Introduction
Chronic obstructive pulmonary disease (COPD) is
characterized by persistent airflow limitation to and from the
lung, which has become a growing global health issue (1,2). In
addition, air pollution and genetic factors are important risk
factors for COPD (3-6).
However, currently available therapeutic options for COPD,
including methylxanthines, β2-agonists, anticholinergics,
corticosteroids and phosphodiesterase 4 inhibitors, were not able
to completely halt the progression of COPD pathogenesis nor reduce
the mortality rate (7-9).
Therefore, there remains to be an urgent demand to explore novel
treatment strategies to prevent the development of COPD.
Although the pathogenesis of COPD has not been fully
elucidated, it has been proposed that epithelial-mesenchymal
transition (EMT) and fibrogenesis may be involved (10,11).
During EMT, epithelial cells gradually lose their epithelial
phenotype and acquire typical mesenchymal characteristics (12-15).
These are characterized by enhanced mitotic ability and
extracellular matrix production, accompanied by the upregulation of
N-cadherin, slug and α-SMA expression and the downregulation of
E-cadherin expression (12-15).
The main pathological changes that occur during fibrosis are
increases in fibrous connective tissue generation in the organ
tissue, decreases in parenchymal cells (16). Continuous EMT and fibrosis
progression can lead to the destruction of organ structure and
functional decline (17). This
serves to be one of the main mechanism of small airway narrowing
and is now considered to be the most important mechanism of COPD
progression (18). Epithelial cell
fibrogenesis process is frequently accompanied with upregulation of
fibrogenesis-related protein expression, such as collagen IV and
fibronectin 1 (FN1) (19).
Previous studies have provided evidence that EMT is active in the
airway of smokers, particularly in patients with COPD who smoke,
suggesting that smoking-induced EMT can contribute to the
pathogenesis of COPD (15,20).
Patients with COPD and acute obstructive pulmonary
disease were frequently found to have vitamin D deficiency
(21). Vitamin D (calcitonin)
deficiency was reported to be positively associated with the
severity of COPD (21). In
addition, patients with COPD or more severe disease were found to
have lower serum vitamin D levels (22). A previous study has suggested that
treatment with vitamin D in patients with COPD and vitamin D
deficiency can reduce the risk of moderate-to-severe disease
(23). Furthermore, a recent study
on patients with COPD caused by COVID-19 showed that although
vitamin D deficiency is not a determinant of disease severity,
supplementation does confer a positive role in alleviation
(24). Cigarette smoke extract
(CSE) can inhibit vitamin D-induced vitamin D receptor (VDR)
translocation (25). Mathyssen
et al (26) previously
documented that the use of vitamin D supplements may reduce
CSE-mediated cellular inflammatory responses by upregulating
cathelicidin expression in bronchial epithelial cells. However, the
underlying molecular mechanism remain unknown (26). In another previous study, although
vitamin D deficiency exacerbated pulmonary fibrosis and EMT
(27), no significant associations
between the two conditions could be found. Vitamin supplements have
been found to delay the process of fibrosis (28). However, the role of vitamins in
CSE-induced fibrogenesis in bronchial epithelial cells remains
unclear.
Club cell protein 16 (CC16) is the most abundant
protein in the bronchoalveolar lavage fluid (29,30).
It is encoded by the secretoglobin family 1A member 1 gene
and serves an anti-inflammatory and antioxidant role in vivo
(29,30). Previous studies have shown that
CC16 mainly exerts anti-inflammatory effects in smoke-exposed
lungs, such that COPD has been associated with CC16 deficiency
(31,32). Smokers and patients with COPD were
found to exhibit reduced airway CC16 immunostaining, which
decreased further with increasing COPD severity (31-33).
Exposing mice to CSE was found to reduce the airway expression of
CC16(34). Therefore, CC16 is
becoming of interest as a target molecule for the treatment of
COPD. CC16 has been associated with the occurrence and development
of COPD, with the severity and prognosis of the disease (34). The dynamic changes in CC16
expression can be used to predict changes in the condition of
patients with COPD and to evaluate the clinical outcome. Combined
with the comprehensive analysis of other common clinical
indicators, the length of the stay in hospital can be shortened as
analyzed by linear correlation analysis and multiple linear
regression analysis (33,35,36).
Therefore, the present study hypothesized that CC16 is associated
with lung fibrogenesis during COPD. However, the effects of vitamin
D3 on the expression of CC16 after CSE exposure and its underlying
molecular mechanism remain unclear.
In COPD, the bronchial epithelium is the first
immune barrier triggered by cigarette smoke (37). The present study aimed to
investigate the effect of vitamin D3 supplementation on EMT and
fibrogenesis in bronchial epithelial cells after CSE treatment. In
addition, the role of CC16 in these processes and the expression of
EMT and fibrogenesis-related markers were detected.
Materials and methods
Bioinformatics Methods
The interaction between CC16 and FN1 was analyzed
using the Search Tool for the Retrieval of Interacting
Genes/Proteins (STRING) database (version 11.5; https://string-db.org/cgi/input.pl).
Bronchial epithelial cell culture
The 16-HBE cells were purchased from the American
Type Culture Collection and were cultured in DMEM (Sigma-Aldrich;
Merck KGaA) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin G sodium and 100 µg/ml
streptomycin sulfate (Invitrogen; Thermo Fisher Scientific, Inc.).
They were cultured at 37˚C in a humidified incubator with 5%
CO2. The cells were cultured until they reached 70-80%
confluence.
CC16 short hairpin (sh) RNA
The pLentiLox 3.7 lentiviral plasmid shRNAs
targeting CC16 and scramble control shRNA were purchased from
Hanbio Biotechnology Co., Ltd. The cells were seeded into a 96-well
plate for 24 h at 37˚C until 70-80% confluence, before the cells
were transfected with the shRNAs (50 nM) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols. The
efficiency of transfection was determined using reverse
transcription-quantitative PCR (RT-qPCR) and western blot analyzes
after 48 h of transfection. The sequences were as follows:
ShRNA-CC16-1, sense 5'-CCAGAGAAAGCATCATTAA-3', antisense
5'-TTAATGATGCTTTCTCTGG-3'; shRNA-CC16-2, sense
5'-CCCAAAGCTCACTGTGTAA-3', antisense 5'-TTACACAGTGAGCTTTGGG-3';
shRNA-NC, sense 5'-GATCCCCTTCTCCGAACG-3', antisense
5'-AGCTAAAAATTCTCCGAAC-3'.
Preparation of CSE
The smoke from 15 lit cigarettes (Sichuan China
Tobacco Industry Co., Ltd.) was slowly inhaled into a 50 ml
syringe, and then injected it into DMEM pre-heated in a 37˚C water
bath (38). The pH of DMEM was
adjusted to 7.4 and sterilized using a 0.22-µm filter (EMD
Millipore) and stored at -80˚C. Serum-free DMEM was used to dilute
100% CSE to the required CSE concentrations (5, 10 and 20%). Cells
were treated with vitamin D3 (250, 500 or 1,000 nM; Sigma-Aldrich;
Merck KGaA) or vehicle (0.1% ethanol) for 30 min prior to 24 h
treatment at 37˚C with CSE and vitamin D3 or vehicle, after shRNA
transfection (when it was required) (26).
Cell Counting Kit (CCK)-8 assay
Cell viability was performed using CCK-8 assay. A
total of 1x104 16-HBE cells/well were seeded into
96-well plates and pre-treated with CSE followed by vitamin D3. In
total, 10 µl CCK-8 reagent (Abcam) was added into each well and the
samples were incubated at 37˚C for 1-4 h. Subsequently, the
absorbance in each well was measured at 460 nm using a microplate
spectrophotometer (Bio-Rad Laboratories, Inc.).
RT-qPCR
Total RNA was extracted from the cells using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.).
The concentration was assessed using a Nanodrop® 2000
spectrophotometer (Thermo Fisher Scientific, Inc.) before being
reverse transcribed into cDNA. cDNA was synthesized using the
PrimeScript™ RT kit (cat. no. RR037A; Takara Bio, Inc.) under the
following conditions: 15 min at 37˚C and 5 sec at 85˚C. The
reactions were performed using SYBR Green Taq Mix (cat. no. RR096A;
Takara Bio, Inc.) in a StepOnePlus™ PCR system (Thermo Fisher
Scientific, Inc.) under the following conditions: 45˚C for 3 min,
95˚C for 10 sec, 40 cycles at 95˚C for 15 sec then 58˚C for 1 min.
