upregulated in most types of tumors and
participates in tumor immune evasion by suppressing macrophage
efferocytosis of tumor cells and reducing tumor angiogenesis
through interaction with thrombospondin-1 (14,15).
It has been reported that the blockade of CD47 signaling
significantly suppresses tumor cell survival and facilitates
Dox-mediated antitumor effects in vivo (16-19).
In addition, blockade of CD47 signaling can increase graft survival
in recipients transplanted with CD47-knockout donor grafts
(20). A previous study also
revealed that CD47 is involved in the development of cardiovascular
diseases. For example, CD47 expression was found to be upregulated
in patients with clinical pulmonary hypertension, which was
associated with pulmonary arterial vasculopathy and dysfunction
(21). Furthermore, a lack or
blockade of CD47 signaling was reported to significantly attenuate
pulmonary hypertension and myocyte hypertrophy in mice, which was
accompanied by reduced expression of histone deacetylase 3 (HDAC3)
and elevated expression of transcription factor c-Myc (21,22).
In addition, the beneficial effects have been observed in animal
models with ischemia-reperfusion injury, in which blocking CD47
signaling by small interfering (si)RNA or CD47 gene deficiency can
effectively attenuate myocardial damage and increase the clearance
of apoptotic cardiomyocytes (23,24).
Studies in mice with isoproterenol-induced cardiac hypertrophy also
showed these beneficial effects, in which anti-CD47 neutralizing
antibody (aCD47) effectively suppressed cardiac hypertrophy and
cardiac fibrosis (25).
Therefore, CD47 plays a critical role in the
development of cardiovascular diseases. However, it is not fully
understood whether CD47 signaling participates in the development
of DCM in mice and the underlying molecular mechanisms remain
elusive. Recently, Feliz-Mosquea et al (16) reported that targeting CD47 enhanced
Dox-induced growth delay of tumors, while protecting cardiac tissue
viability and function in mice. These beneficial effects were
associated with the increased activation of protective autophagy
and autophagic disposal of DOX-damaged mitochondria by
aCD47(16). This dual role in
protecting cardiac tissues and improving antitumor effects was also
observed in mice treated with phosphoinositide 3-kinase γ (PI3Kγ)
inhibitor (26). In the present
study, we aimed to explore the expression and role of CD47 in a
mouse model with DCM. The results showed that blockade of CD47
signaling by aCD47 significantly attenuated myocardial
cytotoxicity, which was associated with attenuated myocardial
inflammation, cardiomyocyte early apoptosis and activation of p38
MAPK signaling. Therefore, CD47 appears to be a promising
therapeutic target in DCM.
Materials and methods
Chemicals and reagents
Dox was purchased from MedChemExpress LLC. Anti-CD47
neutralizing antibody (aCD47) was purchased from Bio X Cell. ELISA
kits for mouse tumor necrosis factor (TNF)-α and interleukin (IL-6)
were purchased from R&D Systems. BCA assay kit was purchased
from Thermo Fisher Scientific, Inc. Antibodies for flow cytometry
staining, including purified rat anti-mouse CD16/CD32 (mouse Fc
block; cat. no. 553141), APC-anti-mouse CD11b (cat. no. 101211),
FITC-anti-mouse CD47 (cat. no. 127503) and PE-Cy7-anti-mouse Ly6G
(cat. no. 560601) were purchased from BD Biosciences and BioLegend.
The PE-Annexin V apoptosis detection kit was purchased from
BioLegend. Anti-cardiac troponin T monoclonal antibody (cTnT,
13-11, (cat. no. MA5-12960), anti-collagen I (cat. no. ab21286),
anti-Bax (cat. no. ab3191), anti-Bcl-2 (cat. no. ab16904),
anti-cleaved caspase-1 and -3 (cat. nos. 9661 and 89332), and
anti-p-p38 MAPK antibodies (cat. no. 9211) were purchased from
Thermo Fisher Scientific, Abcam and Cell signaling, respectively.
Cy3- or FITC-conjugated secondary antibodies were purchased from
Jackson ImmunoResearch Laboratories (cat. nos. 711-165-152 and
715-165-150). Lactate dehydrogenase (LDH) activity assay kit was
purchased from Beijing Solarbio Science & Technology.
Mice and treatment
A total of 63 male C57BL/6 mice (wild-type; 8-12
weeks of age; weight 18-22 g) were purchased from Shanghai Model
Organisms Center (Shanghai, China), and housed in an animal
facility at 23˚C (room temperature). All mice were maintained under
a constant 12 h light-dark cycle and a standard mouse diet with
ad libitum access to food and water. During the animal
experiments, a mouse model of DCM was established by
intraperitoneal (i.p.) administration of 5, 10 and 20 mg/kg Dox
respectively for consecutive 4 weeks, according to the previous
protocols with some modifications (1,27).
For therapeutic treatment, murine DCM were i.p. treated with
different doses of aCD47 (3.5, 7 and 14 mg/kg) weekly in 200 µl
volume, according to a previous protocol with some modifications
(17). Mice injected with PBS,
aCD47 (7 mg/kg), or both Dox and goat IgG isotype in the same
volume were used as the PBS, aCD47 and IgG/Dox control groups,
respectively. The mice were sacrificed under anesthesia by i.p.
injection of 75 mg/kg pentobarbital 4 weeks after treatment. Blood
(150 µl) was collected via cardiac puncture and the hearts were
removed for analysis. All animals were housed and treated according
to the guidelines of the Institutional Animal Care and Use
Committee of Fudan University, Zhongshan Hospital (Shanghai,
China). The study protocol was approved by the Animal Experimental
Ethics Committee of Zhongshan Hospital, Fudan University (Shanghai,
China).
Echocardiography
A high-resolution micro-ultrasound system equipped
with a 30-MHz probe (RMVTM 707b) was used for transthoracic
echocardiography on mice. Briefly, the mice were anesthetized with
1.5% isoflurane and hearts were imaged in the two-dimensional
parasternal short-axis view. M-mode echocardiogram of the
mid-ventricle was recorded. The following measurements were
obtained during both systole and diastole: heart rate, ejection
fraction (EF), left ventricular fractional shortening (LVFS), left
ventricular internal diameter (LVID), left ventricular posterior
wall thickness (LVPW) and inter-ventricular septal thickness
(IVS).
Heart histology and
immunohistology
Heart tissues were fixed by 4% paraformaldehyde and
processed for sectioning after paraffin embedding. Hematoxylin and
eosin (H&E) and Masson staining were performed by standard
protocols routinely used in our laboratory. The area of
cardiomyocytes in H&E-stained heart tissues was semi-quantified
by manual counting of center nuclei localized cardiac myofibers.
Briefly, H&E-stained sections were viewed under a contrast
microscope with x200 magnification. The entire cardiomyocytes were
marked, and its area was measured. At least 10 randomly selected
fields per section were marked and counted. The expression levels
of collagen I and Bax in heart tissues were analyzed by
immunostaining. Briefly, heart sections were incubated with 10%
goat serum for 1 h, followed by incubation with 0.5% Triton X-100
for 10 min. Then, the sections were incubated with antibodies
against collagen I and Bax (1:300 dilution, cat. nos. ab21286 and
ab3191) overnight, followed by incubation with Cy3-conjugated
secondary antibody (1:500 dilution, cat. no. 711-165-152). Nuclei
were stained with 4',6-diamidino-2-phenylindole (DAPI). The stained
sections were visualized under fluorescence microscope and
semi-quantified by ImageJ software, v. 1.8.0 (National Institutes
of Health).