The primer sequences used were as follows: CC16 forward,
5'-CAGAGACGGAACCAGAGACG-3' and reverse, 5'-CAGATCTCTGCAGAAGCGGA-3'
and GAPDH forward, 5'-GGAGCGAGATCCCTCCAAAAT-3' and reverse,
5'-GGCTGTTGTCATACTTCTCATGG-3'. Gene expression was evaluated using
the 2-ΔΔCq method (39)
using GAPDH as a reference gene.
Western blot analysis
The total protein was extracted from the cells using
RIPA lysate buffer (Beyotime Institute of Biotechnology) containing
protease inhibitors, phosphatase inhibitors and
phenylmethylsulfonyl fluoride (all from Beyotime Institute of
Biotechnology). The protein concentration was determined using a
BCA protein determination kit (Beyotime Institute of
Biotechnology). A total of 30 µg protein per lane extract was
separated using 6-12% SDS-PAGE and transferred onto PVDF membranes.
Subsequently, the PVDF membranes were blocked with TBS containing
5% BSA (Sigma-Aldrich; Merck KGaA) for 2 h at room temperature,
before being incubated overnight at 4˚C with the following primary
antibodies: E-cadherin (1:1,000; cat. no. 14472; Cell Signaling
Technology, Inc.), N-cadherin (1:1,000; cat. no. 13116; Cell
Signaling Technology, Inc.), slug (1:1,000; cat. no. 9585; Cell
Signaling Technology, Inc.), α-smooth muscle actin (1:2,000; α-SMA;
cat. no. NBP2-78836; Novus Biologicals, LLC), collagen Ⅳ (1:1,000;
cat. no. ab6586; Abcam), FN1 (1:1,000; cat. no. 26836; Cell
Signaling Technology, Inc.), TGF-β1 (1:2,000; cat. no. sc-130348;
Santa Cruz Biotechnology, Inc.), SMAD3 (1:1,000; cat. no. 9523;
Cell Signaling Technology, Inc.), phosphorylated (p-) SMAD3
(1:1,000; cat. no. 9520; Cell Signaling Technology, Inc.) and CC16
(5 µg/ml; cat. no. RD181022220-01; BioVender). The membranes were
then incubated with the goat anti-mouse IgG H&L HRP-conjugated
secondary antibodies (1:20,000; cat. no. ab205719; Abcam) and goat
anti-rabbit IgG H&L (1:50,000; cat. no. ab205718; Abcam) at
room temperature for 2 h. Afterwards, they were washed for 25 min
with TBS-0.1% Tween-20. After using an enhanced chemiluminescence
reagent (Thermo Fisher Scientific, Inc.), the protein bands were
detected using a Bio-Rad Imaging system (Bio-Rad Laboratories,
Inc.) and analyzed using ImageJ software (version 1.43; National
Institutes of Health).
Immunofluorescence staining
The cells were fixed in 4% paraformaldehyde
(Beyotime Institute of Biotechnology) for 1 h at room temperature,
washed in PBS and blocked for 15 min in QuickBlock™ Blocking buffer
for immunol staining (Beyotime Institute of Biotechnology) at room
temperature. The samples were then incubated with primary
antibodies overnight at 4˚C. The following primary antibodies used
targeted E-cadherin (1:200; cat. no. 14472; Cell Signaling
Technology, Inc.), N-cadherin (1:200; cat. no. 13116; Cell
Signaling Technology, Inc.), collagen Ⅳ (1:500; cat. no. ab6586;
Abcam). Subsequently, the samples were incubated with corresponding
Alexa Fluor® 488-conjugated secondary antibodies (Goat
Anti-Rabbit IgG H&L; 1:1,000; cat. no. 150077; Abcam and Goat
Anti-Mouse IgG H&L; 1:1,000; cat. no. 150113; Abcam) for 1 h at
room temperature after washing with PBS. Then these samples were
incubated for 5 min at room temperature with DAPI (1 µg/ml; cat.
no. D1306; Invitrogen; Thermo Fisher Scientific, Inc.), and
observations were performed using a fluorescence microscope
(magnification, x100; Olympus Corporation) and were analyzed with
ImageJ version 1.43 software.
Statistical analysis
Statistical analysis was performed using GraphPad
8.0 (GraphPad Software, Inc.). Data were expressed as means ± SEM
of three independent experiments. Comparisons were performed using
one-way analysis of variance with Tukey-Kramer post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
CES reduces cell viability and induces
EMT
After treating the 16-HBE cells with different
concentrations of CSE (5, 10 and 20%) for 24 h, cell viability was
decreased in a dose-dependent manner, comparing with that in the
control group (Fig. 1A). CSE at a
concentration of 20% was used for subsequent experiments. Western
blot analysis revealed that the protein expression level of
E-cadherin was decreased, whilst the protein expression levels of
N-cadherin, slug and α-SMA were increased, in a dose-dependent
manner following CSE treatment (Fig.
1B and C). The results of
immunofluorescence were consistent with those in the western blot
analysis results, revealing a decrease in E-cadherin protein
expression and an increase in N-cadherin protein expression
compared with those in the control group (Fig. 1D). These results suggest that CSE
promoted the transformation of these 16-HBE epithelial cells into
mesenchymal-like cells.
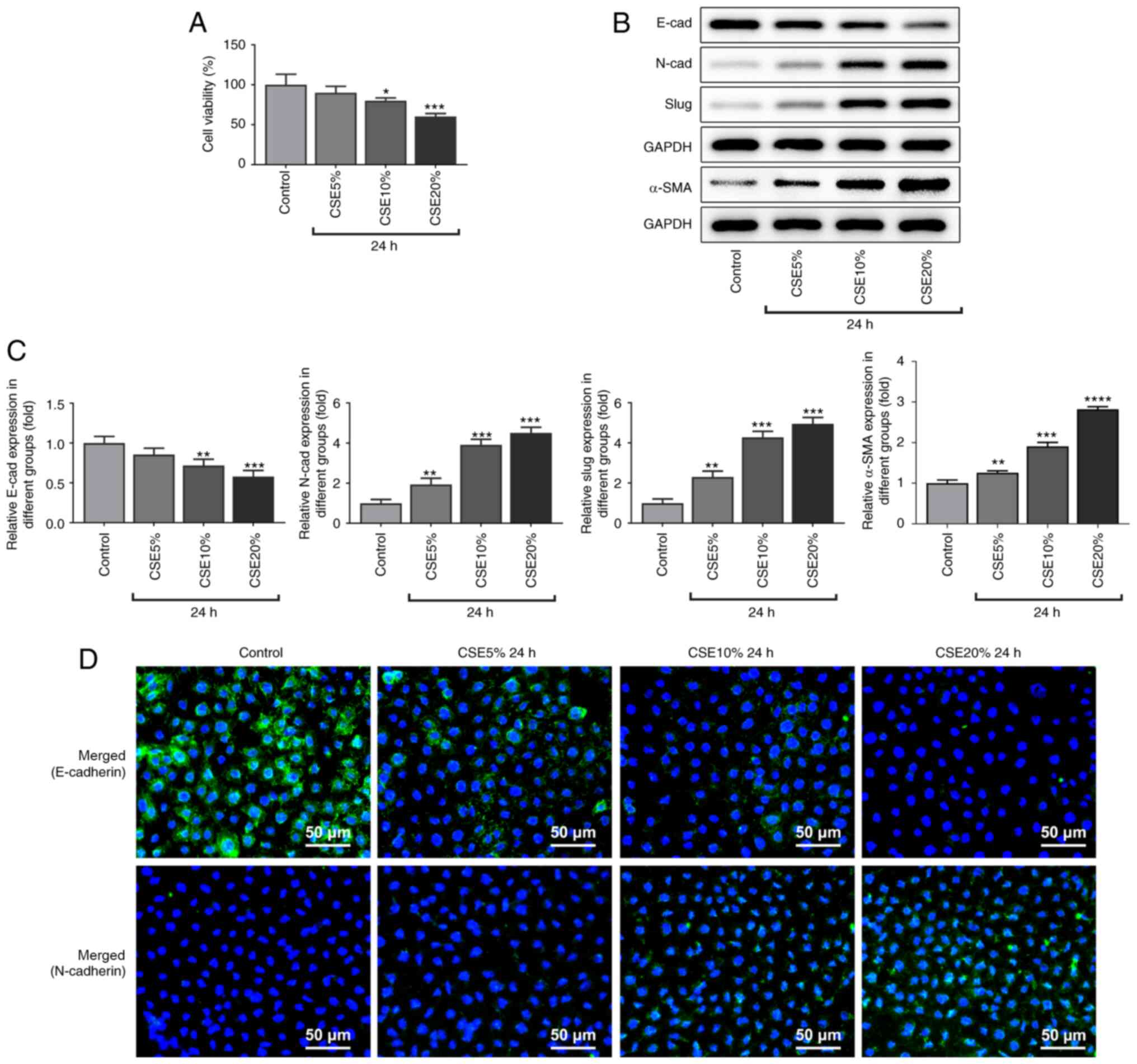 | Figure 1Protein expression levels of
E-cadherin, N-cadherin, slug and α-SMA and the viability of 16-HBE
cells following treatment with CSE. (A) Cell viability. (B) Protein
expression levels of E-cadherin, N-cadherin, slug and α-SMA were
measured by western blotting, (C) which were quantified. (D)
Immunofluorescence staining of E-cadherin and N-cadherin. Scale
bar, 50 µm. *P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001 vs.
Control. CSE, cigarette smoke extract; α-SMA, α-smooth muscle
actin. |
CSE induces fibrogenesis in 16-HBE
cells
It was first found that CC16 interacted with FN1,
which is associated with fibrogenesis (40), from analysis using the STRING
database (Fig. 2A). Therefore,
western blot analysis was used to measure the protein expression
level of fibrogenesis-related proteins. Compared with that in the
control group, the protein expression level of CC16 in 16-HBE cells
were decreased following CSE treatment. Furthermore, the protein
expression levels of the fibrogenesis-related proteins collagen IV
and FN1, in addition to those in the TGF-β1/SMAD3 signaling
pathway, were increased in the CSE treatment groups in a
dose-dependent manner compared with that in the control group
(Fig. 2B). Immunofluorescence
results also showed that the expression level of collagen IV was
also increased in the CSE treatment group compared with that in the
control group (Fig. 2C). In
summary, these results suggest that CSE induced fibrogenesis in the
16-HBE cell line.
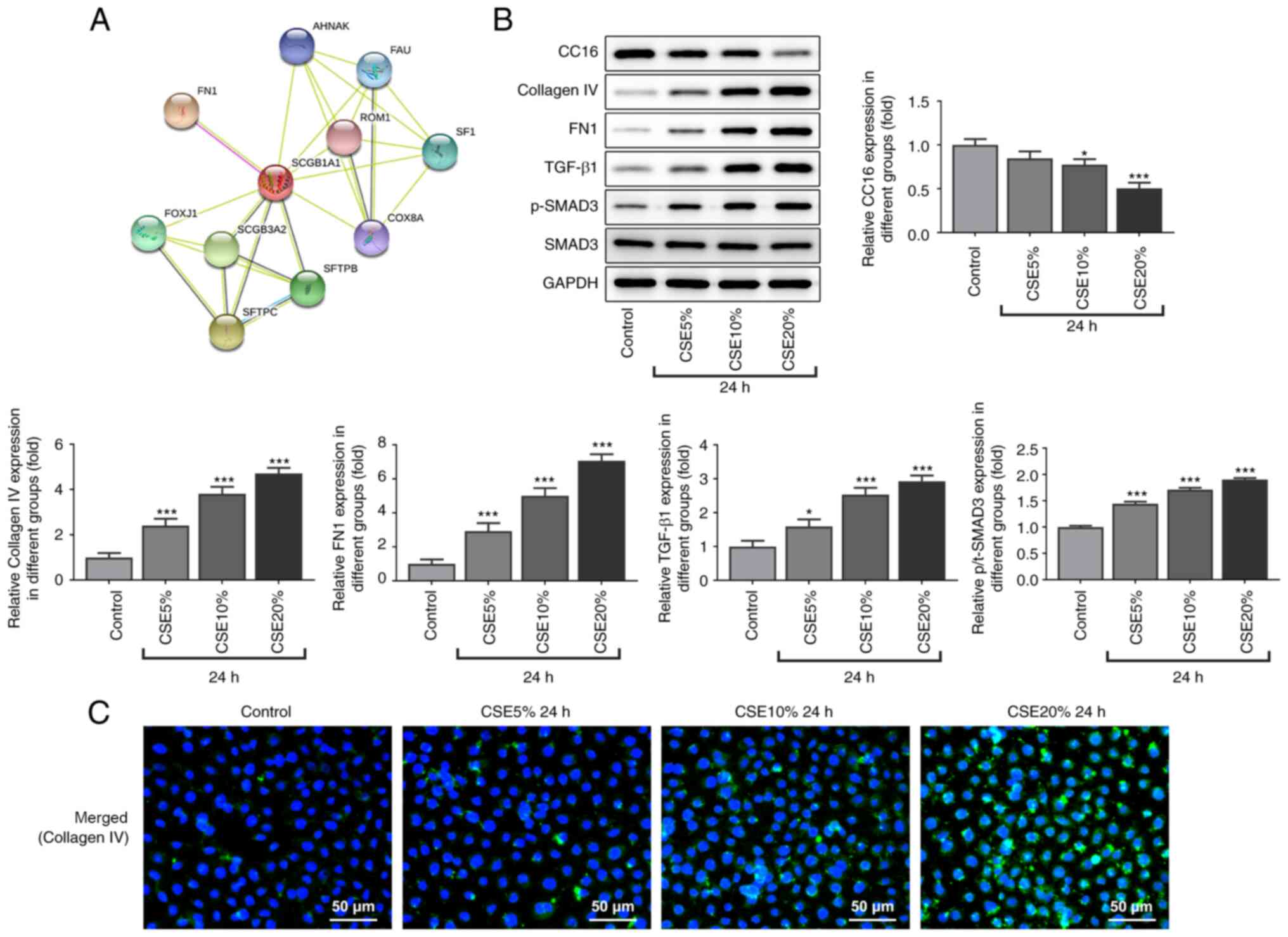 | Figure 2Effect of CSE on fibrogenesis-related
protein expression in 16-HBE cells. (A) Interactions between CC16
and FN1 were predicted using the Search Tool for the Retrieval of
Interacting Genes/Proteins (STRING) database. (B) Western blot
analysis of CC16, collagen Ⅳ, FN1, TGF-β1, p-SMAD3 and SMAD3
protein expression levels. (C) 16-HBE cells were stained for
collagen Ⅳ (green), whilst the cell nuclei were stained with DAPI
(blue). Scale bar, 50 µm. *P<0.05 and
***P<0.001 vs. Control. CSE, cigarette smoke extract;
p-, phosphorylated; CC16, club cell protein 16; FN1, fibronectin
1. |
Supplementation of vitamin D3 inhibits
EMT caused by CSE in the 16-HBE cells
Vitamin D3 treatment did not appear to cause
toxicity to 16-HBE cells treated with 20% CSE, instead increasing
cell viability (Fig. 3A). Western
blot analysis found that the protein expression level of E-cadherin
was increased, whilst the protein expression levels of N-cadherin
and slug were decreased following supplementation with vitamin D3,
compared with those in the 20% CSE treatment alone group for 24 h
(Fig. 3B and C). The results from immunofluorescence
were consistent with those in western blot analysis (Fig. 3C), suggesting that vitamin D3
supplementation can reverse EMT induced by CSE.