Analysis of cytokines and LDH
release
The expression of TNF-α and IL-6 in heart protein
extracts and serum were measured by ELISA, according to the
manufacturer's instructions. Briefly, 96-well Nunc
MaxiSorp™ flat-bottom plates (Thermo Fisher Scientific,
Inc.) were coated with 2 µg/ml capture antibody in coating buffer
(0.1 M carbonate, pH 9.5) overnight. After incubation with blocking
buffer [3% bovine serum albumin (BSA) in PBS], the plates were
incubated with protein samples for 2 h, followed by incubation with
0.2 µg/ml biotin-conjugated detection antibody and streptavidin at
the recommended concentration. After washed with washing buffer
(PBS supplemented with 3% BSA and 0.05% Tween-20), the plates were
developed using substrate TMB (3,3',5,5'-tetramethylbenzidine). The
reaction was halted by 2N H2SO4, and
absorbance was read by a spectrometer at 450 nm. LDH release was
measured by using commercial kits (cat. no. BC0680), according to
the manufacturer's instructions.
Flow cytometry analysis
Single cell suspensions were incubated with an
antibody cocktail containing APC-anti-CD11b, FITC-anti-CD47 and
PE-Cy7-anti-Ly6G for 30 min at room temperature. For blockade of Fc
receptors on monocyte/macrophages and neutrophils during flow
cytometric analysis, the cells were pre-incubated with 0.5 µg
purified rat anti-CD16/CD32 for 5 min on ice prior to staining with
antibody cocktail. Apoptotic cells were detected by PE-Annexin V
apoptosis detection kit (BioLegend; cat. no. 640934), according to
the manufacturer's recommendations. All stained cells were analyzed
on a BD FACSAria™ III instrument (BD Biosciences) and
FlowJo software, v. 8.8.4 (FlowJo LLC).
Cardiomyocyte isolation and
immunostaining
Neonatal cardiomyocytes were isolated and cultured
as previously described with some modifications (28). Briefly, wild-type C57BL/6 neonatal
mice (1-3 g weight) were obtained from the mother 2-7 days after
delivery and sacrificed by cervical dislocation. The hearts were
collected and digested using 1 mg/ml collagenase A for 1 h at 37˚C.
Primary cardiomyocytes were enriched by a pre-plating approach to
remove contaminated cells before seeding into cell culture plates.
The enriched cardiomyocytes were identified using a cTnT antibody
and 95% purity of the cells was obtained. After 2 days in culture,
cardiomyocytes were pre-treated with 1 µg/ml aCD47 for 1 h,
followed by incubation with 10 µM Dox for 24 h. Anti-Bax (cat. no.
ab3191), anti-Bcl-2 (cat. no. ab16904), anti-cleaved caspase-1 and
-3 (cat. nos. 9661 and 89332), and anti-p-p38 MAPK (cat. no. 9211)
antibodies (1:300 dilution) were used as primary antibodies.
Cy3-conjugated anti-rabbit or mouse IgG (1:500 dilution; cat. nos.
711-165-152 and 715-165-150) were used as secondary antibodies.
Nuclei were stained with DAPI. The positively stained cells (red)
were visualized under a fluorescence microscope. Images were
captured in 5 randomly selected fields at x200 magnification, and
positively stained cells were semi-quantified by ImageJ software,
v. 1.8.0. after images were inverted.
Western blot analysis
Protein expression of collagen I, Bax, Bcl-2,
pro-caspase-1, cleaved caspase-1 and cleaved caspase-3 in the heart
tissues and cells were analyzed via western blotting. Briefly, 40
µg protein samples were resolved on 10% SDS-PAGE gel. After running
for 1 h at 100 V, the resolved protein was transferred onto a
polyvinylidene fluoride membrane (EMD Millipore). Blots were then
blocked with 3% non-fat milk for 30 min, followed by incubation
with the indicated primary antibodies (1:1,000) for 2 h. The blots
incubated with anti-GAPDH antibody (1:1,000 dilution; cat. no.
ab9485) was used as a loading control. After washing with 1X
Tris-buffered saline buffer with 0.05% Tween-20 (TBST), the blots
were incubated with HRP-conjugated secondary antibody (1:1,000
dilution; cat. nos. 615-035-214 and 711-035-152) for 1 h. After
washing with 1X TBST three times, immune reactivity was visualized
using the enhanced chemiluminescent reagent (ECL) (Beyotime
Institute of Biotechnology). Band densitometric intensity was
semi-quantified by ImageJ software.
Statistical analysis
Results are presented as mean ± standard error,
n=5-7. All data were first tested for normal distribution using the
Shapiro-Wilk test (GraphPad Prism version 8.0.2, GraphPad Software,
Inc.). Datasets of multiple groups passing the Shapiro-Wilk test
were analyzed by one-way ANOVA followed by Tukey's multiple
comparisons. Data that did not pass the Shapiro-Wilk test were
analyzed by Mann Whitney test. P<0.05 was considered to be a
statistically significant difference.
Results
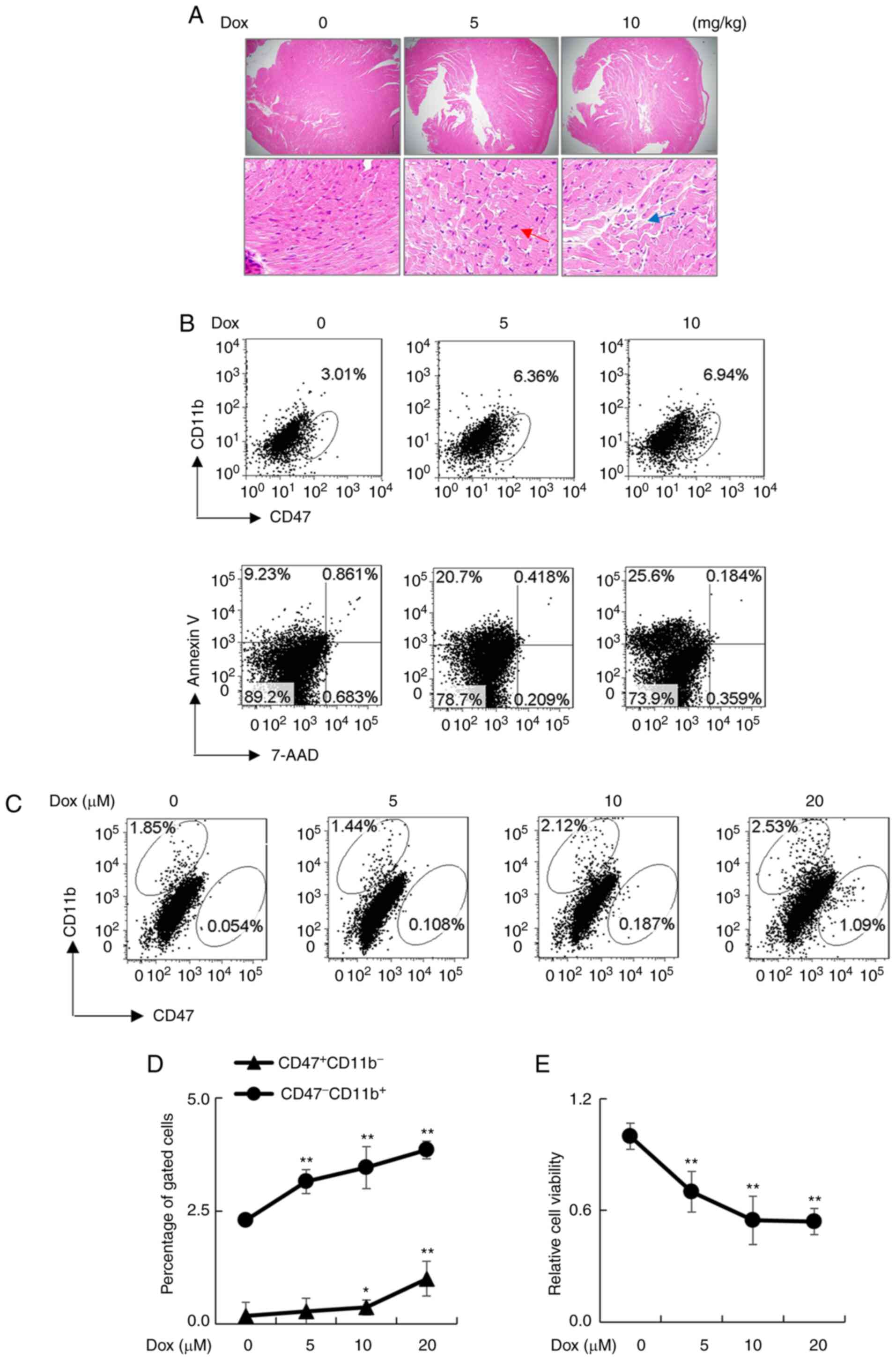
CD47 is upregulated in the heart
tissues of Dox-treated mice
Murine models of Dox-induced DCM were established by
administration of 5 or 10 mg/kg Dox i.p. once a week for 4 weeks.