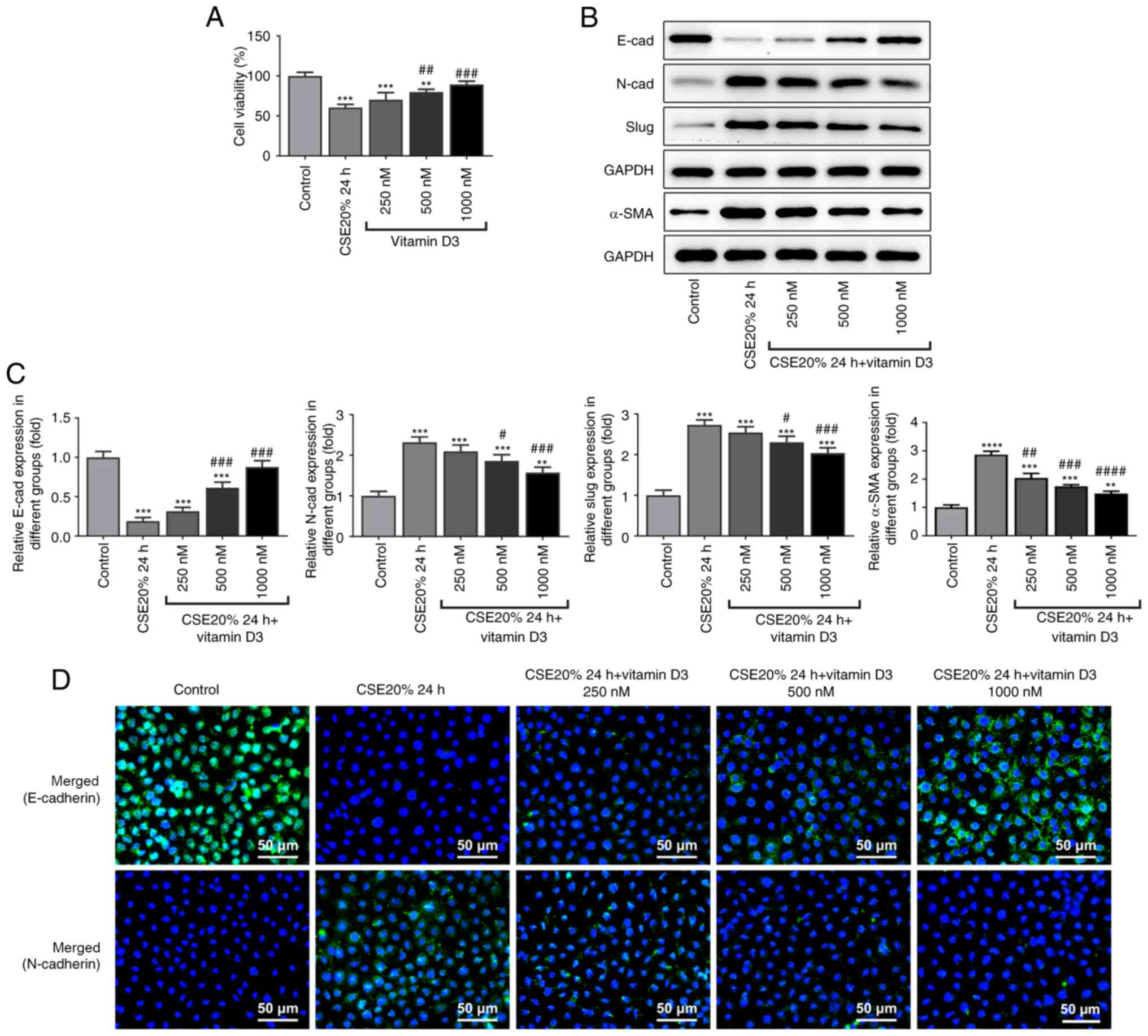 | Figure 3Effect of vitamin D3 on E-cadherin,
N-cadherin, slug and α-SMA protein expression and viability in
CSE-induced 16-HBE cells. (A) Cell viability. (B) Protein
expression levels of E-cadherin, N-cadherin, slug and α-SMA were
measured by western blotting, (C) which were quantified. (D)
Immunofluorescence staining showing E-cadherin and N-cadherin
expression. Scale bar, 50 µm. **P<0.01,
***P<0.001 and ****P<0.0001 vs.
Control. #P<0.05, ##P<0.01,
###P<0.001 and ####P<0.0001 vs. CSE 20%
24 h. CSE, cigarette smoke extract; α-SMA, α-smooth muscle
actin. |
Vitamin D3 supplementation inhibits
fibrogenesis caused by CSE in the 16-HBE cell line
Compared with that in the CSE alone group, the
protein expression level of CC16 was increased following
supplementation with vitamin D3. Western blot analysis was used to
also measure the protein expression level of fibrogenesis-related
proteins. The results showed that compared with that in the CSE
alone group, the protein expression level of fibrogenesis-related
proteins collagen IV, FN1, TGF-β1 and SMAD phosphorylation were
decreased following administration with vitamin D3 (Fig. 4A). Immunofluorescence results
showed that compared with that in the CSE alone group, the protein
expression level of collagen IV was inhibited following
supplementation with vitamin D3 (Fig.
4B). These results suggest that vitamin D3 can inhibit the
CSE-induced fibrogenesis of bronchial epithelial cells (Fig. 4B).
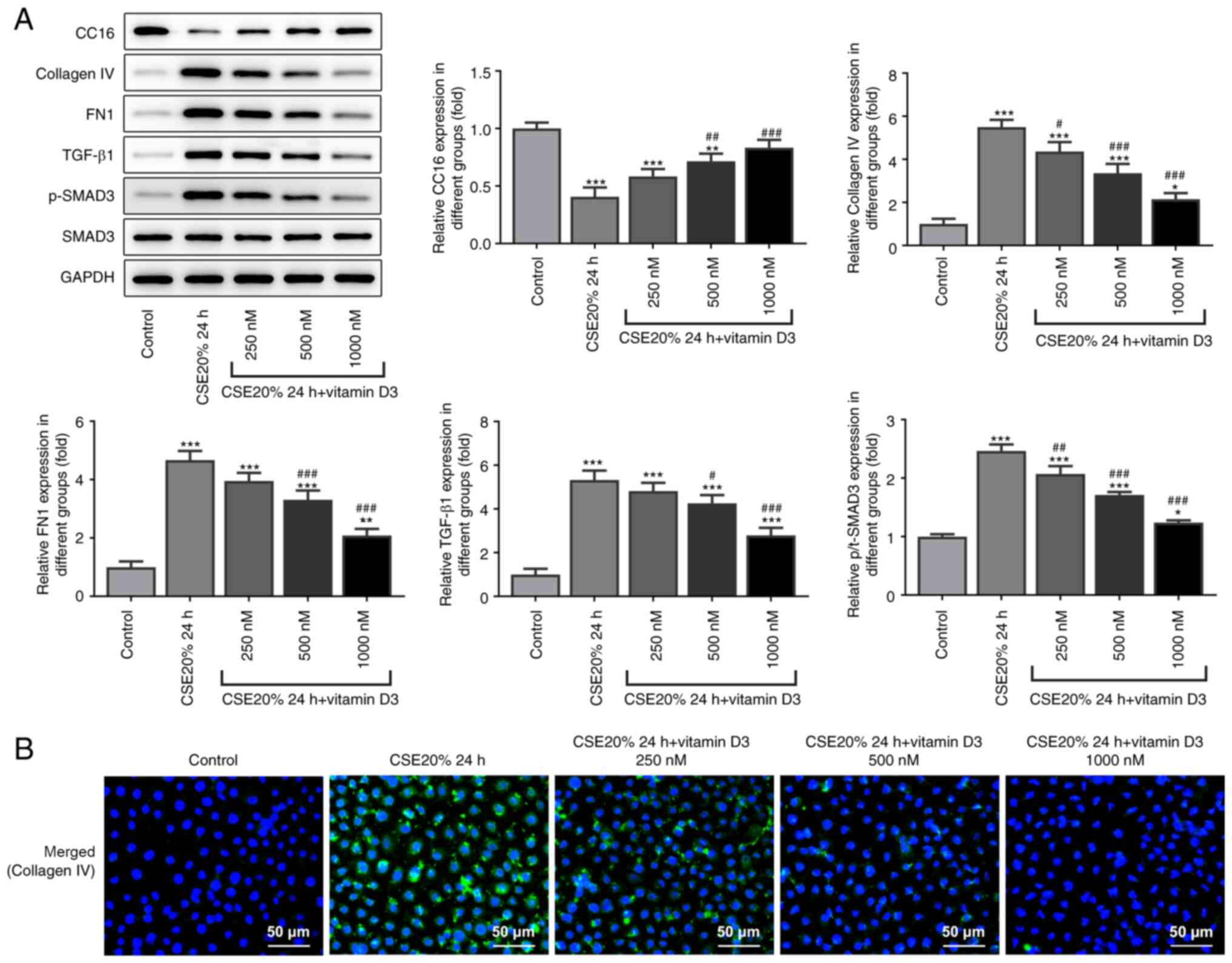 | Figure 4Effects of vitamin D3 on
fibrogenesis-related protein expression levels in CSE-induced
16-HBE cells. (A) Western blot analysis of CC16, collagen Ⅳ, FN1,
TGF-β1, p-SMAD3 and SMAD3 protein expression. (B) 16-HBE cells were
stained for collagen Ⅳ (green) whereas all cell nuclei were stained
with DAPI (blue). Scale bar, 50 µm. *P<0.05,
**P<0.01 and ***P<0.001 vs. Control
group. #P<0.05, ##P<0.01 and
###P<0.001 vs. CSE 20% 4 h. CSE, cigarette smoke
extract; p-, phosphorylated; CC16, club cell protein 16; FN1,
fibronectin 1. |
Inhibitory effects of vitamin D3 on
EMT and fibrogenesis is weakened following transfection with shRNAs
targeting CC16 in the 16-HBE cells
To assess the role of CC16 in fibrogenesis and EMT,
specific shRNAs were used to inhibit the expression level of CC16
in the 16-HBE cell line. Compared with that in the scrambled shRNA
control group, both shRNA-CC16-1 and shRNA-CC16-2 significantly
reduced CC16 expression in the 16-HBE cell line, with shRNA-CC16-1
being more potent (Fig. 5A).