After Dox treatment, we observed the increased number of
cardiomyocytes in heart tissues of the treated mice, that was
identified by center nuclei localized cells (red arrow) and broken
cardiac myofibers (blue arrow) (Fig.
1A). In addition, Dox treatment upregulated CD47 expression in
CD11b- cardiac myofibers and elevated the percentage of
Annexin V+7-AAD- early apoptotic cells in a
dose-dependent manner (Fig. 1B).
The detrimental effects were further observed in primary
cardiomyocytes isolated from wild-type neonatal mice, in which the
expression of CD47 in CD11b- cardiomyocytes was
increased in a Dox concentration-dependent manner, as determined by
flow cytometry analysis (Fig. 1C
and D). Consistently, cell
viability was gradually reduced in a Dox concentration-dependent
manner (Fig. 1E). The results
indicate the role of Dox in the upregulation of CD47 and the
induction of cytotoxic effects.
aCD47 significantly reduces the
severity of DCM
To further define whether blockade of CD47 signaling
affects the development of DCM, mice were treated with 7 mg/kg
aCD47 (i.p.) once a week in conjunction with 10 mg/kg Dox. We did
not observe any abnormal heart morphology between the mice treated
with PBS control and aCD47 alone, indicating that aCD47 had no
obvious side effects on mice 4 weeks after aCD47 treatment.
However, there was increased left ventricular chamber (LV) with
thinned posterior wall in the Dox-treated mice, that was
effectively reversed in the aCD47 co-treated mice, compared to the
IgG-treated control [Fig. 2A
(upper panel)]. M-mode non-invasive transthoracic echocardiography
analysis revealed that both left ventricular ejection fraction
(LVEF) and left ventricular fractional shortening (LVFS) were
significantly improved in mice after aCD47 co-treatment, compared
with the IgG-treated control mice, indicating successful
establishment of murine DCM and beneficial effects of aCD47 on
recovery of heart function in murine DCM (Fig. 2B and C). Further histological analysis of the
heart tissues showed that aCD47 co-treatment significantly reduced
hypertrophic cardiomyocytes (red arrow) and broken cardiac
myofibers (patchy area, blue arrow), compared with the IgG-treated
mice [Fig. 2A (upper panels) and
D]. In addition, aCD47 co-treatment effectively reduced
interstitial fibrosis (yellow arrow) and the expression of collagen
I (green) in the heart tissues, compared with the IgG-treated
murine DCM [Fig. 2A and D (lower panels)]. The suppressive effects
of aCD47 on the expression of collagen I in the heart tissues of
murine DCM were further confirmed via western blot analysis
(Fig. 2E and F). Additional flow cytometry analysis
also confirmed that infiltration of CD11b+ macrophages
were significantly reduced in the heart tissues of murine DCM after
aCD47 treatment (Fig. 2G and
H). The results indicated that
aCD47 effectively suppressed the development of murine DCM, in
association with the reduced interstitial fibrosis and infiltration
of inflammatory cells in the heart tissues.
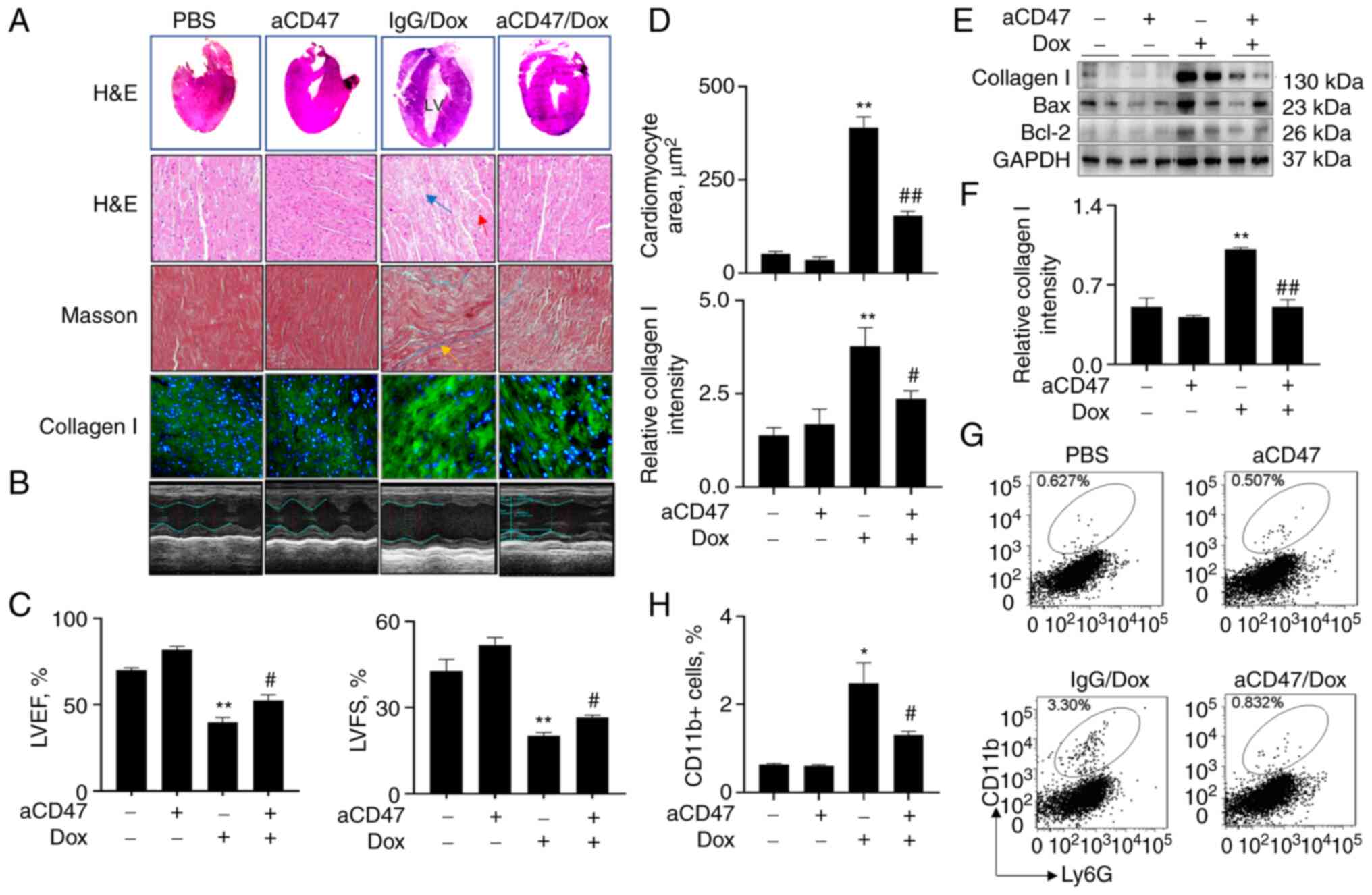 | Figure 2aCD47 significantly reduces the
severity of Dox-induced dilated cardiomyopathy (DCM) in mice. Adult
mice (aCD47/Dox group) were i.p. administered with both 7 mg/kg
aCD47 and 10 mg/kg Dox once a week for 4 weeks. Mice treated with
both IgG isotype and Dox (IgG/Dox group) or PBS and aCD47 alone
(PBS and aCD47 groups) were controls. (A) Hematoxylin and eosin
(H&E) staining for mouse heart tissues. Representative gross
morphology and phase contrast microscope images with x200
magnification (upper two panels). Masson staining for interstitial
fibrosis and immunostaining for collagen I (lower two panels).