According to western blot analysis, CC16 knockdown markedly reduced
the protein expression level of E-cadherin whilst promoting the
protein expression levels of N-cadherin, slug and α-SMA, compared
with those in the vitamin D3 treatment group (Fig. 5B). Subsequent western blot analysis
revealed that knocking down CC16 expression led to significant
increases in the protein expression levels of fibrogenesis-related
proteins compared with those in the vitamin D3 treatment group
(Fig. 5C). These results suggest
that after transfection with shRNA-CC16-1, the inhibitory effects
of vitamin D3 on EMT and fibrogenesis were reversed in the 16-HBE
cell line. In addition, these suppressive effects of vitamin D3 on
CSE-induced EMT and fibrogenesis was at least partially mediated by
CC16.
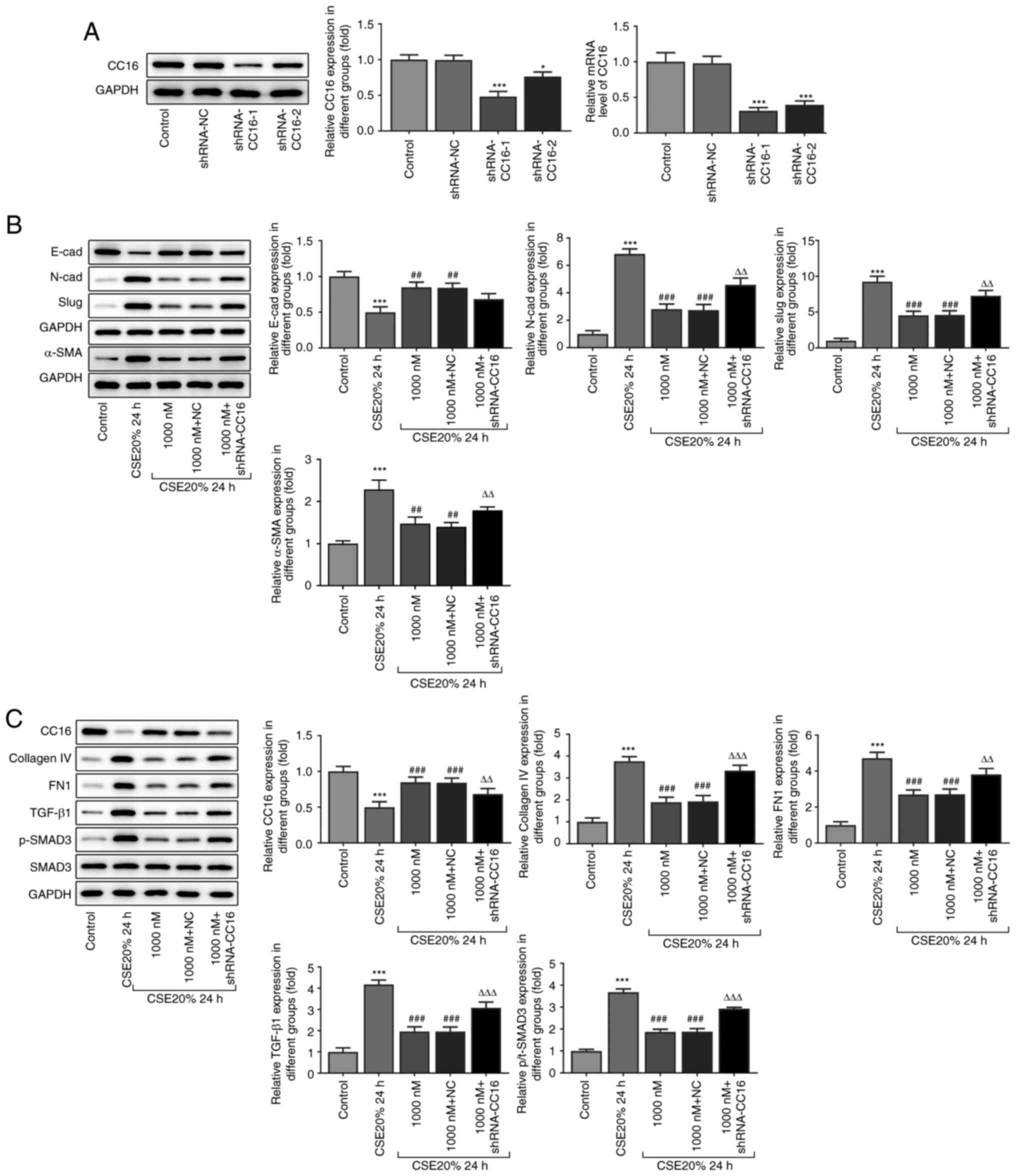 | Figure 5Effect of vitamin D3 on fibrogenesis-
and epithelial-mesenchymal transition-related protein expression in
CSE-induced 16-HBE cells after CC16 knockdown. (A) Western blotting
and reverse transcription-quantitative PCR analysis of protein and
mRNA expression levels of CC16 in 16-HBE cells following
transfection with shRNA-CC16. The protein expression levels of (B)
E-cadherin, N-cadherin, slug and α-SMA, in addition to (C) CC16,
collagen Ⅳ, FN1, TGF-β1, p-SMAD3 and SMAD3 were measured using
western blotting and quantified. *P<0.05 and
***P<0.001 vs. Control. ##P<0.01 and
###P<0.001 vs. CSE 20% 24 h. ∆∆P<0.01
and ∆∆∆P<0.001 vs. 1,000 nM + NC. shRNA, short
hairpin RNA; NC, negative control; p-, phosphorylated; CC16, club
cell protein 16; CSE, cigarette smoke extract; α-SMA, α-smooth
muscle actin; FN1, fibronectin 1. |
Discussion
COPD is a type of chronic bronchitis and/or
emphysema with the pathological characteristics of airflow
obstruction, which can further develop into common chronic diseases
such as pulmonary heart disease and respiratory failure (41). COPD is associated with aberrant
inflammatory reactions caused by harmful gases and harmful
particles (42). Among these
harmful factors, cigarette smoke considered to be one of the most
common harmful particles that can cause COPD (43). Metal substances (such as Arsenic,
cadmium and lead) and tobacco derivatives contained within
cigarette smoke increases the risk of lung adenocarcinoma, such
that ~6 million deaths are caused by tobacco use every year
worldwide (44-46).
Previous studies have shown that smoking contributes to lung
remodeling prior to COPD, where CSE induces bronchial epithelial
damage by increasing the susceptibility to respiratory infections
(47-49).
During this process, cells tend to undergo EMT and
anchor-independent growth (50).
In the present study, 16-HBE cells exposed to CSE exhibited EMT
characteristics in a dose-dependent manner, with the highest
N-cadherin, slug and α-SMA protein expression levels and the lowest
E-cadherin protein expression levels being observed following 20%
CSE exposure. Previous studies have found signs of peribronchiolar
fibrosis in the small airways of patients with COPD, which EMT
appearing to be involved in this process (47,51).