Representative images with x200 magnification. Red arrow, cells
with centrally localized nuclei; blue arrow, broken and patchy
myofibers; yellow arrow, interstitial fibrosis; green, collagen I.
(B) Representative echocardiograms of mice 4 weeks after treatment.
(C) Quantitative analysis of echocardiographic measurements. Left
ventricular ejection fraction (LVEF); left ventricular fractional
shortening (LVFS). (D) Quantitative analysis of cardiomyocyte area
after H&E staining (upper panel; Mann-Whitney test) and
fluorescence intensity of collagen I-positive fibers in heart
tissues after immunostaining by ImageJ software. Data are presented
as relative fluorescence intensity of positively stained cells over
untreated controls. (E) Western blot analysis for the expression of
collagen I, Bax and Bcl-2 in the cardiac tissues of treated mice.
GAPDH was internal loading control. One representative blot. (F)
Band densitometric intensity was semi-quantified by ImageJ
software. (G) Flow cytometry analysis for the infiltrating
CD11b+ macrophages in cardiac tissues. (H) The
infiltrating CD11b+ macrophages in cardiac tissues were
measured by flow cytometry and quantitatively analyzed. All
quantitative data are presented as mean ± standard error.
*P<0.05, **P<0.01 vs. PBS group;
#P<0.05, ##P<0.01 vs. IgG/Dox group
(n=5-7). Two-way ANOVA with Tukey's multiple comparison's test,
except where it is indicated otherwise. aCD47, anti-CD47
neutralizing antibody; Dox, doxorubicin. |
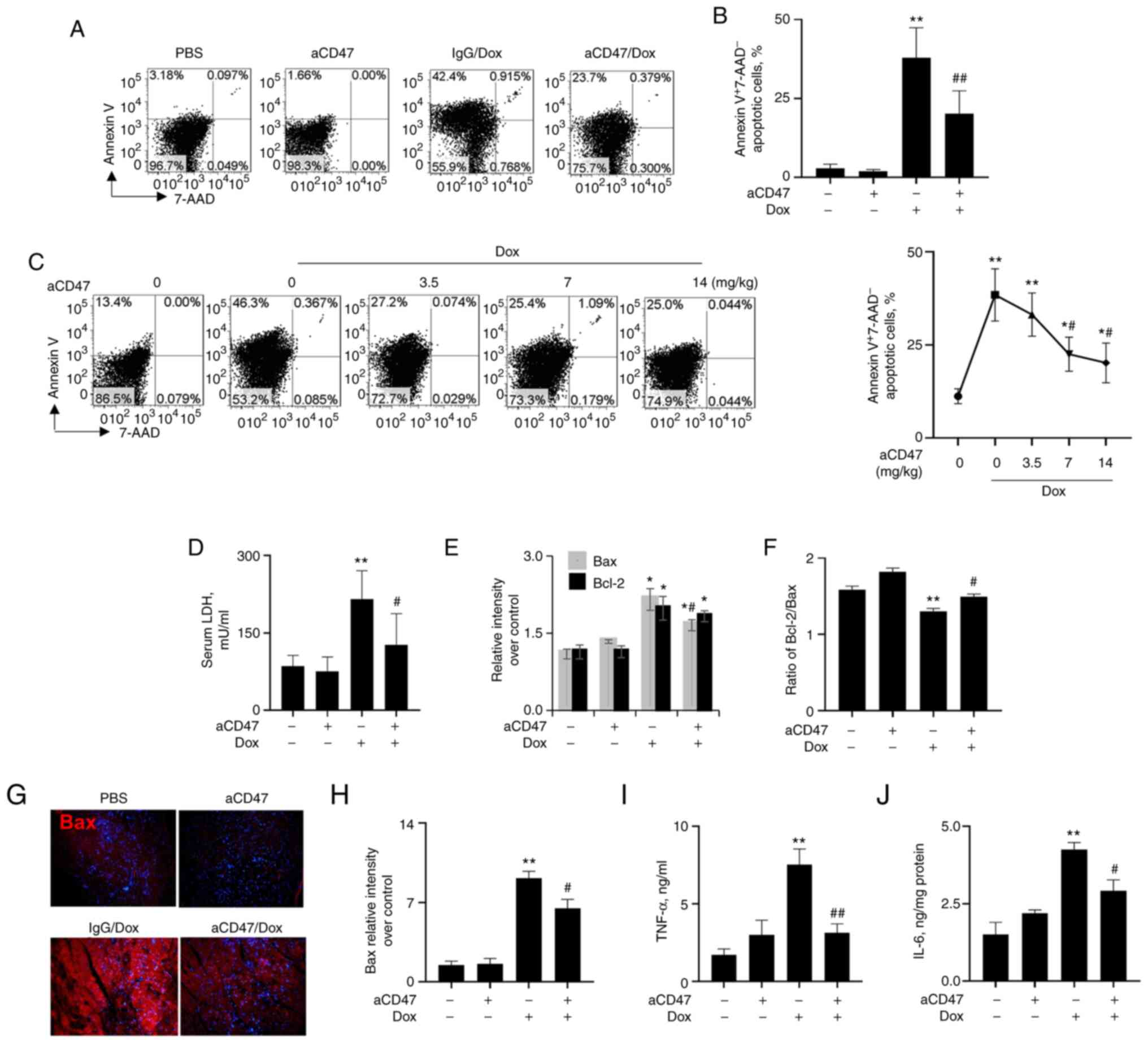
aCD47 suppresses cardiac myofiber
early apoptosis in mice after Dox treatment
To further investigate the effects of aCD47 on
cardiac myofiber apoptosis in murine DCM, mice were treated with
Dox in conjunction with different doses of aCD47. Heart tissues
were collected 4 weeks after co-treatment. We found an average of
40% Annexin V+7-AAD- early apoptotic cardiac
myofibers in the heart tissues of Dox-treated mice. There was no
obvious Annexin V+7-AAD- early apoptotic
cardiac myofibers in the heart tissues of mice treated with aCD47
alone, indicating no potential side effects of aCD47 in mice. In
addition, we observed that aCD47 co-treatment exhibited effective
suppressive effects on early apoptosis of cardiac myofiber in
murine DCM (Fig. 3A and B). aCD47 suppressed early apoptosis of
cardiac myofiber in murine DCM at a dose-dependent manner with a
plateau at weekly doses of aCD47 over 7 mg/kg aCD47 (Fig. 3C). In addition, we observed that
LDH release was significantly attenuated in the aCD47-treated mice,
compared with the IgG-treated controls (Fig. 3D). Further analysis via western
blotting indicated that Dox effectively upregulated the expression
of both Bax and Bcl-2, and these were significantly reduced by
aCD47 co-treatment, with more reduced expression of Bax than Bcl-2
and increased ratio of Bcl-2/Bax after aCD47 co-treatment (Figs. 2E and 3E and F). These results were further
demonstrated by immunostaining of heart tissues, in which the
expression of both Bax (Fig. 3G
and H) and Bcl-2 (data not shown)
was upregulated by Dox, which was reversed by aCD47 co-treatment.