The present study observed that the expression of
fibrogenesis-related proteins collagen IV and FN1 were
significantly upregulated in 16-HBE cells after being treated with
20% CSE for 24 h. These results suggest that CSE can induce EMT and
fibrogenesis in bronchial epithelial cells.
According to analysis using the STRING database,
CC16 is associated with the fibrosis-related protein FN1. CC16 is a
major protein that is secreted by club cells in the bronchial
epithelium and is eliminated by the kidneys (30). After observing this association
between CC16 and fibrogenesis, the expression levels of CC16 was
measured after CSE exposure. Previous study reported that CC16 can
exert anti-inflammatory properties in smoke-exposed lungs of mice
and proposed that COPD may be associated with CC16 deficiency
(33). In the present study,
downregulation of CC16 expression in CSE treated 16-HBE cells was
also observed.
Over the past decade, a number of studies have shown
that vitamins serve an important role in COPD (52-54).
Vitamin A is essential for the preservation and integrity of the
lung epithelium (55). Compared
with that in the healthy group, the levels and intake of vitamin A
in patients with COPD was significantly reduced whilst serum
vitamin A levels were also found to be positively associated with
the severity of COPD (56). In a
previous clinical study that involved Korean patients with COPD,
vitamin C was found to exert protective effects against COPD
(57). In addition, another
previous study hypothesized that the protective effects of vitamin
C against COPD was due to its antioxidant effects (52). Vitamin B12, vitamin E and vitamin K
were also found to serve key roles in inhibiting the occurrence and
development of COPD (58-61).
However, accumulating evidence has found that vitamin D, which is a
class of fat-soluble vitamins that includes vitamin D3 and D2, can
exert a role in COPD (62,63). Its reported physiological effects
include regulation of calcium and phosphorus metabolism, cell
proliferation and differentiation, immune regulation and promotion
of bone growth (64). Therefore,
vitamin D has been of interest for the potential treatment and
prevention of various diseases, such as chronic kidney disease and
cystic fibrosis (65-67).
In a previous in vivo study, vitamin D3 supplementation was
found to alleviate lung injury in rats with COPD whilst also
reducing cell apoptosis in the lung tissues (68). Clinical studies have also shown
that vitamin D3 supplementation can alleviate moderate or severe
COPD to reduce the incidence of upper respiratory tract infections
(23-69).
Furthermore, CSE has been reported to inhibit vitamin D-induced VDR
translocation (25), but the
vitamin D3/VDR axis can inhibit emphysema in patients with COPD
(70). However, the specific
mechanism of the protective effects of vitamin D3 against COPD
remains unclear. Therefore, the present study investigated the
effects of vitamin D3 on CSE-induced EMT and fibrogenesis in 16-HBE
cells.
A previous study found that combining aerobic
exercise with vitamin D3 supplementation can upregulate the levels
of serum CC16 in patients with lung injury caused by smoking
(71). However, the specific
mechanism was not elucidated (71). Compared with the findings reported
by Mathyssen et al (26),
this previous study found no clear association between vitamin D3
supplementation and the degree of EMT of bronchial epithelial cells
(71). In the present study, EMT
and fibrogenesis was induced in 16-HBE cells following exposure to
CSE, which were alleviated by vitamin D3 in a dose-dependent
manner. In addition, to verify the association between Vitamin D3
and CC16 in this process, CC16 shRNA was used to knock down its
expression. The results showed that after CC16 expression was
reduced, the protective effects of vitamin D3 on 16-HBE cells were
significantly reversed, with EMT and fibrogenesis restored. This
suggests that vitamin D3 can mediate protective effects by
increasing the protein expression of CC16. This provides a novel
mechanism to further the understanding of the protective role of
vitamin D3 against COPD. However, further investigation is
required.
During EMT, the TGF-β1/SMAD signaling pathway serves
an important role (72). A
previous study has shown that smoking can increase TGF-β1
production (73). The levels of
EMT and TGF-β in the airway epithelial cells of patients with COPD
were associated with the severity of peribronchial fibrosis and
airway obstruction (74). In the
present study, upregulation of TGF-β1 expression was observed in
CSE-treated 16-HBE cells. A previous study also suggested that the
TGF-β1/SMAD2/3 pathway can be a potential therapeutic target for
EMT in malignant tumor cells derived from epithelial cells
(75). Compared with that in
heathy individuals, the expression level of TGF-β1 and its
downstream signal, SMAD2/3 was found to be significantly increased
in the airways of patients with COPD (73). The present study showed that SMAD3
was activated in 16-HBE cells exposed to CSE. After treating the
cells with vitamin D3, the expression of TGF-β1 and the activation
of SMAD3 was partially inhibited whilst the expression of CC16 was
upregulated.
Bronchial epithelial cells were used for the present
study. However, in vivo models must be applied for
verification. In addition, it is of importance to investigate
further the exact mechanistic relationship between vitamin D3 and
CC16.
In conclusion, vitamin D3 supplementation could
inhibit CSE-mediated EMT and fibrogenesis by increasing the protein
expression levels of CC16. After CC16 expression was knocked down
using shRNA, the inhibitory effects of vitamin D3 on EMT and
fibrogenesis was reversed, suggesting that vitamin D3 protected the
bronchial epithelial cells by inhibiting EMT and fibrogenesis. To
the best of our knowledge, the present study was the first to
suggest that vitamin D3 can serve a inhibitory role in EMT and
fibrogenesis by regulating CC16. This may furthering the
understanding of the mechanism underlying the effects exerted by
vitamin D3 supplementation in preventing the development of
COPD.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HF guided the project, analyzed the data and wrote
the manuscript. YM conceived the technical details and designed the
experiments. HF and YM performed the experiments. YM and HF confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regional mortality from 235 causes of death for 20
age groups in 1990 and 2010: A systematic analysis for the global
burden of disease study 2010. Lancet. 380:2095–2128.
2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pauwels RA and Rabe KF: Burden and
clinical features of chronic obstructive pulmonary disease (COPD).
Lancet. 364:613–620. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Churg A, Brauer M, del Carmen Avila-Casado
M, Fortoul TI and Wright JL: Chronic exposure to high levels of
particulate air pollution and small airway remodeling. Environ
Health Perspect. 111:714–718. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Brakema EA, Tabyshova A, Kasteleyn MJ,
Molendijk E, van der Kleij RMJJ, van Boven JFM, Emilov B,
Akmatalieva M, Mademilov M, Numans ME, et al: High COPD prevalence
at high altitude: Does household air pollution play a role? Eur
Respir J. 53(1801193)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hall R, Hall IP and Sayers I: Genetic risk
factors for the development of pulmonary disease identified by
genome-wide association. Respirology. 24:204–214. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Barrera C, Rocchi S, Degano B, Soumagne T,
Laurent L, Bellanger AP, Laplante JJ, Millon L, Dalphin JC and
Reboux G: Microbial exposure to dairy farmers' dwellings and COPD
occurrence. Int J Environ Health Res. 29:387–399. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vasques F, Camporota L and Barrett NA:
Nonantibiotic pharmacological treatment of severe chronic
obstructive pulmonary disease exacerbations. Semin Respir Crit Care
Med. 41:842–850. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim V and Criner GJ: Chronic bronchitis
and chronic obstructive pulmonary disease. Am J Respir Crit Care
Med. 187:228–237. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Matera MG, Calzetta L, Rogliani P, Cesario
A and Cazzola M: New treatments for COPD in the elderly. Curr Pharm
Des. 20:5968–5982. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Eapen MS, Sharma P, Thompson IE, Lu W,
Myers S, Hansbro PM and Sohal SS: Heparin-binding epidermal growth
factor (HB-EGF) drives EMT in patients with COPD: Implications for
disease pathogenesis and novel therapies. Lab Invest. 99:150–157.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Barnes PJ: Small airway fibrosis in COPD.