In addition, we observed the significantly elevated expression of
pro-inflammatory cytokines, including TNF-α and IL-6 in the heart
tissues of Dox-treated mice, which was effectively attenuated by
aCD47 co-treatment (Fig. 3I and
J). Therefore, aCD47 attenuated
the severity of DCM, in association with the reduced cardiac
myofiber early apoptosis and expression of pro-inflammatory
cytokines in heart tissues.
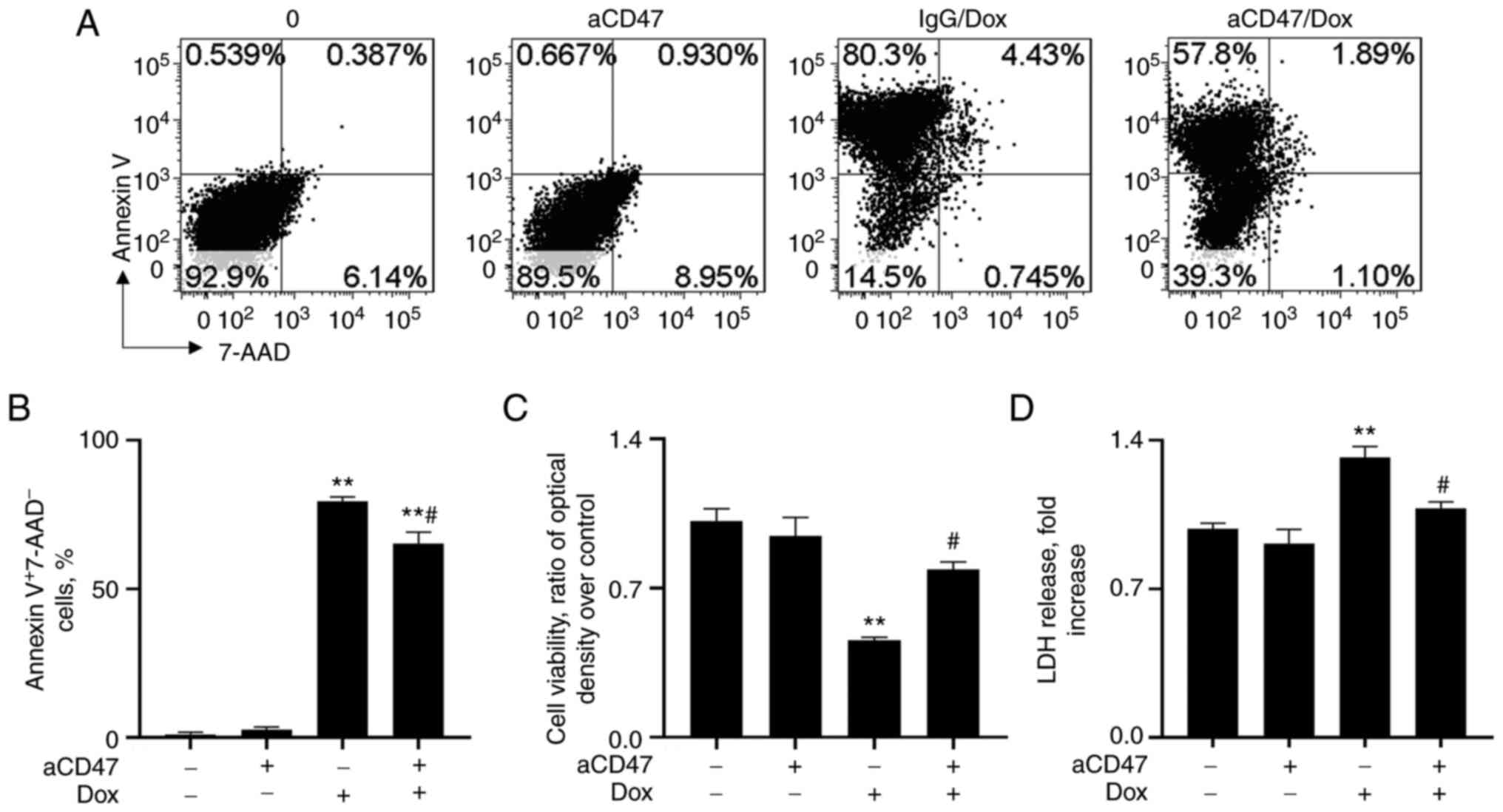
aCD47 reduces cardiomyocyte early
apoptosis in vitro
To investigate whether the reduced severity of DCM
by aCD47 was caused by targeting CD47 on cardiomyocytes, primary
cardiomyocytes from neonatal mice were treated with 1 µg/ml aCD47,
in conjunction with 10 µM Dox for 24 h. The results of flow
cytometry analysis showed that Dox treatment effectively increased
Annexin V+7-AAD- cardiomyocyte early
apoptosis, which was significantly reversed by pre-treatment with
aCD47 (Fig. 4A and B). Consistently, aCD47 co-treatment
effectively improved cell viability and attenuated LDH release into
the cell supernatants, compared with the IgG co-treated control
cells (Fig. 4C and D). The results indicated the direct
anti-apoptotic effects of aCD47 on cardiomyocytes.
aCD47 reduces cardiomyocyte early
apoptosis by suppressing the expression of Bax and p38 MAPK
signaling
Immunostaining showed that the isolated
cardiomyocytes were stained positive for cTnT, a
cardiomyocyte-specific marker, confirming that the isolated cells
were indeed cardiomyocytes [Fig.
5A (upper panel, green)]. Treatment with aCD47 and Dox did not
change the expression of cTnT, indicating no effects of aCD47 on
cardiomyocyte proliferation and differentiation. However, as
expected, we observed the increased expression of cleaved
caspase-1, cleaved caspase-3 and Bax in the Dox-treated
cardiomyocytes, which was effectively reversed by aCD47
pre-treatment [Fig. 5A (middle
panel) and B]. The results were further confirmed by western blot
analysis, in which aCD47 pre-treatment effectively suppressed
Dox-induced upregulation of cleaved caspase-1, cleaved caspase-3
and Bax (Fig. 5C). The expression
of Bcl-2 was upregulated in cardiomyocytes by treatment of Dox
alone or in conjunction with both Dox and aCD47. aCD47 and Dox
co-treatment induced increased upregulation of Bcl-2 when compared
with Dox treatment alone (Fig.
5C), indicating the synergistic effects of aCD47 and Dox in
increasing the expression of Bcl-2. The effects induced an
increased ratio of Bcl-2/Bax in the aCD47 cotreated
cardiomyocytes.
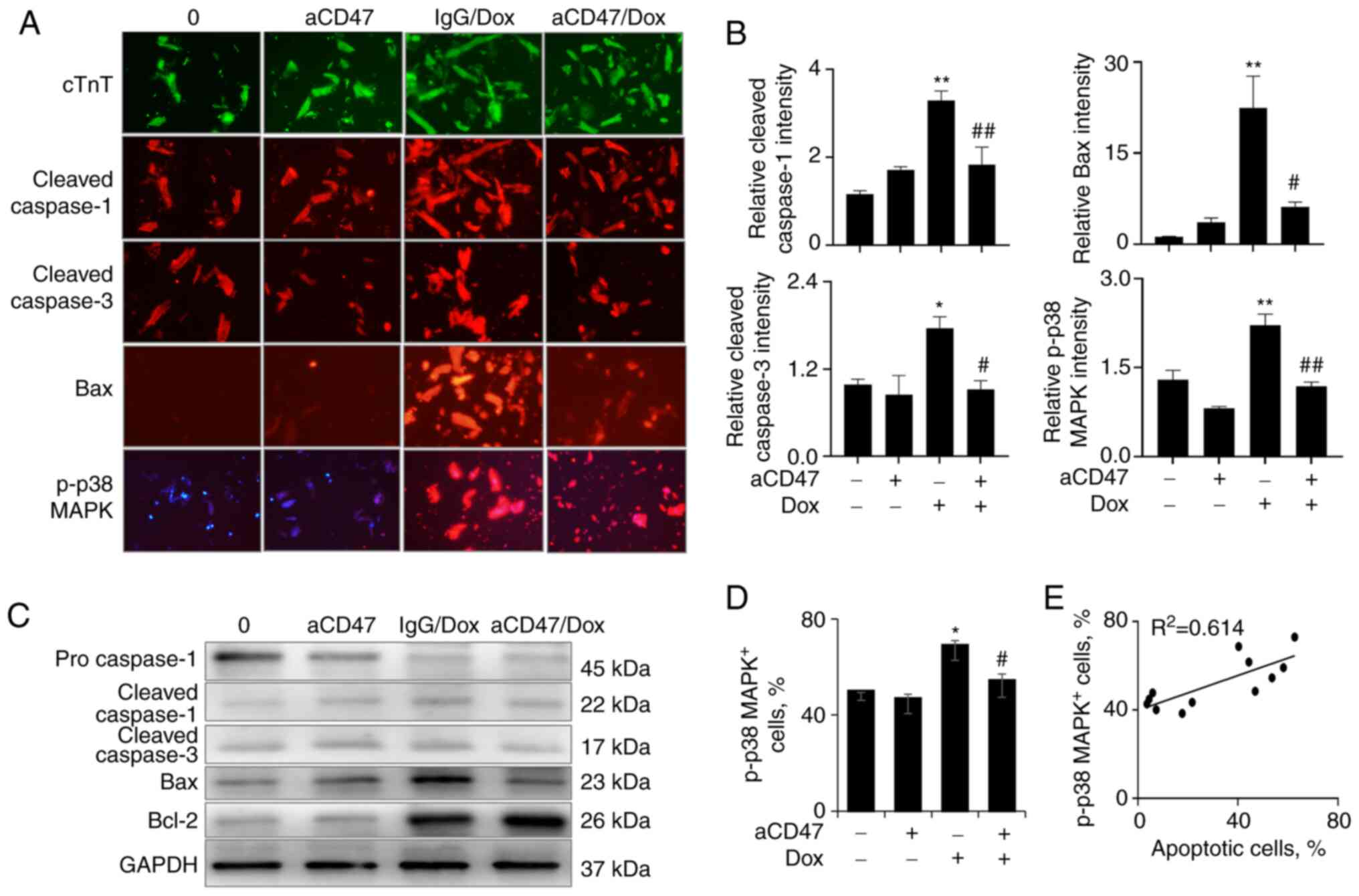 | Figure 5aCD47 reduces the expression of Bax
and p-p38 MAPK in Dox-treated cardiomyocytes. (A) Immunostaining
for the expression of cTnT, cleaved caspase-1/3, Bax and p-p38 MAPK
in the treated cardiomyocytes. Cardiomyocytes were identified as
cTnT-positive cells (green). Red, cells positively stained for
cleaved caspase-1/3, Bax and p-p38 MAPK. Representative images with
x200 magnification. (B) Semi-quantitative analysis of positively
stained cells by ImageJ software. Data are presented as the
relative intensity of positively stained cells over untreated
controls. (C) Western blot analysis for the expression of
pro-caspase-1, cleaved caspase-1, cleaved caspase-3, Bax and Bcl-2
in the treated cardiomyocytes. GAPDH was internal loading control.
One representative blot. (D) p-p38 MAPK+ cells after Dox
and aCD47 treatment were analyzed by flow cytometry. Data are
presented as the percentage of p-p38 MAPK+ cells. (E)
Association between the percentage of p-p38 MAPK+ cells
and apoptotic cardiomyocytes after treatment. Bar plot data in all
panels are represented as mean ± standard error. n=3.
*P<0.05, **P<0.01 vs. 0 group;
#P<0.05, ##P<0.01 vs. IgG/Dox group.
Two-way ANOVA followed by a Tukey's multiple comparison's test.
aCD47, anti-CD47 neutralizing antibody; Dox, doxorubicin; p-,
phosphorylated; cTnT, cardiac troponin T. |
To further investigate the downstream signaling
pathway of aCD47-mediated suppression of cardiomyocyte early
apoptosis, the activation of p38 MAPK in the treated cells was
further measured. The results by immunostaining [Fig. 5A (lower panel) and B (right lower
panel)] indicated that Dox significantly increased p-p38 MAPK,
which was significantly reversed by co-treatment with aCD47 in the
aCD47/Dox group. Further analysis by flow cytometry confirmed the
attenuated percentage of p-38 MAPK+ cells in aCD47/Dox
co-treated cells, compared to Dox alone-treated controls (Fig. 5D). There was a positive association
between the percentage of p-p38 MAPK+ cells and
cardiomyocyte early apoptosis (Fig.
5E). These results indicate that p38 MAPK signaling was
involved in the aCD47-mediated protection against Dox-induced
cardiomyocyte early apoptosis.
Discussion
CD47 is expressed at low levels in normal human
tissues. However, recent studies have shown that the expression of
CD47 is increased in atherosclerosis (29), pathogen-infected cells (30) and tumors (18,31).
The upregulated expression of CD47 on cells can interact with
signal regulatory protein-a (SIRP-a) on macrophages or natural
killer cells, subsequently suppressing phagocytosis of macrophages
and reducing the clearance of apoptotic cells, dead cells and tumor
cells. Therefore, targeting CD47 is a promising therapeutic
approach to improving tissue repair and inflammation resolution. It
was recently reported that blocking CD47 activity by aCD47 or CD47
deficiency effectively suppressed isoproterenol-induced cardiac
hypertrophy and protected cardiomyocytes from
hypoxia/reoxygenation-induced cardiac injury by suppressing cell
apoptosis, as well as improving autophagic flux and autophagic
clearance (23,25).
Consistent with the upregulated CD47 expression in
atherosclerosis and pulmonary hypertension (21,29,32),
the results of the present study showed that CD47 expression was
increased in the mice with Dox-induced dilated cardiomyopathy
(DCM), suggesting the possible involvement of CD47 in the
pathogenesis of murine DCM.
To further define the role of CD47 in the
development of DCM, CD47 activity was blocked by i.p.
administration of aCD47 to murine models of DCM for 4 weeks. The
results revealed that aCD47 significantly reduced the severity of
murine DCM, as evidenced by the reduced destruction of cardiac
myofibers and formation of interstitial fibrosis in the myocardium.
The beneficial effects were accompanied by reduced infiltration of
macrophages and cardiac myofiber early apoptosis, as compared with
the murine DCM treated with IgG isotype control. In addition, LDH
release and the expression of pro-inflammatory cytokines, including
IL-6 and TNF-α, were significantly reduced in aCD47-treated mice,
confirming the anti-inflammatory effect of aCD47 in murine DCM. The
reduced infiltration of inflammatory cells may contribute to the
lower expression of pro-inflammatory cytokines and mediators in
vivo. Supporting the anti-apoptotic and anti-inflammatory role
of aCD47 in murine DCM, similar beneficial effects have also been
observed in other animal models, such as vascular inflammation
(33), isoproterenol-induced heart
hypertrophy (25),
hypoxia/reoxygenation injury (23)
and allograft transplantation (20). Therefore, CD47 is a promising
target in the treatment of cardiovascular diseases. As there were
no obvious effects of aCD47 on cardiac histology and myofiber
apoptosis in the aCD47 alone-treated mice, it may be inferred that
there are no toxic effects associated with the potential use of
aCD47 in humans in the future. However, it should be noted that the
study was limited by lack of time-point study for aCD47 therapeutic
effects in vivo. In addition, an actual tumor model should
be used in future study, as the tumor microenvironment may affect
the therapeutic effects of aCD47 in vivo. A
cardiomyocyte-specific CD47 knockout mouse model may be used to
further dissect the role and underlying molecular mechanisms of
CD47 in the pathogenesis of murine DCM.