Int J Biochem Cell Biol. 116(105598)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Clapéron A, Mergey M, Ho-Bouldoires TH,
Vignjevic D, Wendum D, Chrétien Y, Merabtene F, Frazao A, Paradis
V, Housset C, et al: EGF/EGFR axis contributes to the progression
of cholangiocarcinoma through the induction of an
epithelial-mesenchymal transition. J Hepatol. 61:325–332.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Han YT, Chen XH, Gao H, Ye JL and Wang CB:
Physcion inhibits the metastatic potential of human colorectal
cancer SW620 cells in vitro by suppressing the transcription factor
SOX2. Acta Pharmacol Sin. 37:264–275. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kannan A, Krishnan A, Ali M, Subramaniam
S, Halagowder D and Sivasithamparam ND: Caveolin-1 promotes gastric
cancer progression by up-regulating epithelial to mesenchymal
transition by crosstalk of signalling mechanisms under hypoxic
condition. Eur J Cancer. 50:204–215. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hou W, Hu S, Li C, Ma H, Wang Q, Meng G,
Guo T and Zhang J: Cigarette smoke induced lung barrier
dysfunction, EMT, and tissue remodeling: A possible link between
COPD and lung cancer. Biomed Res Int. 2019(2025636)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nicholson AG, Colby TV and Wells AU:
Histopathological approach to patterns of interstitial pneumonia in
patient with connective tissue disorders. Sarcoidosis Vasc Diffuse
Lung Dis. 19:10–17. 2002.PubMed/NCBI
|
|
17
|
Wynn TA and Ramalingam TR: Mechanisms of
fibrosis: Therapeutic translation for fibrotic disease. Nat Med.
18:1028–1040. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Hogg JC, Pare PD and Hackett TL: The
contribution of small airway obstruction to the pathogenesis of
chronic obstructive pulmonary disease. Physiol Rev. 97:529–552.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kyung SY, Kim DY, Yoon JY, Son ES, Kim YJ,
Park JW and Jeong SH: Sulforaphane attenuates pulmonary fibrosis by
inhibiting the epithelial-mesenchymal transition. BMC Pharmacol
Toxicol. 19(13)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sohal SS, Mahmood MQ and Walters EH:
Clinical significance of epithelial mesenchymal transition (EMT) in
chronic obstructive pulmonary disease (COPD): Potential target for
prevention of airway fibrosis and lung cancer. Clin Transl Med.
3(33)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Islam S, Sarkar NK, Mujahid AA, Bennoor
KS, Hossain SS, Attar MM, Jahan R, Hossain MA, Chowdhury HA and Ali
L: Association of serum vitamin D (25OHD) level with acute
exacerbation of chronic obstructive pulmonary disease. Mymensingh
Med J. 28:441–448. 2019.PubMed/NCBI
|
|
22
|
Baneen U and Naseem S: Correlation of
severity of chronic obstructive pulmonary disease with serum
vitamin-D level. J Family Med Prim Care. 8:2268–2277.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Martineau AR, James WY, Hooper RL, Barnes
NC, Jolliffe DA, Greiller CL, Islam K, McLaughlin D, Bhowmik A,
Timms PM, et al: Vitamin D3 supplementation in patients with
chronic obstructive pulmonary disease (ViDiCO): A multicentre,
double-blind, randomised controlled trial. Lancet Respir Med.
3:120–130. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chaabouni M, Feki W, Chaabouni K and
Kammoun S: Vitamin D supplementation to prevent COVID-19 in
patients with COPD: A research perspective. Adv Respir Med.
88:364–365. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Uh ST, Koo SM, Kim YK, Kim KU, Park SW,
Jang AS, Kim DJ, Kim YH and Park CS: Inhibition of vitamin d
receptor translocation by cigarette smoking extracts. Tuberc Respir
Dis (Seoul). 73:258–265. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mathyssen C, Serre J, Sacreas A, Everaerts
S, Maes K, Verleden S, Verlinden L, Verstuyf A, Pilette C,
Gayan-Ramirez G, et al: Vitamin D modulates the response of
bronchial epithelial cells exposed to cigarette smoke extract.
Nutrients. 11(2138)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li SR, Tan ZX, Chen YH, Hu B, Zhang C,
Wang H, Zhao H and Xu DX: Vitamin D deficiency exacerbates
bleomycin-induced pulmonary fibrosis partially through aggravating
TGF-β/smad2/3-mediated epithelial-mesenchymal transition. Respir
Res. 20(266)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tzilas V, Bouros E, Barbayianni I,
Karampitsakos T, Kourtidou S, Ntassiou M, Ninou I, Aidinis V,
Bouros D and Tzouvelekis A: Vitamin D prevents experimental lung
fibrosis and predicts survival in patients with idiopathic
pulmonary fibrosis. Pulm Pharmacol Ther. 55:17–24. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Almuntashiri S, Zhu Y, Han Y, Wang X,
Somanath PR and Zhang D: Club cell secreted protein cc16: Potential
applications in prognosis and therapy for pulmonary diseases. J
Clin Med. 9(4039)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Egron C, Labbé A, Rochette E, Mulliez A,
Bernard A and Flore A: Urinary club cell protein 16 (CC16): Utility
of its assay during acute bronchiolitis. Pediatr Pulmonol.
55:490–495. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pang M, Liu HY, Li T, Wang D, Hu XY, Zhang
XR, Yu BF, Guo R and Wang HL: Recombinant club cell protein 16
(CC16) ameliorates cigarette smokeinduced lung inflammation in a
murine disease model of COPD. Mol Med Rep. 18:2198–2206.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhu L, Di PY, Wu R, Pinkerton KE and Chen
Y: Repression of CC16 by cigarette smoke (CS) exposure. PLoS One.
10(e0116159)2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Laucho-Contreras ME, Polverino F,
Tesfaigzi Y, Pilon A, Celli BR and Owen CA: Club cell protein 16
(CC16) augmentation: A potential disease-modifying approach for
chronic obstructive pulmonary disease (COPD). Expert Opin Ther
Targets. 20:869–883. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Laucho-Contreras ME, Polverino F, Gupta K,
Taylor KL, Kelly E, Pinto-Plata V, Divo M, Ashfaq N, Petersen H,
Stripp B, et al: Protective role for club cell secretory protein-16
(CC16) in the development of COPD. Eur Respir J. 45:1544–1556.
2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rong B, Fu T, Gao W, Li M, Rong C, Liu W
and Liu H: Reduced serum concentration of CC16 is associated with
severity of chronic obstructive pulmonary disease and contributes
to the diagnosis and assessment of the disease. Int J Chron
Obstruct Pulmon Dis. 15:461–470. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zemans RL, Jacobson S, Keene J, Kechris K,
Miller BE, Tal-Singer R and Bowler RP: Multiple biomarkers predict
disease severity, progression and mortality in COPD. Respir Res.
18(117)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Danov O, Wolff M, Bartel S, Böhlen S,
Obernolte H, Wronski S, Jonigk D, Hammer B, Kovacevic D, Reuter S,
et al: Cigarette smoke affects dendritic cell populations,
epithelial barrier function, and the immune response to viral
infection with H1N1. Front Med (Lausanne). 7(571003)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li D, Hu J, Wang T, Zhang X, Liu L, Wang
H, Wu Y, Xu D and Wen F: Silymarin attenuates cigarette smoke
extract-induced inflammation via simultaneous inhibition of
autophagy and ERK/p38 MAPK pathway in human bronchial epithelial
cells. Sci Rep. 6(37751)2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Miguel V, Ramos R, García-Bermejo L,
Rodríguez-Puyol D and Lamas S: The program of renal fibrogenesis is
controlled by microRNAs regulating oxidative metabolism. Redox
Biol. 40(101851)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
López-Campos JL, Tan W and Soriano JB:
Global burden of COPD. Respirology. 21:14–23. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Raherison C and Girodet PO: Epidemiology
of COPD. Eur Respir Rev. 18:213–221. 2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Vij N, Chandramani-Shivalingappa P, Van
Westphal C, Hole R and Bodas M: Cigarette smoke-induced autophagy
impairment accelerates lung aging, COPD-emphysema exacerbations and
pathogenesis. Am J Physiol Cell Physiol. 314:C73–C87.
2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Pinto E, Cruz M, Ramos P, Santos A and
Almeida A: Metals transfer from tobacco to cigarette smoke:
Evidences in smokers' lung tissue. J Hazard Mater. 325:31–35.
2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Paumgartten FJR, Gomes-Carneiro MR and de
Oliveira AC: The impact of tobacco additives on cigarette smoke
toxicity: A critical appraisal of tobacco industry studies. Cad
Saude Publica. 33 (Suppl 3)(e00132415)2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
D'Angelo D, Ahluwalia IB, Pun E, Yin S,
Palipudi K and Mbulo L: Current cigarette smoking, access, and
purchases from retail outlets among students aged 13-15
years-global youth tobacco survey, 45 countries, 2013 and 2014.