At present, it remains unknown whether aCD47
suppresses DCM through directly targeting cardiomyocytes or
indirectly suppressing the immune responses. To further address
this issue, an in vitro experiment was performed in the
current study, in which primary cardiomyocytes were treated with
Dox and aCD47, alone or in combination. Consistent with the in
vivo results, the increased cardiomyocyte early apoptosis and
LDH release were observed after Dox treatment. However, aCD47
pre-treatment effectively reversed Dox-induced cardiomyocyte early
apoptosis and LDH release, confirming the direct targeting of aCD47
on cardiomyocytes. Thus, Dox treatment upregulated the expression
of CD47 and participated in the pathogenesis of murine DCM, and the
detrimental effects were attenuated by blocking CD47 signaling with
aCD47. Further analysis also indicated that Dox effectively
upregulated the expression of both pro-apoptotic Bax and
anti-apoptotic Bcl-2. However, Bax, but not Bcl-2, was effectively
reversed by aCD47 pre-treatment in vitro, indicating the
direct anti-apoptotic effect of aCD47 in Dox-treated
cardiomyocytes. These results were consistent with previously
reported results, in which cocaine treatment upregulated the
expression of both Bcl-2 and Bax protein, with an increased ratio
of Bax/Bcl-2 in neuronal cells (34). Thus, we speculate that the
increased expression of Bcl-2 may play a protective feedback role
in Dox-induced cardiomyocyte early apoptosis and aCD47 may protect
cardiomyocytes from early apoptosis via increasing the ratio of
Bcl-2/Bax.
In addition, the present study showed the increased
phosphorylation of p38 MAPK in Dox-treated cardiomyocytes, but
aCD47 pre-treatment effectively reduced the activation of p38 MAPK.
As p38 MAPK signaling is associated with cardiomyocyte activation
and apoptosis as previously reported (3,35-37),
we concluded that aCD47 may suppress cardiomyocyte early apoptosis
through blocking CD47 downstream p38 MAPK signaling pathway, and
subsequently reducing the expression of pro-apoptotic protein Bax
in cardiomyocytes.
It should not be excluded that aCD47 may target CD47
on other cell types, such as immune, vascular, epithelial and stem
cells, improving angiogenesis and renal tubular epithelial cell
renewal (12,13). For example, CD47 was highly
expressed in animal models of pulmonary hypertension (PH), and
blockade of CD47 signaling by CD47 antibody was able to effectively
attenuate PH-associated cardiopulmonary pathological changes and
enhance cell renewal (21). Thus,
it was hypothesized that the improved cardiomyocyte renewal may be
involved in the protective effects of aCD47 in murine DCM. It will
be investigated further in the future.
Apoptotic and dead cells prevent tissue repair by
stimulating local tissue inflammation. Effective clearance of
apoptotic and dead cells may facilitate local inflammation
resolution and tissue repair. It was previously reported that
blockade of CD47 signaling effectively reduced atherosclerosis
through improving the clearance of diseased vascular tissues
(29,38). Therefore, it is hypothesized that
aCD47 effectively attenuated DCM, possibly through improving the
clearance of apoptotic cardiac myofibers and inflammatory cells in
the heart tissues of murine DCM.
Taken together, the findings of the in vivo
and in vitro experiments performed in the present study
provide evidence that aCD47 attenuated DCM in mice, by suppressing
cardiac myofiber early apoptosis and the p38 MAPK signaling
pathway. CD47 may be a useful therapeutic target for Dox-induced
DCM.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by a research grant from the
Natural Science Foundation of Shanghai (19ZR1409000) to ZLJ.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH designed and performed the experiments. LC
generated the hypothesis and designed the study. ZJ was responsible
for generating the hypothesis, performing the experiments, writing
the manuscript and was responsible for all directions of the work.
All authors confirm the authenticity of all the raw data. All
authors read and approved the final manuscript for publication.
Ethics approval and consent to
participate
The study protocol was approved by the Animal
Experimental Ethics Committee of Zhongshan Hospital, Fudan
University (approval number 20130039; 26/02/2020).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu Y, Zhang W, Hu T, Ni J, Xu B and Huang
W: A doxorubicin-induced murine model of dilated cardiomyopathy in
vivo. J Vis Exp: May 16, 2020 (Epub ahead of print). doi:
10.3791/61158.
|
|
2
|
Rocca C, Scavello F, Colombo B, Gasparri
AM, Dallatomasina A, Granieri MC, Amelio D, Pasqua T, Cerra MC,
Tota B, et al: Physiological levels of chromogranin A prevent
doxorubicin-induced cardiotoxicity without impairing its anticancer
activity. FASEB J. 33:7734–7747. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang S, Ding L, Ji H, Xu Z, Liu Q and
Zheng Y: The role of p38 MAPK in the development of diabetic
cardiomyopathy. Int J Mol Sci. 17(1037)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kankeu C, Clarke K, Passante E and Huber
HJ: Doxorubicin-induced chronic dilated cardiomyopathy-the
apoptosis hypothesis revisited. J Mol Med (Berl). 95:239–248.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cheng X, Liu D, Xing R, Song H, Tian X,
Yan C and Han Y: Orosomucoid 1 attenuates doxorubicin-induced
oxidative stress and apoptosis in cardiomyocytes via Nrf2
signaling. Biomed Res Int. 2020(5923572)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Song T, Yao Y, Wang T, Huang H and Xia H:
Tanshinone IIA ameliorates apoptosis of myocardiocytes by
up-regulation of miR-133 and suppression of caspase-9. Eur J
Pharmacol. 815:343–350. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yao Y, Xu X, Zhang G, Zhang Y, Qian W and
Rui T: Role of HMGB1 in doxorubicin-induced myocardial apoptosis
and its regulation pathway. Basic Res Cardiol.
107(267)2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zeng C, Duan F, Hu J, Luo B, Huang B, Lou
X, Sun X, Li H, Zhang X, Yin S and Tan H: NLRP3
inflammasome-mediated pyroptosis contributes to the pathogenesis of
non-ischemic dilated cardiomyopathy. Redox Biol.
34(101523)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li J, Wang PY, Long NA, Zhuang J, Springer
DA, Zou J, Lin Y, Bleck CKE, Park JH, Kang JG and Hwang PM: p53
prevents doxorubicin cardiotoxicity independently of its
prototypical tumor suppressor activities. Proc Natl Acad Sci USA.
116:19626–19634. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mizutani H, Tada-Oikawa S, Hiraku Y,
Kojima M and Kawanishi S: Mechanism of apoptosis induced by
doxorubicin through the generation of hydrogen peroxide. Life Sci.
76:1439–1453. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Reeve JL, Szegezdi E, Logue SE, Ní
Chonghaile T, O'Brien T, Ritter T and Samali A: Distinct mechanisms
of cardiomyocyte apoptosis induced by doxorubicin and hypoxia
converge on mitochondria and are inhibited by Bcl-xL. J Cell Mol
Med. 11:509–520. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rogers NM, Zhang ZJ, Wang JJ, Thomson AW
and Isenberg JS: CD47 regulates renal tubular epithelial cell
self-renewal and proliferation following renal ischemia
reperfusion. Kidney Int. 90:334–347. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ghimire K, Li Y, Chiba T, Julovi SM, Li J,
Ross MA, Straub AC, O'Connell PJ, Rüegg C, Pagano PJ, et al: CD47
promotes age-associated deterioration in angiogenesis, blood flow
and glucose homeostasis. Cells. 9(1695)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kaur S, Bronson SM, Pal-Nath D, Miller TW,
Soto-Pantoja DR and Roberts DD: Functions of thrombospondin-1 in
the tumor microenvironment. Int J Mol Sci. 22(4570)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rath GM, Schneider C, Dedieu S, Rothhut B,
Soula-Rothhut M, Ghoneim C, Sid B, Morjani H, El Btaouri H and
Martiny L: The C-terminal CD47/IAP-binding domain of
thrombospondin-1 prevents camptothecin- and doxorubicin-induced
apoptosis in human thyroid carcinoma cells. Biochim Biophys Acta.
1763:1125–1134. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Feliz-Mosquea YR, Christensen AA, Wilson
AS, Westwood B, Varagic J, Meléndez GC, Schwartz AL, Chen QR,
Mathews Griner L, Guha R, et al: Combination of anthracyclines and
anti-CD47 therapy inhibit invasive breast cancer growth while
preventing cardiac toxicity by regulation of autophagy. Breast
Cancer Res Treat. 172:69–82. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lo J, Lau EY, So FT, Lu P, Chan VS, Cheung
VC, Ching RH, Cheng BY, Ma MK, Ng IO and Lee TK: Anti-CD47 antibody
suppresses tumour growth and augments the effect of chemotherapy
treatment in hepatocellular carcinoma. Liver Int. 36:737–745.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tong B and Wang M: CD47 is a novel potent
immunotherapy target in human malignancies: Current studies and
future promises. Future Oncol. 14:2179–2188. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Veillette A and Chen J: SIRPα-CD47 immune
checkpoint blockade in anticancer therapy. Trends Immunol.
39:173–184. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen M, Wang Y, Wang H, Sun L, Fu Y and
Yang YG: Elimination of donor CD47 protects against vascularized
allograft rejection in mice. Xenotransplantation.
26(e12459)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rogers NM, Sharifi-Sanjani M, Yao M,
Ghimire K, Bienes-Martinez R, Mutchler SM, Knupp HE, Baust J,
Novelli EM, Ross M, et al: TSP1-CD47 signaling is upregulated in
clinical pulmonary hypertension and contributes to pulmonary
arterial vasculopathy and dysfunction. Cardiovasc Res. 113:15–29.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sharifi-Sanjani M, Shoushtari AH, Quiroz
M, Baust J, Sestito SF, Mosher M, Ross M, McTiernan CF, St Croix
CM, Bilonick RA, et al: Cardiac CD47 drives left ventricular heart
failure through Ca2+-CaMKII-regulated induction of
HDAC3. J Am Heart Assoc. 3(e000670)2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li Y, Zhao K, Zong P, Fu H, Zheng Y, Bao
D, Yin Y, Chen Q, Lu L, Dai Y, et al: CD47 deficiency protects
cardiomyocytes against hypoxia/reoxygenation injury by rescuing
autophagic clearance. Mol Med Rep. 19:5453–5463. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang HB and Yang J, Ding JW, Chen LH, Li
S, Liu XW, Yang CJ, Fan ZX and Yang J: RNAi-mediated
down-regulation of CD47 protects against
ischemia/reperfusion-induced myocardial damage via activation of
eNOS in a rat model. Cell Physiol Biochem. 40:1163–1174.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li Y, Chen X, Li P, Xiao Q, Hou D and Kong
X: CD47 antibody suppresses isoproterenol-induced cardiac
hypertrophy through activation of autophagy. Am J Transl Res.
12:5908–5923. 2020.PubMed/NCBI
|
|
26
|
Li M, Sala V, De Santis MC, Cimino J,
Cappello P, Pianca N, Di Bona A, Margaria JP, Martini M, Lazzarini
E, et al: Phosphoinositide 3-kinase gamma inhibition protects from
anthracycline cardiotoxicity and reduces tumor growth. Circulation.
138:696–711. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hu C, Zhang X, Wei W, Zhang N, Wu H, Ma Z,
Li L, Deng W and Tang Q: Matrine attenuates oxidative stress and
cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via
maintaining AMPKα/UCP2 pathway. Acta Pharm Sin B. 9:690–701.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rui T, Cepinskas G, Feng Q and Kvietys PR:
Delayed preconditioning in cardiac myocytes with respect to
development of a proinflammatory phenotype: Role of SOD and NOS.
Cardiovasc Res. 59:901–911. 2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kojima Y, Volkmer JP, McKenna K, Civelek
M, Lusis AJ, Miller CL, Direnzo D, Nanda V, Ye J, Connolly AJ, et
al: CD47-blocking antibodies restore phagocytosis and prevent
atherosclerosis. Nature. 536:86–90. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tal MC, Torrez Dulgeroff LB, Myers L, Cham
LB, Mayer-Barber KD, Bohrer AC, Castro E, Yiu YY, Lopez Angel C,
Pham E, et al: Upregulation of CD47 is a host checkpoint response
to pathogen recognition. mBio. 11:e01293–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Deuse T, Hu X, Agbor-Enoh S, Jang MK,
Alawi M, Saygi C, Gravina A, Tediashvili G, Nguyen VQ, Liu Y, et
al: The SIRPα-CD47 immune checkpoint in NK cells. J Exp Med.
218(e20200839)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Novelli EM, Little-Ihrig L, Knupp HE,
Rogers NM, Yao M, Baust JJ, Meijles D, St Croix CM, Ross MA, Pagano
PJ, et al: Vascular TSP1-CD47 signaling promotes sickle
cell-associated arterial vasculopathy and pulmonary hypertension in
mice. Am J Physiol Lung Cell Mol Physiol. 316:L1150–L1164.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jarr KU, Nakamoto R, Doan BH, Kojima Y,
Weissman IL, Advani RH, Iagaru A and Leeper NJ: Effect of CD47
blockade on vascular inflammation. N Engl J Med. 384:382–383.
2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xiao D and Zhang L: Upregulation of Bax
and Bcl-2 following prenatal cocaine exposure induces apoptosis in
fetal rat brain. Int J Med Sci. 5:295–302. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cao Y, Ruan Y, Shen T, Huang X, Li M, Yu
W, Zhu Y, Man Y, Wang S and Li J: Astragalus polysaccharide
suppresses doxorubicin-induced cardiotoxicity by regulating the
PI3k/Akt and p38MAPK pathways. Oxid Med Cell Longev.
2014(674219)2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xuan T, Wang D, Lv J, Pan Z, Fang J, Xiang
Y, Cheng H, Wang X and Guo X: Downregulation of Cypher induces
apoptosis in cardiomyocytes via Akt/p38 MAPK signaling pathway. Int
J Med Sci. 17:2328–2337. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zuo G, Ren X, Qian X, Ye P, Luo J, Gao X,
Zhang J and Chen S: Inhibition of JNK and p38 MAPK-mediated
inflammation and apoptosis by ivabradine improves cardiac function
in streptozotocin-induced diabetic cardiomyopathy. J Cell Physiol.
234:1925–1936. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gerlach BD, Marinello M, Heinz J, Rymut N,
Sansbury BE, Riley CO, Sadhu S, Hosseini Z, Kojima Y, Tang DD, et
al: Resolvin D1 promotes the targeting and clearance of necroptotic
cells. Cell Death Differ. 27:525–539. 2020.PubMed/NCBI View Article : Google Scholar
|