MMWR Morb Mortal Wkly Rep. 65:898–901. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Xu H, Ling M, Xue J, Dai X, Sun Q, Chen C,
Liu Y, Zhou L, Liu J, Luo F, et al: Exosomal microRNA-21 derived
from bronchial epithelial cells is involved in aberrant
epithelium-fibroblast cross-talk in COPD induced by cigarette
smoking. Theranostics. 8:5419–5433. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Amatngalim GD, Schrumpf JA, Dishchekenian
F, Mertens TC, Ninaber DK, van der Linden AC, Pilette C, Taube C,
Hiemstra PS, van der Does AM, et al: Aberrant epithelial
differentiation by cigarette smoke dysregulates respiratory host
defence. Eur Respir J. 51(1701009)2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Willey JC, Grafstrom RC, Moser CE Jr,
Ozanne C, Sundquvist K and Harris CC: Biochemical and morphological
effects of cigarette smoke condensate and its fractions on normal
human bronchial epithelial cells in vitro. Cancer Res.
47:2045–2049. 1987.PubMed/NCBI
|
|
50
|
Vaz M, Hwang SY, Kagiampakis I, Phallen J,
Patil A, O'Hagan HM, Murphy L, Zahnow CA, Gabrielson E, Velculescu
VE, et al: Chronic cigarette smoke-induced epigenomic changes
precede sensitization of bronchial epithelial cells to single-step
transformation by KRAS mutations. Cancer Cell. 32:360–376.
2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Milara J, Peiró T, Serrano A and Cortijo
J: Epithelial to mesenchymal transition is increased in patients
with COPD and induced by cigarette smoke. Thorax. 68:410–420.
2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Pirabbasi E, Shahar S, Manaf ZA, Rajab NF
and Manap RA: Efficacy of ascorbic acid (Vitamin C)
and/N-Acetylcysteine (NAC) supplementation on nutritional and
antioxidant status of male chronic obstructive pulmonary disease
(COPD) patients. J Nutr Sci Vitaminol (Tokyo). 62:54–61.
2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Piscaer I, Wouters EFM, Vermeer C,
Janssens W, Franssen FME and Janssen R: Vitamin K deficiency: The
linking pin between COPD and cardiovascular diseases? Respir Res.
18(189)2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhu M, Wang T, Wang C and Ji Y: The
association between vitamin D and COPD risk, severity, and
exacerbation: An updated systematic review and meta-analysis. Int J
Chron Obstruct Pulmon Dis. 11:2597–2607. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Biesalski HK and Nohr D: Importance of
vitamin-A for lung function and development. Mol Aspects Med.
24:431–440. 2003.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Caram LM, Amaral RA, Ferrari R, Tanni SE,
Correa CR, Paiva SAR and Godoy I: Serum vitamin A and inflammatory
markers in individuals with and without chronic obstructive
pulmonary disease. Mediators Inflamm. 2015(862086)2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Park HJ, Byun MK, Kim HJ, Kim JY, Kim YI,
Yoo KH, Chun EM, Jung JY, Lee SH and Ahn CM: Dietary vitamin C
intake protects against COPD: The Korea national health and
nutrition examination survey in 2012. Int J Chron Obstruct Pulmon
Dis. 11:2721–2728. 2016.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Liu JT, Luo B, He XT, Li LY and Xu SG: The
protective effects of vitamin E on lung injury caused by high
temperature and PM2.5 in COPD rats. Zhongguo Ying Yong Sheng Li Xue
Za Zhi. 35:293–296. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
59
|
Peh HY, Tan WSD, Chan TK, Pow CW, Foster
PS and Wong WSF: Vitamin E isoform gamma-tocotrienol protects
against emphysema in cigarette smoke-induced COPD. Free Radic Biol
Med. 110:332–344. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Paulin FV, Zagatto AM, Chiappa GR and
Muller PT: Addition of vitamin B12 to exercise training improves
cycle ergometer endurance in advanced COPD patients: A randomized
and controlled study. Respir Med. 122:23–29. 2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Piscaer I, van den Ouweland JMW,
Vermeersch K, Reynaert NL, Franssen FM, Keene S, Wouters EF,
Janssens W, Vermeer C and Janssen R: Low vitamin K status is
associated with increased elastin degradation in chronic
obstructive pulmonary disease. J Clin Med. 8(1116)2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Jolliffe DA, Greenberg L, Hooper RL,
Mathyssen C, Rafiq R, de Jongh RT, Camargo CA, Griffiths CJ,
Janssens W and Martineau AR: Vitamin D to prevent exacerbations of
COPD: systematic review and meta-analysis of individual participant
data from randomised controlled trials. Thorax. 74:337–345.
2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Milne S and Sin DD: Vitamin D deficiency
in COPD: Biomarker, treatable trait, or just a common comorbidity?
Chest. 157:755–756. 2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Gil Á, Plaza-Diaz J and Mesa MD: Vitamin
D: Classic and novel actions. Ann Nutr Metab. 72:87–95.
2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Chau YY and Kumar J: Vitamin D in chronic
kidney disease. Indian J Pediatr. 79:1062–1068. 2012.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Chesdachai S and Tangpricha V: Treatment
of vitamin D deficiency in cystic fibrosis. J Steroid Biochem Mol
Biol. 164:36–39. 2016.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Tripkovic L, Lambert H, Hart K, Smith CP,
Bucca G, Penson S, Chope G, Hyppönen E, Berry J, Vieth R and
Lanham-New S: Comparison of vitamin D2 and vitamin D3
supplementation in raising serum 25-hydroxyvitamin D status: A
systematic review and meta-analysis. Am J Clin Nutr. 95:1357–1364.
2012.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Chen L, Yuan X, Zou L, Peng J and Hu X:
Effects of 1,25-Dihydroxyvitamin D3 on the prevention of chronic
obstructive pulmonary disease (COPD) in rats exposed to air
pollutant particles less than 2.5 micrometers in diameter (PM2.5).
Med Sci Monit. 24:356–362. 2018.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Hyun DG, Oh YM, Lee SW, Lee SD and Lee JS:
Clinical phenotypes, comorbidities, and exacerbations according to
serum 25-OH vitamin D and plasma fibrinogen levels in chronic
obstructive pulmonary disease. J Korean Med Sci.
34(e195)2019.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Hu G, Dong T, Wang S, Jing H and Chen J:
Vitamin D3-vitamin D receptor axis suppresses pulmonary emphysema
by maintaining alveolar macrophage homeostasis and function.
EBioMedicine. 45:563–577. 2019.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Nikniaz L, Ghojazadeh M, Nateghian H,
Nikniaz Z, Farhangi MA and Pourmanaf H: The interaction effect of
aerobic exercise and vitamin D supplementation on inflammatory
factors, anti-inflammatory proteins, and lung function in male
smokers: A randomized controlled trial. BMC Sports Sci Med Rehabil.
13(102)2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Mahmood MQ, Reid D, Ward C, Muller HK,
Knight DA, Sohal SS and Walters EH: Transforming growth factor
(TGF) β1 and smad signalling pathways: A likely key to
EMT-associated COPD pathogenesis. Respirology. 22:133–140.
2017.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Wang L, Meng J, Wang C, Yang C, Wang Y and
Li Y and Li Y: Hydrogen sulfide alleviates cigarette smoke-induced
COPD through inhibition of the TGF-β1/smad pathway. Exp Biol Med
(Maywood). 245:190–200. 2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Gohy ST, Hupin C, Fregimilicka C, Detry
BR, Bouzin C, Chevronay HG, Lecocq M, Weynand B, Ladjemi MZ,
Pierreux CE, et al: Imprinting of the COPD airway epithelium for
dedifferentiation and mesenchymal transition. Eur Respir J.
45:1258–1272. 2015.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Camara J and Jarai G:
Epithelial-mesenchymal transition in primary human bronchial
epithelial cells is Smad-dependent and enhanced by fibronectin and
TNF-alpha. Fibrogenesis Tissue Repair. 3(2)2010.PubMed/NCBI View Article : Google Scholar
|



















